Toward Versatile Transient Electronics: Electrospun Biocompatible Silk Fibroin/Carbon Quantum Dot-Based Green-Emission, Water-Soluble Piezoelectric Nanofibers
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Electrospinning of G-CQD/PVA/SF Nanofibers
2.3. Characterization of Materials
2.4. Cytocompatibility of Hybrid Nanofibers
2.5. Fabrication of PENGs
2.6. Test of Devices
2.7. Monitoring of Human Physiology
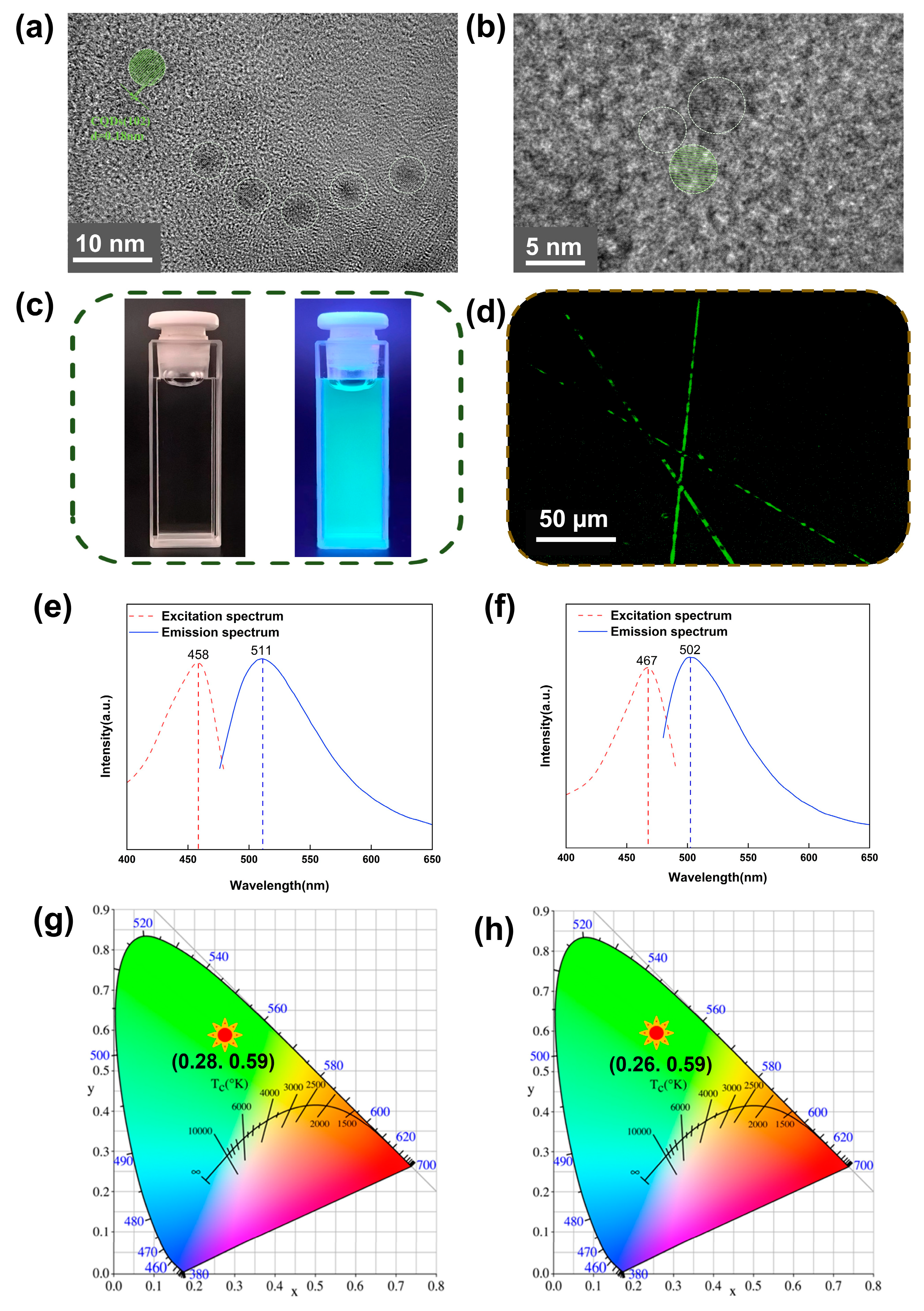
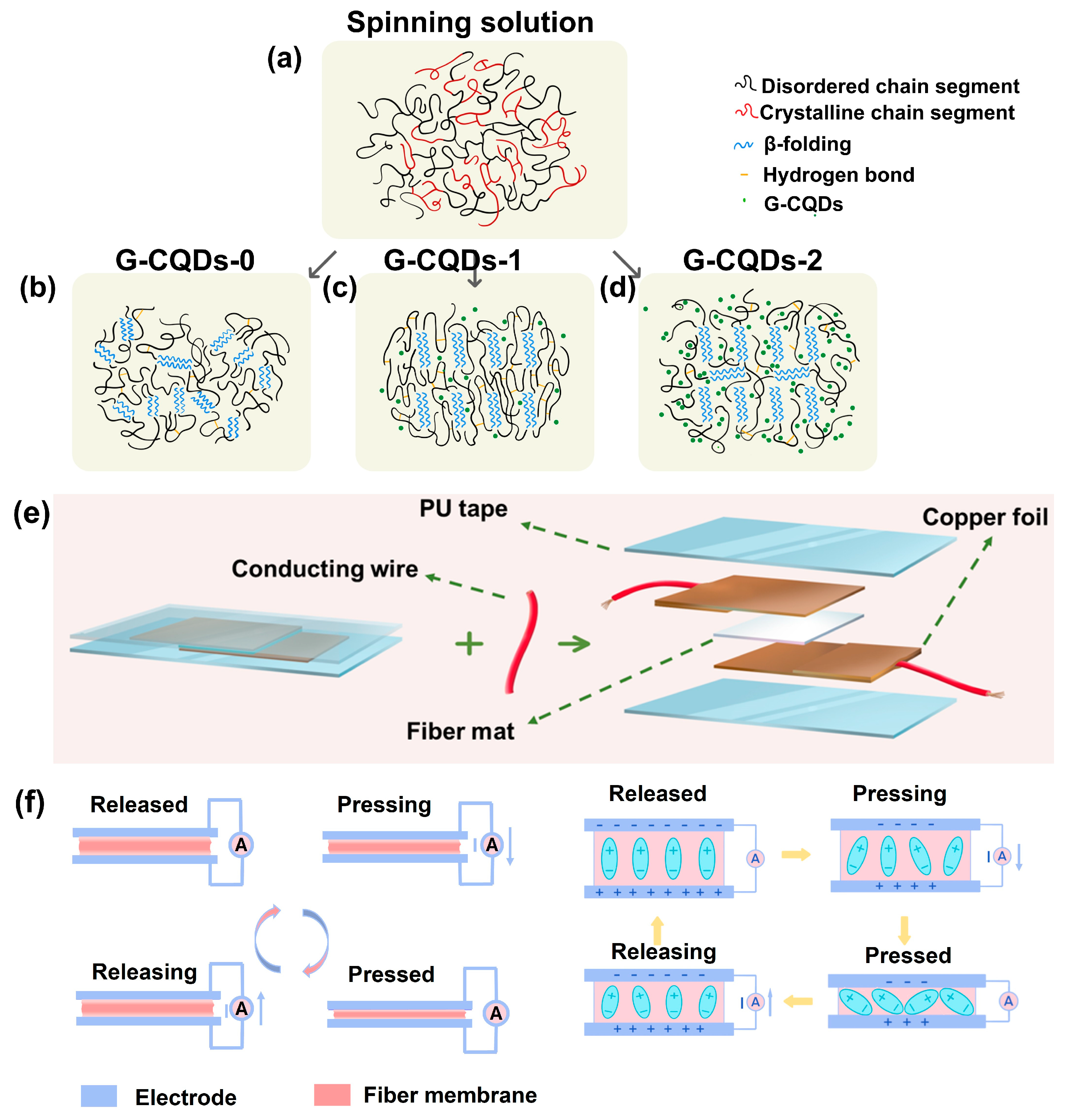
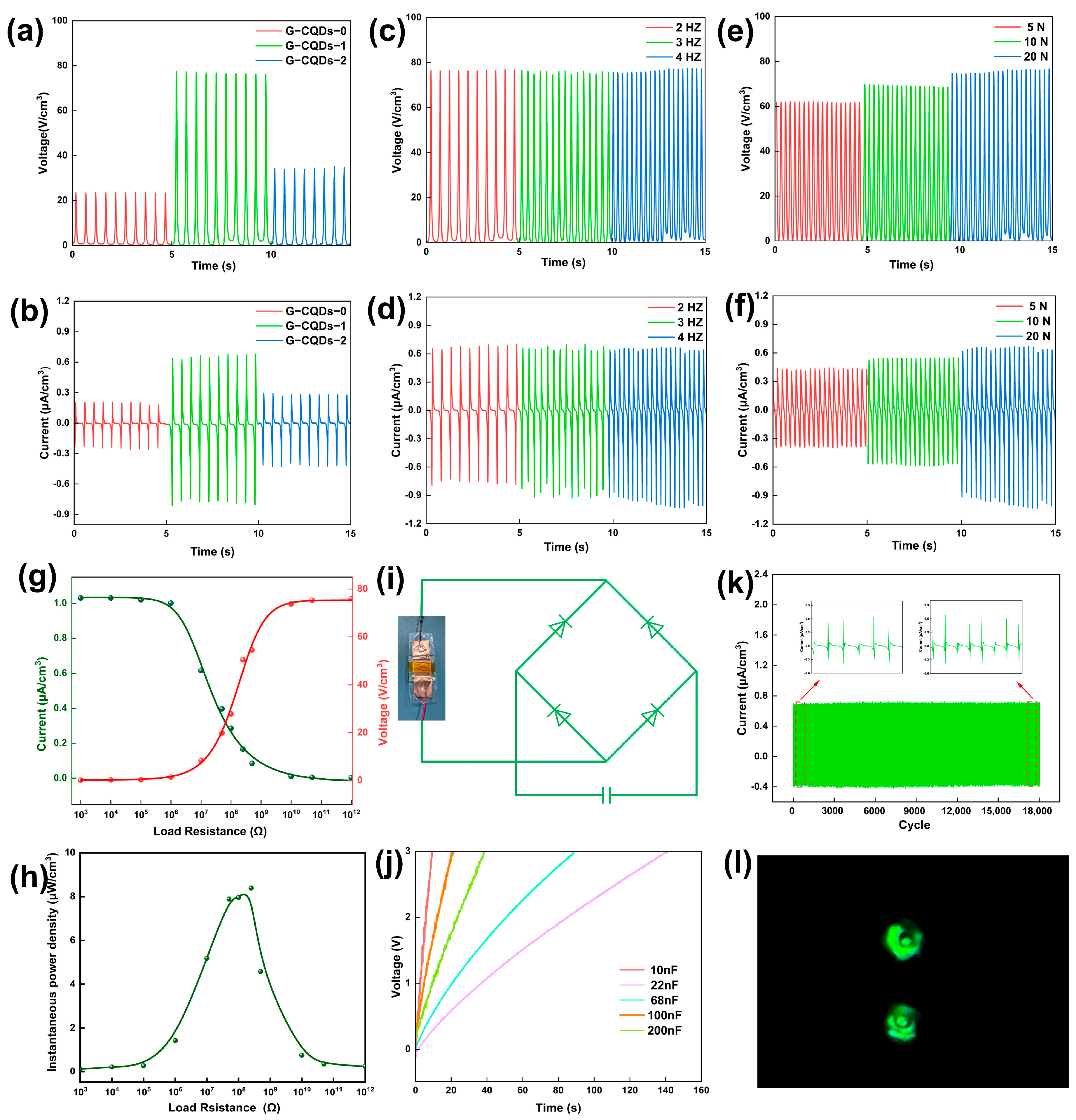
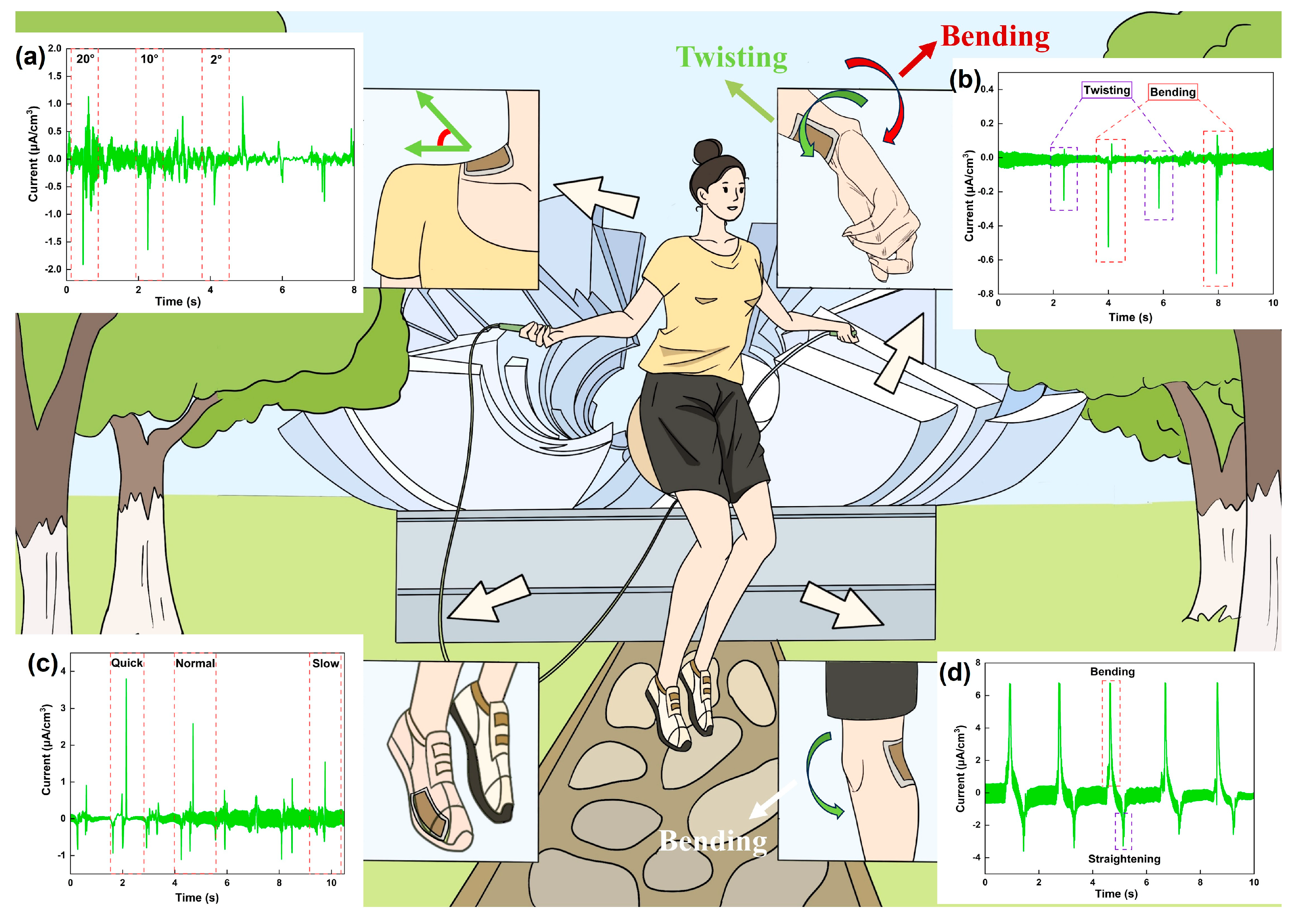
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hwang, S.-W.; Tao, H.; Kim, D.-H.; Cheng, H.; Song, J.-K.; Rill, E.; Brenckle, M.A.; Panilaitis, B.; Won, S.M.; Kim, Y.-S.; et al. A Physically Transient Form of Silicon Electronics. Science 2012, 337, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Guan, M.; Liu, J.; Lin, H.-C.; Pfattner, R.; Shaw, L.; McGuire, A.F.; Huang, T.-C.; Shao, L.; Cheng, K.-T.; et al. Biocompatible and totally disintegrable semiconducting polymer for ultrathin and ultralightweight transient electronics. Proc. Natl. Acad. Sci. USA 2017, 114, 5107–5112. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-K.; Yin, L.; Bettinger, C. The emergence of transient electronic devices. MRS Bull. 2020, 45, 87–95. [Google Scholar] [CrossRef]
- Luo, Y.; Abidian, M.R.; Ahn, J.-H.; Akinwande, D.; Andrews, A.M.; Antonietti, M.; Bao, Z.; Berggren, M.; Berkey, C.A.; Bettinger, C.J.; et al. Technology Roadmap for Flexible Sensors. ACS Nano 2023, 17, 5211–5295. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, H.; Liu, Z.; Li, Z.; Tian, J.; Shi, B.; Zou, Y.; Ouyang, H.; Zhao, C.; Zhao, L.; et al. Fully bioabsorbable natural-materials-based triboelectric nanogenerators. Adv. Mater. 2018, 30, 1801895. [Google Scholar] [CrossRef]
