Effect of Multiple Phosphorus-Nitrogen Flame Retardant on the Properties of PA66
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
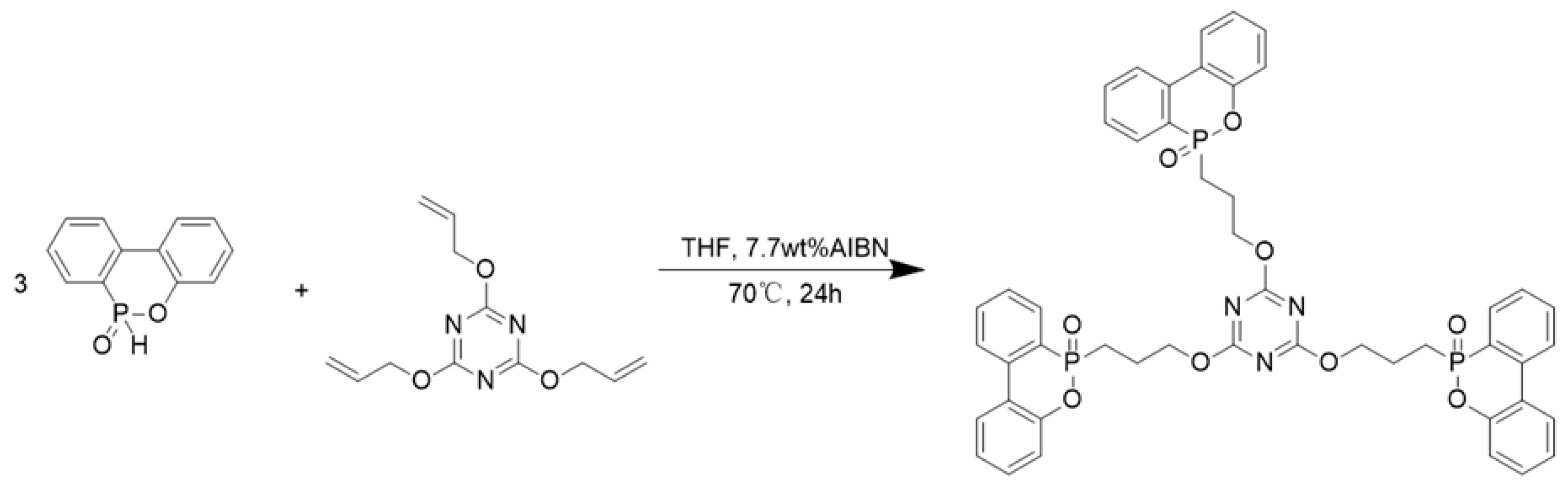
2.2. Synthesis of DT
