An Electrospun DFO-Loaded Microsphere/SAIB System Orchestrates Angiogenesis–Osteogenesis Coupling via HIF-1α Activation for Vascularized Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
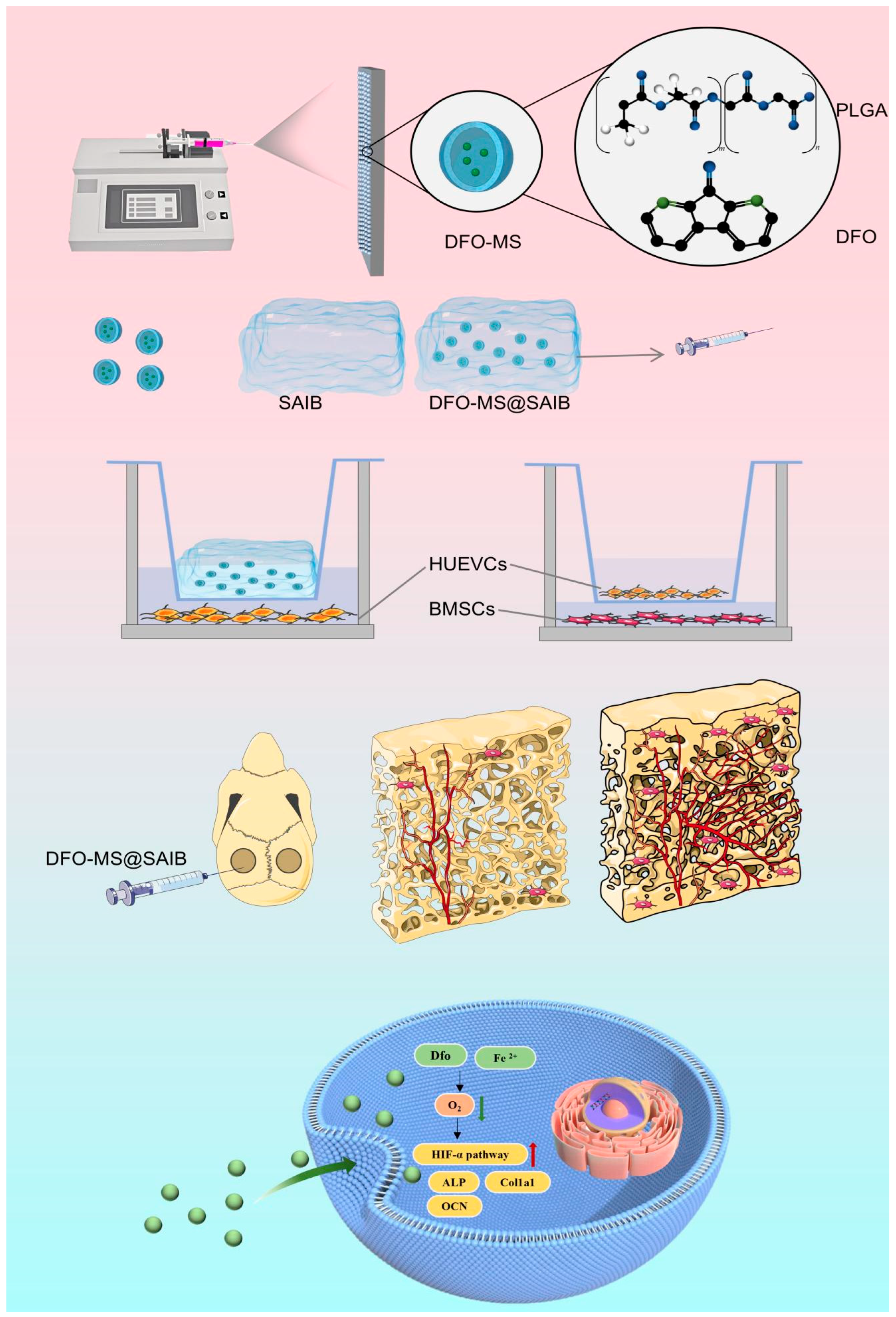
2.1. Preparation of DFO-MS and DFO-MS@SAIB
2.2. Scanning Electron Microscopy (SEM)
2.3. Drug-Encapsulation Efficacy (EE) and Drug-Loading Efficacy (LE)
2.4. Determination of the Contact Angle
2.5. In Vitro Degradation
2.6. Fourier-Transform Infrared Spectroscopy (FTIR)
2.7. In Vitro Release Analysis
2.8. Dynamic Shear Rheological Characterization
2.9. Cytocompatibility and Proliferation Assessment
2.10. In Vitro Angiogenic Evaluation of HUVECs
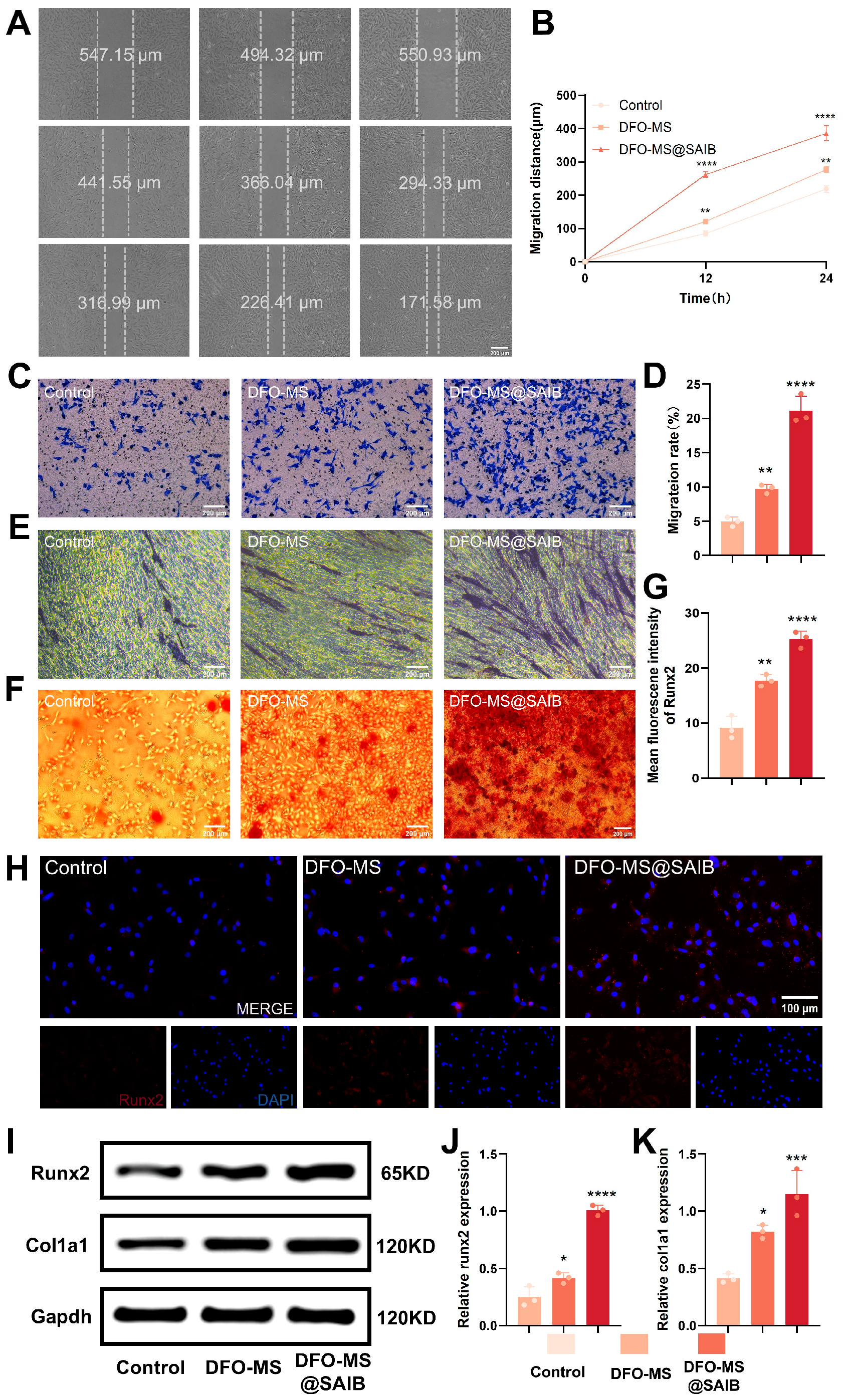
2.11. Wound Healing and Transwell Migration Assays
2.12. Alkaline Phosphatase (ALP) and Alizarin Red Staining (ARS)
2.13. Fluorescence Immunostaining
2.14. Western Blotting
2.15. In Vivo Bone Regeneration Experiments
3. Results and Discussion
3.1. Characterization of DFO-MS and DFO-MS@SAIB
3.2. Characterization of DFO-MS@SAIB
3.3. Dose Selection Based on Endothelial Angiogenesis and Viability
3.4. In Vitro Osteogenesis of BMSCs in Endothelial Co-Culture Systems
3.5. In Vivo Osteogenesis in Calvarial Defects
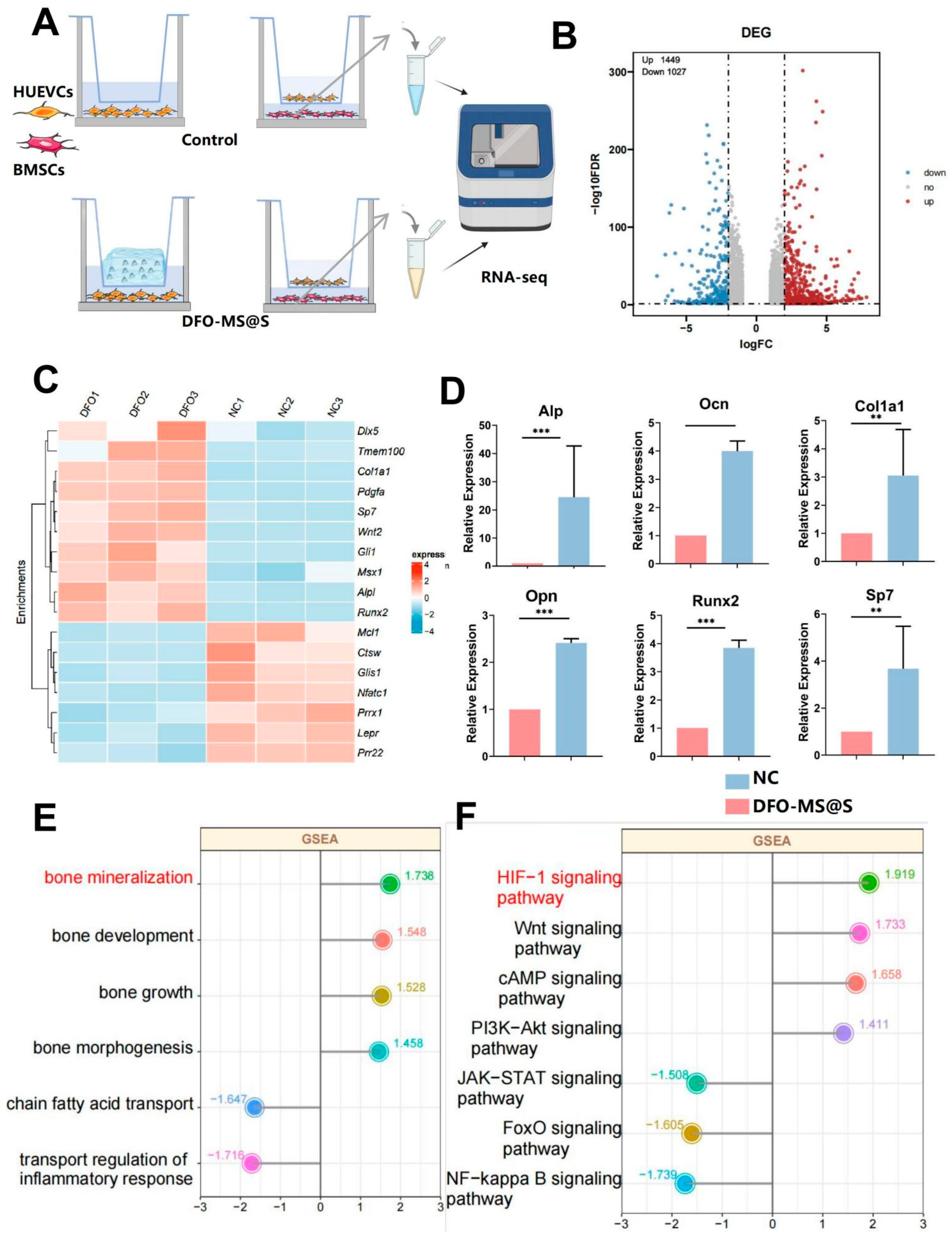
3.6. RNA-Seq Analysis of BMSCs and HUVECs Treated with DFO-MS@S
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Fan, M.; Zhang, Y. Revolutionizing bone defect healing: The power of mesenchymal stem cells as seeds. Front. Bioeng. Biotechnol. 2024, 12, 1421674. [Google Scholar] [CrossRef] [PubMed]
- Gage, J.; Liporace, A.; Egol, A.; McLaurin, M. Management of Bone Defects in Orthopedic Trauma. Bull. NYU Hosp. Jt. Dis. 2018, 76, 4–8. [Google Scholar]
