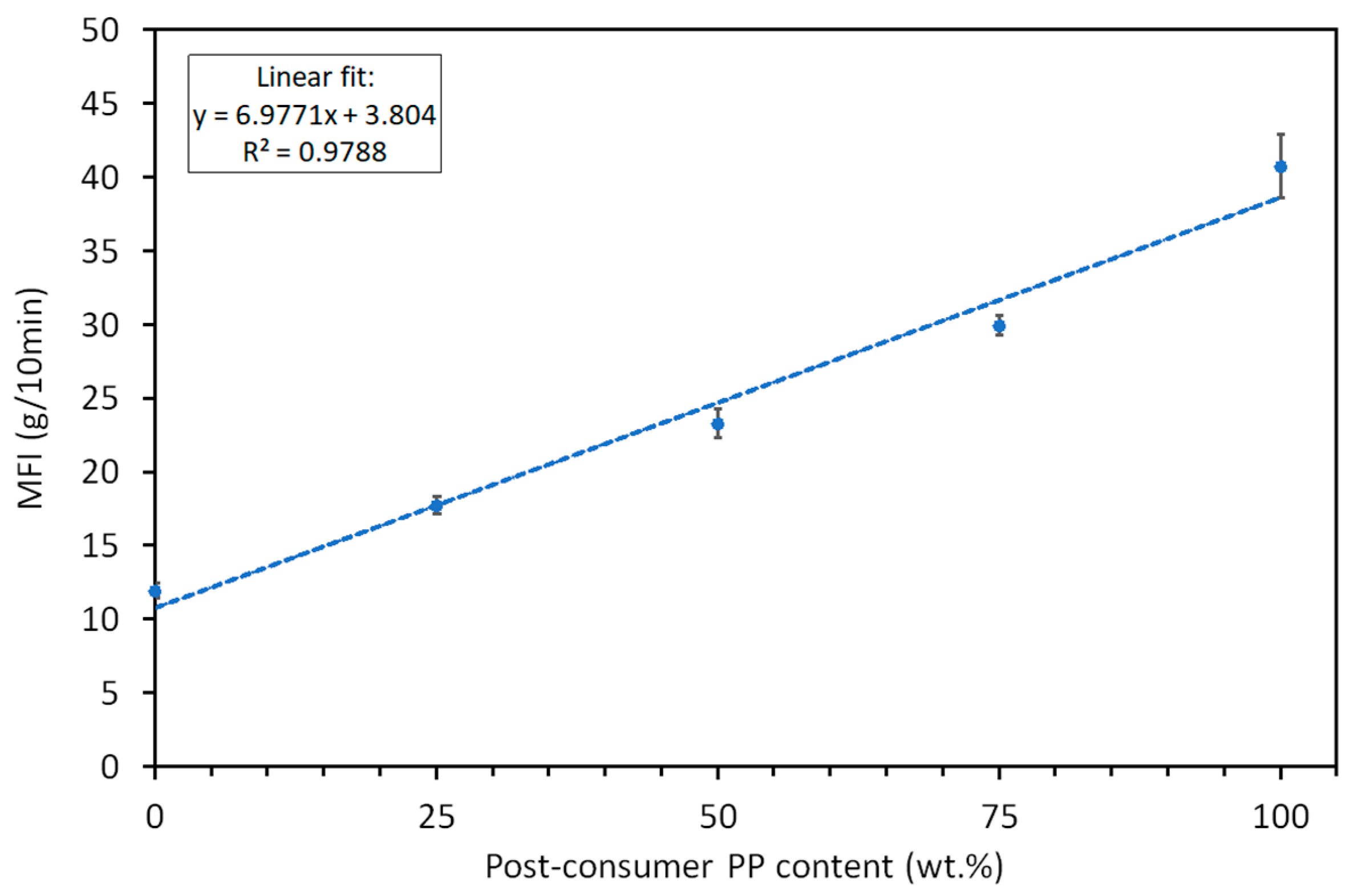
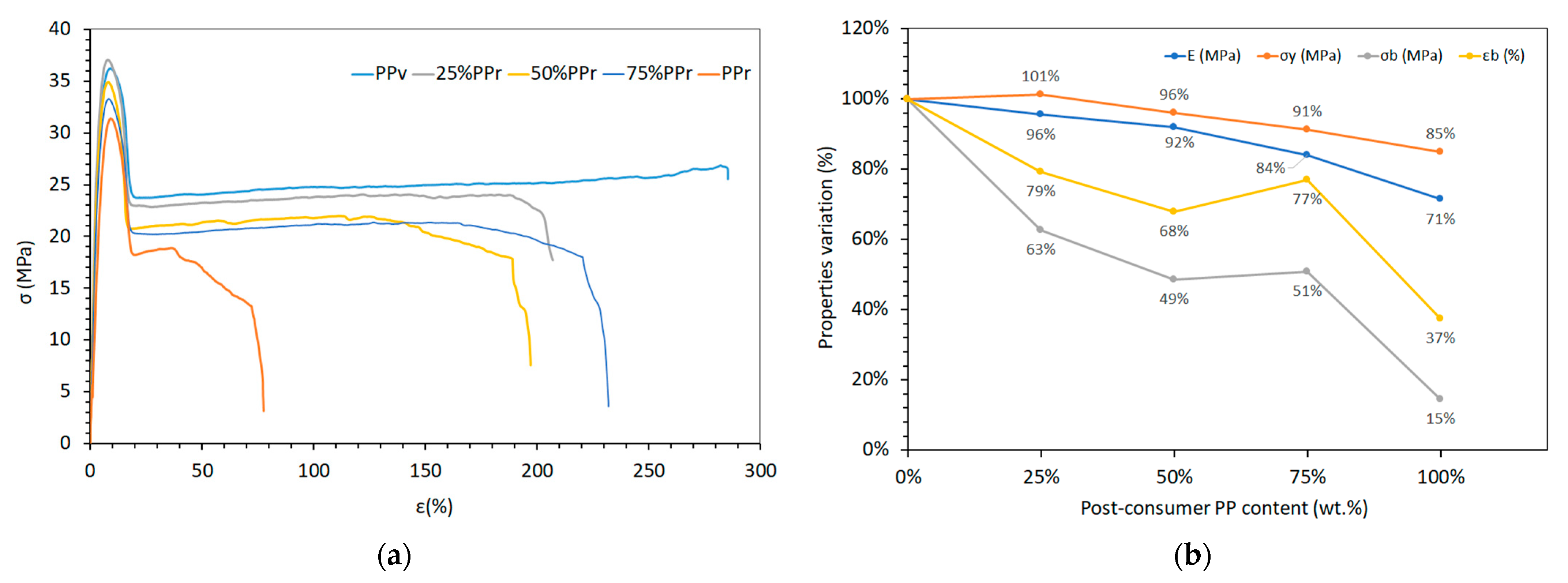
3.2.1. Thermal Transitions and Crystallinity Analysis
The thermal behaviour of PPᵥ and its blends with PP
r was evaluated by DSC, and the respective values are exemplified in
Figure 2 and may be consulted in
Table 3, where H
c (J/g) and T
c (°C) are, respectively, crystallization enthalpy and temperature; H
f (J/g) and T
f (°C) are, respectively, fusion enthalpy and temperature; and χ (%) is the crystallinity degree.
Table 4 shows how the thermal properties of the blends and PP
r vary in comparison to PP
v.
The fusion enthalpy decreased slightly with increasing PP
r content, from 101.59 ± 2.06 (J/g) for PPᵥ to 92.04 ± 1.37 (J/g) for 100% PP
r, accompanied by a minor reduction in the degree of crystallinity (from 49.08% to 44.46%). However, the fusion temperature remained relatively stable across all compositions, ranging from 163.61 ± 0.12 °C (PPᵥ) to 162.63 ± 0.09 °C (PP
r), indicating that the crystalline structure of polypropylene does not undergo significant alteration. Baldini et al. [
24] reported similar melting behaviour; however, their study observed a double crystallization peak during cooling, attributed to polyethylene contamination, common in post-consumer polymer waste.
