A Study of the Dielectric Relaxation of Nitrile–Butadiene Rubber, Ethylene–Propylene–Diene Monomer, and Fluoroelastomer Polymers with a Self-Developed Deconvolution Analysis Program
Abstract
1. Introduction
2. Relaxation Process and Model Analysis
2.1. α Relaxation
2.2. α′ Relaxation (Normal Mode Relaxation)
2.3. β and γ Relaxations (Secondary Relaxation)
2.4. Interfacial Relaxation (MWS Relaxation)
2.5. DC Conduction Process
2.6. Models for Analyzing the Relaxation Process
3. Methods
3.1. Dielectric Spectroscopy
3.2. Differential Scanning Calorimetry
4. Development of Self-Developed Deconvolution Analysis Program
- Weighted fitting between the real and imaginary parts of permittivity spectra to emphasize dominant spectral contributions;
- Global fitting across temperature series under physically meaningful constraints (e.g., shared activation energy or Arrhenius/VFT behavior);
- User-controllable fitting accuracy achieved by adjusting convergence thresholds, iteration limits, and optimization tolerances.
5. Results and Discussion
5.1. NBR
- (i).
- The temperature range for β relaxation: 233–269 K.
- (ii).
- The temperature range for γ relaxation: 233–269 K.
- (iii).
- The temperature range for α relaxation: 233–341 K.
- (iv).
- The temperature range for α’ relaxation: 254–404 K.
- (v).
- The temperature range for DC conduction contribution: 324–404 K.
5.2. EPDM
- (i).
- The temperature range for α relaxation: 233 K to 302 K.
- (ii).
- The temperature range for β relaxation: 233 K to 305 K.
- (iii).
- The temperature range for αβ merged relaxation: 305 K to 404 K.
- (iv).
- The temperature range for the DC conduction contribution: 308 K to 404 K.
- (v).
- The temperature range for MWS relaxation: 233 K to 404 K.
5.3. FKM
- (i).
- The temperature range for β relaxation: 233 K to 308 K.
- (ii).
- The temperature range for α relaxation: 268 K to 308 K.
- (iii).
- The temperature range for MWS relaxation: 268 K to 373 K.
- (iv).
- The temperature range for the DC conduction contribution: 283 K to 373 K.
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rodgers, B. Rubber Compounding: Chemistry and Applications; CRC Press: New York, NY, USA, 2015. [Google Scholar]
- Vijayaram, T.R. A technical review on rubber. Int. J. Des. Manuf. Technol. 2009, 3, 25–37. [Google Scholar] [CrossRef]
- Azahar, N.M.; Hassan, N.A.; Jaya, R.P.; Kadir, M.A.A.; Yunus, N.Z.M.; Mahmud, M.Z.H. An overview on natural rubber application for asphalt modification. Int. J. Agric. For. Plant. 2016, 2, 212–218. [Google Scholar]
- Myhre, M.; Saiwari, S.; Dierkes, W.; Noordermeer, J. Rubber recycling: Chemistry, processing, and applications. Rubber Chem. Technol. 2012, 85, 408–449. [Google Scholar] [CrossRef]
- Yoon, B.; Kim, J.Y.; Hong, U.; Oh, M.K.; Kim, M.; Han, S.B.; Nam, J.-D.; Suhr, J. Dynamic viscoelasticity of silica-filled styrene-butadiene rubber/polybutadiene rubber (SBR/BR) elastomer composites. Compos. Part B Eng. 2020, 187, 107865. [Google Scholar] [CrossRef]
- Liu, Z. Rational design of high-performance nanomaterials for electric vehicle tires. Highlights Sci. Eng. Technol. 2023, 62, 110–118. [Google Scholar] [CrossRef]
- Liao, L.; Zuo, Y.; Meng, H.; Liao, X. Research on the technology of noise reduction in hybrid electric vehicle with composite materials. Adv. Mech. Eng. 2018, 10, 1687814018766916. [Google Scholar] [CrossRef]
- Sancho-Turrado, A.; Lopez, J.A.R.; Calleja, A.J. Improved composite for tires of urban electric vehicles. In Proceedings of the 2013 International Conference on New Concepts in Smart Cities: Fostering Public and Private Alliances (SmartMILE), Gijon, Spain, 11–13 December 2013; pp. 1–6. [Google Scholar]
- Rahnavard, M.; Motlagh, G.H.; Heidarian, J.; Mobarake, M.D. Improving heat ageing, mechanical properties, and thermal conductivity of silicon rubber by surface modification of iron oxide and field testing of silicon rubber O-rings. J. Pet. Sci. Technol. 2023, 13, 17–27. [Google Scholar]
- Yamabe, J.; Nishimura, S. Influence of fillers on hydrogen penetration properties and blister fracture of rubber composites for O-ring exposed to high-pressure hydrogen gas. Int. J. Hydrogen Energy 2009, 34, 1977–1989. [Google Scholar] [CrossRef]
- Mao, H.; Zou, H.; Liu, W.; Zhuang, X.; Xing, B. Preparation and application research of composites with low vacuum outgassing and excellent electromagnetic sealing performance. e-Polymers 2024, 24, 170–177. [Google Scholar] [CrossRef]
- Zhou, C.; Zheng, J.; Gu, C.; Zhao, Y.; Liu, P. Sealing performance analysis of rubber O-ring in high-pressure gaseous hydrogen based on finite element method. Int. J. Hydrogen Energy 2017, 42, 11996–12004. [Google Scholar] [CrossRef]
- Jung, J.-K.; Kim, K.-T.; Chung, N.-K.; Baek, U.-B.; Nahm, S.-H. Characterizing the diffusion property of hydrogen sorption and desorption processes in several spherical-shaped polymers. Polymers 2022, 14, 1468. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, M.L.; Barchenko, V.T.; Lisenkov, A.A.; Kostrin, D.K.; Babinov, N.A. Gas permeation through vacuum materials: Mass-spectrometry measurement system. Vak. Forsch. Prax. 2015, 27, 26–29. [Google Scholar] [CrossRef]
- Chea, S.; Luengchavanon, M.; Anancharoenwong, E.; Techato, K.-a.; Jutidamrongphan, W.; Chaiprapat, S.; Niyomwas, S.; Marthosa, S. Development of an O-ring from NR/EPDM filled silica/CB hybrid filler for use in a solid oxide fuel cell testing system. Polym. Test. 2020, 88, 106568. [Google Scholar] [CrossRef]
- Rivera, M. Elastomers in space and in other high vacuum environments. Rubber Chem. Technol. 1966, 39, 1127–1140. [Google Scholar] [CrossRef]
- Koga, A.; Uchida, K.; Yamabe, J.; Nishimura, S. Evaluation on high-pressure hydrogen decompression failure of rubber O-ring using design of experiments. Int. J. Automot. Eng. 2011, 2, 123–129. [Google Scholar] [CrossRef]
- Jin, L.; Che, L. Experimental study on stress relaxation of rubber O-ring for mechanical seal. J. Phys. Conf. Ser. 2024, 2691, 012015. [Google Scholar] [CrossRef]
- Yang, H.; Yao, X.; Zheng, Z.; Liu, Y. Graphene rubber composites integrated sealing rings for monitoring contact pressure and the aging process. Compos. Part A Appl. Sci. Manuf. 2019, 118, 171–178. [Google Scholar] [CrossRef]
- Jung, J.K.; Lee, J.H.; Kim, Y.W.; Chung, N.K. Development of portable gas sensing system for measuring gas emission concentration and diffusivity using commercial manometric sensors in gas exposed polymers: Application to pure gases, H2, He, N2, O2 and Ar. Sens. Actuators B Chem. 2024, 418, 136240. [Google Scholar] [CrossRef]
- Jung, J.K.; Lee, J.H. High-performance hydrogen gas sensor system based on transparent coaxial cylinder capacitive electrodes and a volumetric analysis technique. Sci. Rep. 2024, 14, 1967. [Google Scholar] [CrossRef]