2.3. Preparation of Flame-Retardant PA66
2.4. Characterization
3. Results
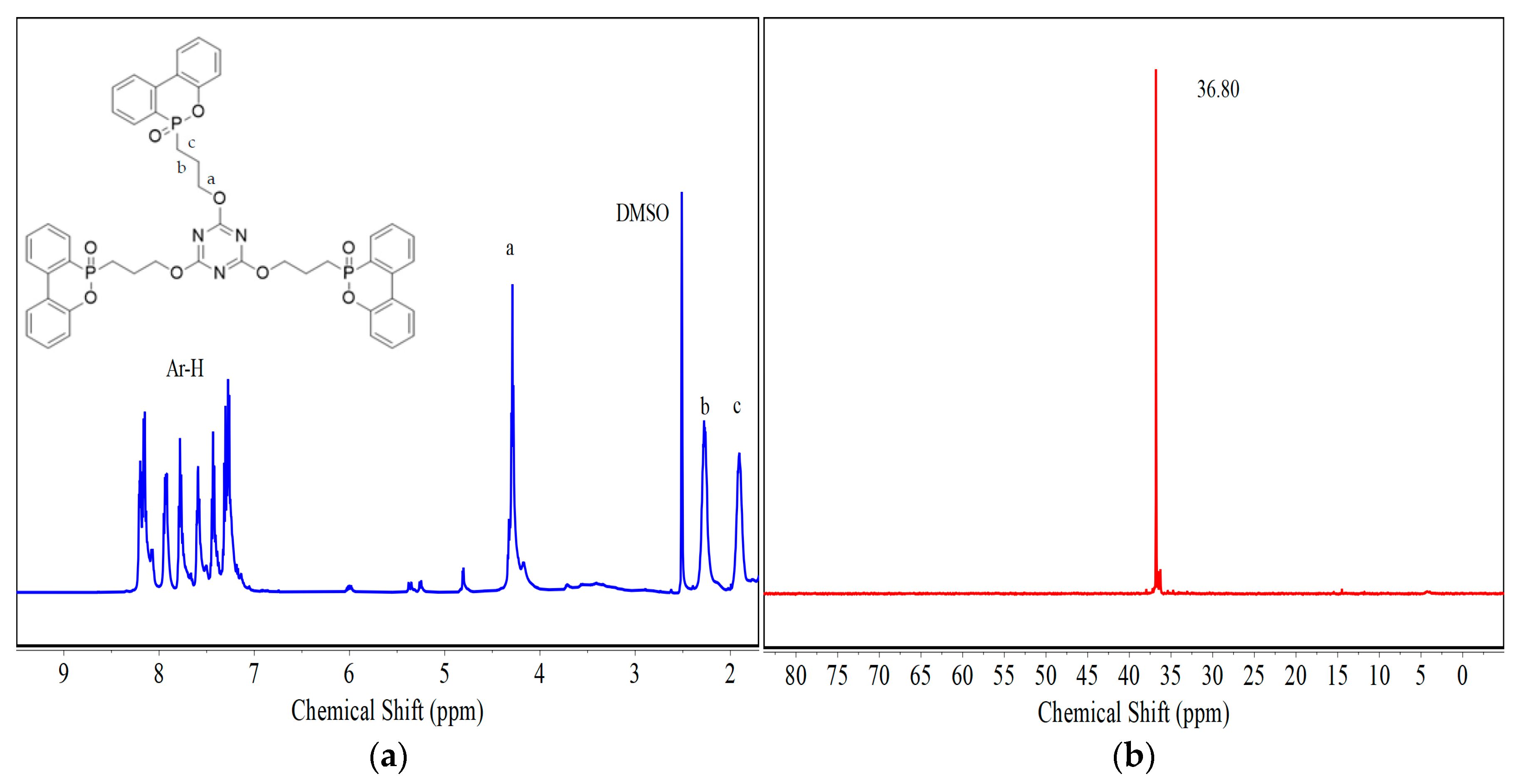
