Design and Preparation of Inherently Photostable Poly(Butylene Adipate-Co-Terephthalate) by Chemically Bonding UV-Stabilizing Moieties in Molecular Chains
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
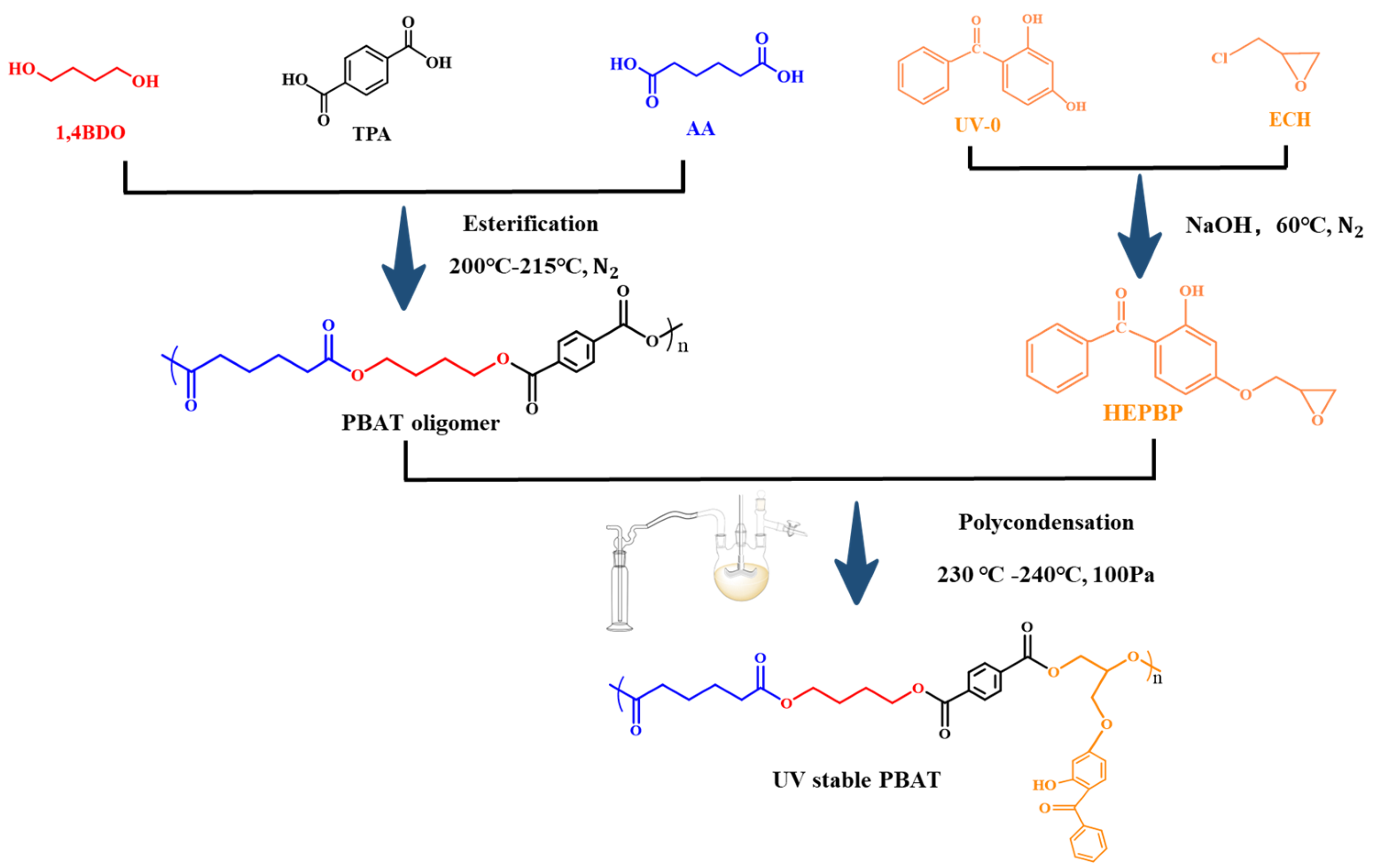
2.2. Synthesis of Reactive Light Stabilizer
2.3. Synthesis of UV-Stable PBAT
2.4. Preparation of PBAT + UV-0 Blends
2.5. Characterization
2.6. Accelerated Aging Test
2.7. Solvent Extraction Test
2.8. In Situ FTIR Accelerated Aging Evaluation
3. Analysis and Discussion
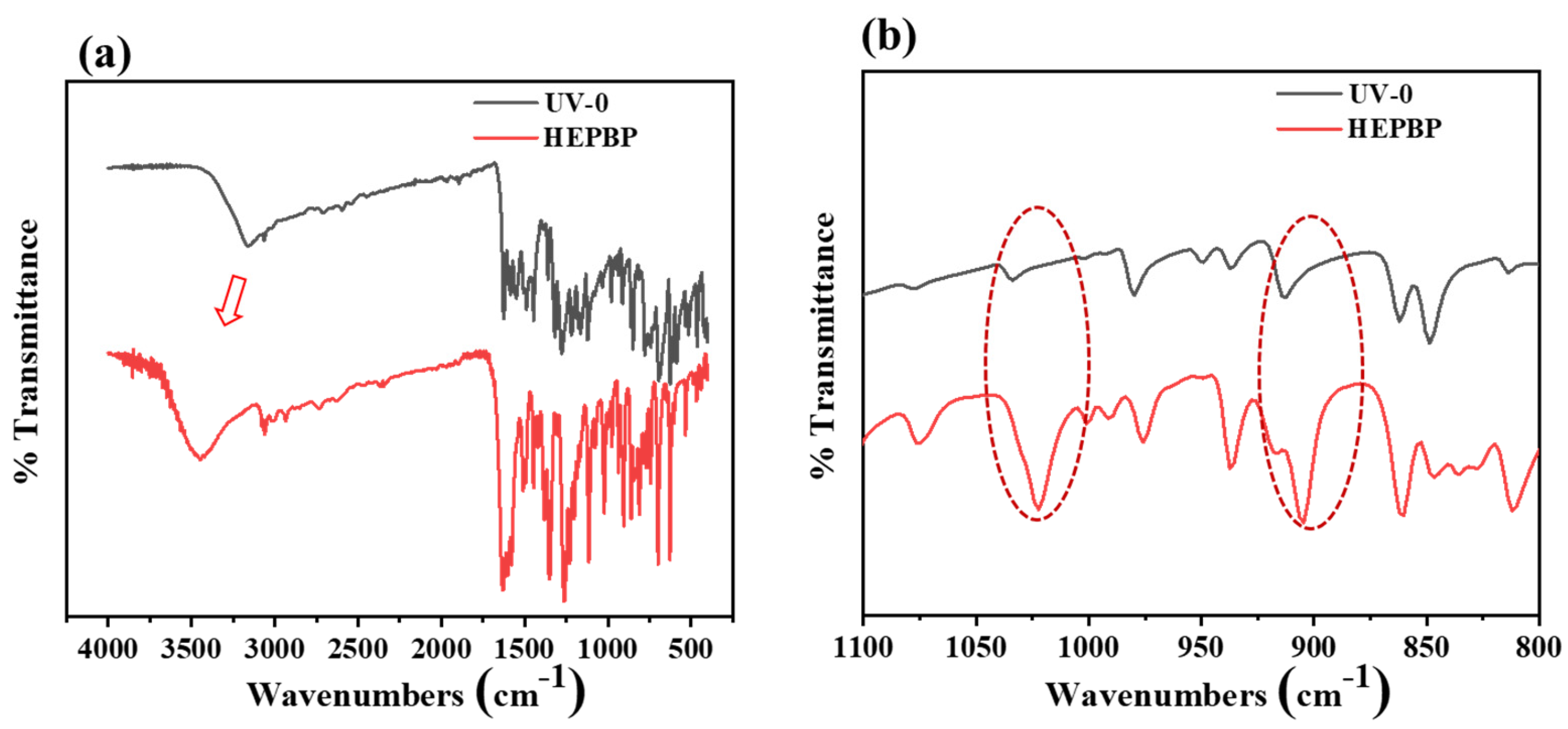
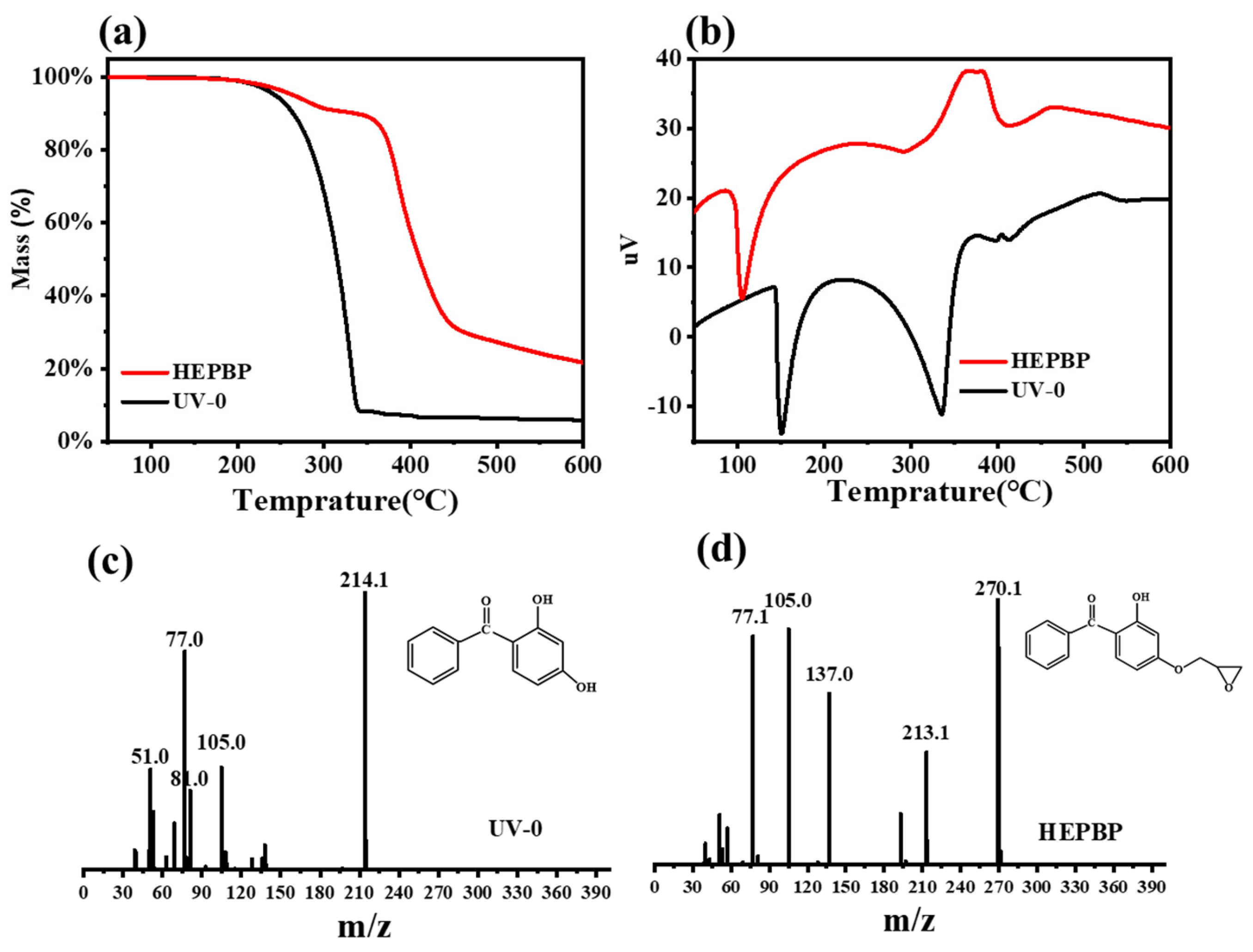
3.1. Synthesis and Characterization of HEPBP UV Absorber
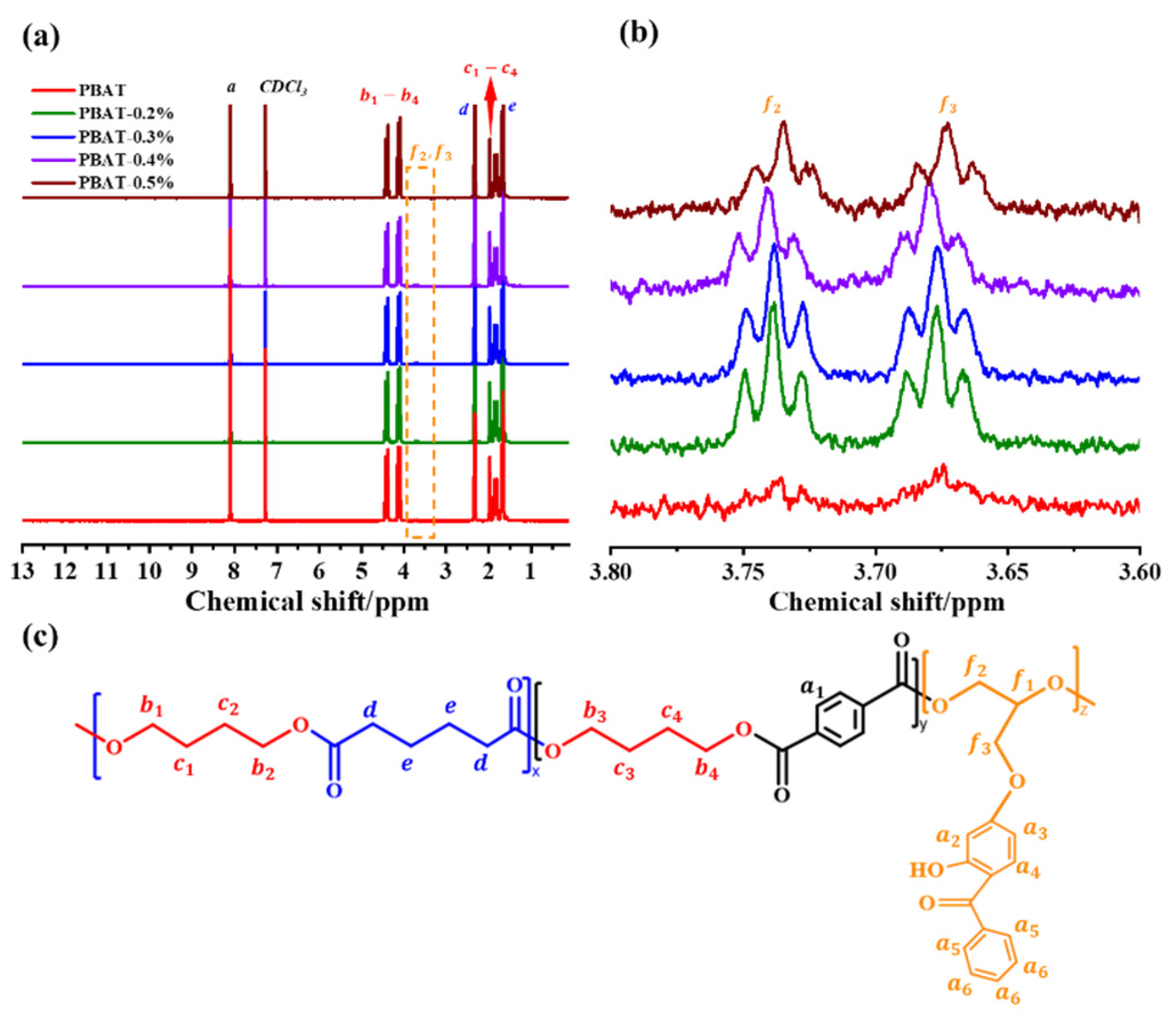
3.2. Molecular Chemical Structure of UV-Stable PBAT
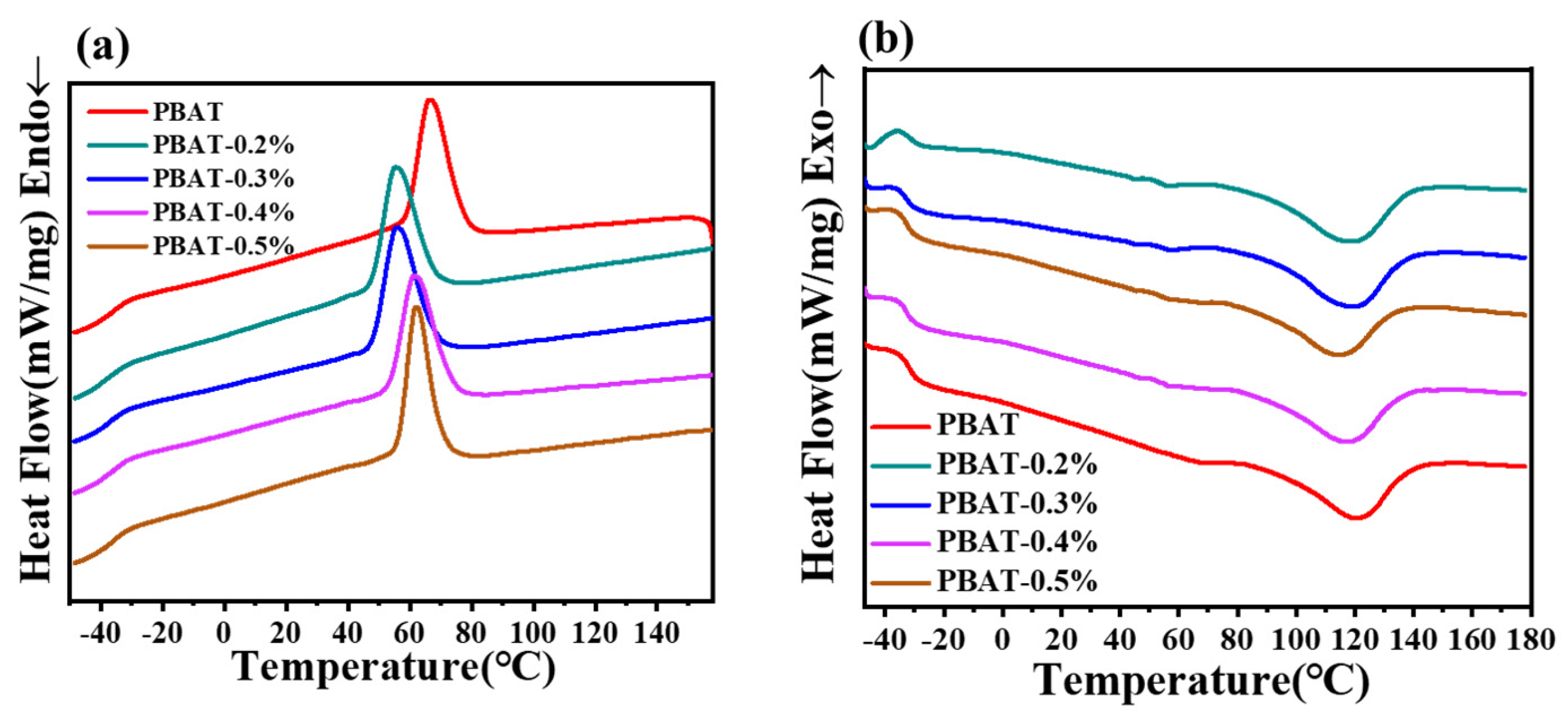
3.3. Thermal and Crystallization Properties
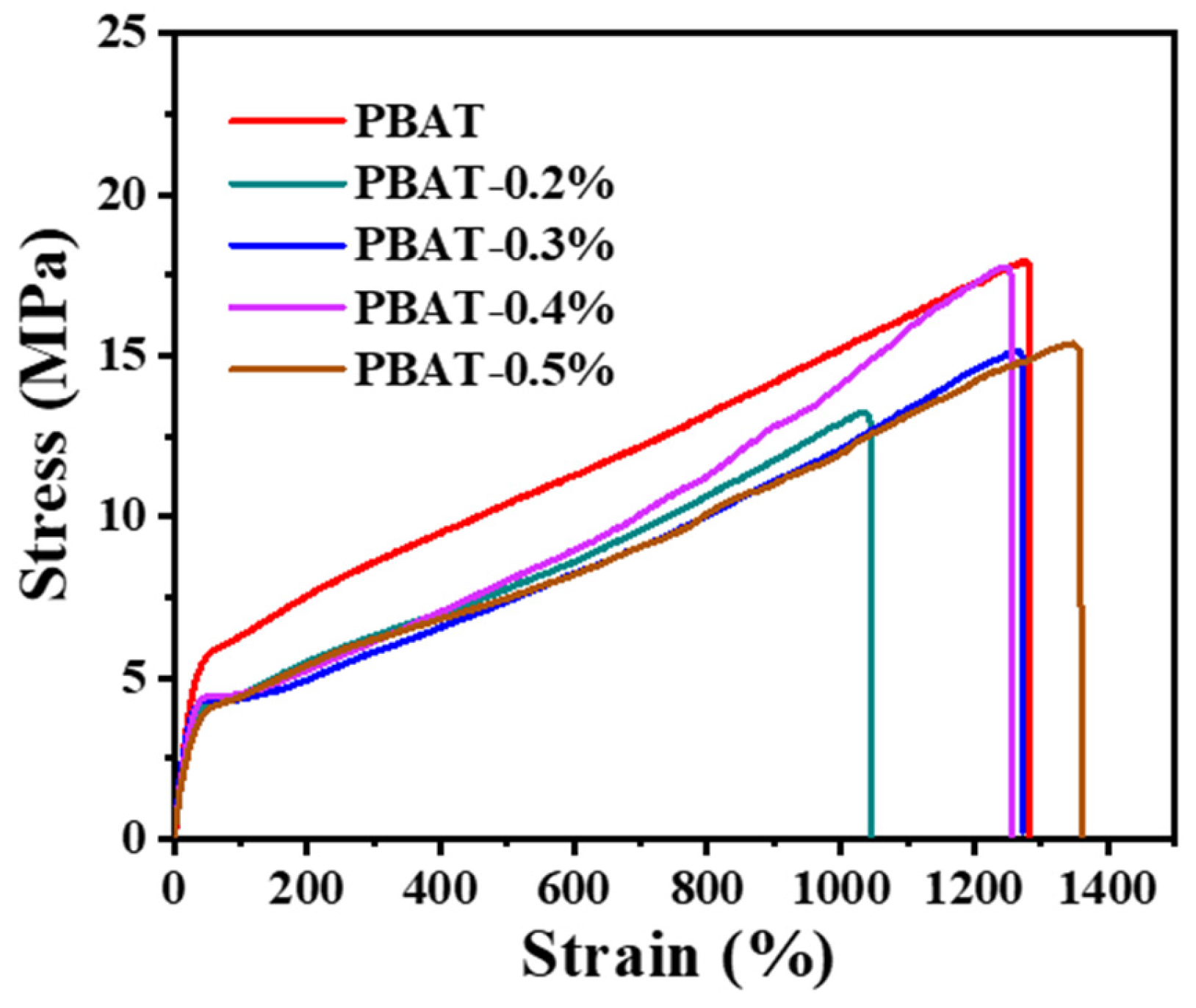
3.4. Mechanical Property
3.5. Rheological Properties
3.6. Enzymatic Hydrolysis
3.7. In Situ FTIR Accelerated Aging Evaluation
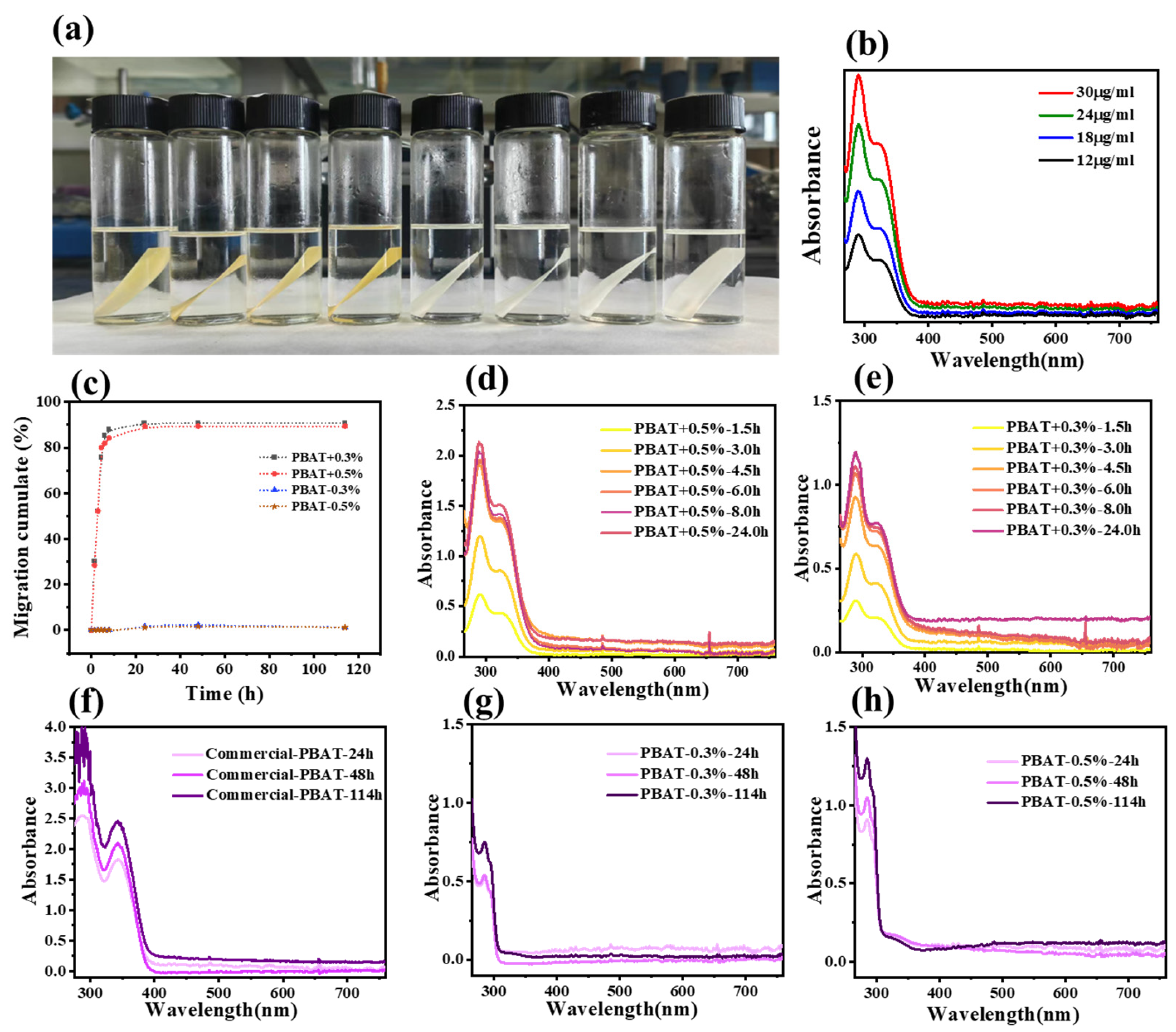
3.8. Solvent Extraction Test
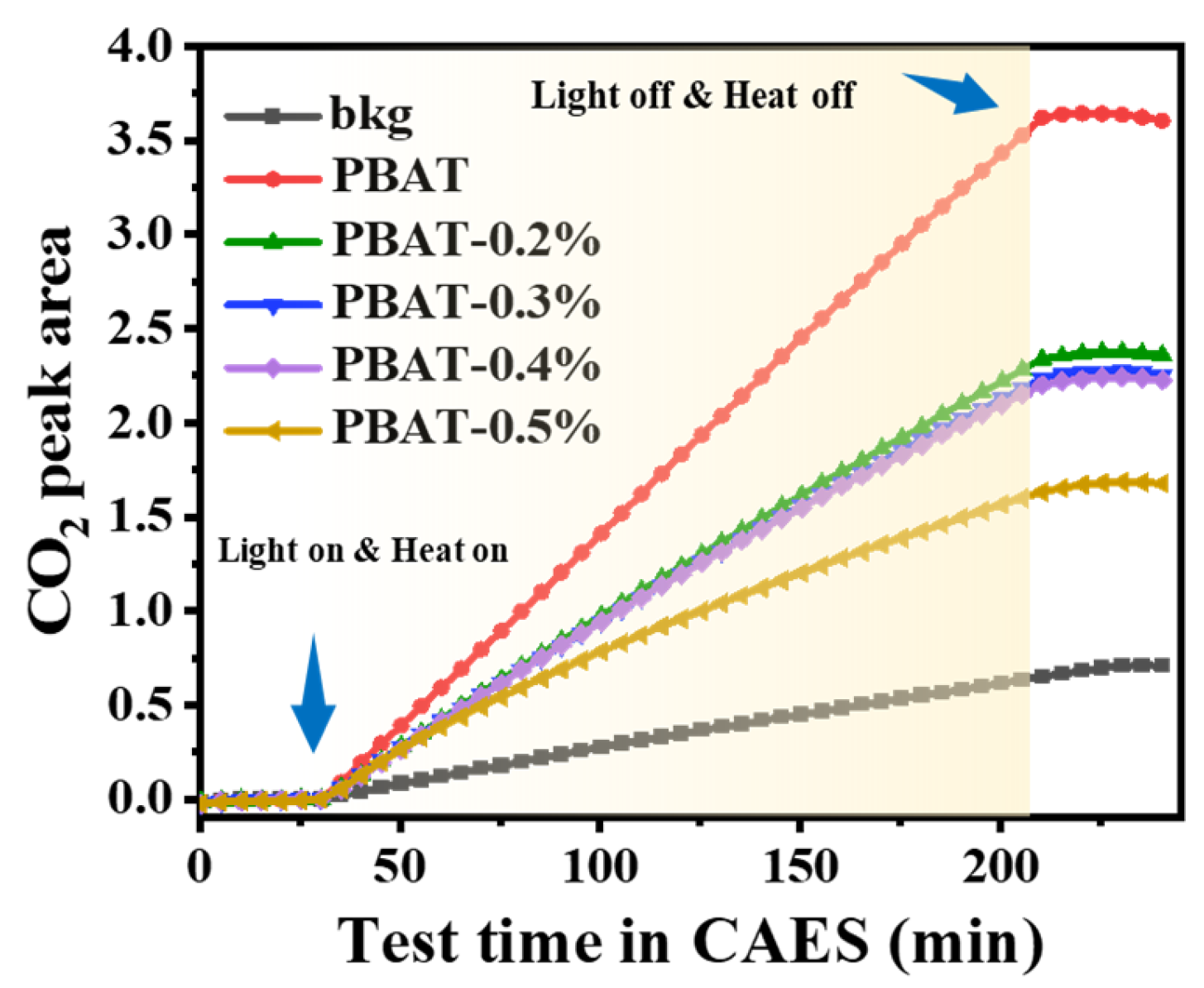
3.9. Accelerated Aging Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iwata, T. Biodegradable and Bio-Based Polymers: Future Prospects of Eco-Friendly Plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, B.-H. Poly(butylene succinate) and its copolymers: Research, development and industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef]
