Oxidative Decomposition of Poly(phenylene sulfide) Composites Under Fast Elevation of Temperature
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Thermogravimetric Analysis (TGA)
2.3. Preliminary Tests for the Measurements at High Rates
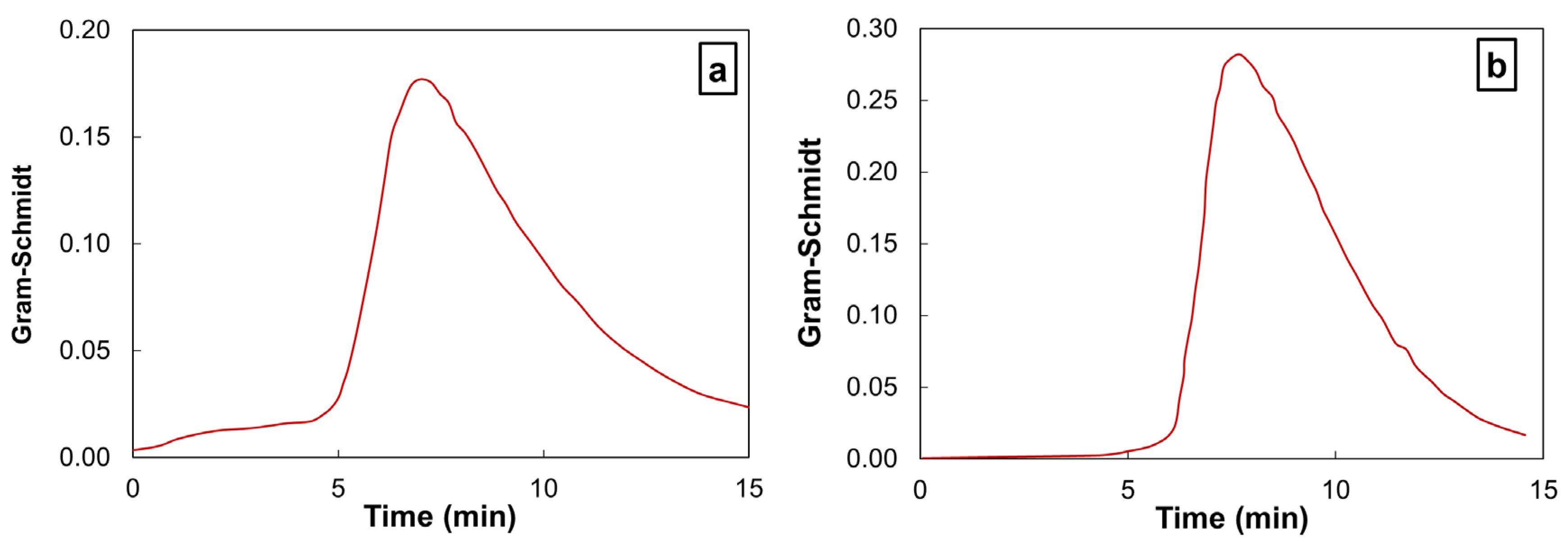
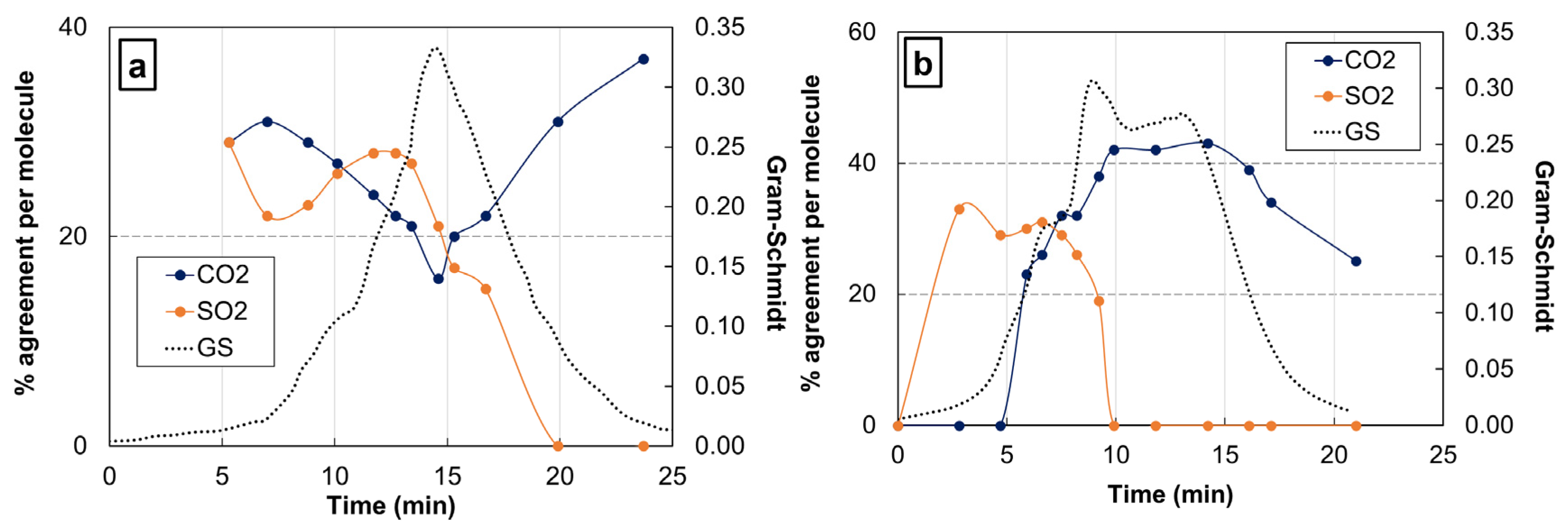
2.4. Coupling of TGA Methods with Fourier-Transform Infrared Spectroscopy
3. Results and Discussion
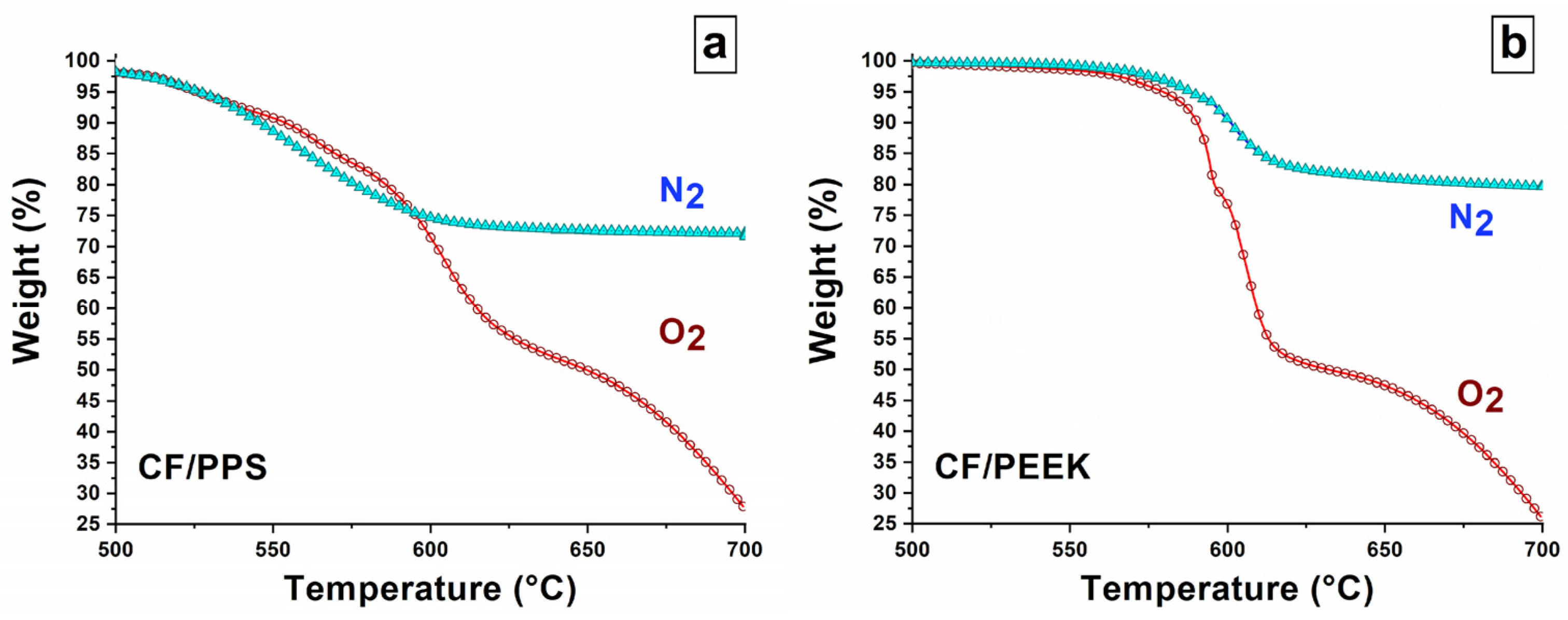
3.1. General Features Regarding the Thermal Decomposition Under Oxygen
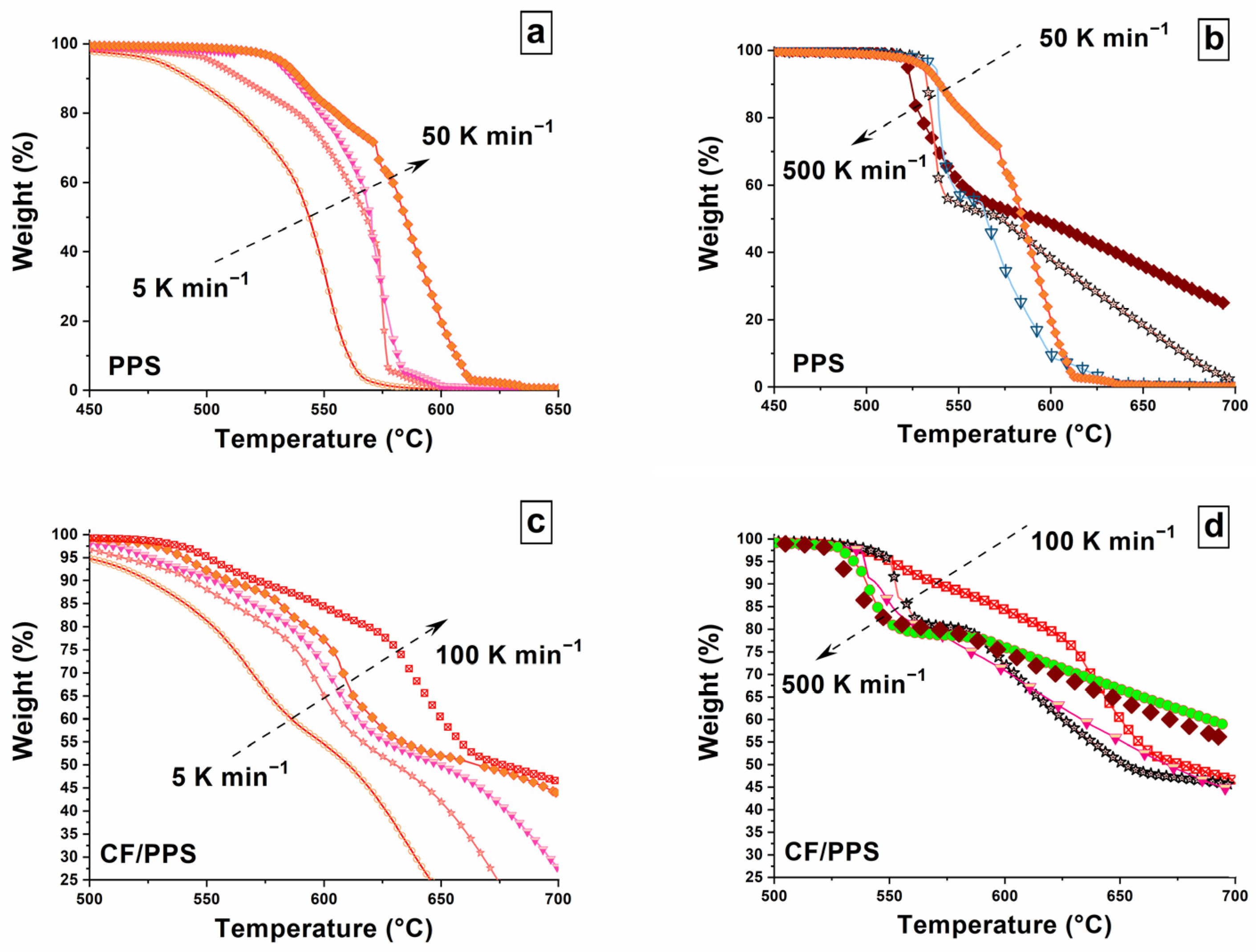
3.2. Decomposition Signatures for Fast Heating Rates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Delichatsios, M.A.; Fateh, T.; Suzanne, M.; Ukleja, S. Characterization of flammability and fire resistance of carbon fibre reinforced thermoset and thermoplastic composite materials. J. Loss Prev. Process Ind. 2017, 50, 275–282. [Google Scholar] [CrossRef]
- Schuhler, E.; Coppalle, A.; Vieille, B.; Yon, J.; Carpier, Y. Behaviour of aeronautical polymer composite to flame: A comparative study of thermoset– and thermoplastic–based laminate. Polym. Degrad. Stabil. 2018, 152, 105–115. [Google Scholar] [CrossRef]
- Christopher, N.S.J.; Cotter, J.L.; Knight, G.J.; Wright, W.W. Thermal degradation of poly(phenylene sulfide) and perfluoropoly(phenylene sulfide). J. Appl. Polym. Sci. 1968, 12, 863–870. [Google Scholar] [CrossRef]
- Peters, O.A.; Still, R.H. The thermal degradation of poly(phenylene sulphide)—Part 1. Polym. Degrad. Stab. 1993, 42, 41–48. [Google Scholar] [CrossRef]
- Li, X.-G.; Huang, M.-R.; Bai, H.; Yang, Y.-L. High-resolution thermogravimetry of polyphenylene sulfide film under four atmospheres. J. Appl. Polym. Sci. 2002, 83, 2053–2059. [Google Scholar] [CrossRef]