- Ramasamy, S.K.; Kusumbe, A.P.; Wang, L.; Adams, R.H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 2014, 507, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.K.; Kusumbe, A.P.; Schiller, M.; Zeuschner, D.; Bixel, M.G.; Milia, C.; Gamrekelashvili, J.; Limbourg, A.; Medvinsky, A.; Santoro, M.M.; et al. Blood flow controls bone vascular function and osteogenesis. Nat. Commun. 2016, 7, 13601. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Huang, C.; Duan, D.; Lou, A.; Guo, Y.; Xiao, T.; Wei, J.; Liu, S.; Wang, Z.; Yang, Q.; et al. Injectable temperature-sensitive hydrogel system incorporating deferoxamine-loaded microspheres promotes H-type blood vessel-related bone repair of a critical size femoral defect. Acta Biomater. 2022, 153, 108–123. [Google Scholar] [CrossRef]
- Cao, L.; Wang, J.; Hou, J.; Xing, W.; Liu, C. Vascularization and bone regeneration in a critical sized defect using 2-N,6-O-sulfated chitosan nanoparticles incorporating BMP-2. Biomaterials 2014, 35, 684–698. [Google Scholar] [CrossRef]
- Li, X.L.; Zhao, Y.Q.; Miao, L.; An, Y.X.; Wu, F.; Han, J.Y.; Han, J.Y.; Tay, F.R.; Mu, Z.; Jiao, Y.; et al. Strategies for promoting neurovascularization in bone regeneration. Mil. Med. Res. 2025, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- de Silva, L.; Bernal, P.N.; Rosenberg, A.; Malda, J.; Levato, R.; Gawlitta, D. Biofabricating the vascular tree in engineered bone tissue. Acta Biomater. 2023, 156, 250–268. [Google Scholar] [CrossRef]
- Shim, S.S. Physiology of blood circulation of bone. J. Bone Jt. Surg. 1968, 50, 812–824. [Google Scholar] [CrossRef]
- Sivan, U.; De Angelis, J.; Kusumbe, A.P. Role of angiocrine signals in bone development, homeostasis and disease. Open Biol. 2019, 9, 190144. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.N.; Cammisa, F.P., Jr.; Sandhu, H.S.; Diwan, A.D.; Girardi, F.P.; Lane, J.M. The biology of bone grafting. J. Am. Acad. Orthop. Surg. 2005, 13, 77–86. [Google Scholar] [CrossRef]
- Wong, S.K.; Yee, M.M.F.; Chin, K.Y.; Ima-Nirwana, S. A Review of the Application of Natural and Synthetic Scaffolds in Bone Regeneration. J. Funct. Biomater. 2023, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, J.; Wang, Y.; Han, W.; Wei, Y.; Hu, Y.; Liang, Z.; Lian, X.; Huang, D. Hydroxyapatite/Polyurethane Scaffolds for Bone Tissue Engineering. Tissue. Eng. Part B Rev. 2024, 30, 60–73. [Google Scholar] [CrossRef]