It should be noted, however, that in the present study, the blend containing 25% recycled polypropylene (25%PP
r) exhibited slightly higher melting temperature, melting enthalpy, and hence, degree of crystallinity compared to the PP
v (
Table 3 and
Table 4). This behaviour can be attributed to residual crystalline fragments and particulate contamination within the recycled material, which may act as heterogeneous nucleating agents when blended at a relatively low proportion (25%) with virgin PP [
37]. These nucleating sites facilitate the crystallization process by promoting faster and more efficient organization of polymer chains during cooling. Furthermore, slight chain scission occurring during the recycling process may enhance molecular mobility, thereby favouring crystallization at low content of the recycled PP [
38]. These combined effects result in an initial increase in crystallinity at low PP
r content before the negative impact of accumulated degradation becomes more pronounced at higher PP
r percentages. In contrast, Stoian et al. [
17] reported a slight increase in crystallinity with increasing amounts of recycled PP derived from raffia. This discrepancy may be attributed to the inherently higher crystallinity of the raffia-based rPP compared to the virgin PP used in their study.
During the cooling cycle, the recrystallization enthalpy (H
c) decreased with higher PP
r content. Notably, the recrystallization temperature (T
c) of the blends increased approximately 10%, from 112.3 °C for PPᵥ to 124.2 °C for PPᵣ. This shift suggests that the recycled material promotes earlier nucleation during cooling, likely due to the presence of heterogeneous nucleation sites generated during prior processing cycles [
39]. This trend is consistent with observations by Infurna et al. [
40], where the crystallization temperature increase with the gradual addition of PPr was attributed to a higher proportion of shorter polymeric chains.
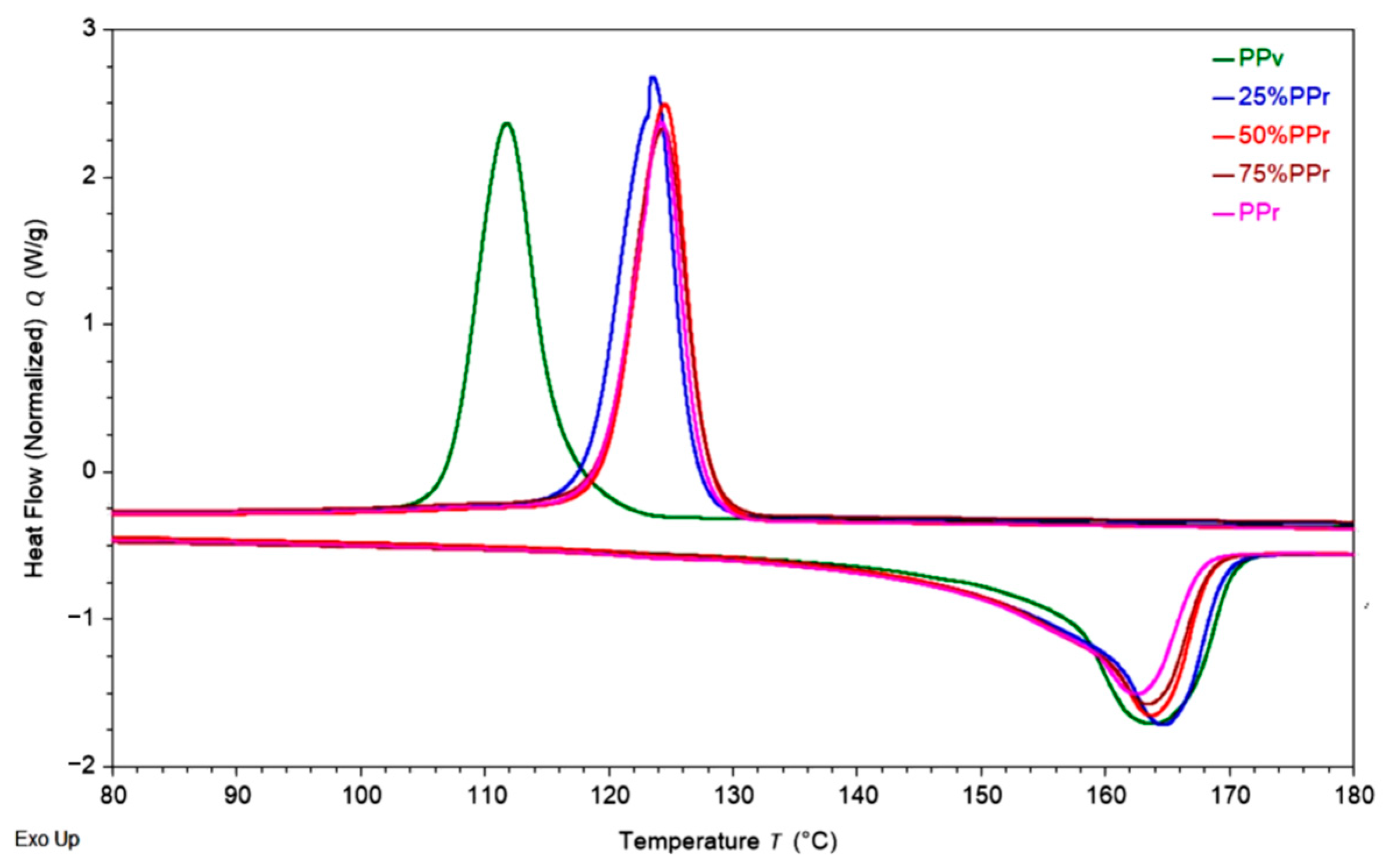
Overall, the incorporation of recycled post-consumer polypropylene into virgin material does not significantly affect the thermal transitions and crystallinity. It is also worth noting that, as shown in
Figure 2, virgin PP, PP
r, and their blends each exhibit a single melting and crystallization peak with no additional thermal transitions, indicating high purity across all samples. However, a slightly asymmetric recrystallization peak, observed for the 25% PP