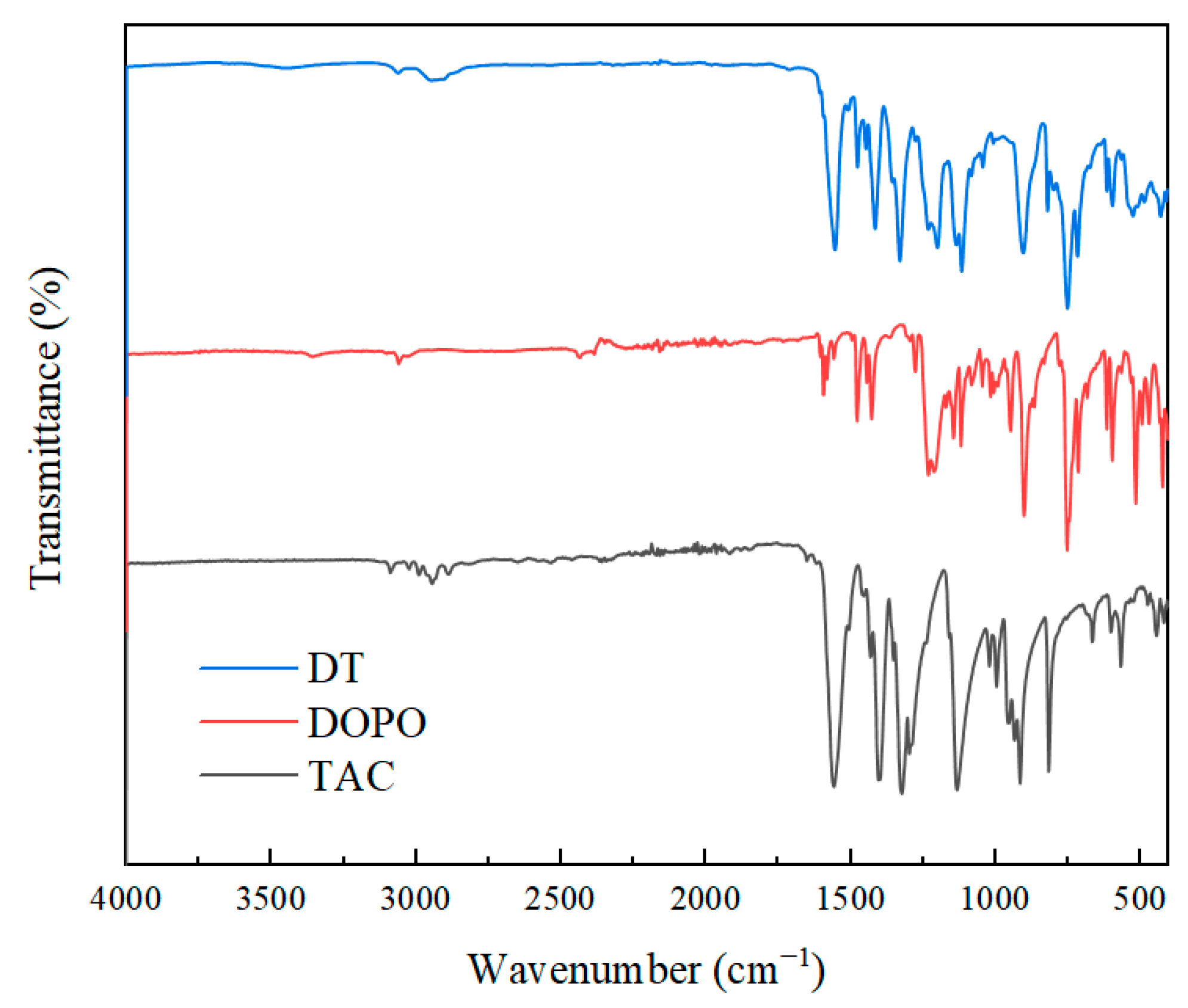
3.1. Structural Characterization of DT
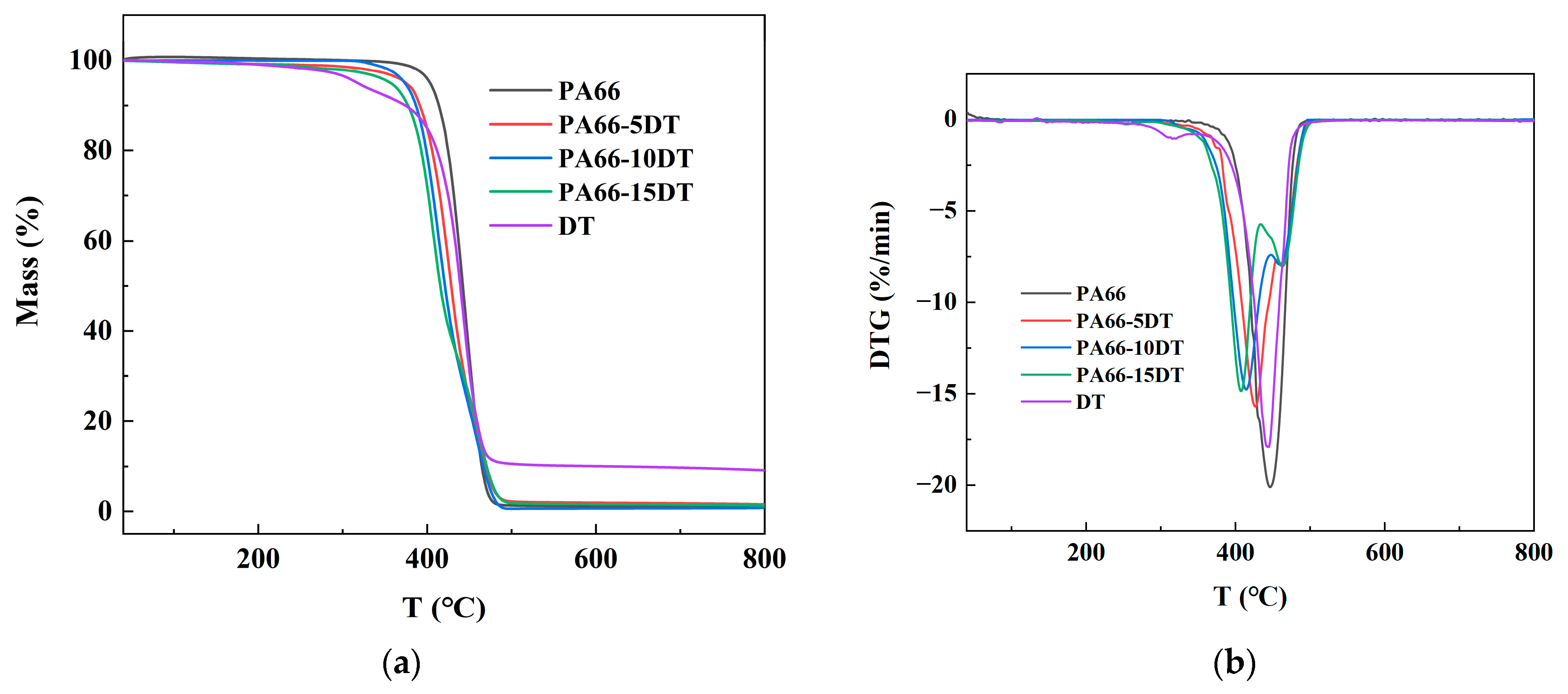