- Geng, Y.; Duan, H.; Xu, L.; Witman, N.; Yan, B.; Yu, Z.; Wang, H.; Tan, Y.; Lin, L.; Li, D.; et al. BMP-2 and VEGF-A modRNAs in collagen scaffold synergistically drive bone repair through osteogenic and angiogenic pathways. Commun. Biol. 2021, 4, 82. [Google Scholar] [CrossRef]
- Bouletreau, P.J.; Warren, S.M.; Spector, J.A.; Peled, Z.M.; Gerrets, R.P.; Greenwald, J.A.; Longaker, M.T. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: Implications for fracture healing. Plast. Reconstr. Surg. 2002, 109, 2384–2397. [Google Scholar] [CrossRef]
- Lee, S.S.; Kim, J.H.; Jeong, J.; Kim, S.H.L.; Koh, R.H.; Kim, I.; Bae, S.; Lee, H.; Hwang, N.S. Sequential growth factor releasing double cryogel system for enhanced bone regeneration. Biomaterials 2020, 257, 120223. [Google Scholar] [CrossRef]
- Legrand, J.M.D.; Martino, M.M. Growth Factor and Cytokine Delivery Systems for Wound Healing. Cold Spring Harb. Perspect. Biol. 2022, 14, a041234. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Fang, J.; Zhong, C.; Wang, M.; Ren, F. Spatiotemporal Delivery of pBMP2 and pVEGF by a Core-Sheath Structured Fiber-Hydrogel Gene-Activated Matrix Loaded with Peptide-Modified Nanoparticles for Critical-Sized Bone Defect Repair. Adv. Healthc. Mater. 2022, 11, e2201096. [Google Scholar] [CrossRef]
- Francis, G.L.; McNamara, P.J.; Filsell, O.H.; Ballard, F.J. Plasma half-lives of native and modified insulin-like growth factor-I in lambs. J. Endocrinol. 1988, 117, 183–189. [Google Scholar] [CrossRef]
- Nauth, A.; Giles, E.; Potter, B.K.; Nesti, L.J.; O’Brien, F.P.; Bosse, M.J.; Anglen, J.O.; Mehta, S.; Ahn, J.; Miclau, T.; et al. Heterotopic ossification in orthopaedic trauma. J. Orthop. Trauma. 2012, 26, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, P.; Yu, F.; Luo, G.; Qing, L.; Tang, J. HIF-1alpha Regulates Bone Homeostasis and Angiogenesis, Participating in the Occurrence of Bone Metabolic Diseases. Cells 2022, 11, 3552. [Google Scholar] [CrossRef]