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Zubkiewicz, A.; Szymczyk, A.; Sablong, R.J.; Soccio, M.; Guidotti, G.; Siracusa, V.; Lotti, N. Bio-based aliphatic/aromatic poly(trimethylene furanoate/sebacate) random copolymers: Correlation between mechanical, gas barrier performances and compostability and copolymer composition. Polym. Degrad. Stab. 2022, 195, 109800. [Google Scholar] [CrossRef]
- Moustafa, H.; El Kissi, N.; Abou-Kandil, A.I.; Abdel-Aziz, M.S.; Dufresne, A. PLA/PBAT Bionanocomposites with Antimicrobial Natural Rosin for Green Packaging. ACS Appl. Mater. Interfaces 2017, 9, 20132–20141. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.; Hong, W.; Yu, G.; Du, Q.; Guo, S.; Li, C. Preparation and improving mechanism of PBAT/PPC-based micro-layer biodegradable mulch film with excellent water resistance and mechanical properties. Polymer 2024, 291, 126614. [Google Scholar] [CrossRef]
- Xie, J.; Yan, Y.; Fan, S.; Min, X.; Wang, L.; You, X.; Jia, X.; Waterhouse, G.I.N.; Wang, J.; Xu, J. Prediction Model of Photodegradation for PBAT/PLA Mulch Films: Strategy to Fast Evaluate Service Life. Environ. Sci. Technol. 2022, 56, 9041–9051. [Google Scholar] [CrossRef]
- Li, C.; Zhang, C.; Zhao, H.; Zhang, H.; Wang, X.; Han, B. Grafted UV absorber as voltage stabilizer against electrical degradation and breakdown in cross-linked polyethylene for high voltage cable insulation. Polym. Degrad. Stab. 2021, 185, 109498. [Google Scholar] [CrossRef]
- Shin, S.; Sim, E.; Lee, W.; Paik, H.-J.; Yu, Y.; Ahn, D. Synthesis and reactivity of novel cinnamonitrile derivatives as reactive UV stabilizers for enhanced light protection and performance of coatings. Polym. Degrad. Stab. 2022, 201, 109969. [Google Scholar] [CrossRef]