3.2. Thermal Stability
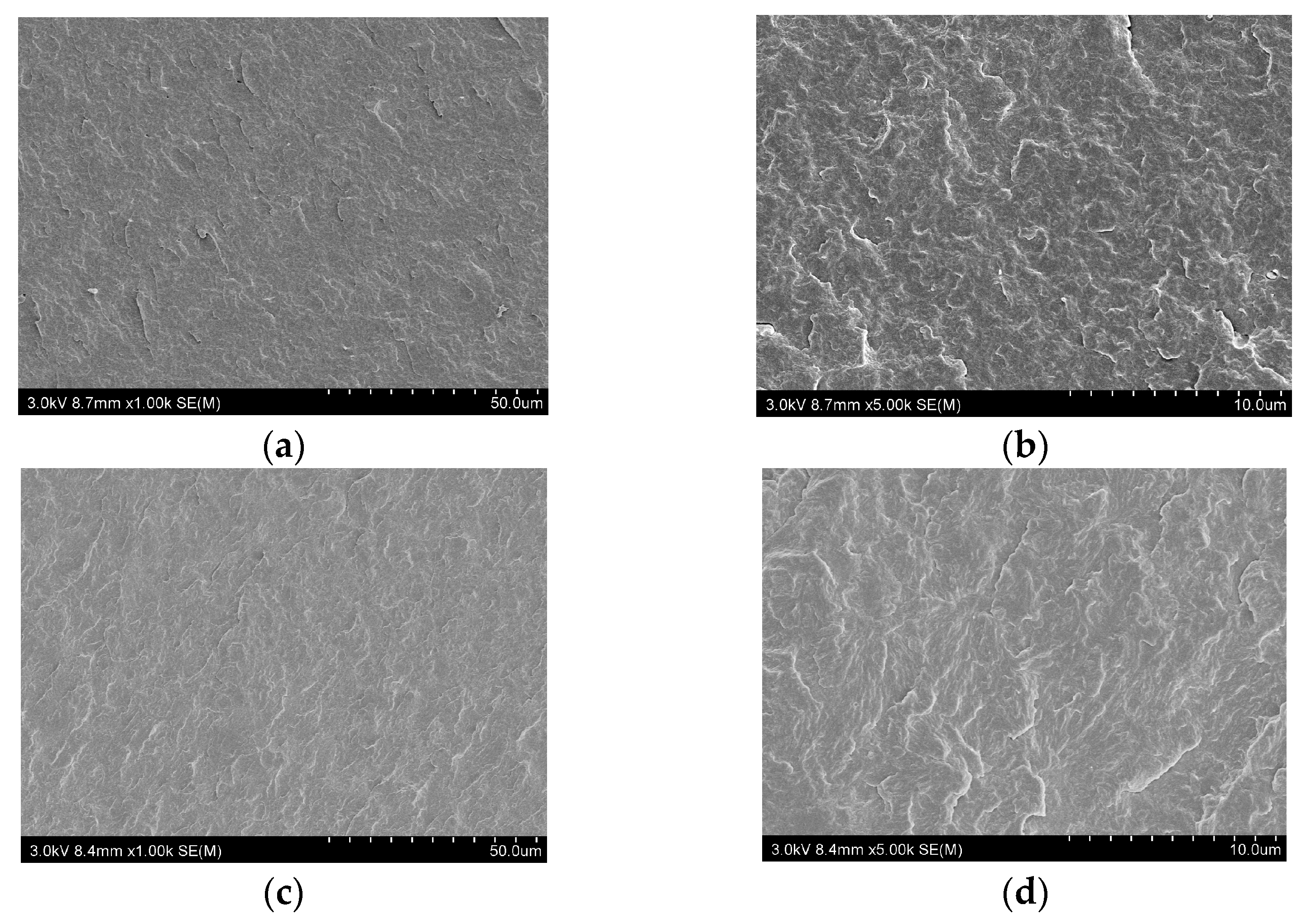
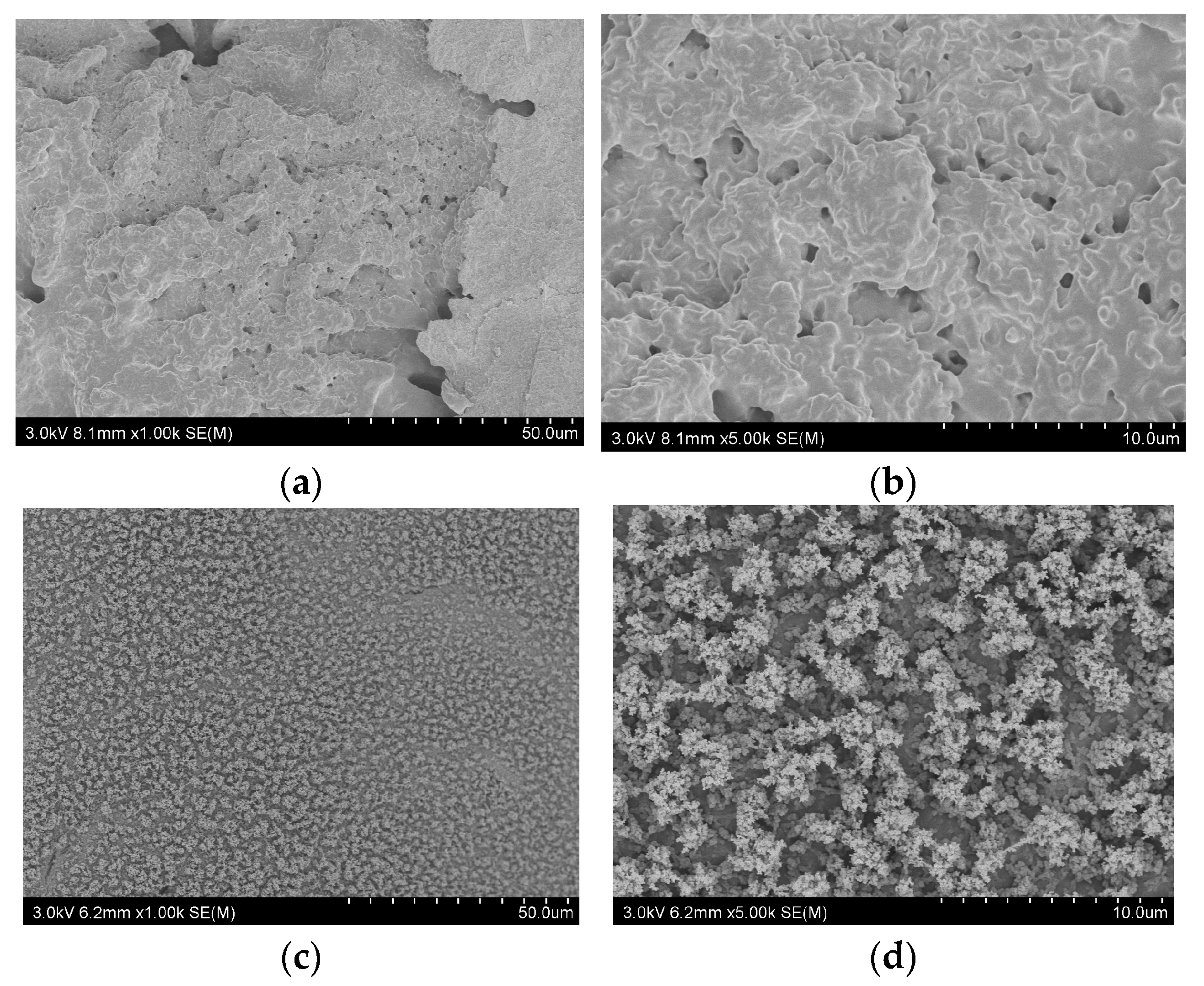