- Jian, M.; Zhang, Y.; Liu, Z. Natural Biopolymers for Flexible Sensing and Energy Devices. Chin. J. Polym. Sci. 2020, 38, 459–490. [Google Scholar] [CrossRef]
- Yang, F.; Li, J.; Long, Y.; Zhang, Z.; Wang, L.; Sui, J.; Dong, Y.; Wang, Y.; Taylor, R.; Ni, D.; et al. Wafer-scale heterostructured piezoelectric bio-organic thin films. Science 2021, 373, 337–342. [Google Scholar] [CrossRef]
- Wang, C.; Lu, L.; Li, W.; Shao, D.; Zhang, C.; Lu, J.; Yang, W. Green-in-green biohybrids as transient biotriboelectric nanogenerators. iScience 2022, 25, 105494. [Google Scholar] [CrossRef]
- Lu, L.; Wang, C.; Liu, Z.; Lai, Y.; Li, W.; Shao, D.; Lu, J.; Yang, W. Natural bioproducts’ hybridization creates transient dynamic electret nanogenerators. J. Mater. Chem. C 2023, 11, 11034–11045. [Google Scholar] [CrossRef]
- Wang, C.; Lu, L.; Liu, Z.; Lai, Y.; Li, J.; Xia, Z.; Cao, J.; Wang, X.; Lu, J.; Yang, W. Green Carbon Dots Illuminate Biogenic Nanohybrids toward Soft, Piezo/Photoactive, and Physically Transient Nanogenerators. ACS Sustain. Chem. Eng. 2023, 11, 14646–14658. [Google Scholar] [CrossRef]
- Liu, Z.; Lai, Y.; Li, J.; Xia, Z.; Lu, L.; Wang, C.; Huang, B.; Pan, C.; Wen, J.; Yang, W.; et al. Natural biopolymer/CuInS2 quantum dot-based red emissive, physically transient, and dynamically self-polarized piezoelectrets. Compos. Commun. 2024, 48, 101919. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, X.; Fu, Y.; Jin, Y.; Weng, Y.; Bian, X.; Chen, X. Degradation Behaviors of Polylactic Acid, Polyglycolic Acid, and Their Copolymer Films in Simulated Marine Environments. Polymers 2024, 16, 1765. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Hu, C.; Liang, A.; Liu, X.; Fang, M.; Yang, S.; Zhang, Y.; Li, M. Preparation and Properties of Antibacterial Silk Fibroin Scaffolds. Polymers 2023, 15, 4581. [Google Scholar] [CrossRef]
- Zou, Y.; Guo, W.; Lu, X.; Sun, Z.; Li, L. A novel water-induced two-way shape memory polymer based on poly (L-lactic acid)/silk fibroin composites. Compos. Commun. 2024, 46, 101822. [Google Scholar] [CrossRef]