- Vieille, B.; Lefebvre, C.; Coppalle, A. Post fire behavior of carbon fibers Polyphenylene Sulfide-and epoxy-based laminates for aeronautical applications: A comparative study. Mater. Des. 2014, 63, 56–68. [Google Scholar] [CrossRef]
- Vieille, B.; Ernault, E.; Delpouve, N.; Pujols Gonzalez, J.-D.; Esposito, A.; Dargent, E.; Le Pluart, L.; Delbreilh, L. On the improvement of thermo-mechanical behavior of carbon/polyphenylene sulfide laminated composites upon annealing at high temperature. Compos. Part B Eng. 2021, 216, 108858. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J. Enhancement of the flame retardant properties of PPS-based composites via the addition of melamine-coated CaAl-LDH fire-retardant filler. Eur. Polym. J. 2023, 201, 112584. [Google Scholar] [CrossRef]
- Cai, W.; You, J.; Wang, W.; Chen, H.; Liu, L.; Ma, Y.; Huang, J.; Zheng, Y.; Lai, Y. Double-hindered phenolic SiO2 composites with excellent oxidation resistance and thermal stability for enhanced thermal oxidation stability of PPS. Chem. Eng. J. 2024, 487, 150662. [Google Scholar] [CrossRef]
- Fujihara, K.; Huang, Z.-M.; Ramakrishna, S.; Hamada, H. Influence of processing conditions on bending property of continuous carbon fiber reinforced PEEK composites. Compos. Sci. Technol. 2004, 64, 2525–2534. [Google Scholar] [CrossRef]
- Saleem, A.; Frormann, L.; Iqbal, A. High performance thermoplastic composites: Study on the mechanical, thermal, and electrical resistivity properties of carbon fiber-reinforced polyetheretherketone and polyethersulphone. Polym. Compos. 2007, 28, 785–796. [Google Scholar] [CrossRef]