r blend (
Figure 2), suggests a more complex nucleation process. This asymmetry may result from the moderate recycled content acting as a nucleating agent, promoting multiple crystallization events. Such behaviour could indicate the formation of a finer and more heterogeneous crystalline morphology, driven by the presence of the recycled fraction. Additionally, the slight increase in crystallinity suggests a potential improvement in mechanical properties, which will be confirmed upon further assessment.
The comparison between the thermal properties of extruded recycled polypropylene (PP
r) and its non-extruded counterpart (rPPcw) [
23] highlights the influence of prior homogenization on crystallization behaviour. While both materials show similar melting temperatures, rPPcw exhibits a higher melting enthalpy (99.05 ± 0.54 (J/g)) and crystallinity (48.00%) compared to PP
r (92.04 ± 1.37 (J/g); 44.46%). This increase in crystallinity may be attributed to residual ordered structures or crystal fragments preserved in the rPPcw due to the absence of additional thermal and shear processing steps during extrusion [
37,
39]. The crystallization temperature (T
c) of rPPcw is slightly lower (122.23 °C) than that of PP
r (124.19 °C), suggesting that although the crystallinity is higher, nucleation may have occurred later or more gradually. This behaviour may result from less uniform chain distribution or incomplete homogenization of rPPcw, as the material was directly processed from reground flakes. These findings suggest that the homogenization by extrusion process can disrupt some of the existing crystalline domains, reducing overall crystallinity and sequential stiffness of the recycled PP but improving its ductility, as it was verified by mechanical testing further discussed ahead.
3.2.2. Oxidation Induction Time
Polypropylene is prone to oxidative degradation due to tertiary carbon atoms in its backbone, which are easily attacked by free radicals, especially under heat or UV exposure. It leads to chain scission, resulting in embrittlement and loss of mechanical properties [
41,
42]. Therefore, OIT analysis is essential for understanding how blending with virgin PP can attenuate oxidative degradation.