- Du, S.; Tan, Y.; Chen, J.; Wei, Y.; Qu, Z.; Li, J.; Zhang, J.; Zhou, W. Robust and washable silk fiber-based electrochemical biosensor for high-performance sensing of hydrogen peroxide. Compos. Commun. 2024, 52, 102122. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, H.; Leow, W.R.; Cai, Y.; Loh, X.J.; Han, M.; Chen, X. Silk Fibroin for Flexible Electronic Devices. Adv. Mater. 2015, 28, 4250–4265. [Google Scholar] [CrossRef]
- DeBari, M.K.; Abbott, R.D. Microscopic considerations for optimizing silk biomaterials. WIREs Nanomed. Nanobiotechnol. 2018, 11, e1534. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xia, K.; Zhang, Y.; Kaplan, D.L. Silk-Based Advanced Materials for Soft Electronics. Acc. Chem. Res. 2019, 52, 2916–2927. [Google Scholar] [CrossRef]
- Wang, R.; Sui, J.; Wang, X. Natural Piezoelectric Biomaterials: A Biocompatible and Sustainable Building Block for Biomedical Devices. ACS Nano 2022, 16, 17708–17728. [Google Scholar] [CrossRef]
- Yucel, T.; Cebe, P.; Kaplan, D.L. Structural Origins of Silk Piezoelectricity. Adv. Funct. Mater. 2011, 21, 779–785. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, B.; Liu, X.; Shi, L.; Zhu, J.; Wei, D.; Zhong, J.; Sun, G.; He, D. Facile method to prepare silk fibroin/hyaluronic acid films for vascular endothelial growth factor release. Carbohydr. Polym. 2016, 143, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-J.; Shi, H.-H.; Zheng, L.; Zhang, H.; Cha, Y.-Y.; Ruan, H.-X.; Zhang, Y.; Zhang, X.-C. A new cancellous bone material of silk fibroin/cellulose dual network composite aerogel reinforced by nano-hydroxyapatite filler. Int. J. Biol. Macromol. 2021, 182, 286–297. [Google Scholar] [CrossRef]