- Jung, J.K.; Kim, K.-T.; Lee, J.H.; Baek, U.B. Effective and low-cost gas sensor based on a light intensity analysis of a webcam image: Gas enriched polymers under high pressure. Sens. Actuators B Chem. 2023, 393, 134258. [Google Scholar] [CrossRef]
- Jung, J.K.; Kim, K.-T.; Baek, U.B. Simultaneous three-channel measurements of hydrogen diffusion with light intensity analysis of images by employing webcam. Curr. Appl. Phys. 2022, 37, 19–26. [Google Scholar] [CrossRef]
- Jung, J.; Kim, G.; Gim, G.; Park, C.; Lee, J. Determination of gas permeation properties in polymer using capacitive electrode sensors. Sensors 2022, 22, 1141. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.W.; Kim, D.J.; Chung, N.K.; Jung, J.K. Comparison of two methods for measuring the temperature dependence of H2 permeation parameters in nitrile butadiene rubber polymer composites blended with fillers: The volumetric analysis method and the differential pressure method. Polymers 2024, 16, 280. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-W.; Jung, J.-K. Investigation of the gas permeation properties using the volumetric analysis technique for polyethylene materials enriched with pure gases under high pressure: H2, He, N2, O2 and Ar. Polymers 2023, 15, 4019. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.K.; Lee, J.H.; Jang, J.S.; Chung, N.K.; Park, C.Y.; Baek, U.B.; Nahm, S.H. Characterization technique of gases permeation properties in polymers: H2, He, N2 and Ar gas. Sci. Rep. 2022, 12, 3328. [Google Scholar] [CrossRef]
- Jung, J.K.; Kim, K.-T.; Chung, K.S. Two volumetric techniques for determining the transport properties of hydrogen gas in polymer. Mater. Chem. Phys. 2022, 276, 125364. [Google Scholar] [CrossRef]
- Jung, J.K.; Kim, I.G.; Chung, K.S.; Baek, U.B. Analyses of permeation characteristics of hydrogen in nitrile butadiene rubber using gas chromatography. Mater. Chem. Phys. 2021, 267, 124653. [Google Scholar] [CrossRef]
- Jung, J.K.; Kim, I.G.; Jeon, S.K.; Kim, K.-T.; Baek, U.B.; Nahm, S.H. Volumetric analysis technique for analyzing the transport properties of hydrogen gas in cylindrical-shaped rubbery polymers. Polym. Test. 2021, 99, 107147. [Google Scholar] [CrossRef]
- Jung, J.K.; Kim, I.G.; Kim, K.T.; Ryu, K.S.; Chung, K.S. Evaluation techniques of hydrogen permeation in sealing rubber materials. Polym. Test. 2021, 93, 107016. [Google Scholar] [CrossRef]
- Jung, J.K.; Kim, I.G.; Chung, K.S.; Kim, Y.-I.; Kim, D.H. Determination of permeation properties of hydrogen gas in sealing rubbers using thermal desorption analysis gas chromatography. Sci. Rep. 2021, 11, 17092. [Google Scholar] [CrossRef]
- Jung, J.K.; Jeon, S.K.; Kim, K.-T.; Lee, C.H.; Baek, U.B.; Chung, K.S. Impedance spectroscopy for in situ and real-time observations of the effects of hydrogen on nitrile butadiene rubber polymer under high pressure. Sci. Rep. 2019, 9, 13035. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, Y.W.; Chung, N.K.; Kang, H.M.; Moon, W.J.; Chan Choi, M.; Jung, J.K. Multiphase modeling of pressure-dependent hydrogen diffusivity in fractal porous structures of acrylonitrile butadiene rubber-carbon black composites with different fillers. Polymer 2024, 311, 127552. [Google Scholar] [CrossRef]
- Lee, C.H.; Jung, J.K.; Kim, K.S.; Kim, C.J. Hierarchical channel morphology in O-rings after two cycling exposures to 70 MPa hydrogen gas: A case study of sealing failure. Sci. Rep. 2024, 14, 5319. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.K.; Lee, J.H.; Jeon, S.K.; Tak, N.H.; Chung, N.K.; Baek, U.B.; Lee, S.H.; Lee, C.H.; Choi, M.C.; Kang, H.M.; et al. Correlations between H2 permeation and physical/mechanical properties in ethylene propylene diene monomer polymers blended with carbon black and silica fillers. Int. J. Mol. Sci. 2023, 24, 2865. [Google Scholar] [CrossRef]
- Moon, Y.I.; Lee, H.E.; Jung, J.K.; Han, H.W. Direct visualization of carbon black aggregates in nitrile butadiene rubber by THz near-field microscope. Sci. Rep. 2023, 13, 7846. [Google Scholar] [CrossRef]
- Jung, J.K.; Baek, U.B.; Lee, S.H.; Choi, M.C.; Bae, J.W. Hydrogen gas permeation in peroxide-crosslinked ethylene propylene diene monomer polymer composites with carbon black and silica fillers. J. Polym. Sci. 2023, 61, 460–471. [Google Scholar] [CrossRef]
- Jung, J.K.; Lee, C.H.; Baek, U.B.; Choi, M.C.; Bae, J.W. Filler influence on H2 permeation properties in sulfur-crosslinked ethylene propylene diene monomer polymers blended with different concentrations of carbon black and silica fillers. Polymers 2022, 14, 592. [Google Scholar] [CrossRef]
- Jung, J.K.; Lee, C.H.; Son, M.S.; Lee, J.H.; Baek, U.B.; Chung, K.S.; Choi, M.C.; Bae, J.W. Filler effects on H2 diffusion behavior in nitrile butadiene rubber blended with carbon black and silica fillers of different concentrations. Polymers 2022, 14, 700. [Google Scholar] [CrossRef]
- Jung, J.K.; Baek, U.B.; Nahm, S.H.; Chung, K.S. Hydrogen sorption and desorption properties in rubbery polymer. Mater. Chem. Phys. 2022, 279, 125745. [Google Scholar] [CrossRef]
- Kang, H.; Bae, J.; Lee, J.; Yun, Y.; Jeon, S.; Chung, N.; Jung, J.; Baek, U.; Lee, J.; Kim, Y.; et al. The synergistic effect of carbon black/carbon nanotube hybrid fillers on the physical and mechanical properties of EPDM composites after exposure to high-pressure hydrogen gas. Polymers 2024, 16, 1065. [Google Scholar] [CrossRef]
- Chai, A.B.; Andriyana, A.; Verron, E.; Johan, M.R.; Haseeb, A.S.M.A. Development of a compression test device for investigating interaction between diffusion of biodiesel and large deformation in rubber. Polym. Test. 2011, 30, 867–875. [Google Scholar] [CrossRef]
- Rowland, S.M.; Robertson, J.; Xiong, Y.; Day, R.J. Electrical and material characterization of field-aged 400 kV silicone rubber composite insulators. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 375–383. [Google Scholar] [CrossRef]
- Dick, T.A.; dos Santos, L.A. In situ synthesis and characterization of hydroxyapatite/natural rubber composites for biomedical applications. Mater. Sci. Eng. C 2017, 77, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Song, K. Interphase characterization in rubber nanocomposites. In Progress in Rubber Nanocomposites; Thomas, S., Maria, H.J., Eds.; Elsevier Inc.: Cambridge, MA, USA, 2017; pp. 115–152. [Google Scholar]
- Bandyopadhyay, A.; Bhowmick, A.K.; De Sarkar, M. Synthesis and characterization of acrylic rubber/silica hybrid composites prepared by sol-gel technique. J. Appl. Polym. Sci. 2004, 93, 2579–2589. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, M.B.; Sagar, S.; Maqsood, A. Fabrication and characterization of multiwalled carbon nanotubes/silicone rubber composites. J. Appl. Polym. Sci. 2012, 128, 2439–2446. [Google Scholar] [CrossRef]