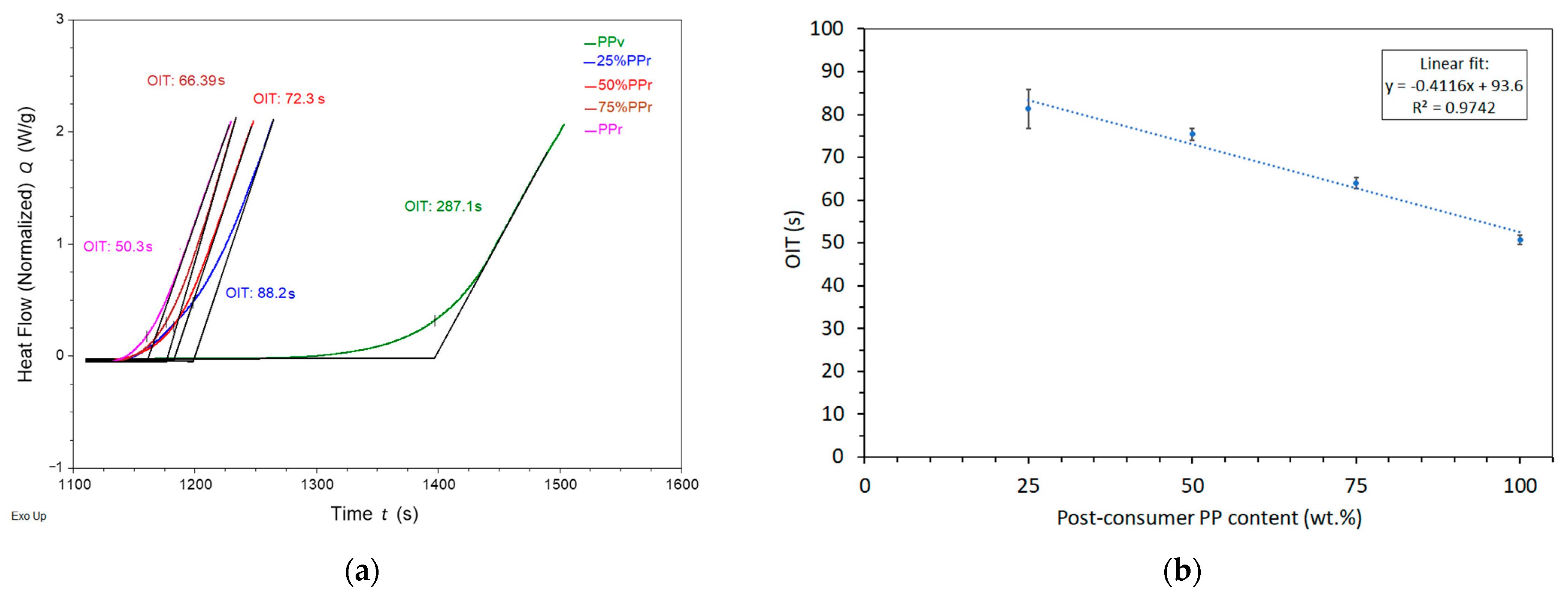
As shown in
Table 5 and exemplified in
Figure 3a, virgin polypropylene’s oxidation induction time was 277.6 ± 55.3 s, while adding just 25% PP
r led to a sharp decrease to 81.3 ± 6.9 s, representing a reduction of approximately 71%. Further increases in PP
r content continued to reduce the OIT, with values of 75.4 ± 7.4 s, 64.0 ± 6.9 s, and 50.8 ± 8.3 s for 50%, 75%, and 100% PP
r, respectively. These results indicate that recycled polypropylene contains a significantly lower amount of antioxidant stabilizers, which are typically consumed during the material’s prior thermal and mechanical processing cycles. The loss of antioxidants and the existence of oxidative breakdown products like peroxides and carbonyl compounds lower the material’s resistance to further oxidation attack [
23,
37].
Furthermore, recycled PP may contain residual catalysts and metal contaminants, which can act as pro-oxidants, enhancing the degradation rate. Such impurities exacerbate thermo-oxidative degradation of PP [
43]. The presence of small amounts of previously degraded material may also trigger localized catalytic effects, accelerating the onset of oxidation and contributing to a reduced OIT. Therefore, OIT should be interpreted as a relative indicator of oxidative stability under controlled conditions rather than a direct predictor of long-term service life.
