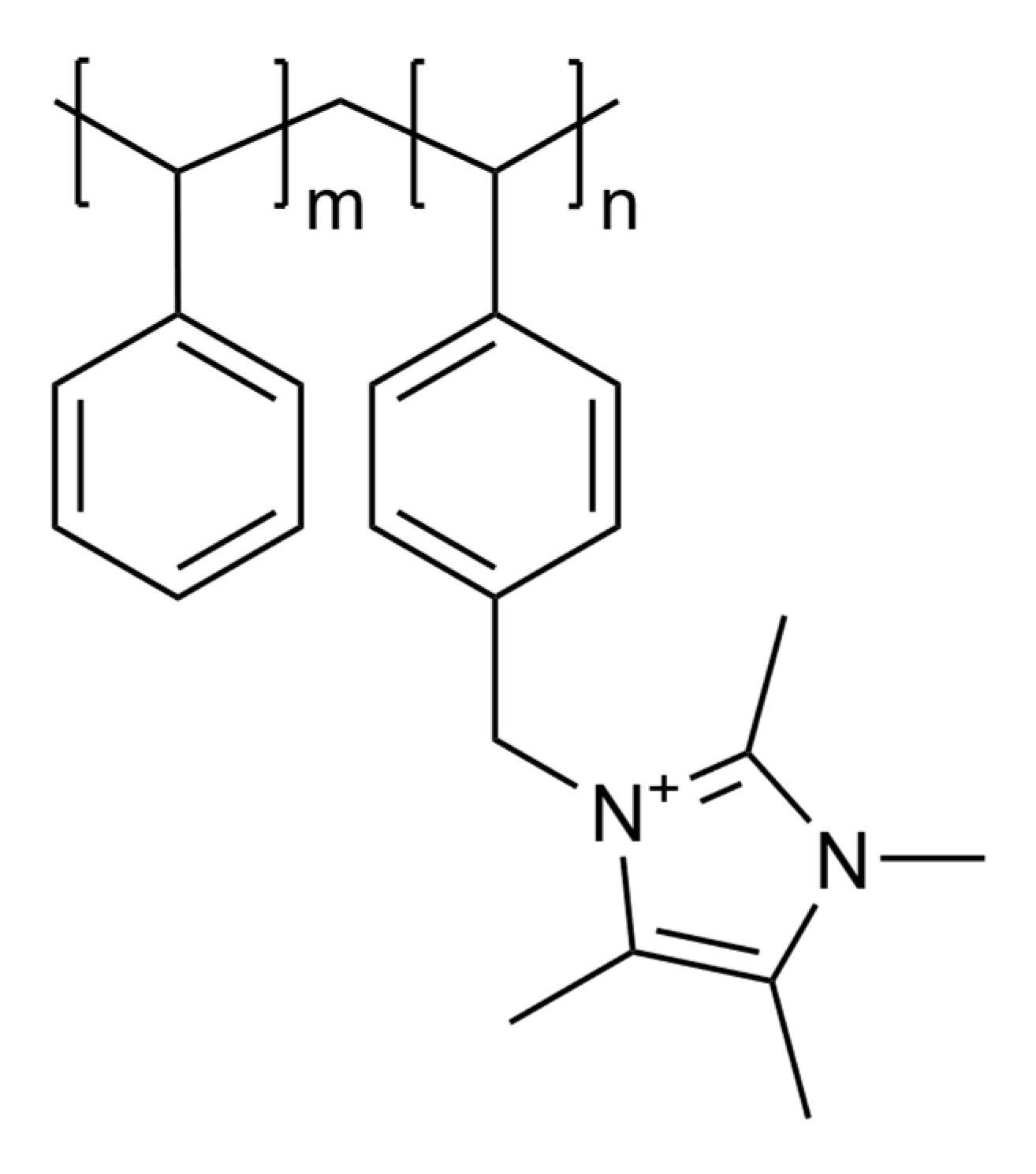
An Assessment of Anion Exchange Membranes for CO2 Capture Processes: A Focus on Fumasep® and Sustainion®
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
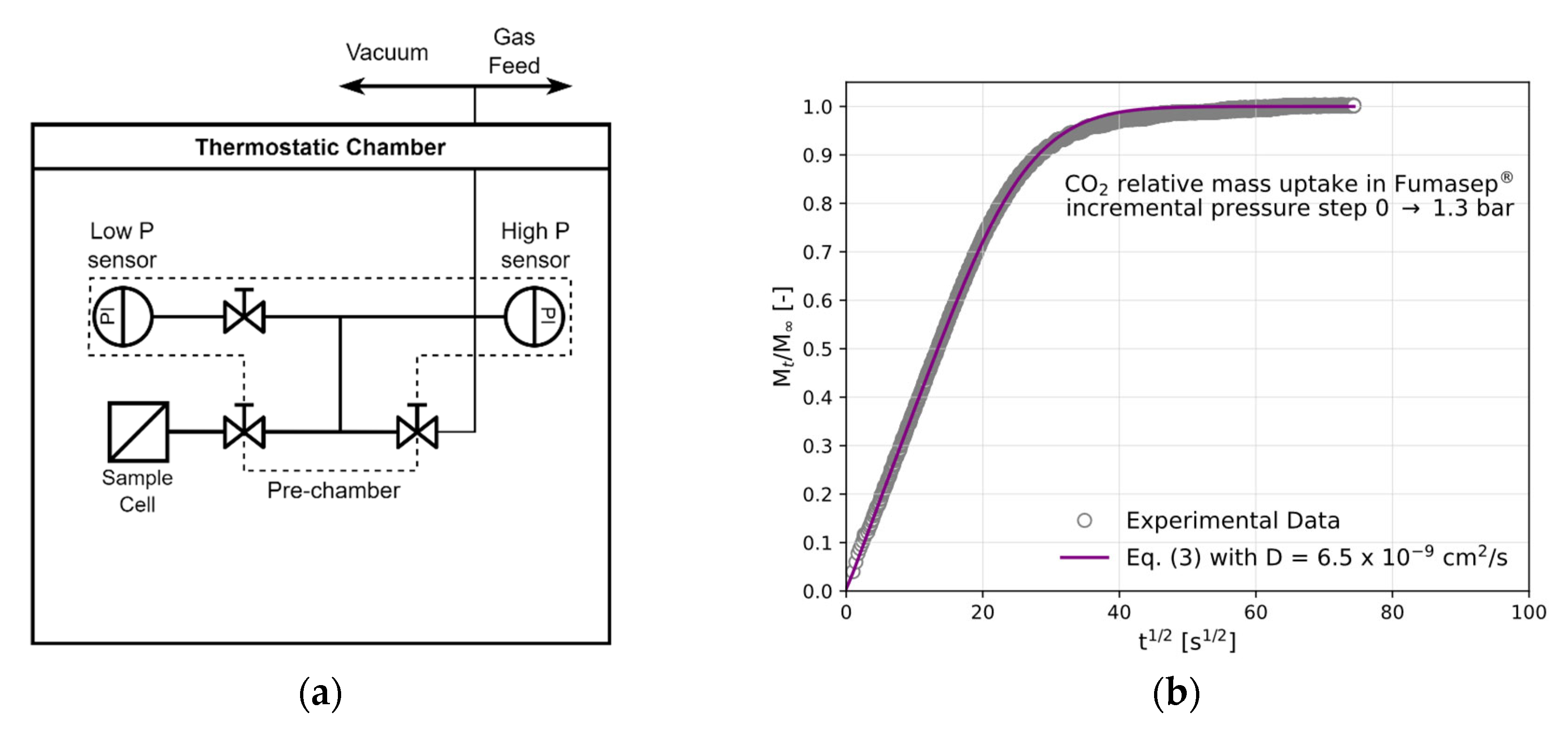
2.2. Gas Sorption
3. Results
3.1. The CO2 and CH4 Sorption Isotherms
3.2. CO2 and CH4 Diffusion
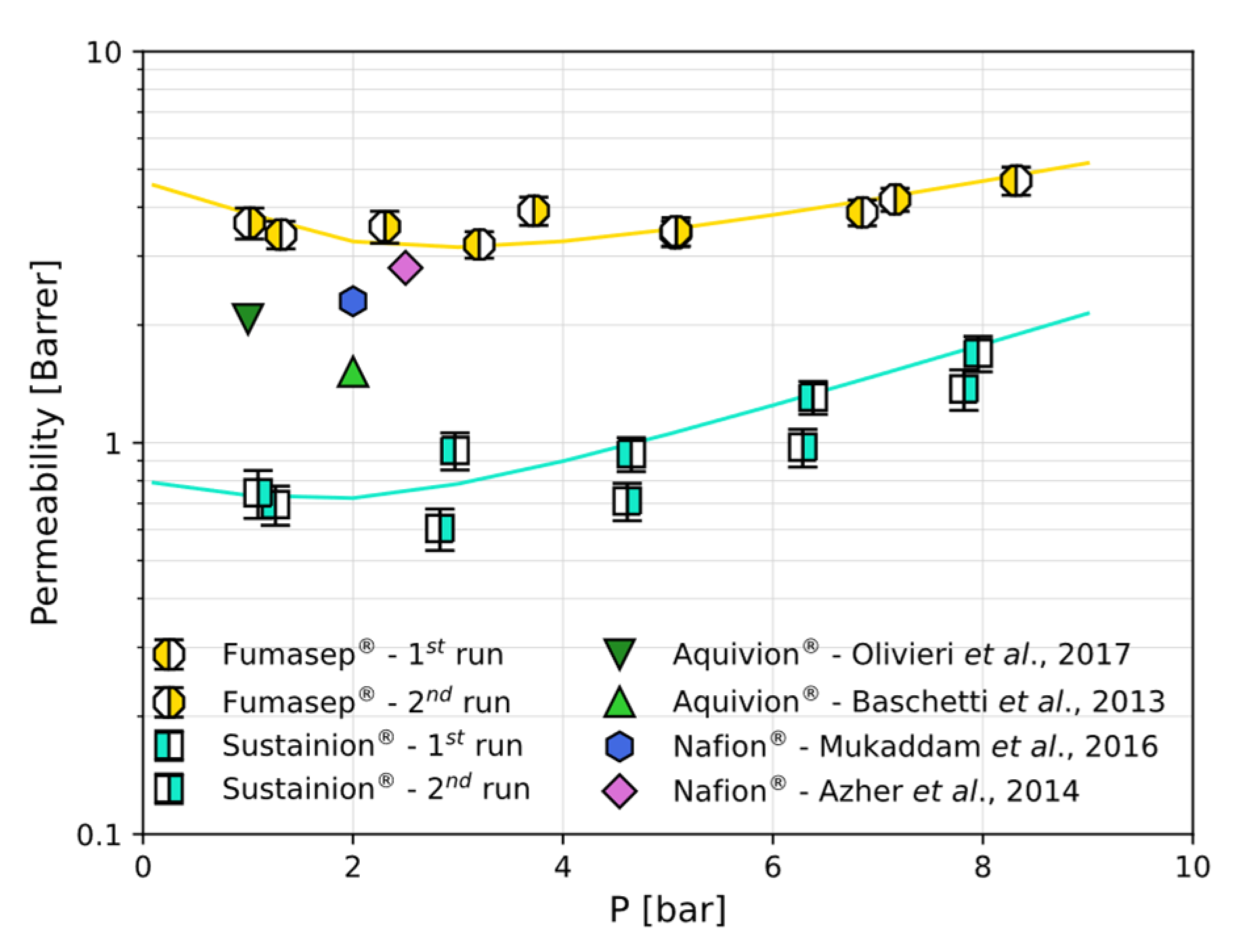
3.3. CO2 and CH4 Permeation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power Plant Post-Combustion Carbon Dioxide Capture: An Opportunity for Membranes. J. Membr. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Ferrari, M.-C.; Amelio, A.; Nardelli, G.M.; Costi, R. Assessment on the Application of Facilitated Transport Membranes in Cement Plants for CO2 Capture. Energies 2021, 14, 4772. [Google Scholar] [CrossRef]
- Fujikawa, S.; Selyanchyn, R.; Kunitake, T. A New Strategy for Membrane-Based Direct Air Capture. Polym. J. 2021, 53, 111–119. [Google Scholar] [CrossRef]
- Castel, C.; Bounaceur, R.; Favre, E. Membrane Processes for Direct Carbon Dioxide Capture From Air: Possibilities and Limitations. Front. Chem. Eng. 2021, 3, 668867. [Google Scholar] [CrossRef]
- Luis, P.; Van Gerven, T.; Van Der Bruggen, B. Recent Developments in Membrane-Based Technologies for CO2 Capture. Prog. Energy Combust. Sci. 2012, 38, 419–448. [Google Scholar] [CrossRef]
- Drioli, E.; Stankiewicz, A.I.; Macedonio, F. Membrane Engineering in Process Intensification—An Overview. J. Membr. Sci. 2011, 380, 1–8. [Google Scholar] [CrossRef]
- Wang, H.; Yan, J.; Song, W.; Jiang, C.; Wang, Y.; Xu, T. Ion Exchange Membrane Related Processes towards Carbon Capture, Utilization and Storage: Current Trends and Perspectives. Sep. Purif. Technol. 2022, 296, 121390. [Google Scholar] [CrossRef]