- Chaikumpollert, O.; Yamamoto, Y.; Suchiva, K.; Kawahara, S. Mechanical properties and cross-linking structure of cross-linked natural rubber. Polym. J. 2012, 44, 772–777. [Google Scholar] [CrossRef]
- Jung, J.K.; Lee, J.H.; Park, J.Y.; Jeon, S.K. Modeling of the time-dependent H2 emission and equilibrium time in H2-enriched polymers with cylindrical, spherical and sheet shapes and comparisons with experimental investigations. Polymers 2024, 16, 2158. [Google Scholar] [CrossRef]
- Zedler, Ł.; Colom, X.; Saeb, M.R.; Formela, K. Preparation and characterization of natural rubber composites highly filled with brewers’ spent grain/ground tire rubber hybrid reinforcement. Compos. Part B Eng. 2018, 145, 182–188. [Google Scholar] [CrossRef]
- Qiu, X.; Yin, H.; Xing, Q. Research progress on fatigue life of rubber materials. Polymers 2022, 14, 4592. [Google Scholar] [CrossRef]
- Genovese, A.; D’Angelo, G.A.; Sakhnevych, A.; Farroni, F. Review on friction and wear test rigs: An overview on the state of the art in tyre tread friction evaluation. Lubricants 2020, 8, 91. [Google Scholar] [CrossRef]
- Jeon, S.K.; Jung, J.K.; Chung, N.K.; Baek, U.B.; Nahm, S.H. Investigation of physical and mechanical characteristics of rubber materials exposed to high-pressure hydrogen. Polymers 2022, 14, 2233. [Google Scholar] [CrossRef] [PubMed]
- Valentín, J.L.; Carretero-González, J.; Mora-Barrantes, I.; Chassé, W.; Saalwächter, K. Uncertainties in the determination of cross-link density by equilibrium swelling experiments in natural rubber. Macromolecules 2008, 41, 4717–4729. [Google Scholar] [CrossRef]
- Karabork, F.; Pehlivan, E.; Akdemir, A. Characterization of styrene butadiene rubber and microwave devulcanized ground tire rubber composites. J. Polym. Eng. 2014, 34, 543–554. [Google Scholar] [CrossRef]
- Soma, P.; Tada, N.; Uchida, M.; Nakahara, K.; Taga, Y. A fracture mechanics approach for evaluating the effects of heat aging on fatigue crack growth of vulcanized natural rubber. J. Solid Mech. Mater. Eng. 2010, 4, 727–737. [Google Scholar] [CrossRef]
- Rattanasom, N.; Prasertsri, S.; Ruangritnumchai, T. Comparison of the mechanical properties at similar hardness level of natural rubber filled with various reinforcing-fillers. Polym. Test. 2009, 28, 8–12. [Google Scholar] [CrossRef]
- Jung, J.K. Review of developed methods for measuring gas uptake and diffusivity in polymers enriched by pure gas under high pressure. Polymers 2024, 16, 723. [Google Scholar] [CrossRef]
- Jung, J.K.; Kim, I.G.; Kim, K.T. Evaluation of hydrogen permeation characteristics in rubbery polymers. Curr. Appl. Phys. 2021, 21, 43–49. [Google Scholar] [CrossRef]
- Jung, J.K.; Kim, I.G.; Chung, K.S.; Baek, U.B. Gas chromatography techniques to evaluate the hydrogen permeation characteristics in rubber: Ethylene propylene diene monomer. Sci. Rep. 2021, 11, 4859. [Google Scholar] [CrossRef]
- Choi, B.-L.; Jung, J.K.; Baek, U.B.; Choi, B.-H. Effect of functional fillers on tribological characteristics of acrylonitrile butadiene rubber after high-pressure hydrogen exposures. Polymers 2022, 14, 861. [Google Scholar] [CrossRef]
- Jung, J.K.; Kim, I.G.; Kim, K.-T.; Baek, U.B.; Nahm, S.H. Novel volumetric analysis technique for characterizing the solubility and diffusivity of hydrogen in rubbers. Curr. Appl. Phys. 2021, 26, 9–15. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, J.K. Development of image-based water level sensor with high-resolution and low-cost using image processing algorithm: Application to outgassing measurements from gas-enriched polymer. Sensors 2024, 24, 7699. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.K.; Lee, J.H.; Jeon, S.K.; Baek, U.B.; Lee, S.H.; Lee, C.H.; Moon, W.J. H2 uptake and diffusion characteristics in sulfur-crosslinked ethylene propylene diene monomer polymer composites with carbon black and silica fillers after high-pressure hydrogen exposure reaching 90 MPa. Polymers 2023, 15, 162. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.K.; Kim, K.T.; Baek, U.B.; Nahm, S.H. Volume dependence of hydrogen diffusion for sorption and desorption processes in cylindrical-shaped polymers. Polymers 2022, 14, 756. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jung, J.K.; Baek, U.B.; Chun, K.S. Development of measurement technology for uptake and diffusivity of hydrogen gas in rubber materials using volumetric analysis. Trans. Korean Hydrog. New Energy Soc. 2022, 33, 67–76. [Google Scholar] [CrossRef]
- Chakraborty, S.; Bandyopadhyay, S.; Ameta, R.; Mukhopadhyay, R.; Deuri, A.S. Application of FTIR in characterization of acrylonitrile-butadiene rubber (nitrile rubber). Polym. Test. 2007, 26, 38–41. [Google Scholar] [CrossRef]
- Noriman, N.Z.; Ismail, H.; Rashid, A.A. Characterization of styrene butadiene rubber/recycled acrylonitrile-butadiene rubber (SBR/NBRr) blends: The effects of epoxidized natural rubber (ENR-50) as a compatibilizer. Polym. Test. 2010, 29, 200–208. [Google Scholar] [CrossRef]
- Irez, A.B.; Bayraktar, E.; Miskioglu, I. Design and mechanical-physical properties of epoxy-rubber based composites reinforced with nanoparticles. Procedia Eng. 2017, 184, 486–496. [Google Scholar] [CrossRef]
- Lim, L.P.; Juan, J.C.; Huang, N.M.; Goh, L.K.; Leng, F.P.; Loh, Y.Y. Enhanced tensile strength and thermal conductivity of natural rubber graphene composite properties via rubber-graphene interaction. Mater. Sci. Eng. B 2019, 246, 112–119. [Google Scholar] [CrossRef]
- Kralevich, M.L.; Koenig, J.L. FTIR analysis of silica-filled natural rubber. Rubber Chem. Technol. 1998, 71, 300–309. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Natarajan, R.K.; Kala, A. FTIR spectra and mechanical strength analysis of some selected rubber derivatives. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 68, 323–330. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization behavior of poly(ε-caprolactone)/layered double hydroxide nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Khimi, S.R.; Pickering, K.L. A new method to predict optimum cure time of rubber compound using dynamic mechanical analysis. J. Appl. Polym. Sci. 2013, 131. [Google Scholar] [CrossRef]
- Kararli, T.T.; Hurlbut, J.B.; Needham, T.E. Glass–rubber transitions of cellulosic polymers by dynamic mechanical analysis. J. Pharm. Sci. 1990, 79, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Berridi, M.J.; González, N.; Mugica, A.; Bernicot, C. Pyrolysis-FTIR and TGA techniques as tools in the characterization of blends of natural rubber and SBR. Thermochim. Acta 2006, 444, 65–70. [Google Scholar] [CrossRef]
- Lee, C.H.; Park, S.-H.; Jung, J.K.; Ryu, K.-S.; Nahm, S.H.; Kim, J.; Chen, Y. 11B nuclear magnetic resonance study of boron nitride nanotubes prepared by mechano-thermal method. Solid State Commun. 2005, 134, 419–423. [Google Scholar] [CrossRef]
- Bueche, F. Tensile strength of rubbers. J. Polym. Sci. 1957, 24, 189–200. [Google Scholar] [CrossRef]