- Patel, P.; Hull, T.R.; Lyon, R.E.; Stoliarov, S.I.; Walters, R.N.; Crowley, S.; Safronava, N. Investigation of the thermal decomposition and flammability of PEEK and its carbon and glass–fibre composites. Polym. Degrad. Stabil. 2011, 96, 12–22. [Google Scholar] [CrossRef]
- Lu, C.; Xu, N.; Zheng, T.; Zhang, X.; Lv, H.; Lu, X.; Xiao, L.; Zhang, D. The optimization of process parameters and characterization of high-performance CF/PEEK composites prepared by flexible CF/PEEK plain weave fabrics. Polymers 2018, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Zheng, S.; Song, P.; Zhang, X.; Wang, C. Development of mechanical and thermal properties of PEEK composites by incorporating inorganic particles modified phosphates. Compos. Part B Eng. 2021, 212, 108715. [Google Scholar] [CrossRef]
- Vieille, B.; Coppalle, A.; Schuhler, E.; Chaudhary, A.; Alia, A.; Delpouve, N.; Bourdet, A. Influence of kerosene flame on fire–behaviour and mechanical properties of hybrid carbon glass fibers reinforced PEEK composite laminates. Compos. Struct. 2022, 279, 114786. [Google Scholar] [CrossRef]
- Tadini, P.; Grange, N.; Chetehouna, K.; Gascoin, N.; Senave, S.; Reynaud, I. Thermal degradation analysis of innovative PEKK-based carbon composites for high temperature aeronautical components. Aerosp. Sci. Technol. 2017, 65, 106–116. [Google Scholar] [CrossRef]
- Vieille, B.; Coppalle, A.; Le Pluart, L.; Schuhler, E.; Chaudhary, A.; Rijal, B.; Alia, A.; Delpouve, N. Kerosene flame behaviour of C/PEKK composite laminates: Influence of exposure time and laminates lay-up on residual mechanical properties. Compos. Part B Eng. 2021, 222, 109046. [Google Scholar] [CrossRef]
- Vieille, B.; Coppalle, A.; Le Pluart, L.; Schuhler, E.; Chaudhary, A.; Rijal, B.; Alia, A.; Delpouve, N.; Bourdet, A. Influence of a flame-retardant on the fire-behaviour and the residual mechanical properties of C/PEKK composite laminates exposed to a kerosene flame. Compos. Part A Appl. Sci. Manuf. 2022, 152, 106720. [Google Scholar] [CrossRef]