- Xing, J.; Yuan, D.; Xie, H.; Wang, N.; Liu, H.; Yang, L.; Xu, Y.; Liu, Z. Preparation of Efficient Ultraviolet-Protective Transparent Coating by Using a Titanium-Containing Hybrid Oligomer. ACS Appl. Mater. Interfaces 2021, 13, 5592–5601. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, S.; Zhou, S.; Hou, S.; Lu, Q. Perspectives on the Next Generation of Sunscreen: Safe, Broadband, and Long-Term Photostability. ACS Mater. Lett. 2019, 1, 336–343. [Google Scholar] [CrossRef]
- Botta, L.; Titone, V.; Teresi, R.; Scarlata, M.C.; Lo Re, G.; La Mantia, F.P.; Lopresti, F. Biocomposite PBAT/lignin blown films with enhanced photo-stability. Int. J. Biol. Macromol. 2022, 217, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, C.; Donnadio, A.; Quaglia, G.; Latterini, L.; Viseras, C.; Ambrogi, V. Halloysite-Doped Zinc Oxide for Enhanced Sunscreening Performance. ACS Appl. Nano Mater. 2019, 2, 6575–6584. [Google Scholar] [CrossRef]
- Maheshwari, N.; Thakur, S.; Neogi, P.; Neogi, S. UV resistance and fire retardant property enhancement of unsaturated polyester composite. Polym. Bull. 2015, 72, 1433–1447. [Google Scholar] [CrossRef]
- Pfaendner, R. How will additives shape the future of plastics? Polym. Degrad. Stab. 2006, 91, 2249–2256. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Lim, Y.W.; Jana, S.; Rusli, W.; Zhao, W.; Choo, S.S.S.; Luo, H.-K. Low-Migration Macromolecular Benzophenones as UV Absorbers for Food Packaging Polymers. ACS Appl. Polym. Mater. 2023, 5, 6932–6940. [Google Scholar] [CrossRef]