- Debye, P. Zur theorie der spezifischen wärmen. Ann. Phys. 1912, 344, 789–839. [Google Scholar] [CrossRef]
- Böhmer, R.; Gainaru, C.; Richert, R. Structure and dynamics of monohydroxy alcohols—Milestones towards their microscopic understanding, 100 years after Debye. Phys. Rep. 2014, 545, 125–195. [Google Scholar] [CrossRef]
- Hill, N.E. Dielectric Properties and Molecular Behaviour; The Van Nostrand Series in Physical Chemistry; UT Back-in-Print Service; Van Nostrand Reinhold Company: New York, NY, USA, 2006. [Google Scholar]
- Saltas, V.; Vallianatos, F.; Gidarakos, E. Charge transport in diatomaceous earth studied by broadband dielectric spectroscopy. Appl. Clay Sci. 2013, 80–81, 226–235. [Google Scholar] [CrossRef]
- George, D.K.; Charkhesht, A.; Vinh, N.Q. New terahertz dielectric spectroscopy for the study of aqueous solutions. Rev. Sci. Instrum. 2015, 86, 123105. [Google Scholar] [CrossRef]
- Baxter, J.B.; Guglietta, G.W. Terahertz spectroscopy. Anal. Chem. 2011, 83, 4342–4368. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.; García-Sánchez, M.F.; M’Peko, J.-C.; Ruiz-Salvador, A.R.; Rodríguez-Gattorno, G.; Echevarría, Y.; Fernández-Gutierrez, F. An elementary picture of dielectric spectroscopy in solids: Physical basis. J. Chem. Educ. 2003, 80, 1062. [Google Scholar] [CrossRef]
- Tonkoshkur, A.S.; Glot, A.B.; Ivanchenko, A.V. Basic models in dielectric spectroscopy of heterogeneous materials with semiconductor inclusions. Multidiscip. Model. Mater. Struct. 2017, 13, 36–57. [Google Scholar] [CrossRef]
- Jonscher, A.K. Dielectric relaxation in solids. J. Phys. D Appl. Phys. 1999, 32, R57–R70. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance Spectroscopy: Emphasizing Solid Materials and Systems; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Irvine, J.T.S.; Sinclair, D.C.; West, A.R. Electroceramics: Characterization by impedance spectroscopy. Adv. Mater. 1990, 2, 132–138. [Google Scholar] [CrossRef]
- Kremer, F.; Vallerien, S.U.; Zentel, R. Dielectric spectroscopy: Fashionable again. Adv. Mater. 1990, 2, 145–147. [Google Scholar] [CrossRef]
- Asami, K.; Yonezawa, T.; Wakamatsu, H.; Koyanagi, N. Dielectric spectroscopy of biological cells. Bioelectrochem. Bioenerg. 1996, 40, 141–145. [Google Scholar] [CrossRef]
- Poplavko, Y. Dielectric Spectroscopy of Electronic Materials: Applied Physics of Dielectrics; Woodhead Publishing Series in Electronic and Optical Materials; Woodhead Publishing: Cambridge, MA, USA, 2021. [Google Scholar]
- Lee, S.B.; Smith, R.L.; Inomata, H.; Arai, K. Coaxial probe and apparatus for measuring the dielectric spectra of high pressure liquids and supercritical fluid mixtures. Rev. Sci. Instrum. 2000, 71, 4226–4230. [Google Scholar] [CrossRef]
- Gabriel, S.; Lau, R.W.; Gabriel, C. The dielectric properties of biological tissues: II. Measurements in the frequency range 10 Hz to 20 GHz. Phys. Med. Biol. 1996, 41, 2251–2269. [Google Scholar] [CrossRef]
- Markus, P.; Martínez-Tong, D.E.; Papastavrou, G.; Alegria, A. Effect of environmental humidity on the ionic transport of poly(ethylene oxide) thin films, investigated by local dielectric spectroscopy. Soft Matter 2020, 16, 3203–3208. [Google Scholar] [CrossRef]
- Kuchler, F.; Farber, R.; Franck, C.M. Humidity and temperature effects on the dielectric properties of PET film. In Proceedings of the 2020 IEEE Electrical Insulation Conference (EIC), Knoxville, TN, USA, 22 June–3 July 2020; pp. 179–183. [Google Scholar]
- Gennaro, A.; Rosa, A.S.; Cornelis, P.; Pfeiffer, H.; Disalvo, E.A.; Wagner, P.; Wübbenhorst, M. A compact device for simultaneous dielectric spectroscopy and microgravimetric analysis under controlled humidity. Rev. Sci. Instrum. 2019, 90, 125106. [Google Scholar] [CrossRef] [PubMed]
- Zafar, R.; Gupta, N. Dielectric spectroscopy of epoxy-based barium titanate nanocomposites: Effect of temperature and humidity. IET Nanodielectr. 2020, 3, 20–27. [Google Scholar] [CrossRef]
- Bibi, F.; Guillaume, C.; Vena, A.; Gontard, N.; Sorli, B. Wheat gluten, a bio-polymer layer to monitor relative humidity in food packaging: Electric and dielectric characterization. Sens. Actuators A Phys. 2016, 247, 355–367. [Google Scholar] [CrossRef]
- Zouaoui, M.J.; Nait-Ali, B.; Glandut, N.; Smith, D.S. Effect of humidity on the dielectric constant and electrical impedance of mesoporous zirconia ceramics. J. Eur. Ceram. Soc. 2016, 36, 163–169. [Google Scholar] [CrossRef]
- Soltani, R.; David, E.; Lamarre, L. Study on the effect of humidity on dielectric response and partial discharges activity of machine insulation materials. In Proceedings of the 2009 IEEE Electrical Insulation Conference, Montreal, QC, Canada, 31 May–3 June 2009; pp. 343–347. [Google Scholar]
- Lipare, A.Y.; Vasambekar, P.N.; Vaingankar, A.S. Dielectric behavior and a.c. resistivity study of humidity sensing ferrites. Mater. Chem. Phys. 2003, 81, 108–115. [Google Scholar] [CrossRef]
- Wolf, M.; Gulich, R.; Lunkenheimer, P.; Loidl, A. Relaxation dynamics of a protein solution investigated by dielectric spectroscopy. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2012, 1824, 723–730. [Google Scholar] [CrossRef]
- Yew, J.H.; Saha, T.K.; Thomas, A.J. Impact of temperature on the frequency domain dielectric spectroscopy for the diagnosis of power transformer insulation. In Proceedings of the 2006 IEEE Power Engineering Society General Meeting, Montreal, QC, Canada, 18–22 June 2006; p. 7. [Google Scholar]
- Paraskevas, C.D.; Vassiliou, P.; Dervos, C.T. Temperature dependent dielectric spectroscopy in frequency domain of high-voltage transformer oils compared to physicochemical results. IEEE Trans. Dielectr. Electr. Insul. 2005, 13, 539–546. [Google Scholar] [CrossRef]
- Petzelt, J.; Kozlov, G.V.; Volkov, A.A. Dielectric spectroscopy of paraelectric soft modes. Ferroelectrics 1987, 73, 101–123. [Google Scholar] [CrossRef]
- Nahm, S.H. Use of dielectric spectroscopy for real-time in-situ reaction monitoring. J. Coat. Technol. Res. 2006, 3, 257–265. [Google Scholar] [CrossRef]
- Diani, J.; Liu, Y.; Gall, K. Finite strain 3D thermoviscoelastic constitutive model for shape memory polymers. Polym. Eng. Sci. 2006, 46, 486–492. [Google Scholar] [CrossRef]
- Sorichetti, P.; Matteo, C.; Lambri, O.; Manguzzi, G.; Salvatierra, L.; Herrero, O. Structural changes in EPDM subjected to ageing in high voltage transmission lines. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 1170–1182. [Google Scholar] [CrossRef]
- Majumder, P.S.; Bhowmick, A.K. Structure-property relationship of electron-beam-modified EPDM rubber. J. Appl. Polym. Sci. 2000, 77, 323–337. [Google Scholar] [CrossRef]
- Erukhimovich, I.; de la Cruz, M.O. Phase equilibrium and charge fractionation in polyelectrolyte solutions. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 3003–3009. [Google Scholar] [CrossRef]