- Li, M.; Yang, K.; Abdinejad, M.; Zhao, C.; Burdyny, T. Advancing Integrated CO2 Electrochemical Conversion with Amine-Based CO2 Capture: A Review. Nanoscale 2022, 14, 11892–11908. [Google Scholar] [CrossRef]
- Rehberger, H.; Rezaei, M.; Aljabour, A. Challenges and Opportunities of Choosing a Membrane for Electrochemical CO2 Reduction. Membranes 2025, 15, 55. [Google Scholar] [CrossRef]
- Pimlott, D.J.D.; Kim, Y.; Berlinguette, C.P. Reactive Carbon Capture Enables CO2 Electrolysis with Liquid Feedstocks. Acc. Chem. Res. 2024, 57, 1007–1018. [Google Scholar] [CrossRef]
- Li, M.; Irtem, E.; Iglesias Van Montfort, H.-P.; Abdinejad, M.; Burdyny, T. Energy Comparison of Sequential and Integrated CO2 Capture and Electrochemical Conversion. Nat Commun 2022, 13, 5398. [Google Scholar] [CrossRef]
- Wang, T.; Lackner, K.S.; Wright, A. Moisture Swing Sorbent for Carbon Dioxide Capture from Ambient Air. Environ. Sci. Technol. 2011, 45, 6670–6675. [Google Scholar] [CrossRef]
- Prajapati, A.; Sartape, R.; Rojas, T.; Dandu, N.K.; Dhakal, P.; Thorat, A.S.; Xie, J.; Bessa, I.; Galante, M.T.; Andrade, M.H.S.; et al. Migration-Assisted, Moisture Gradient Process for Ultrafast, Continuous CO2 Capture from Dilute Sources at Ambient Conditions. Energy Environ. Sci. 2022, 15, 680–692. [Google Scholar] [CrossRef]
- Wade, J.L.; Lopez Marques, H.; Wang, W.; Flory, J.; Freeman, B. Moisture-Driven CO2 Pump for Direct Air Capture. J. Membr. Sci. 2023, 685, 121954. [Google Scholar] [CrossRef]
- Liu, S.; Hu, J.; Zhang, F.; Zhu, J.; Shi, X.; Wang, L. Robust Enhancement of Direct Air Capture of CO2 Efficiency Using Micro-Sized Anion Exchange Resin Particles. Sustainability 2024, 16, 3601. [Google Scholar] [CrossRef]
- Kaneko, Y.; Lackner, K.S. Kinetic Model for Moisture-Controlled CO2 Sorption. Phys. Chem. Chem. Phys. 2022, 24, 21061–21077. [Google Scholar] [CrossRef]