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic–inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef]
- Mahdavian, M.; Yari, H.; Ramezanzadeh, B.; Bahlakeh, G.; Hasani, M. Immobilization of ultraviolet absorbers on graphene oxide nanosheets to be utilized as a multifunctional hybrid UV-blocker: A combined density functional theory and practical application. Appl. Surf. Sci. 2018, 447, 135–151. [Google Scholar] [CrossRef]
- He, J.; Li, M.; Li, D.; Bao, B.; Xue, M.; Huang, Y.; Xu, Y.; Chen, G.; Dai, L. Fabrication of azobenzene non-covalent bonding grafting graphene composite and its application in weathering and corrosion resistant polyurethane coating. Polym. Degrad. Stab. 2022, 206, 110157. [Google Scholar] [CrossRef]
- Zhong, B.; Tang, Y.; Chen, Y.; Luo, Y.; Jia, Z.; Jia, D. Improvement of UV aging resistance of PBAT composites with silica-immobilized UV absorber prepared by a facile method. Polym. Degrad. Stab. 2023, 211, 110337. [Google Scholar] [CrossRef]
- Fischer, J.; Metzsch-Zilligen, E.; Zou, M.; Pfaendner, R. A novel class of high molecular weight multifunctional antioxidants for polymers based on thiol-ene click reaction. Polym. Degrad. Stab. 2020, 173, 109099. [Google Scholar] [CrossRef]
- Lin, C.; Hu, H.; Zhu, H.; Luan, Q.; Li, Z.; Wang, J.; Zhu, J. Disulfide-Driven On-Demand Degradation of the PBAT Copolymer: Stable Comprehensive Performance, Long-Term Storage, and Redox-Induced Degradation. Macromolecules 2025, 58, 4170–4182. [Google Scholar] [CrossRef]