- Nayak, S.; Chaki, T.K.; Khastgir, D. Dielectric relaxation and viscoelastic behavior of polyurethane–titania composites: Dielectric mixing models to explain experimental results. Polym. Bull. 2017, 74, 369–392. [Google Scholar] [CrossRef]
- Airinei, A.; Asandulesa, M.; Stelescu, M.D.; Tudorachi, N.; Fifere, N.; Bele, A.; Musteata, V. Dielectric, thermal and water absorption properties of some EPDM/Flax fiber composites. Polymers 2021, 13, 2555. [Google Scholar] [CrossRef]
- Min, D.; Li, S.; Hirai, N.; Ohki, Y. Dielectric spectroscopic analysis of degradation in ethylene-propylene-diene copolymer. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 3620–3630. [Google Scholar] [CrossRef]
- Schönhals, A.; Szymoniak, P. Dynamics of Composite Materials; Springer International Publishing: Cham, Switzerland, 2022. [Google Scholar]
- Eid, M.A.M.; El-Nashar, D.E. Filling effect of silica on electrical and mechanical properties of EPDM/NBR blends. Polym. Plast. Technol. Eng. 2006, 45, 675–684. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, Q.; Liu, T.; Lei, Y.; Chen, C. Preparation and relaxation dynamics of ethylene–propylene–diene rubber/clay nanocomposites with crosslinking interfacial design. J. Appl. Polym. Sci. 2018, 135, 45553. [Google Scholar] [CrossRef]
- Chailan, J.F.; Boiteux, G.; Chauchard, J.; Pinel, B.; Seytre, G. Viscoelastic and dielectric study of thermally aged ethylene—Propylene diene monomer (EPDM) compounds. Polym. Degrad. Stab. 1995, 47, 397–403. [Google Scholar] [CrossRef]
- Abd-El-Messieh, S.L.; El-Sabbagh, S.; Abadir, I.F. Dielectric relaxation and mechanical investigation of ethylene propylene diene monomer rubber with some crosslinking additives. J. Appl. Polym. Sci. 1999, 73, 1509–1519. [Google Scholar] [CrossRef]
- Dash, B.K.; Achary, P.G.R.; Nayak, N.C.; Choudhary, R.N.P. Dielectric relaxation behavior of exfoliated graphite nanoplatelet-filled EPDM vulcanizates. J. Electron. Mater. 2017, 46, 563–572. [Google Scholar] [CrossRef]
- Nayak, N.C.; Dash, B.K.; Parida, B.N.; Padhee, R. Dielectric relaxation behavior of exfoliated graphite nanoplatelets filled ethylene vinyl acetate copolymer and ethylene propylene diene terpolymer blend. J. Mater. Sci. Mater. Electron. 2018, 29, 1955–1963. [Google Scholar] [CrossRef]
- Barkoula, N.M.; Alcock, B.; Cabrera, N.O.; Peijs, T. Fatigue Properties of Highly Oriented Polypropylene Tapes and All-Polypropylene Composites. Polym. Polym. Compos 2008, 16, 101–113. [Google Scholar] [CrossRef]
- Mocellini, R.R.; Lambri, O.A.; Matteo, C.L.; Sorichetti, P.A. Dielectric properties and viscoelastic response in two-phase polymers. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 982–993. [Google Scholar] [CrossRef]
- Tiwari, A.; Sen, V.; Dhakate, S.R.; Mishra, A.P.; Singh, V. Synthesis, characterization, and hoping transport properties of HCl doped conducting biopolymer-co-polyaniline zwitterion hybrids. Polym. Adv. Technol. 2008, 19, 909–914. [Google Scholar] [CrossRef]
- Ismail, M.N.; Turky, G.M. Effect of fillers and vulcanizing systems on the physicomechanical and electrical properties of EPDM vulcanizates. Polym. Plast. Technol. Eng. 2001, 40, 635–652. [Google Scholar] [CrossRef]
- Younan, A.F.; Choneim, A.M.; Tawfik, A.A.A.; Abd-El-Nour, K.N. Electrical and physical properties of ethylene—Propylene—Diene monomer (EPDM) rubber loaded with semi-reinforcing furnace black. Polym. Degrad. Stab. 1995, 49, 215–222. [Google Scholar] [CrossRef]
- Celette, N.; Stevenson, I.; David, L.; Davenas, J.; Vigier, G.; Seytre, G. Irradiation effects on the relaxation behaviour of EPDM elastomers. Polym. Int. 2004, 53, 495–505. [Google Scholar] [CrossRef]
- Ajroldi, G.; Pianca, M.; Fumagalli, M.; Moggi, G. Fluoroelastomers-dependence of relaxation phenomena on composition. Polymer 1989, 30, 2180–2187. [Google Scholar] [CrossRef]
- Moni, G.; Jose, T.; Rajeevan, S.; Mayeen, A.; Rejimon, A.; Sarath, P.S.; George, S.C. Influence of exfoliated graphite inclusion on the thermal, mechanical, dielectric and solvent transport characteristics of fluoroelastomer nanocomposites. J. Polym. Res. 2020, 27, 72. [Google Scholar] [CrossRef]
- Moni, G.; Mayeen, A.; Mohan, A.; George, J.J.; Thomas, S.; George, S.C. Ionic liquid functionalised reduced graphene oxide fluoroelastomer nanocomposites with enhanced mechanical, dielectric and viscoelastic properties. Eur. Polym. J. 2018, 109, 277–287. [Google Scholar] [CrossRef]
- Balachandran, N.A.; Philip, K.; Rani, J. Effect of expanded graphite on thermal, mechanical and dielectric properties of ethylene–propylene–diene terpolymer/hexa fluoropropylene–vinylidinefluoride dipolymer rubber blends. Eur. Polym. J. 2013, 49, 247–260. [Google Scholar] [CrossRef]
- Starkweather, H.W.; Avakian, P.; Fontanella, J.J.; Wintersgill, M.C. Dielectric properties of polymers based on hexafluoropropylene. J. Therm. Anal. 1996, 46, 785–794. [Google Scholar] [CrossRef]
- Tamir, E.; Srebnik, S.; Sidess, A. Prediction of the relaxation modulus of a fluoroelastomer using molecular dynamics simulation. Chem. Eng. Sci. 2020, 225, 115786. [Google Scholar] [CrossRef]
- Zabelina, A.N.; Glushak, M.I.; Shcherbinin, A.S.; Khoroshavina, Y.V.; Ramsh, A.S.; Kurliand, S.K. Dielectric spectroscopy study of butadiene–nitrile rubbers. Russ. J. Phys. Chem. A 2020, 94, 119–124. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Natarajan, R.; Kala, A.; Jagannathan, R. Dielectric studies of some rubber materials at microwave frequencies. Indian J. Pure Appl. Phys. 2008, 46, 733–737. [Google Scholar]
- Yang, D.; Tian, M.; Dong, Y.; Liu, H.; Yu, Y.; Zhang, L. Disclosed dielectric and electromechanical properties of hydrogenated nitrile–butadiene dielectric elastomer. Smart Mater. Struct. 2012, 21, 035017. [Google Scholar] [CrossRef]
- Adachi, H.; Adachi, K.; Kotaka, T. Effects of cross-linking density and of stretching on dielectric α-relaxation in poly(acrylonitrile-co-butadiene) rubber. Polym. J. 1980, 12, 329–334. [Google Scholar] [CrossRef][Green Version]
- Böhm, M.; Soden, W.V.; Heinrich, W.; Yehia, A.A. Influence of crosslinking on mechanical and dielectric properties of nitrile-butadiene-rubber. Colloid Polym. Sci. 1987, 265, 295–303. [Google Scholar] [CrossRef]
- Garraza, A.L.R.; Sorichetti, P.; Marzocca, A.J.; Matteo, C.L.; Monti, G.A. Influence of the microstructure of vulcanized polybutadiene rubber on the dielectric properties. Polym. Test. 2011, 30, 657–662. [Google Scholar] [CrossRef]
- Yang, D.; Kong, X.; Ni, Y.; Gao, D.; Yang, B.; Zhu, Y.; Zhang, L. Novel nitrile-butadiene rubber composites with enhanced thermal conductivity and high dielectric constant. Compos. Part A Appl. Sci. Manuf. 2019, 124, 105447. [Google Scholar] [CrossRef]