- He, Z.; Zhao, T.; Zhou, X.; Liu, Z.; Huang, H. Sequential Order of the Secondary Structure Transitions of Proteins under External Perturbations: Regenerated Silk Fibroin under Thermal Treatment. Anal. Chem. 2017, 89, 5534–5541. [Google Scholar] [CrossRef] [PubMed]
- Rani, U.A.; Ng, L.Y.; Ng, C.Y.; Mahmoudi, E. A review of carbon quantum dots and their applications in wastewater treatment. Adv. Colloid Interface Sci. 2020, 278, 102124. [Google Scholar] [CrossRef]
- Guan, X.; Li, Z.; Geng, X.; Lei, Z.; Karakoti, A.; Wu, T.; Kumar, P.; Yi, J.; Vinu, A. Emerging Trends of Carbon-Based Quantum Dots: Nanoarchitectonics and Applications. Small 2023, 19, e2207181. [Google Scholar] [CrossRef]
- Ma, S.; Jin, L.; Huang, X.; Riziotis, C.; Huang, R.; Zhang, C.; Lu, J.; Yang, W. Nanogenerators Begin to Light Up: A Novel Poling-Free Piezoelectric System with Multicolor Photoluminescence as an Efficient Mechatronics Development Platform. Adv. Mater. Interfaces 2018, 5, 1800587. [Google Scholar] [CrossRef]
- He, X.; Wang, C.; Huang, X.; Jin, L.; Chu, X.; Xie, M.; Nie, Y.; Xu, Y.; Peng, Z.; Zhang, C.; et al. Carbon Nanolights in Piezopolymers are Self-Organizing Toward Color Tunable Luminous Hybrids for Kinetic Energy Harvesting. Small 2020, 16, e1905703. [Google Scholar] [CrossRef]
- Huang, X.; Jin, L.; Wang, C.; Xu, Y.; Peng, Z.; Xie, M.; Zhang, C.; Yang, W.; Lu, J. Electrospun luminescent piezo webs as self-powered sensing platform for small accelerations at low frequency. Compos. Commun. 2020, 20, 100348. [Google Scholar] [CrossRef]
- Yang, R.; Qin, Y.; Li, C.; Dai, L.; Wang, Z.L. Characteristics of output voltage and current of integrated nanogenerators. Appl. Phys. Lett. 2009, 94, 022905. [Google Scholar] [CrossRef]
- Curry, E.J.; Le, T.T.; Das, R.; Ke, K.; Santorella, E.M.; Paul, D.; Chorsi, M.T.; Tran, K.T.M.; Baroody, J.; Borges, E.R.; et al. Biodegradable nanofiber-based piezoelectric transducer. Proc. Natl. Acad. Sci. USA 2020, 117, 214–220. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, T.; Yao, H.; Yang, F. PIXE analysis of silk. J. Appl. Polym. Sci. 2015, 66, 405–408. [Google Scholar] [CrossRef]