- Pedoto, G.; Grandidier, J.-C.; Gigliotti, M.; Vinet, A. Characterization and modelling of the PEKK thermomechanical and creep behavior above the glass transition temperature. Mech. Mater. 2022, 166, 104189. [Google Scholar] [CrossRef]
- Lisa, G.; Hamciuc, C.; Hamciuc, E.; Tudorachi, N. Thermal and thermo-oxidative stability and probable degradation mechanism of some polyetherimides. J. Anal. Appl. Pyrolysis 2016, 118, 144–154. [Google Scholar] [CrossRef]
- Miao, W.; Chen, H.; Pan, Z.; Pei, X.; Li, L.; Li, P.; Liu, J.; Zhai, J.; Pan, H. Enhancement thermal stability of polyetherimide-based nanocomposites for applications in energy storage. Compos. Sci. Technol. 2021, 201, 108501. [Google Scholar] [CrossRef]
- Zhu, L.; Li, N.; Childs, P.R.N. Light-weighting in aerospace component and system design. Propuls. Power Res. 2018, 7, 103–119. [Google Scholar] [CrossRef]
- Chetehouna, K.; Grange, N.; Gascoin, N.; Lemée, L.; Reynaud, I.; Senave, S. Release and flammability evaluation of pyrolysis gases from carbon-based composite materials undergoing fire conditions. J. Anal. Appl. Pyrolysis. 2018, 134, 136–142. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, Y.; Song, H.; Huang, C. Residual strength and damage mechanisms of laminated carbon fiber reinforced polymer under thermal environments and laser irradiations. Polym. Eng. Sci. 2018, 58, 2311–2319. [Google Scholar] [CrossRef]
- Levchik, S.; Wilkie, C.A. Char formation. In Fire Retardancy of Polymeric Materials; Grand, A.F., Wilkie, C.A., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2000; pp. 171–216. [Google Scholar]