- Sturniolo, S.; Pieruccini, M.; Corti, M.; Rigamonti, A. Probing α-relaxation with nuclear magnetic resonance echo decay and relaxation: A study on nitrile butadiene rubber. Solid State Nucl. Magn. Reson. 2013, 51–52, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, G.; Zhu, W.; Chen, C.; Fu, Y.; Zhang, Z.; Li, H. Study of relaxations in epoxy/rubber composites by thermally stimulated depolarization current and dielectric spectroscopy. Front. Chem. 2022, 10, 874685. [Google Scholar] [CrossRef] [PubMed]
- Ardi, M.S.; Dick, W.; Kubát, J. Time-domain dielectric relaxation of poly(methyl methacrylate) and nitrile rubber. Colloid Polym. Sci. 1993, 271, 739–747. [Google Scholar] [CrossRef]
- Khalaf, A.I.; Ward, A.A.; El-Kader, A.E.A.; El-Sabbagh, S.H. Effect of selected vegetable oils on the properties of acrylonitrile-butadiene rubber vulcanizates. Polimery 2015, 60, 43–56. [Google Scholar] [CrossRef]
- Gaca, M.; Pietrasik, J.; Zaborski, M.; Okrasa, L.; Boiteux, G.; Gain, O. Effect of zinc oxide modified silica particles on the molecular dynamics of carboxylated acrylonitrile-butadiene rubber composites. Polymers 2017, 9, 645. [Google Scholar] [CrossRef]
- Roland, C.M. Electrical and dielectric properties of rubber. Rubber Chem. Technol. 2016, 89, 32–53. [Google Scholar] [CrossRef]
- Pietrasik, J.; Gaca, M.; Zaborski, M.; Okrasa, L.; Boiteux, G.; Gain, O. Studies of molecular dynamics of carboxylated acrylonitrile-butadiene rubber composites containing in situ synthesized silica particles. Eur. Polym. J. 2009, 45, 3317–3325. [Google Scholar] [CrossRef]
- Crossley, J. Dielectric relaxation of 1-alkenes. J. Chem. Phys. 1973, 58, 5315–5318. [Google Scholar] [CrossRef]
- Vo, L.T.; Anastasiadis, S.H.; Giannelis, E.P. Dielectric study of poly(styrene-co-butadiene) composites with carbon black, silica, and nanoclay. Macromolecules 2011, 44, 6162–6171. [Google Scholar] [CrossRef]
- Huang, M.; Tunnicliffe, L.B.; Thomas, A.G.; Busfield, J.J.C. The glass transition, segmental relaxations and viscoelastic behaviour of particulate-reinforced natural rubber. Eur. Polym. J. 2015, 67, 232–241. [Google Scholar] [CrossRef]
- Souillard, C.; Chazeau, L.; Cavaillé, J.-Y.; Brun, S.; Schach, R. On the β relaxations in poly(butadiene) and poly(styrene-butadiene) rubbers. Polymer 2019, 168, 236–245. [Google Scholar] [CrossRef]
- Pissis, P.; Fragiadakis, D.; Kanapitsas, A.; Delides, K. Broadband dielectric relaxation spectroscopy in polymer nanocomposites. Macromol. Symp. 2008, 265, 12–20. [Google Scholar] [CrossRef]
- Souillard, C.; Cavaillé, J.-Y.; Chazeau, L.; Schach, R. Dynamic mechanical relaxation of cross-linked styrene-butadiene polymers containing free chains: Possibility of reptation. Polymer 2014, 55, 5218–5225. [Google Scholar] [CrossRef]
- Abd-El-Messieh, S.L.; Abd-El-Nour, K.N. Effect of curing time and sulfur content on the dielectric relaxation of styrene butadiene rubber. J. Appl. Polym. Sci. 2003, 88, 1613–1621. [Google Scholar] [CrossRef]
- Xiang, K.; Wu, S.; Huang, G.; Zheng, J.; Huang, J.; Li, G. Relaxation behavior and time-temperature superposition (TTS) profiles of thermally aged styrene-butadiene rubber (SBR). Macromol. Res. 2014, 22, 820–825. [Google Scholar] [CrossRef]
- Wu, S.; Tang, Z.; Guo, B.; Zhang, L.; Jia, D. Effects of interfacial interaction on chain dynamics of rubber/graphene oxide hybrids: A dielectric relaxation spectroscopy study. RSC Adv. 2013, 3, 14549–14559. [Google Scholar] [CrossRef]
- Jungk, J.; Klueppel, M.; Meier, J.G. Dielectric relaxation spectroscopy of precipitated silica and elastomer-silica composites. KGK Rubberpoint 2009, 62, 319–325. [Google Scholar]
- Kumar, R.P.; Thomas, S. Dielectric properties of short sisal fibre reinforced SBR composites. Sci. Eng. Compos. Mater. 1999, 8, 311–326. [Google Scholar] [CrossRef]
- Hanna, F.F.; Yehia, A.A.; Abou-Bakr, A.F. Dielectric properties of styrene—Butadiene rubber/silicon dioxide mixtures. Br. Polym. J. 1973, 5, 83–90. [Google Scholar] [CrossRef]
- Gambino, T.; Alegría, A.; Arbe, A.; Colmenero, J.; Malicki, N.; Dronet, S. Modeling the high frequency mechanical relaxation of simplified industrial polymer mixtures using dielectric relaxation results. Polymer 2020, 187, 122051. [Google Scholar] [CrossRef]
- Ortega, L.; Cerveny, S.; Sill, C.; Isitman, N.A.; Rodriguez-Garraza, A.L.; Meyer, M.; Westermann, S.; Schwartz, G.A. The effect of vulcanization additives on the dielectric response of styrene-butadiene rubber compounds. Polymer 2019, 172, 205–212. [Google Scholar] [CrossRef]
- Ward, A. Dielectric and Mechanical Properties of Filled Rubbers in Dependence on Stress Amplitude and Temperature. Ph.D. Thesis, Cairo University, Giza, Egypt, 2003. [Google Scholar]
- Cerveny, S.; Bergman, R.; Schwartz, G.A.; Jacobsson, P. Dielectric α- and β-relaxations in uncured styrene butadiene rubber. Macromolecules 2002, 35, 4337–4342. [Google Scholar] [CrossRef]
- Renukappa, N.M.; Siddaramaiah; Sudhaker Samuel, R.D.; Sundara Rajan, J.; Lee, J.H. Dielectric properties of carbon black: SBR composites. J. Mater. Sci. Mater. Electron. 2009, 20, 648–656. [Google Scholar] [CrossRef]
- Janik, P.; Paluch, M.; Ziolo, J.; Sulkowski, W.; Nikiel, L. Low-frequency dielectric relaxation in rubber. Phys. Rev. E 2001, 64, 042502. [Google Scholar] [CrossRef]
- Lindemann, N.; Schawe, J.E.K.; Lacayo-Pineda, J. Kinetics of the glass transition of styrene-butadiene-rubber: Dielectric spectroscopy and fast differential scanning calorimetry. J. Appl. Polym. Sci. 2021, 138, 49769. [Google Scholar] [CrossRef]
- Moon, Y.I.; Jung, J.K.; Kim, G.H.; Chung, K.S. Observation of the relaxation process in fluoroelastomers by dielectric relaxation spectroscopy. Phys. B Condens. Matter 2021, 608, 412870. [Google Scholar] [CrossRef]
- Moon, Y.I.; Jung, J.K.; Chung, K.S. Dielectric relaxation spectroscopy in synthetic rubber polymers: Nitrile butadiene rubber and ethylene propylene diene monomer. Adv. Mater. Sci. Eng. 2020, 2020, 8406059. [Google Scholar] [CrossRef]
- Jung, J.K.; Moon, Y.I.; Kim, G.H.; Tak, N.H. Characterization of dielectric relaxation process by impedance spectroscopy for polymers: Nitrile butadiene rubber and ethylene propylene diene monomer. J. Spectrosc. 2020, 2020, 8815492. [Google Scholar] [CrossRef]
- Jung, J.K.; Moon, Y.I.; Chung, K.S.; Kim, K.-T. Development of a program for analyzing dielectric relaxation and its application to polymers: Nitrile butadiene rubber. Macromol. Res. 2020, 28, 596–604. [Google Scholar] [CrossRef]
- Jung, J.K.; Il Moon, Y.; Chung, K.S. Dielectric relaxation in a fluoroelastomer and ethylene propylene diene monomer observed by using impedance spectroscopy. J. Korean Phys. Soc. 2020, 76, 416–425. [Google Scholar] [CrossRef]