- Feng, T.; Zhao, X.; Gu, P.; Yang, W.; Wang, C.; Guo, Q.; Long, Q.; Liu, Q.; Cheng, Y.; Li, J.; et al. Adipocyte-derived lactate is a signalling metabolite that potentiates adipose macrophage inflammation via targeting PHD2. Nat. Commun. 2022, 13, 5208. [Google Scholar] [CrossRef]
- Zeng, L.; Tan, L.; Li, H.; Zhang, Q.; Li, Y.; Guo, J. Deferoxamine therapy for intracerebral hemorrhage: A systematic review. PLoS ONE 2018, 13, e0193615. [Google Scholar] [CrossRef]
- Qiu, M.; Li, C.; Cai, Z.; Li, C.; Yang, K.; Tulufu, N.; Chen, B.; Cheng, L.; Zhuang, C.; Liu, Z.; et al. 3D Biomimetic Calcified Cartilaginous Callus that Induces Type H Vessels Formation and Osteoclastogenesis. Adv. Sci. 2023, 10, e2207089. [Google Scholar] [CrossRef]
- Shan, B.H.; Wu, F.G. Hydrogel-Based Growth Factor Delivery Platforms: Strategies and Recent Advances. Adv. Mater. 2024, 36, e2210707. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Su, Y.; Zhang, H.; Liu, N.; Wang, Z.; Gao, X.; Gao, J.; Zheng, A. Poly(lactic-co-glycolic acid) microsphere production based on quality by design: A review. Drug Deliv. 2021, 28, 1342–1355. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, B.; Xia, G.; Choi, S.H. FDA’s Poly (Lactic-Co-Glycolic Acid) Research Program and Regulatory Outcomes. AAPS J. 2021, 23, 92. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, J.; Meng, D.; Yi, Y.; Zhang, T.; Shu, Y.; Wu, X. Electrospun naringin-loaded microsphere/sucrose acetate isobutyrate system promotes macrophage polarization toward M2 and facilitates osteoporotic bone defect repair. Regen. Biomater. 2023, 10, rbad006. [Google Scholar] [CrossRef]
- Filipowska, J.; Tomaszewski, K.A.; Niedzwiedzki, L.; Walocha, J.A.; Niedzwiedzki, T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017, 20, 291–302. [Google Scholar] [CrossRef]