- Sun, X.; Liang, H.; Wang, H.; Meng, N.; Jin, S.; Zhou, N. Silk fibroin/polyvinyl alcohol composite film loaded with antibacterial AgNP/polydopamine-modified montmorillonite; characterization and antibacterial properties. Int. J. Biol. Macromol. 2023, 251, 126368. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S. Water-Soluble Fluorescent Carbon Quantum Dots and Photocatalyst Design. Angew. Chem. Int. Ed. Engl. 2010, 49, 4430–4434. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Pang, H.; Yang, H.B.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew. Chem. Int. Ed. 2013, 52, 7800–7804. [Google Scholar] [CrossRef]
- Xu, Z.-Q.; Yang, L.-Y.; Fan, X.-Y.; Jin, J.-C.; Mei, J.; Peng, W.; Jiang, F.-L.; Xiao, Q.; Liu, Y. Low temperature synthesis of highly stable phosphate functionalized two color carbon nanodots and their application in cell imaging. Carbon 2014, 66, 351–360. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Shayannia, M.; Sajjadi, H.; Motaghitalab, V.; Haghi, A. Effect of multi wall carbon nanotubes on characteristics and morphology of nanofiber scaffolds composited of MWNTs/silk fibroin. Adv. Powder Technol. 2017, 28, 775–784. [Google Scholar] [CrossRef]
- Veronica, A.; Liu, S.; Yang, Z.; Nyein, H.Y.Y.; Hsing, I. Enhancing Piezoelectricity of Silk Fibroin Through In Situ Growth of Metal-Free Perovskite for Organic and Eco-friendly Wearable Bioelectronics. Adv. Mater. Technol. 2023, 9, 2301320. [Google Scholar] [CrossRef]
- Adalı, T.; Uncu, M. Silk fibroin as a non-thrombogenic biomaterial. Int. J. Biol. Macromol. 2016, 90, 11–19. [Google Scholar] [CrossRef]
- Wang, J.; Liang, J.; Sun, L.; Gao, S. PVA/CS and PVA/CS/Fe gel beads’ synthesis mechanism and their performance in cultivating anaerobic granular sludge. Chemosphere 2019, 219, 130–139. [Google Scholar] [CrossRef]