- Wang, L.; Tu, Z.; Liang, J.; Wei, Z. Poly(butylene oxalate-co-terephthalate): A PBAT-like but rapid hydrolytic degradation plastic. J. Hazard. Mater. 2024, 471, 134349. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, Z.; Zhou, Y.; Wang, B.; Chen, J.; Li, J.; Huang, L.; Wang, C. Hydrophilic and Biodegradable PBAT Copolyesters Prepared from Chemically Recycled Resources. ACS Appl. Polym. Mater. 2024, 6, 5235–5245. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, Y.; Peng, Z.; Lim, K.H.; Wang, Q.; Shi, S.; Zheng, J.; Yang, X.; Liu, P.; Wang, W.-J. Synthesis of Poly(butylene adipate terephthalate)-co-poly(glycolic acid) with Enhanced Degradability in Water. Macromolecules 2023, 56, 9207–9217. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, J.; Wu, S.; Qi, Z.; Xu, J.; Guo, B. Synthesis, physical properties and photodegradation of functional poly(butylene succinate) covalently linking UV stabilizing moieties in molecular chains. Colloids Surf. A Physicochem. Eng. Asp. 2017, 524, 160–168. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Shi, L.; Ying, W.B.; Wang, J.; Zhu, J. Modification of Poly(butylene 2,5-furandicarboxylate) with Lactic Acid for Biodegradable Copolyesters with Good Mechanical and Barrier Properties. Ind. Eng. Chem. Res. 2018, 57, 11020–11030. [Google Scholar] [CrossRef]
- Wang, M.; Tashiro, K.; Ozaki, Y. Reinvestigation of the β-to-α Crystal Phase Transition of Poly(butylene adipate) by the Time-Resolved X-ray Scattering and FTIR Spectral Measurements in the Temperature-Jump Process. Macromolecules 2017, 50, 3883–3889. [Google Scholar] [CrossRef]