- Price, D.; Anthony, G.; Carty, P. Introduction: Polymer combustion, condensed phase pyrolysis and smoke formation. In Fire Retardant Materials; Horrocks, A.R., Price, D., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2002; pp. 1–30. [Google Scholar] [CrossRef]
- Pering, G.A.; Farrell, P.V.; Springer, G.S. Degradation of tensile and shear properties of composites exposed to fire or high temperature. J. Compos. Mater. 1989, 14, 54–66. [Google Scholar]
- Yao, F.; Zheng, J.; Qi, M.; Wang, W.; Qi, Z. The thermal decomposition kinetics of poly(ether–ether–ketone) (PEEK) and its carbon fiber composite. Thermochim. Acta. 1991, 183, 91–97. [Google Scholar] [CrossRef]
- Carpier, Y.; Vieille, B.; Delpouve, N.; Dargent, E. Isothermal and anisothermal decomposition of carbon fibres polyphenylene sulfide composites for fire behavior analysis. Fire Safety J. 2019, 109, 102868. [Google Scholar] [CrossRef]
- Montaudo, G.; Puglisi, C.; Samperi, F. Primary thermal degradation processes occurring in poly(phenylenesulfide) investigated by direct pyrolysis–mass spectrometry. J. Polym. Sci. Part A Pol. Chem. 1994, 32, 1807–1815. [Google Scholar] [CrossRef]
- Budgell, D.R.; Day, M.; Cooney, J.D. Thermal degradation of poly(phenylene sulfide) as monitored by pyrolysis—GC/MS. Polym. Degrad. Stabil. 1994, 43, 109–115. [Google Scholar] [CrossRef]
- Hao, J.; Xu, F.; Yang, D.; Wang, B.; Qiao, Y.; Tian, Y. Analytical pyrolysis of biomass using pyrolysis-gas chromatography/mass spectrometry. Renew. Sustain. Energy Rev. 2025, 208, 115090. [Google Scholar] [CrossRef]
- Perng, L.H. Thermal decomposition characteristics of poly(phenylene sulfide) by stepwise Py–GC/MS and TG/MS techniques. Polym. Degrad. Stabil. 2000, 69, 323–332. [Google Scholar] [CrossRef]