- Nolze, G.; Hielscher, R. Orientations—Perfectly colored. J. Appl. Crystallogr. 2016, 49, 1786–1802. [Google Scholar] [CrossRef]
- Su, C.; Shi, G.; Li, X.; Zhang, X.; Müller, A.J.; Wang, D.; Liu, G. Uniaxial and Mixed Orientations of Poly(ethylene oxide) in Nanoporous Alumina Studied by X-ray Pole Figure Analysis. Macromolecules 2018, 51, 9484–9493. [Google Scholar] [CrossRef]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.-R.; Kim, Y.-K.; Kim, M.-H.; Min, D.-H. Behaviors of NIH-3T3 Fibroblasts on Graphene/Carbon Nanotubes: Proliferation, Focal Adhesion, and Gene Transfection Studies. ACS Nano 2010, 4, 6587–6598. [Google Scholar] [CrossRef]
- Di Fiore, P.P.; Pierce, J.H.; Fleming, T.P.; Hazan, R.; Ullrich, A.; King, C.; Schlessinger, J.; Aaronson, S.A. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell 1987, 51, 1063–1070. [Google Scholar] [CrossRef]
- Li, W.; Wang, C.; Shao, D.; Lu, L.; Cao, J.; Wang, X.; Lu, J.; Yang, W. Red carbon dot directed biocrystalline alignment for piezoelectric energy harvesting. Nanoscale 2022, 14, 9031–9044. [Google Scholar] [CrossRef]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W.; et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Shao, Z.; Vollrath, F. Surprising strength of silkworm silk. Nature 2002, 418, 741. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Yucel, T.; Lovett, M.L.; Kaplan, D.L. Silk-based biomaterials for sustained drug delivery. J. Control. Release 2014, 190, 381–397. [Google Scholar] [CrossRef]
- Maiti, S.; Karan, S.K.; Lee, J.; Mishra, A.K.; Khatua, B.B.; Kim, J.K. Bio-waste onion skin as an innovative nature-driven piezoelectric material with high energy conversion efficiency. Nano Energy 2017, 42, 282–293. [Google Scholar] [CrossRef]
- Alluri, N.R.; Raj, N.P.M.J.; Khandelwal, G.; Vivekananthan, V.; Kim, S.-J. Aloe vera: A tropical desert plant to harness the mechanical energy by triboelectric and piezoelectric approaches. Nano Energy 2020, 73, 104767. [Google Scholar] [CrossRef]