- Wang, T.; Lackner, K.S.; Wright, A.B. Moisture-Swing Sorption for Carbon Dioxide Capture from Ambient Air: A Thermodynamic Analysis. Phys. Chem. Chem. Phys. 2013, 15, 504–514. [Google Scholar] [CrossRef]
- Chakraborti, T.; Sharma, R.; Krishnamoorthy, A.N.; Chaudhari, H.; Mamtani, K.; Singh, J.K. Unravelling the Effect of Molecular Interactions on Macroscale Properties in Sustainion Anion Exchange Membrane (AEM) under Hydrated Conditions Using MD Simulations. J. Membr. Sci. 2024, 705, 122887. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, H.; Kutz, R.; Masel, R.I. CO2 Electrolysis to CO and O2 at High Selectivity, Stability and Efficiency Using Sustainion Membranes. J. Electrochem. Soc. 2018, 165, J3371–J3377. [Google Scholar] [CrossRef]
- Jang, E.-S.; Kamcev, J.; Kobayashi, K.; Yan, N.; Sujanani, R.; Talley, S.J.; Moore, R.B.; Paul, D.R.; Freeman, B.D. Effect of Water Content on Sodium Chloride Sorption in Cross-Linked Cation Exchange Membranes. Macromolecules 2019, 52, 2569–2579. [Google Scholar] [CrossRef]
- Kubannek, F.; Zhegur-Khais, A.; Li, S.; Dekel, D.R.; Krewer, U. Model-Based Insights into the Decarbonation Dynamics of Anion-Exchange Membranes. Chem. Eng. J. 2023, 459, 141534. [Google Scholar] [CrossRef]
- ASTM D1434-82(2015)e1; Standard Test Method for Determining Gas Permeability Characteristics of Plastic Film and Sheeting. F02 Committee ASTM International: West Conshohocken, PA, USA, 2015. [CrossRef]
- Ricci, E.; Minelli, M.; De Angelis, M.G. Modelling Sorption and Transport of Gases in Polymeric Membranes across Different Scales: A Review. Membranes 2022, 12, 857. [Google Scholar] [CrossRef] [PubMed]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford Science Publications; Clarendon Press: London, UK; Oxford University Press: Oxford, UK, 1975; ISBN 978-0-19-853411-2. [Google Scholar]