3.3. The Dispersion of the Flame Retardants in PA66 Composites
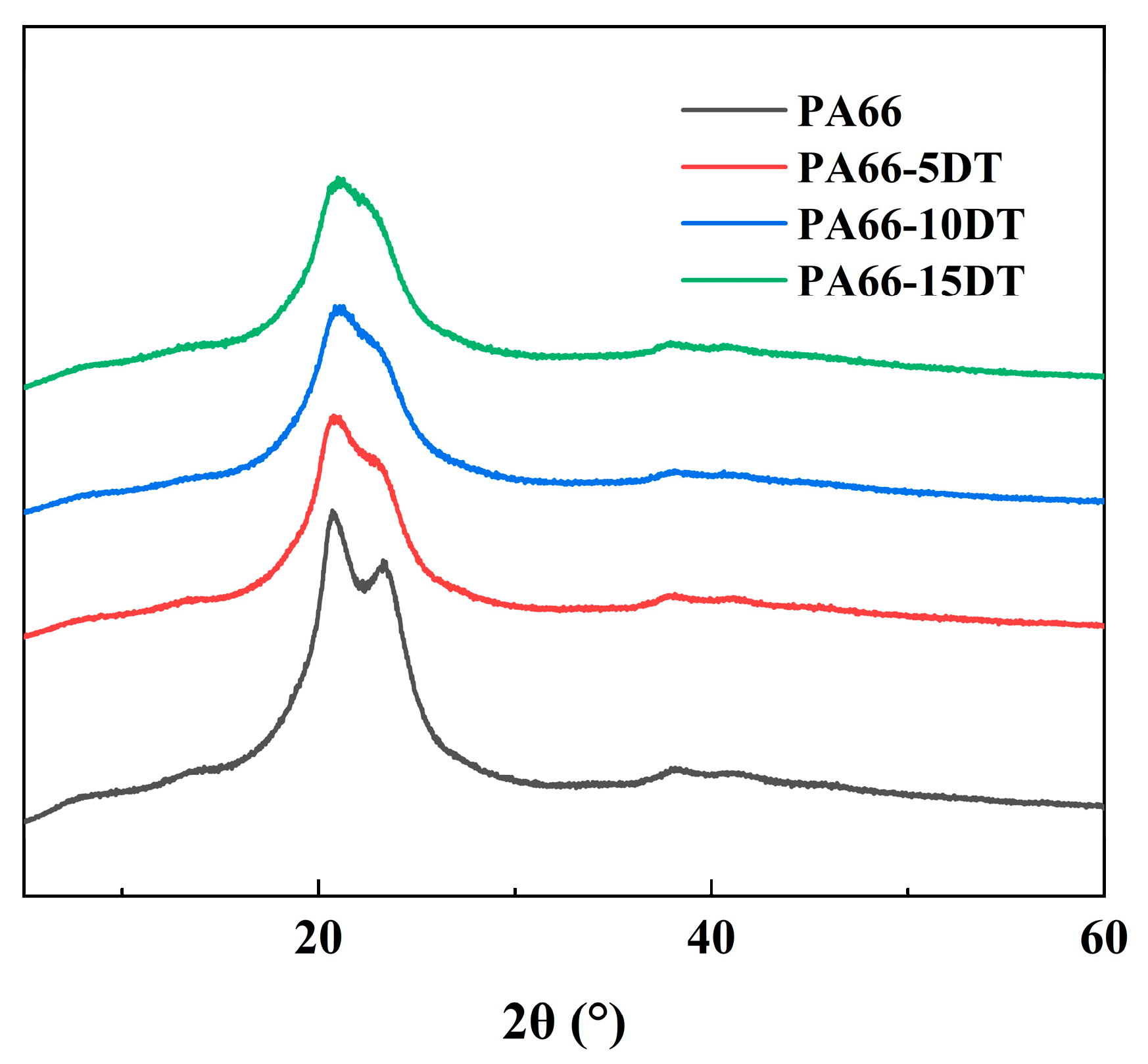