- Kim, G.-H.; Moon, Y.-I.; Jung, J.-K.; Choi, M.-C.; Bae, J.-W. Influence of carbon black and silica fillers with different concentrations on dielectric relaxation in nitrile butadiene rubber investigated by impedance spectroscopy. Polymers 2022, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Miles, P.A.; Westphal, W.B.; Von Hippel, A. Dielectric spectroscopy of ferromagnetic semiconductors. Rev. Mod. Phys. 1957, 29, 279–307. [Google Scholar] [CrossRef]
- Jonscher, A.K. Dielectric characterisation of semiconductors. Solid-State Electron. 1990, 33, 737–742. [Google Scholar] [CrossRef]
- Koltunowicz, T.N. Test station for frequency-domain dielectric spectroscopy of nanocomposites and semiconductors. J. Appl. Spectrosc. 2015, 82, 653–658. [Google Scholar] [CrossRef]
- Nobre, M.A.L.; Lanfredi, S. Dielectric spectroscopy on Bi3Zn2Sb3O14 ceramic: An approach based on the complex impedance. J. Phys. Chem. Solids 2003, 64, 2457–2464. [Google Scholar] [CrossRef]
- Vila, R.; González, M.; Mollá, J.; Ibarra, A. Dielectric spectroscopy of alumina ceramics over a wide frequency range. J. Nucl. Mater. 1998, 253, 141–148. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Baptista, J.L.; Kamba, S.; Petzelt, J. Dielectric spectroscopy of MgTiO3-based ceramics in the 109–1014Hz region. J. Mater. Sci. 1993, 28, 5894–5900. [Google Scholar] [CrossRef]
- Krohns, S.; Lunkenheimer, P.; Ebbinghaus, S.G.; Loidl, A. Broadband dielectric spectroscopy on single-crystalline and ceramic CaCu3Ti4O12. Appl. Phys. Lett. 2007, 91, 022910. [Google Scholar] [CrossRef]
- Tsurumi, T.; Li, J.; Hoshina, T.; Kakemoto, H.; Nakada, M.; Akedo, J. Ultrawide range dielectric spectroscopy of BaTiO3-based perovskite dielectrics. Appl. Phys. Lett. 2007, 91, 182905. [Google Scholar] [CrossRef]
- Yassin, A.Y. Dielectric spectroscopy characterization of relaxation in composite based on (PVA–PVP) blend for nickel–cadmium batteries. J. Mater. Sci. Mater. Electron. 2020, 31, 19447–19463. [Google Scholar] [CrossRef]
- Badot, J.-C.; Lestriez, B.; Dubrunfaut, O. Interest in broadband dielectric spectroscopy to study the electronic transport in materials for lithium batteries. Mater. Sci. Eng. B 2016, 213, 190–198. [Google Scholar] [CrossRef]
- Di Noto, V.; Giffin, G.A.; Vezzù, K.; Piga, M.; Lavina, S. Broadband dielectric spectroscopy: A powerful tool for the determination of charge transfer mechanisms in ion conductors. In Solid State Proton Conductors: Properties and Applications in Fuel Cells; Knauth, P., Vona, M.L.D., Eds.; Wiley: West Sussex, UK, 2012; pp. 109–183. [Google Scholar]
- Hanafusa, H.; Sato, Y. Dielectric properties of polymer-TiO2 composites. Kobunshi Ronbunshu 1978, 35, 277–282. [Google Scholar] [CrossRef]
- Michl, M.; Bauer, T.; Lunkenheimer, P.; Loidl, A. Nonlinear dielectric spectroscopy in a fragile plastic crystal. J. Chem. Phys. 2016, 144, 114506. [Google Scholar] [CrossRef]
- Hedvig, P.; Czvikovszky, T. Dielectric spectroscopic study of wood plastic combinations. Angew. Makromol. Chem. 1972, 21, 79–85. [Google Scholar] [CrossRef]
- Strobl, G.R. The Physics of Polymers: Concepts for Understanding Their Structures and Behavior; Springer: Berlin/Heidelberg, Germany, 1997; pp. 143–190. [Google Scholar]
- Rosen, S.L. Fundamental Principles of Polymeric Materials; Wiley: New York, NY, USA, 1982. [Google Scholar]
- Karak, N. Fundamentals of Polymers: Raw Materials to Finish Products; PHI Learning Pvt Ltd.: New Delhi, India, 2009; pp. 1–30. [Google Scholar]
- Watanabe, H. Dielectric relaxation of type-A polymers in melts and solutions. Macromol. Rapid Commun. 2001, 22, 127–175. [Google Scholar] [CrossRef]
- Nikonorova, N.A.; Yakimansky, A.V.; Smirnov, N.N.; Kudryavtsev, V.V.; Diaz-Calleja, R.; Pissis, P. Dielectric relaxation in copolymethacrylates containing side-chain nonlinear optical chromophores. Polymer 2007, 48, 556–563. [Google Scholar] [CrossRef]
- Ngai, K.L.; Roland, C.M. Chemical structure and intermolecular cooperativity: Dielectric relaxation results. Macromolecules 1993, 26, 6824–6830. [Google Scholar] [CrossRef]
- North, A.M. Dielectric relaxation in polymer solutions. Chem. Soc. Rev. 1972, 1, 49–72. [Google Scholar] [CrossRef]
- Hammami, H.; Arous, M.; Lagache, M.; Kallel, A. Study of the interfacial MWS relaxation by dielectric spectroscopy in unidirectional PZT fibres/epoxy resin composites. J. Alloys Compd. 2007, 430, 1–8. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, X. Investigation of MWS polarization and dc conductivity in polyamide 610 using dielectric relaxation spectroscopy. Eur. Polym. J. 2011, 47, 1031–1038. [Google Scholar] [CrossRef]
- Chanmal, C.; Jog, J. Dielectric relaxation spectroscopy for polymer nanocomposites. In Characterization Techniques for Polymer Nanocomposites; Mittal, V., Ed.; Wiley: Weinheim, Germany, 2012; pp. 167–184. [Google Scholar]
- Mijović, J.; Lee, H.; Kenny, J.; Mays, J. Dynamics in polymer−silicate nanocomposites as studied by dielectric relaxation spectroscopy and dynamic mechanical spectroscopy. Macromolecules 2006, 39, 2172–2182. [Google Scholar] [CrossRef]
- Kremer, F.; Schönhals, A. Broadband Dielectric Spectroscopy; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Runt, J.; Fitzgerald, J.J. Dielectric Spectroscopy of Polymeric Materials: Fundamentals and Applications; American Chemical Society: Washington, DC, USA, 1997. [Google Scholar]
- Vassilikou-Dova, A.; Kalogeras, I.M. Dielectric analysis (DEA). In Thermal Analysis of Polymers: Fundamentals and Applications; Menczel, J.D., Prime, R.B., Eds.; Wiley: Hoboken, NJ, USA, 2009; pp. 497–614. [Google Scholar]
- Stockmayer, W.H. Dielectric dispersion in solutions of flexible polymers. Pure Appl. Chem. 1967, 15, 539–554. [Google Scholar] [CrossRef]
- Block, H. The nature and application of electrical phenomena in polymers. In Electric Phenomena in Polymer Science. Advances in Polymer Science; Abe, A., Albertsson, A.-C., Dusek, K., Genzer, J., Kobayashi, S., Lee, K.-S., Leibler, L., Long, T.E., Manners, I., Möller, M., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 1979; pp. 93–167. [Google Scholar]
- Van Krevelen, D.W.; Te Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: Oxford, UK, 2009; pp. 779–786. [Google Scholar]
- Kakani, S.L. Electronics Theory and Applications; New Age International (P) Ltd.: New Delhi, India, 2005. [Google Scholar]
- Cole, K.S.; Cole, R.H. Dispersion and absorption in dielectrics I. Alternating current characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Davidson, D.W.; Cole, R.H. Dielectric relaxation in glycerine. J. Chem. Phys. 1950, 18, 1417. [Google Scholar] [CrossRef]
- Havriliak, S.; Negami, S. A complex plane representation of dielectric and mechanical relaxation processes in some polymers. Polymer 1967, 8, 161–210. [Google Scholar] [CrossRef]
- Gerstl, C.; Schneider, G.J.; Pyckhout-Hintzen, W.; Allgaier, J.; Richter, D.; Alegría, A.; Colmenero, J. Segmental and normal mode relaxation of poly(alkylene oxide)s studied by dielectric spectroscopy and rheology. Macromolecules 2010, 43, 4968–4977. [Google Scholar] [CrossRef]