- Duthie, X.; Kentish, S.; Powell, C.; Nagai, K.; Qiao, G.; Stevens, G. Operating Temperature Effects on the Plasticization of Polyimide Gas Separation Membranes. J. Membr. Sci. 2007, 294, 40–49. [Google Scholar] [CrossRef]
- Minelli, M. Modeling CO2 Solubility and Transport in Poly(Ethylene Terephthalate) above and below the Glass Transition. J. Membr. Sci. 2014, 451, 305–311. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The Solution-Diffusion Model: A Review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Wijmans, J.G.H.; Baker, R.W. The Solution–Diffusion Model: A Unified Approach to Membrane Permeation. In Materials Science of Membranes for Gas and Vapor Separation; Yampolskii, Y., Pinnau, I., Freeman, B., Eds.; Wiley: Hoboken, NJ, USA, 2006; pp. 159–189. ISBN 978-0-470-85345-0. [Google Scholar]
- Shi, X.; Xiao, H.; Liao, X.; Armstrong, M.; Chen, X.; Lackner, K.S. Humidity Effect on Ion Behaviors of Moisture-Driven CO2 Sorbents. J. Chem. Phys. 2018, 149, 164708. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, H.; Kanamori, K.; Yonezu, A.; Lackner, K.S.; Chen, X. Moisture-Driven CO2 Sorbents. Joule 2020, 4, 1823–1837. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of Permeability/Selectivity Tradeoff Relations in Polymeric Gas Separation Membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Checchetto, R.; De Angelis, M.G.; Minelli, M. Exploring the Membrane-Based Separation of CO2/CO Mixtures for CO2 Capture and Utilisation Processes: Challenges and Opportunities. Sep. Purif. Technol. 2024, 346, 127401. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; te Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure: Their Numerical Estimation and Prediction from Additive Group Contributions, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 978-0-08-054819-7. [Google Scholar]