3.4. Crystallization Properties
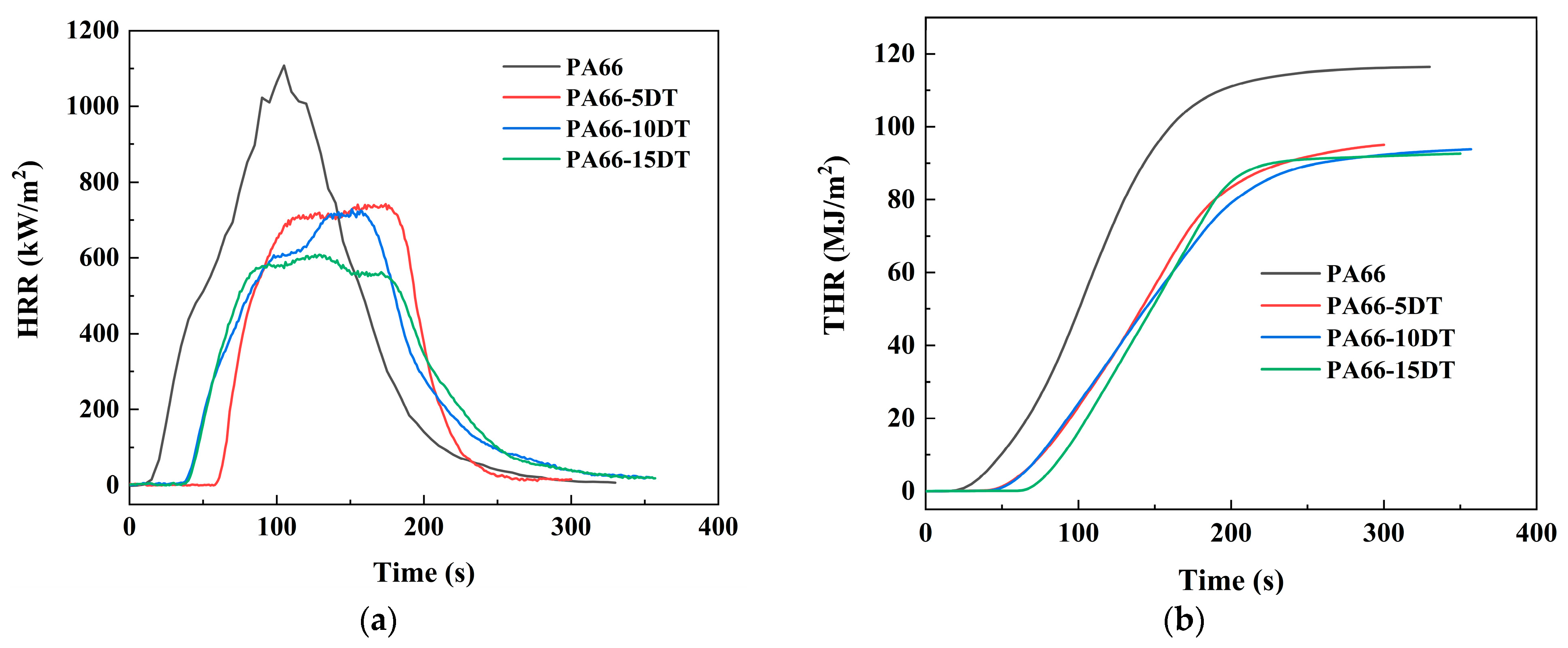