- Hankenson, K.D.; Dishowitz, M.; Gray, C.; Schenker, M. Angiogenesis in bone regeneration. Injury 2011, 42, 556–561. [Google Scholar] [CrossRef]
- Stegen, S.; van Gastel, N.; Carmeliet, G. Bringing new life to damaged bone: The importance of angiogenesis in bone repair and regeneration. Bone 2015, 70, 19–27. [Google Scholar] [CrossRef]
- Schultz, K.; Stuwe, D.; Westhoff, B. Juvenile osteochondrosis and osteonecrosis. Orthopadie 2022, 51, 829–843. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, Z.; Zheng, Z.; Chen, H.; Chen, Y. Nanomaterial-integrated injectable hydrogels for craniofacial bone reconstruction. J. Nanobiotechnol. 2024, 22, 525. [Google Scholar] [CrossRef] [PubMed]
- Gharanizadeh, K.; Sharifi, A.M.; Tayyebi, H.; Heidari, R.; Amiri, S.; Noorigaravand, S. Core decompression combined with local DFO administration loaded on polylactic glycolic acid scaffolds for the treatment of osteonecrosis of the femoral head: A pilot study. BMC Pharmacol. Toxicol. 2023, 24, 44. [Google Scholar] [CrossRef]
- Burin, G.R.M.; Santos, T.C.d.; Battisti, M.A.; Campos, A.M.d.; Ferreira, S.R.S.; Carciofi, B.A.M. Transport properties of hydrophilic compounds in PLGA microspheres. Res. Soc. Dev. 2022, 11, e398111638335. [Google Scholar] [CrossRef]
- Schweitzer, C. Encapsulation of a Small Hydrophilic Drug in Injectable PLGA Microparticles for Treatment of Pancreatic Cancer. In Proceedings of the 7th Inquiry@Queen’s Undergraduate Research Conference Proceedings, 2013. 7th I@Q Conference Proceedings; Available online: https://ojs.library.queensu.ca/index.php/inquiryatqueens/issue/view/708 (accessed on 2 May 2025).
- Jia, P.; Chen, H.; Kang, H.; Qi, J.; Zhao, P.; Jiang, M.; Guo, L.; Zhou, Q.; Qian, N.D.; Zhou, H.B.; et al. Deferoxamine released from poly(lactic-co-glycolic acid) promotes healing of osteoporotic bone defect via enhanced angiogenesis and osteogenesis. J. Biomed. Mater. Res. A 2016, 104, 2515–2527. [Google Scholar] [CrossRef]