- Lee, J.-H.; Heo, K.; Schulz-Schönhagen, K.; Desai, M.S.; Jin, H.-E.; Lee, S.-W. Diphenylalanine Peptide Nanotube Energy Harvesters. ACS Nano 2018, 12, 8138–8144. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Lu, Y.; Tao, X.; Chen, P.; Zhang, Y.; Ren, B.; Xie, F.; Yu, X.; Zhou, X.; Yang, D.; et al. Superelastic wood-based nanogenerators magnifying the piezoelectric effect for sustainable energy conversion. Carbon Energy 2024, 6, e561. [Google Scholar] [CrossRef]
- Maity, K.; Mondal, A.; Saha, M.C. Cellulose Nanocrystal-Based All-3D-Printed Pyro-Piezoelectric Nanogenerator for Hybrid Energy Harvesting and Self-Powered Cardiorespiratory Monitoring toward the Human–Machine Interface. ACS Appl. Mater. Interfaces 2023, 15, 13956–13970. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, K.; Kelly, S.; Nguyen, V.; Wu, Y.; Yang, R. Piezoelectric diphenylalanine peptide for greatly improved flexible nanogenerators. Nano Energy 2018, 51, 317–323. [Google Scholar] [CrossRef]
- Song, X.; Zou, H.; Cao, S.; Jiang, B.; Li, M.; Huang, L.; Zhang, Y.; Yuan, Q. Flexible regenerated cellulose/ZnO based piezoelectric composites fabricated via an efficient one-pot method to load high-volume ZnO with assistance of crosslinking. Chem. Eng. J. 2023, 475, 146184. [Google Scholar] [CrossRef]
- Zhang, G.; Liao, Q.; Ma, M.; Gao, F.; Zhang, Z.; Kang, Z.; Zhang, Y. Uniformly assembled vanadium doped ZnO microflowers/bacterial cellulose hybrid paper for flexible piezoelectric nanogenerators and self-powered sensors. Nano Energy 2018, 52, 501–509. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, X.; Li, D.; Xiao, L.; Chen, J.; Zhou, L.; Chen, J.; Yuan, Q. Large enhancement on performance of flexible cellulose-based piezoelectric composite film by welding CNF and MXene via growing ZnO to construct a “brick-rebar-mortar” structure. Adv. Funct. Mater. 2024, 34, 2408588. [Google Scholar] [CrossRef]