- Roland, C.M.; Bero, C.A. Normal mode relaxation in linear and branched polyisoprene. Macromolecules 1996, 29, 7521–7526. [Google Scholar] [CrossRef][Green Version]
- Kuttich, B.; Lederle, C.; Stühn, B. Water dependence of the dielectric β-relaxation in poly(ɛ-caprolactone). J. Chem. Phys. 2013, 139, 244907. [Google Scholar] [CrossRef]
- Priestley, R.D.; Broadbelt, L.J.; Torkelson, J.M.; Fukao, K. Glass transition and α-relaxation dynamics of thin films of labeled polystyrene. Phys. Rev. E 2007, 75, 061806. [Google Scholar] [CrossRef]
- Pissis, P.; Fragiadakis, D. Dielectric studies of segmental dynamics in epoxy nanocomposites. J. Macromol. Sci. Part B 2007, 46, 119–136. [Google Scholar] [CrossRef]
- Dunn, A.M.; Hofmann, O.S.; Waters, B.; Witchel, E. Cloaking malware with the trusted platform module. In Proceedings of the 20th USENIX Conference on Security (SEC’11), San Francisco, CA, USA, 8–12 August 2011; USENIX Association: Berkeley, CA, USA, 2011; pp. 395–410. [Google Scholar]
- Kyriakos, K.; Raftopoulos, K.N.; Pissis, P.; Kyritsis, A.; Näther, F.; Häußler, L.; Fischer, D.; Vyalikh, A.; Scheler, U.; Reuter, U.; et al. Dielectric and thermal studies of the segmental dynamics of poly(methyl methacrylate)/silica nanocomposites prepared by the sol–gel method. J. Appl. Polym. Sci. 2013, 128, 3771–3781. [Google Scholar] [CrossRef]
- Wübbenhorst, M.; van Koten, E.M.; Jansen, J.C.; Mijs, W.; van Turnhout, J. Dielectric relaxation spectroscopy of amorphous and liquid-crystalline side-chain polycarbonates. Macromol. Rapid Commun. 1997, 18, 139–147. [Google Scholar] [CrossRef]
- Atawa, B.; Correia, N.T.; Couvrat, N.; Affouard, F.; Coquerel, G.; Dargent, E.; Saiter, A. Molecular mobility of amorphous N-acetyl-α-methylbenzylamine and Debye relaxation evidenced by dielectric relaxation spectroscopy and molecular dynamics simulations. Phys. Chem. Chem. Phys. 2019, 21, 702–717. [Google Scholar] [CrossRef]
- Vallerien, S.U.; Kremer, F.; Boeffel, C. Broadband dielectric spectroscopy on side group liquid crystal polymers. Liq. Cryst. 1989, 4, 79–86. [Google Scholar] [CrossRef]
- Vikulova, M.; Tsyganov, A.; Bainyashev, A.; Artyukhov, D.; Gorokhovsky, A.; Muratov, D.; Gorshkov, N. Dielectric properties of PMMA/KCTO(H) composites for electronics components. J. Appl. Polym. Sci. 2021, 138, 51168. [Google Scholar] [CrossRef]
- Yehia, A.A. Conductivity studies on acrylonitrile butadiene rubber loaded with different types of carbon blacks. Int. J. Mater. Methods Technol. 2013, 1, 22–33. [Google Scholar]
- Hiltz, J.A. Characterization of fluoroelastomers by various analytical techniques including pyrolysis gas chromatography/mass spectrometry. J. Anal. Appl. Pyrolysis 2014, 109, 283–295. [Google Scholar] [CrossRef]
- Yeo, Y.-G.; Park, H.-H.; Lee, C.-S. A study on the characteristics of a rubber blend of fluorocarbon rubber and hydrogenated nitrile rubber. J. Ind. Eng. Chem. 2013, 19, 1540–1548. [Google Scholar] [CrossRef]
- De Angelis, M.G.; Sarti, G.C.; Sanguineti, A.; Maccone, P. Permeation, diffusion, and sorption of dimethyl ether in fluoroelastomers. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 1987–2006. [Google Scholar] [CrossRef]
- Kader, M.A.; Bhowmick, A.K. Acrylic rubber-fluorocarbon rubber miscible blends: Effect of curatives and fillers on cure, mechanical, aging, and swelling properties. J. Appl. Polym. Sci. 2003, 89, 1442–1452. [Google Scholar] [CrossRef]
- George, S.; Sushama, C.M.; Nair, A.B. Efficiencies of dipolymer rubber blends (EPDM\FKM) using common weight data envelopment analysis. Mater. Res. 2017, 20, 1722–1728. [Google Scholar] [CrossRef][Green Version]
- Koizumi, N.; Tsunashima, K.; Yano, S. Dielectric relaxation in copolymers of vinylidene fluoride and hexafluoropropylene. J. Polym. Sci. Part B Polym. Lett. 1969, 7, 815–820. [Google Scholar] [CrossRef]
- Kochervinskii, V.V.; Malyshkina, I.A.; Markin, G.V.; Gavrilova, N.D.; Bessonova, N.P. Dielectric relaxation in vinylidene fluoride–hexafluoropropylene copolymers. J. Appl. Polym. Sci. 2007, 105, 1101–1117. [Google Scholar] [CrossRef]
- Axelrod, N.; Axelrod, E.; Gutina, A.; Puzenko, A.; Ishai, P.B.; Feldman, Y. Dielectric spectroscopy data treatment: I. Fre-quency domain. Meas. Sci. Technol. 2004, 15, 755–764. [Google Scholar] [CrossRef]
- Nelder, J.A.; Mead, R. A simplex method for function minimization. Comput. J. 1965, 7, 308–313. [Google Scholar] [CrossRef]












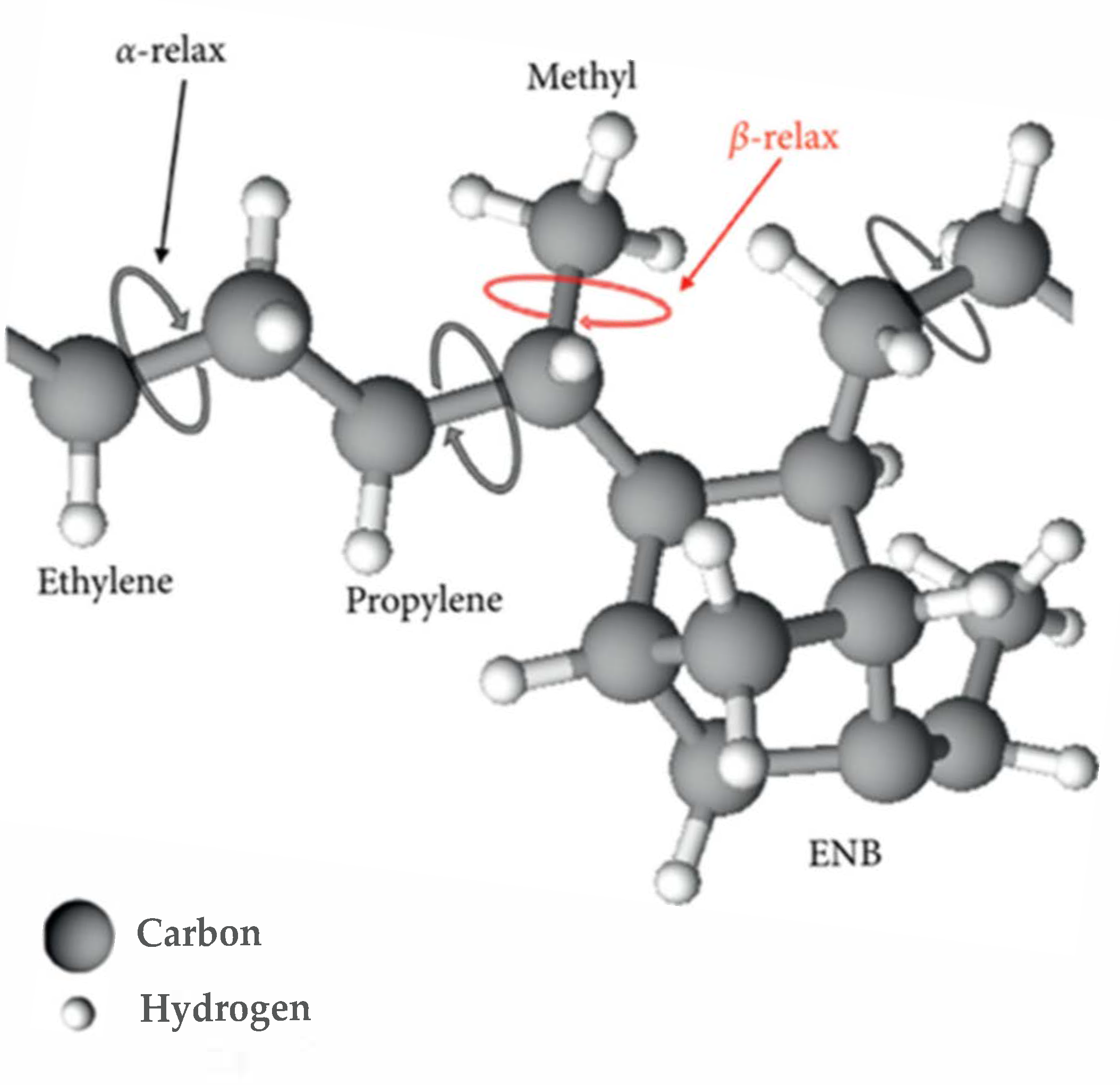


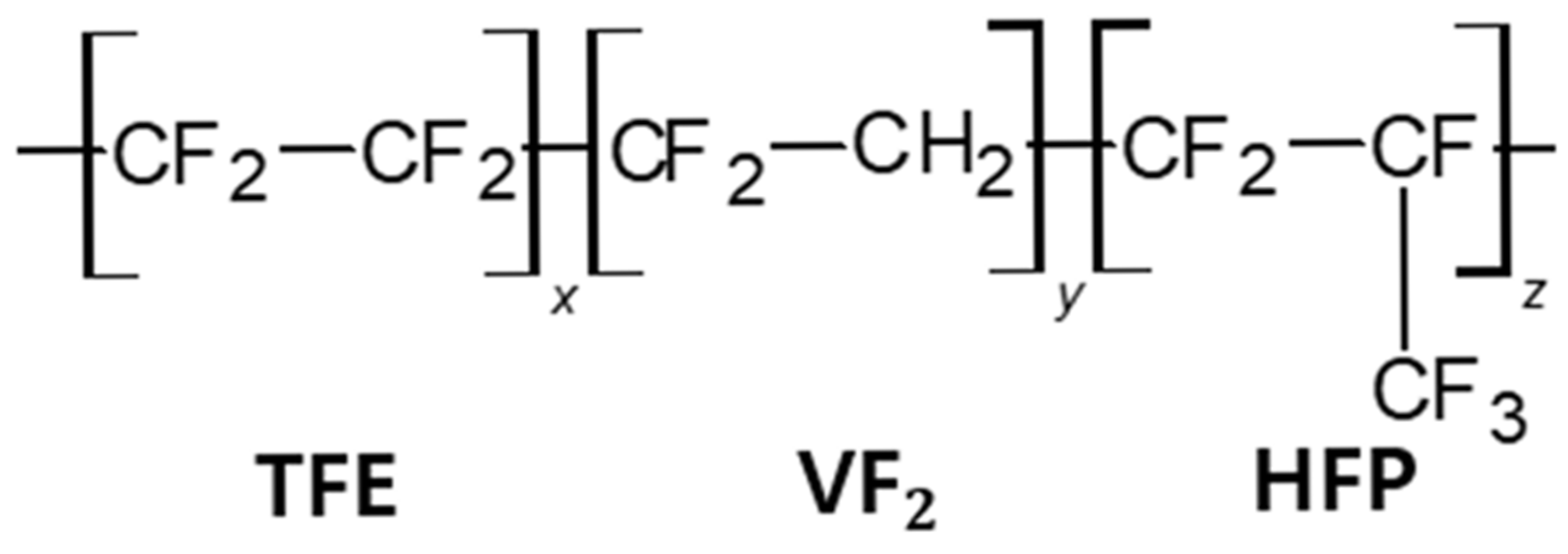





| Chemical Name | Function | (%) |
|---|---|---|
| Acrylonitrile–butadiene rubber | Polymer | 40.0 |
| Carbon black (medium thermal) | Filler reinforcing | 50.0 |
| 1,2-Benzenedicarboxylic acid | Processing aid | 6.0 |
| 2-Benzimidazolethiol | Antioxidant | 2.0 |
| Sulfur | Curing agent | 2.0 |
| Total | 100.0 |
| Chemical Name | Function | (%) |
|---|---|---|
| Ethylene propylene rubber | Polymer | 58.0 |
| Carbon black | Filler reinforcement | 34.0 |
| Zinc oxide | Processing aid | 3.0 |
| Dicumyl peroxide | Antioxidant | 5.0 |
| Total | 100.0 |
| Chemical Name | Function | (%) |
|---|---|---|
| Poly (vinylidene fluoride-co-hexafluoropropylene) | Polymer | 82.0 |
| Carbon black | Filler reinforcement | 14.0 |
| Calcium dihydroxide | Curing agent | 4.0 |
| Total | 100.0 |
| Reference | Ours | [172] | [226] | [133] | [227] |
|---|---|---|---|---|---|
| Ea for β (kJ/mol) | 50.88 | 63.5 | 50.2, 54.4 * | 47 | 49.8, 40.2 |
| Ea for MWS (kJ/mol) | 88.57 | 110 | 151, 81 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, Y.; Kim, G.; Jung, J. A Study of the Dielectric Relaxation of Nitrile–Butadiene Rubber, Ethylene–Propylene–Diene Monomer, and Fluoroelastomer Polymers with a Self-Developed Deconvolution Analysis Program. Polymers 2025, 17, 1539. https://doi.org/10.3390/polym17111539
Moon Y, Kim G, Jung J. A Study of the Dielectric Relaxation of Nitrile–Butadiene Rubber, Ethylene–Propylene–Diene Monomer, and Fluoroelastomer Polymers with a Self-Developed Deconvolution Analysis Program. Polymers. 2025; 17(11):1539. https://doi.org/10.3390/polym17111539
Chicago/Turabian StyleMoon, Youngil, Gyunghyun Kim, and Jaekap Jung. 2025. "A Study of the Dielectric Relaxation of Nitrile–Butadiene Rubber, Ethylene–Propylene–Diene Monomer, and Fluoroelastomer Polymers with a Self-Developed Deconvolution Analysis Program" Polymers 17, no. 11: 1539. https://doi.org/10.3390/polym17111539
APA StyleMoon, Y., Kim, G., & Jung, J. (2025). A Study of the Dielectric Relaxation of Nitrile–Butadiene Rubber, Ethylene–Propylene–Diene Monomer, and Fluoroelastomer Polymers with a Self-Developed Deconvolution Analysis Program. Polymers, 17(11), 1539. https://doi.org/10.3390/polym17111539