- Meares, P. The Diffusion of Gases Through Polyvinyl Acetate1. J. Am. Chem. Soc. 1954, 76, 3415–3422. [Google Scholar] [CrossRef]
- Olivieri, L.; Aboukeila, H.; Giacinti Baschetti, M.; Pizzi, D.; Merlo, L.; Sarti, G.C. Humid Permeation of CO2 and Hydrocarbons in Aquivion® Perfluorosulfonic Acid Ionomer Membranes, Experimental and Modeling. J. Membr. Sci. 2017, 542, 367–377. [Google Scholar] [CrossRef]
- Giacinti Baschetti, M.; Minelli, M.; Catalano, J.; Sarti, G.C. Gas Permeation in Perflurosulfonated Membranes: Influence of Temperature and Relative Humidity. Int. J. Hydrogen Energy 2013, 38, 11973–11982. [Google Scholar] [CrossRef]
- Mukaddam, M.; Litwiller, E.; Pinnau, I. Gas Sorption, Diffusion, and Permeation in Nafion. Macromolecules 2016, 49, 280–286. [Google Scholar] [CrossRef]
- Azher, H.; Scholes, C.A.; Stevens, G.W.; Kentish, S.E. Water Permeation and Sorption Properties of Nafion 115 at Elevated Temperatures. J. Membr. Sci. 2014, 459, 104–113. [Google Scholar] [CrossRef]
- Ma, S.; Skou, E. CO2 Permeability in Nafion® EW1100 at Elevated Temperature. Solid State Ion. 2007, 178, 615–619. [Google Scholar] [CrossRef]
- Ziv, N.; Mondal, A.N.; Weissbach, T.; Holdcroft, S.; Dekel, D.R. Effect of CO2 on the Properties of Anion Exchange Membranes for Fuel Cell Applications. J. Membr. Sci. 2019, 586, 140–150. [Google Scholar] [CrossRef]
- Wang, T.; Wang, X.; Hou, C.; Liu, J. Quaternary Functionalized Mesoporous Adsorbents for Ultra-High Kinetics of CO2 Capture from Air. Sci. Rep. 2020, 10, 21429. [Google Scholar] [CrossRef]




| Material | CO2 | CH4 | ||||
|---|---|---|---|---|---|---|