- Tashiro, K.; Nakai, Y.; Kobayashi, M.; Tadokoro, H. Solid-State Transition of Poly(butylene terephthalate) Induced by Mechanical Deformation. Macromolecules 1980, 13, 137–145. [Google Scholar] [CrossRef]
- Usanase, G.; Fraisse, F.; Taam, M.; Boyron, O. Determination of Short Chain Branching in LLDPE by Rheology. Macromol. Chem. Phys. 2022, 223, 2200150. [Google Scholar] [CrossRef]
- Park, H.E.; Dealy, J.M.; Marchand, G.R.; Wang, J.; Li, S.; Register, R.A. Rheology and Structure of Molten, Olefin Multiblock Copolymers. Macromolecules 2010, 43, 6789–6799. [Google Scholar] [CrossRef]
- An, Z.; Xu, Z.; Ye, Y.; Yang, R. A rapid and highly sensitive evaluation of polymer composite aging with linear correlation to real-time aging. Anal. Chim. Acta 2021, 1169, 338632. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; An, Z.; Ye, Y.; Yang, R. Photoantioxidant versus photodegradant: Action of nanoparticle inorganic fillers in outdoor aging of PP. J. Vinyl Addit. Technol. 2022, 28, 405–417. [Google Scholar] [CrossRef]
- Meng, X.; Ye, Y.; Yang, R. Computational and Experimental Study on the Mechanism of CO2 Production during Photo-Oxidative Degradation of Poly(butylene adipate-co-terephthalate): Differences between PBA and PBT Segments. Macromolecules 2023, 56, 7749–7762. [Google Scholar] [CrossRef]
- Wang, S.; Gao, P.; Han, Q.; Liu, Y.; Feng, L.; Zhang, L. Insights into photoaging behaviors and mechanisms of biodegradable and conventional microplastics in soil. J. Hazard. Mater. 2024, 480, 136418. [Google Scholar] [CrossRef]