The slight improvement in oxidation induction time (OIT) from 41.6 ± 5.5 s [
23] in non-extruded recycled polypropylene (rPPcw) to 50.8 ± 8.3 s in extruded PP
r can be attributed to the homogenization effect of the extrusion process. Extrusion promotes a more uniform distribution of oxidative degradation products and residual antioxidants, reducing localized concentrations of reactive species that would otherwise accelerate degradation. Additionally, thermal and shear conditions during extrusion may partially remove volatile oxidative byproducts and facilitate structural reorganization, slightly enhancing oxidative resistance even without added stabilizers [
44].
Besides the sharp reduction in oxidation induction time (OIT) observed with the incorporation of 25% post-consumer recycled polypropylene (PP
r), the relationship between OIT and recycled content remains linear across the 25% to 100% range (
Figure 3b) in line with the Law of Mixtures, highlighting the strong negative correlation between increasing PP
r content and decreasing OIT at this range.
3.2.3. Thermogravimetric Analysis
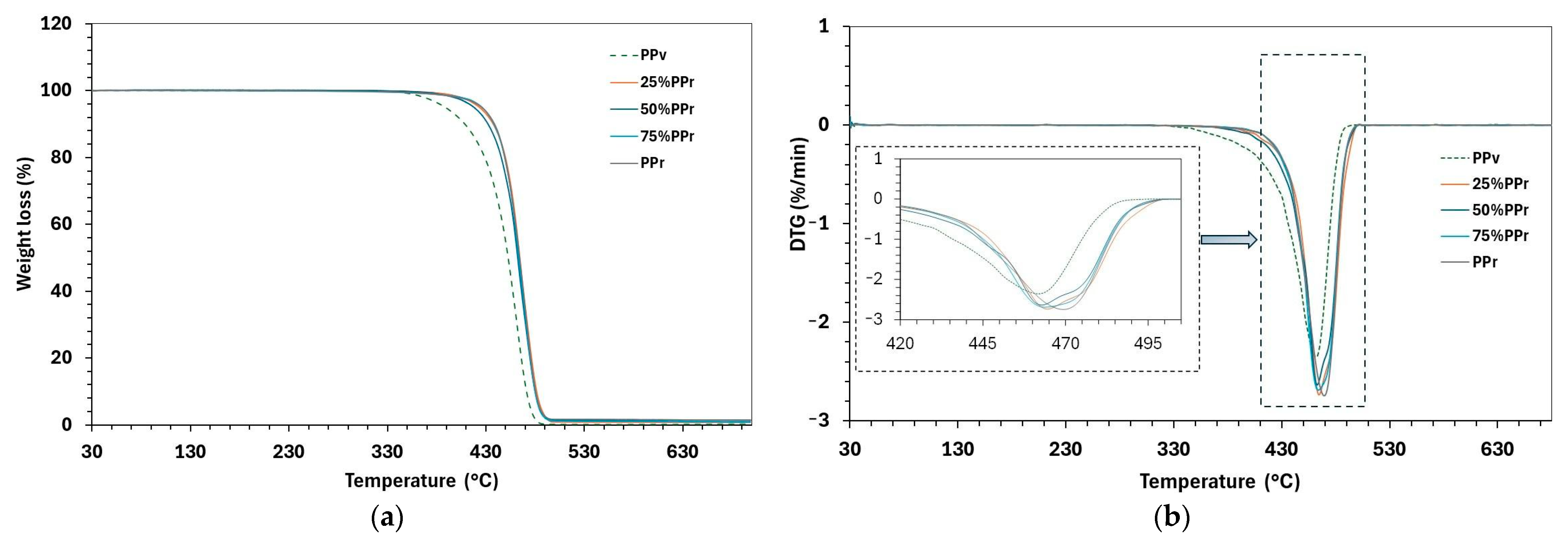
As demonstrated in
Figure 4 and
Table 6, the initial degradation temperature (T
on) of the virgin polypropylene is 325.5 °C, whereas all blends containing recycled PP exhibit a substantially higher T
on around 344 °C (for 25%, 50%, 75% PP
r blends and 100% PP
r). PP
r exhibits significantly higher T
on values, around 344 °C. This represents an increase in approximately 19 °C with the addition of any recycled content, indicating enhanced thermal stability of the blends and PP
r under an inert atmosphere. A similar trend has been reported by Stoian et al., who also reported higher degradation onset in recycled PP and its blends with virgin PP compared to the latter [
17].