- Erickson, K.L. Thermal decomposition mechanisms common to polyurethane, epoxy, poly(diallyl phthalate), polycarbonate and poly(phenylene sulfide). J. Therm. Anal. Calorim. 2007, 89, 427–440. [Google Scholar] [CrossRef]
- Carpier, Y.; Bourdet, A.; Delpouve, N.; Dargent, E.; Vieille, B. Thermal decomposition study of carbon fiber-reinforced polyphenylene sulfide at high heating rates met under fire exposure. J. Therm. Anal. Calorim. 2024, 149, 6039–6050. [Google Scholar] [CrossRef]
- Feih, S.; Mouritz, A.P. Tensile properties of carbon fibres and carbon fibre–polymer composites in fire. Compos. Part A Appl. Sci. Manuf. 2012, 43, 765–772. [Google Scholar] [CrossRef]
- Vanden Poel, G.; Mathot, V.B.F. High-speed/high performance differential scanning calorimetry (HPer DSC): Temperature calibration in the heating and cooling mode and minimization of thermal lag. Thermochim. Acta 2006, 446, 41–54. [Google Scholar] [CrossRef]
- Gros, C.; Tarrieu, J.; Nassiet, V.; Dutarde, E. A study of mechanisms of Poly (PhenyleneSulfide) thermal degradation in air. Adv. Struct. Anal. Adv. Mater. 2010, 112, 10–18. [Google Scholar] [CrossRef]
- Yan, P.; Peng, W.; Yang, F.; Cao, Y.; Xiang, M.; Wu, T.; Fu, Q. Investigation on thermal degradation mechanism of poly(phenylene sulfide). Polym. Degrad. Stabil. 2022, 197, 323–332. [Google Scholar] [CrossRef]
- Martín, M.I.; Rodríguez-Lance, F.; Güemes, A.; Fernández-López, A.; Pérez-Maqueda, L.A.; Perejón, A. On the determination of thermal degradation effects and detection techniques for thermoplastic composites obtained by automatic lamination. Compos. Part A Appl. Sci. Manuf. 2018, 111, 23–32. [Google Scholar] [CrossRef]