- Barakh Ali, S.F.; Dharani, S.; Afrooz, H.; Mohamed, E.M.; Cook, P.; Khan, M.A.; Rahman, Z. Development of Abuse-Deterrent Formulations Using Sucrose Acetate Isobutyrate. AAPS PharmSciTech 2020, 21, 99. [Google Scholar] [CrossRef]
- Schlegel, C.; Liu, K.; Spring, B.; Dietz, S.; Poets, C.F.; Hudalla, H.; Lajqi, T.; Köstlin-Gille, N.; Gille, C. Decreased expression of hypoxia-inducible factor 1α (HIF-1α) in cord blood monocytes under anoxia. Pediatr. Res. 2023, 93, 870–877. [Google Scholar] [CrossRef]
- Yue, X.; Lin, X.; Yang, T.; Yang, X.; Yi, X.; Jiang, X.; Li, X.; Li, T.; Guo, J.; Dai, Y.; et al. Rnd3/RhoE Modulates Hypoxia-Inducible Factor 1α/Vascular Endothelial Growth Factor Signaling by Stabilizing Hypoxia-Inducible Factor 1α and Regulates Responsive Cardiac Angiogenesis. Hypertension 2016, 67, 597–605. [Google Scholar] [CrossRef]
- Song, S.; Zhang, G.; Chen, X.; Zheng, J.; Liu, X.; Wang, Y.; Chen, Z.; Wang, Y.; Song, Y.; Zhou, Q. HIF-1α increases the osteogenic capacity of ADSCs by coupling angiogenesis and osteogenesis via the HIF-1α/VEGF/AKT/mTOR signaling pathway. J. Nanobiotechnol. 2023, 21, 257. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, J.; Wu, S.; Wang, H. Electroacupuncture pretreatment promotes angiogenesis via hypoxia-inducible factor 1α and vascular endothelial growth factor in a rat model of chronic myocardial ischemia. Acup. Med. 2021, 39, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Liu, M.; Jihu, Y.; Zeng, H.; Yao, C.; Yan, H. Hypoxia activates the PI3K/AKT/HIF-1α pathway to promote the anti-inflammatory effect of adipose mesenchymal stem cells. Acta Histochem. 2023, 125, 152042. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, M.; Li, W.; Guo, Y.; Lou, H.; Zhang, J.; Xu, Y.; Zeng, B.; Wen, X.; Ji, X.; et al. CBX7 promotes choroidal neovascularization by activating the HIF-1α/VEGF pathway in choroidal vascular endothelial cells. Exp. Eye Res. 2024, 247, 110057. [Google Scholar] [CrossRef] [PubMed]