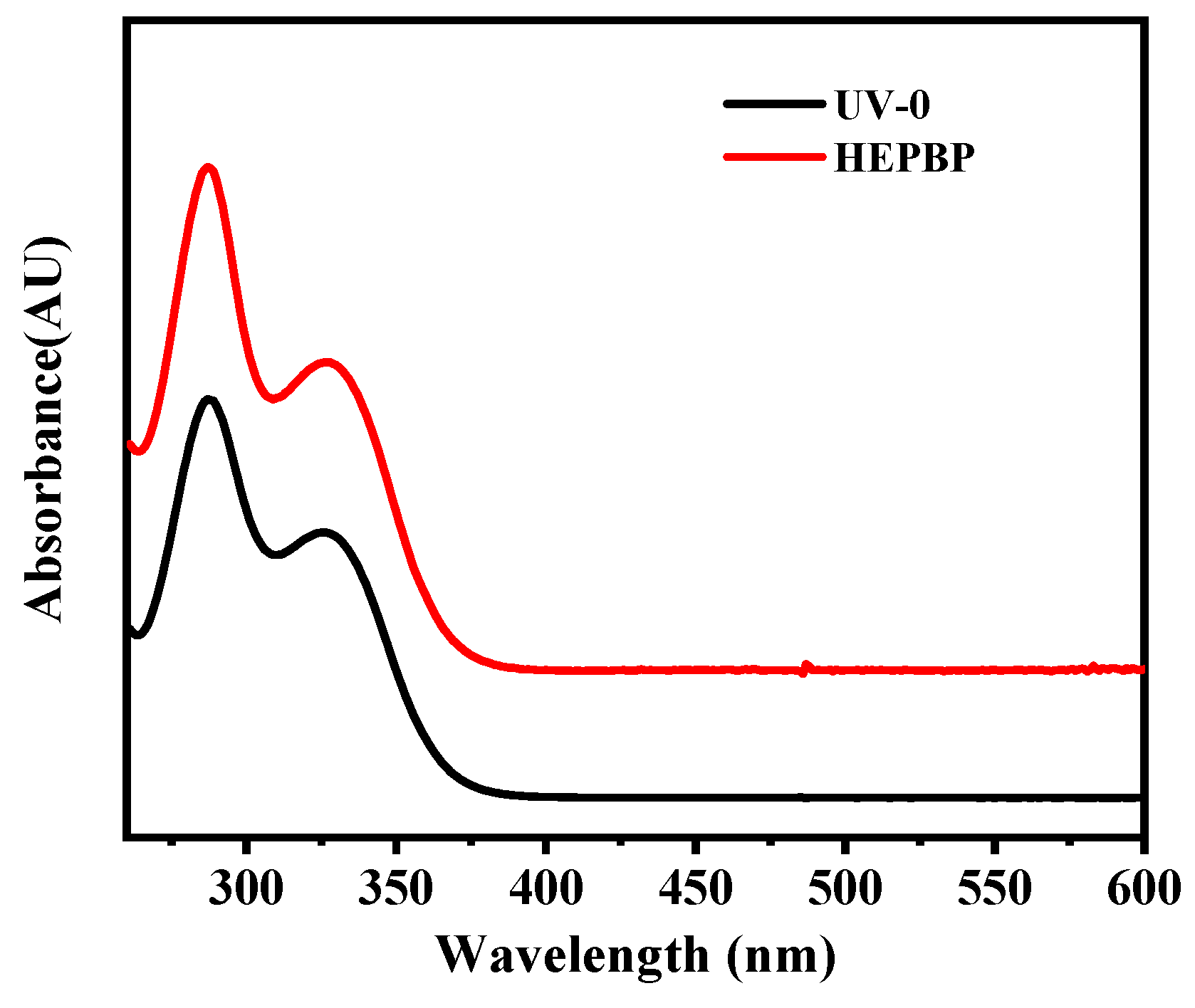



| Sample | ) | |||
|---|---|---|---|---|
| UV-0 | 287 | 0.0626 | 326 | 0.0419 |
| HEPBP | 290 | 0.0922 | 330 | 0.0566 |
| Sample | R | DPI | (dl/g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PBAT | 54.61 | 45.39 | n.d | 2.03 | 1.86 | 1.04 | 70.2 | 52.1 | 1.35 | 1.39 |
| PBAT-0.2% | 55.31 | 44.52 | 0.17 | 1.99 | 1.95 | 0.97 | 69.8 | 51.9 | 1.37 | 1.36 |
| PBAT-0.3% | 54.84 | 44.91 | 0.25 | 2.04 | 1.90 | 1.01 | 68.3 | 48.9 | 1.40 | 1.32 |
| PBAT-0.4% | 54.56 | 45.17 | 0.27 | 2.06 | 1.89 | 1.01 | 70.5 | 50.5 | 1.39 | 1.40 |
| PBAT-0.5% | 54.25 | 45.34 | 0.41 | 2.09 | 1.85 | 1.02 | 68.4 | 50.8 | 1.34 | 1.38 |
| Sample | ||||||
|---|---|---|---|---|---|---|
| PBAT | 66.2 | 19.0 | −32.0 | 120.4 | 16.2 | 14.2 |
| PBAT-0.2% | 55.2 | 17.9 | −32.1 | 118.6 | 15.5 | 13.6 |
| PBAT-0.3% | 55.6 | 18.9 | −33.2 | 118.8 | 15.4 | 13.5 |
| PBAT-0.4% | 61.3 | 18.5 | −32.6 | 117.9 | 13.9 | 12.2 |
| PBAT-0.5% | 62.0 | 17.6 | −33.0 | 114.3 | 13.0 | 11.4 |
| Sample | E (MPa) | (MPa) | EF (%) | (MPa) |
|---|---|---|---|---|
| PBAT | 21.6 ± 0.9 | 5.9 ± 0.3 | 1296 ± 72 | 17.8 ± 2.1 |
| PBAT-0.2% | 18.9 ± 1.1 | 4.4 ± 0.2 | 1035 ± 91 | 13.2 ± 1.4 |
| PBAT-0.3% | 21.1 ± 0.6 | 4.9 ± 0.3 | 1272 ± 85 | 14.8 ± 1.7 |
| PBAT-0.4% | 18.1 ± 0.7 | 5.5 ± 0.4 | 1245 ± 81 | 17.8 ± 2.0 |
| PBAT-0.5% | 17.7 ± 0.6 | 5.1 ± 0.2 | 1347 ± 74 | 15.4 ± 1.9 |
| Sample | (MPa) | EF(%) | EF Retention | |
|---|---|---|---|---|