Capilene, as a commercial-grade polypropylene homopolymer intended for general-purpose applications, is expected to contain a typical antioxidant (AO) package, primarily to protect against oxidative degradation during melt processing and service life. However, recycled PP contains a mixture of polypropylene grades from unknown sources and various end-use applications and may retain residual antioxidants and stabilizers from inherently more stable grades in the recycled mix that delay the onset of thermal degradation [
41]. The recycled fraction imparts enhanced stability to the blends: even at 25% PP
r content, elevating T
on to the same level as 100% recycled PP, suggesting the presence of stabilizers or altered polymer segments in the recycled material dominates the initial decomposition behaviour of the blend, outweighing the crystallinity loss (
Table 3 and
Table 4).
The peak degradation temperature (T
p) corresponds to the temperature of the fastest mass loss (the DTG peak), as shown in detail in the inset of
Figure 4b. All samples show a single prominent degradation peak, with T
p values clustered in the mid-460 °C range. Virgin PP presents a T
p of 463.3 °C, and the T
p of the 25–75% PP
r blends are very similar (within ±1 °C, around 462–465 °C). It indicates that the primary decomposition event occurs at essentially the same temperature for virgin and recycled blends, implying that the fundamental degradation mechanism is unchanged and typical of polypropylene, known to undergo a one-step, random-chain scission thermal decomposition [
38]. Moreover, it also indicates a single-step degradation for all formulations (virgin, recycled, and blends). The presence of recycled content, up to 75%, did not significantly alter the kinetics of this degradation step. The fully recycled PP shows a slightly higher T
p (469.3 °C), about 6 °C above the virgin/blend values, indicating higher thermal stability. This slight shift to a higher peak temperature for the recycled sample implies that once degradation starts, minor cross-linking/branching from its past lifecycle or trace contaminants in the recycled stream, which decomposes at higher temperatures, may influence the peak position [
45]. Moreover, recycled PP may retain residual stabilizers such as phenolic antioxidants from previous processing cycles, which can delay thermal decomposition and increase T
p under inert conditions [
44].
However, the thermal stability observed under inert conditions in TGA analysis does not reflect the oxidative stability of recycled PP and its blends with virgin PP. The oxidation induction time (OIT) results presented in
Table 5 show a clear and significant decline in oxidative resistance with increasing recycled content. While PP
r exhibits a higher T
on and T
p in TGA, it also shows an 82% reduction in OIT compared to PP
v, indicating that it has undergone oxidative degradation during its prior use and recycling. This degradation is likely caused by chain scission and the formation of oxidation-prone functional groups, such as carbonyls [
43]; however, the latter degradation mechanism is mostly absent under nitrogen in TGA analysis. Therefore, while recycled PP thermal decomposition is delayed at higher temperatures under inert conditions, its chemical structure makes it more vulnerable to oxidative attack [
38,
44,
46].
Furthermore, the degradation range (ΔT = Te − Tp), which represents the temperature interval over which most decomposition occurs, supports this interpretation. For virgin PP, Te (end degradation temperature) is about 497.6 °C, resulting in a broad degradation interval of 172.1 °C. In contrast, the blends and recycled PP exhibit slightly higher Te values (501–502 °C) but narrower ΔT ranges −ΔT of approximately 156–158 °C, degrading over a narrower temperature range, likely due to the presence of thermally unstable regions, such as oxidized or low molecular weight segments, resulting from prior thermal and mechanical degradation, and leading to a faster thermal decomposition process.