- Kumagai, S.; Sato, M.; Ma, C.; Nakai, Y.; Kameda, T.; Saito, Y.; Watanabe, A.; Watanabe, C.; Teramae, N.; Yoshioka, T. A comprehensive study into the thermo-oxidative degradation of sulfur-based engineering plastics. J. Anal. Appl. Pyrolysis 2022, 168, 105754. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.S.; Cheng, S.; Qu, B.; Li, A.; Meng, F.; Ji, G. The impact of heating rate on the decomposition kinetics and product distribution of algal waste pyrolysis with in–situ weight measurement. Chem. Eng. J. 2023, 457, 141368. [Google Scholar] [CrossRef]
- Efika, C.E.; Onwudili, J.A.; Williams, P.T. Influence of heating rates on the products of high–temperature pyrolysis of waste wood pellets and biomass model compounds. Waste Manag. 2018, 76, 497–506. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourdet, A.; Carpier, Y.; Dargent, E.; Vieille, B.; Delpouve, N. Oxidative Decomposition of Poly(phenylene sulfide) Composites Under Fast Elevation of Temperature. Polymers 2025, 17, 1560. https://doi.org/10.3390/polym17111560
Bourdet A, Carpier Y, Dargent E, Vieille B, Delpouve N. Oxidative Decomposition of Poly(phenylene sulfide) Composites Under Fast Elevation of Temperature. Polymers. 2025; 17(11):1560. https://doi.org/10.3390/polym17111560
Chicago/Turabian StyleBourdet, Aurélie, Yann Carpier, Eric Dargent, Benoit Vieille, and Nicolas Delpouve. 2025. "Oxidative Decomposition of Poly(phenylene sulfide) Composites Under Fast Elevation of Temperature" Polymers 17, no. 11: 1560. https://doi.org/10.3390/polym17111560
APA StyleBourdet, A., Carpier, Y., Dargent, E., Vieille, B., & Delpouve, N. (2025). Oxidative Decomposition of Poly(phenylene sulfide) Composites Under Fast Elevation of Temperature. Polymers, 17(11), 1560. https://doi.org/10.3390/polym17111560