- Kang, D.; Pikhitsa, P.V.; Choi, Y.W.; Lee, C.; Shin, S.S.; Piao, L.; Park, B.; Suh, K.Y.; Kim, T.I.; Choi, M. Ultrasensitive mechanical crack-based sensor inspired by the spider sensory system. Nature 2014, 516, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Gao, W.; Wang, Z.L. The Triboelectric Nanogenerator as an Innovative Technology toward Intelligent Sports. Adv. Mater. 2021, 33, 2004178. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Z.; Liu, C.; Li, J.; Huang, B.; Pan, C.; Lai, Y.; Liu, Z.; Wu, D.; Liang, S.; Wang, X.; et al. Toward Versatile Transient Electronics: Electrospun Biocompatible Silk Fibroin/Carbon Quantum Dot-Based Green-Emission, Water-Soluble Piezoelectric Nanofibers. Polymers 2025, 17, 1579. https://doi.org/10.3390/polym17111579
Xia Z, Liu C, Li J, Huang B, Pan C, Lai Y, Liu Z, Wu D, Liang S, Wang X, et al. Toward Versatile Transient Electronics: Electrospun Biocompatible Silk Fibroin/Carbon Quantum Dot-Based Green-Emission, Water-Soluble Piezoelectric Nanofibers. Polymers. 2025; 17(11):1579. https://doi.org/10.3390/polym17111579
Chicago/Turabian StyleXia, Zhipei, Chubao Liu, Juan Li, Biyao Huang, Chu Pan, Yu Lai, Zhu Liu, Dongling Wu, Sen Liang, Xuanlun Wang, and et al. 2025. "Toward Versatile Transient Electronics: Electrospun Biocompatible Silk Fibroin/Carbon Quantum Dot-Based Green-Emission, Water-Soluble Piezoelectric Nanofibers" Polymers 17, no. 11: 1579. https://doi.org/10.3390/polym17111579
APA StyleXia, Z., Liu, C., Li, J., Huang, B., Pan, C., Lai, Y., Liu, Z., Wu, D., Liang, S., Wang, X., Yang, W., & Lu, J. (2025). Toward Versatile Transient Electronics: Electrospun Biocompatible Silk Fibroin/Carbon Quantum Dot-Based Green-Emission, Water-Soluble Piezoelectric Nanofibers. Polymers, 17(11), 1579. https://doi.org/10.3390/polym17111579