- Almalki, S.G.; Agrawal, D.K. ERK signaling is required for VEGF-A/VEGFR2-induced differentiation of porcine adipose-derived mesenchymal stem cells into endothelial cells. Stem. Cell Res. Ther. 2017, 8, 113. [Google Scholar] [CrossRef]
- Petrillo, S.; Genova, T.; Chinigo, G.; Roato, I.; Scarpellino, G.; Kopecka, J.; Altruda, F.; Tolosano, E.; Riganti, C.; Mussano, F.; et al. Endothelial Cells Promote Osteogenesis by Establishing a Functional and Metabolic Coupling With Human Mesenchymal Stem Cells. Front. Physiol. 2021, 12, 813547. [Google Scholar] [CrossRef]
- Tsao, C.C.; Baumann, J.; Huang, S.F.; Kindler, D.; Schroeter, A.; Kachappilly, N.; Gassmann, M.; Rudin, M.; Ogunshola, O.O. Pericyte hypoxia-inducible factor-1 (HIF-1) drives blood-brain barrier disruption and impacts acute ischemic stroke outcome. Angiogenesis 2021, 24, 823–842. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, X.; Yuan, X.; Wu, X. An Electrospun DFO-Loaded Microsphere/SAIB System Orchestrates Angiogenesis–Osteogenesis Coupling via HIF-1α Activation for Vascularized Bone Regeneration. Polymers 2025, 17, 1538. https://doi.org/10.3390/polym17111538
Shan X, Yuan X, Wu X. An Electrospun DFO-Loaded Microsphere/SAIB System Orchestrates Angiogenesis–Osteogenesis Coupling via HIF-1α Activation for Vascularized Bone Regeneration. Polymers. 2025; 17(11):1538. https://doi.org/10.3390/polym17111538
Chicago/Turabian StyleShan, Xujia, Xiaoyan Yuan, and Xiaohong Wu. 2025. "An Electrospun DFO-Loaded Microsphere/SAIB System Orchestrates Angiogenesis–Osteogenesis Coupling via HIF-1α Activation for Vascularized Bone Regeneration" Polymers 17, no. 11: 1538. https://doi.org/10.3390/polym17111538
APA StyleShan, X., Yuan, X., & Wu, X. (2025). An Electrospun DFO-Loaded Microsphere/SAIB System Orchestrates Angiogenesis–Osteogenesis Coupling via HIF-1α Activation for Vascularized Bone Regeneration. Polymers, 17(11), 1538. https://doi.org/10.3390/polym17111538