| PBAT-1 | 14.5 ± 0.5 | 920 ± 48 | 67.1% | 48.8% |
| PBAT-2 | 12.6 ± 0.6 | 874 ± 52 | 42.6% | 47.7% |
| PBAT-3 | 10.1 ± 0.7 | 858 ± 65 | 45.1% | 45.5% |
| PBAT-4 | 1.8 ± 0.3 | 35 ± 5 | 8.3% | 2.1% |
| PBAT-5 | 0.1 | 3 | <1% | <1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Ye, Y.; Wang, Y.; Bian, H.; Yuan, J.; Ding, J.; Li, W.; Xu, J.; Guo, B. Design and Preparation of Inherently Photostable Poly(Butylene Adipate-Co-Terephthalate) by Chemically Bonding UV-Stabilizing Moieties in Molecular Chains. Polymers 2025, 17, 1567. https://doi.org/10.3390/polym17111567
Zhang X, Ye Y, Wang Y, Bian H, Yuan J, Ding J, Li W, Xu J, Guo B. Design and Preparation of Inherently Photostable Poly(Butylene Adipate-Co-Terephthalate) by Chemically Bonding UV-Stabilizing Moieties in Molecular Chains. Polymers. 2025; 17(11):1567. https://doi.org/10.3390/polym17111567
Chicago/Turabian StyleZhang, Xinpeng, Yan Ye, Yaqiao Wang, Hongli Bian, Jing Yuan, Jianping Ding, Wanli Li, Jun Xu, and Baohua Guo. 2025. "Design and Preparation of Inherently Photostable Poly(Butylene Adipate-Co-Terephthalate) by Chemically Bonding UV-Stabilizing Moieties in Molecular Chains" Polymers 17, no. 11: 1567. https://doi.org/10.3390/polym17111567
APA StyleZhang, X., Ye, Y., Wang, Y., Bian, H., Yuan, J., Ding, J., Li, W., Xu, J., & Guo, B. (2025). Design and Preparation of Inherently Photostable Poly(Butylene Adipate-Co-Terephthalate) by Chemically Bonding UV-Stabilizing Moieties in Molecular Chains. Polymers, 17(11), 1567. https://doi.org/10.3390/polym17111567