| × 102 mmol g−1 bar−1 | × 101 mmol g−1 | bar−1 | × 102 mmol g−1 bar−1 | |||
| Fumasep® | 4.84 ± 0.04 | 2.42 ± 0.18 | 0.62 ± 0.01 | 0.991 | 1.92 ± 0.24 | 0.841 |
| Sustainion® | 6.19 ± 0.24 | 2.90 ± 0.86 | 0.63 ± 0.11 | 0.969 | 3.10 ± 0.12 | 0.963 |
| Material | × 109 cm2 s−1 | mmol g−1 | |
|---|---|---|---|
| Fumasep® | 6.07 ± 0.19 | 2.34 ± 0.15 | 0.977 |
| Sustainion® | 1.22 ± 0.11 | 2.96 ± 0.31 | 0.921 |
| Material | T °C | P bar | Barrer | × 102 mmol g−1 bar−1 | × 109 cm2 s−1 | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Fumasep® | 30 | 2 | 3.43 | 9.24 | 9.55 | 19.3 * | 4.82 | 4.00 * | This work |
| Sustainion® | 30 | 2 | 0.72 | 11.1 | 2.40 | 14.4 * | 3.59 | 4.00 * | This work |
| Aquivion® | 35 | 1 | 2.07 | - | - | 23.8 | - | - | [35] |
| Nafion® | 35 | 2 | 2.3 | 1.46 | 27.0 | 27.7 | 4.58 | 6.00 | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papchenko, K.; Kentish, S.; De Angelis, M.G. An Assessment of Anion Exchange Membranes for CO2 Capture Processes: A Focus on Fumasep® and Sustainion®. Polymers 2025, 17, 1581. https://doi.org/10.3390/polym17111581
Papchenko K, Kentish S, De Angelis MG. An Assessment of Anion Exchange Membranes for CO2 Capture Processes: A Focus on Fumasep® and Sustainion®. Polymers. 2025; 17(11):1581. https://doi.org/10.3390/polym17111581
Chicago/Turabian StylePapchenko, Kseniya, Sandra Kentish, and Maria Grazia De Angelis. 2025. "An Assessment of Anion Exchange Membranes for CO2 Capture Processes: A Focus on Fumasep® and Sustainion®" Polymers 17, no. 11: 1581. https://doi.org/10.3390/polym17111581
APA StylePapchenko, K., Kentish, S., & De Angelis, M. G. (2025). An Assessment of Anion Exchange Membranes for CO2 Capture Processes: A Focus on Fumasep® and Sustainion®. Polymers, 17(11), 1581. https://doi.org/10.3390/polym17111581