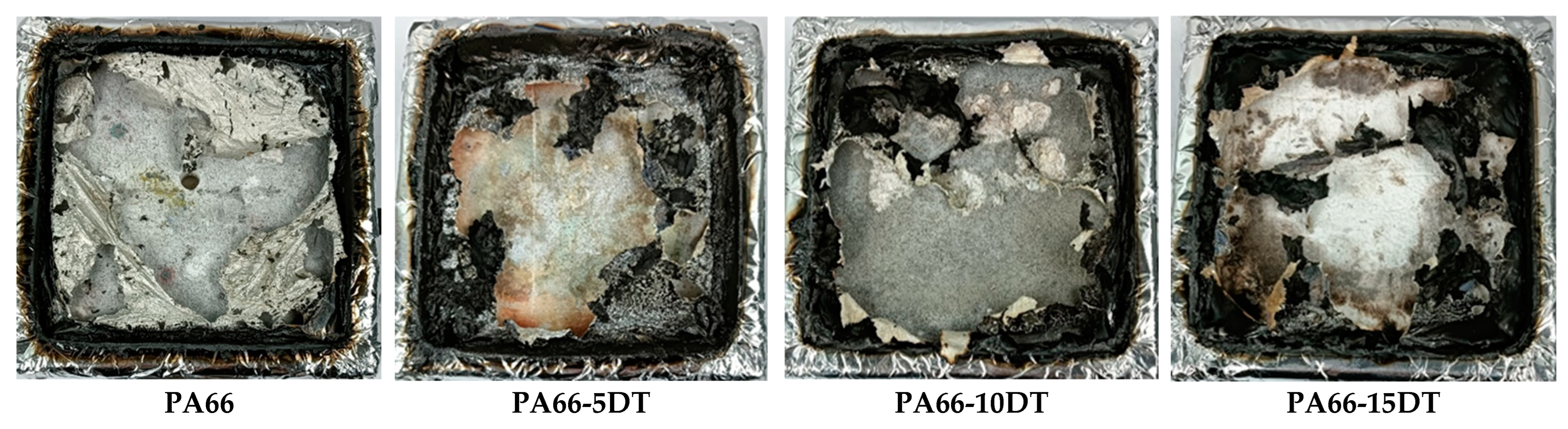
3.5. Flame-Retardant Properties
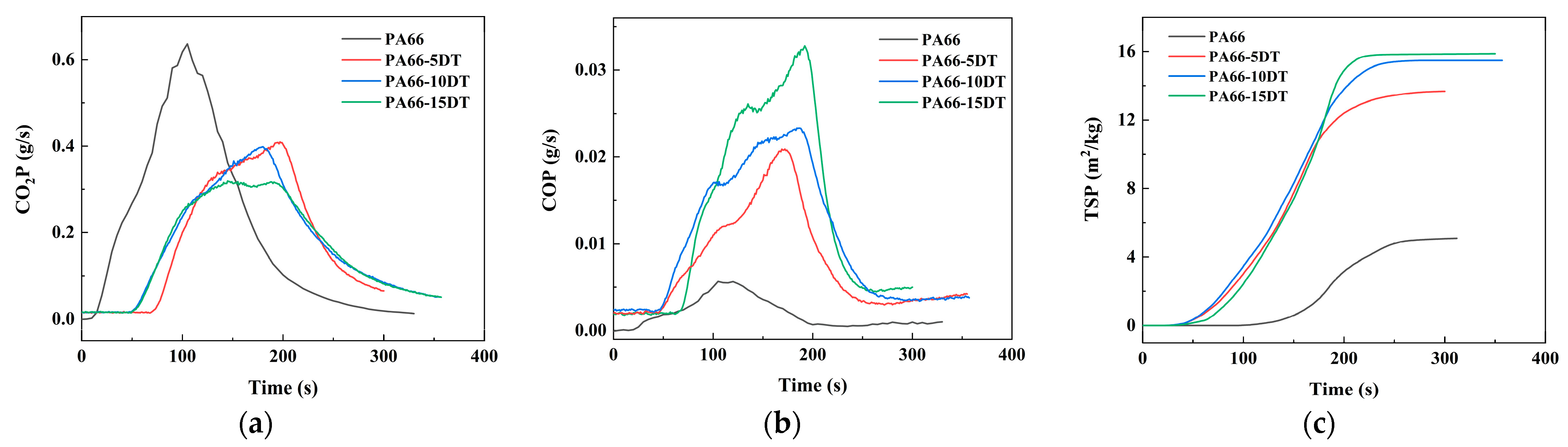
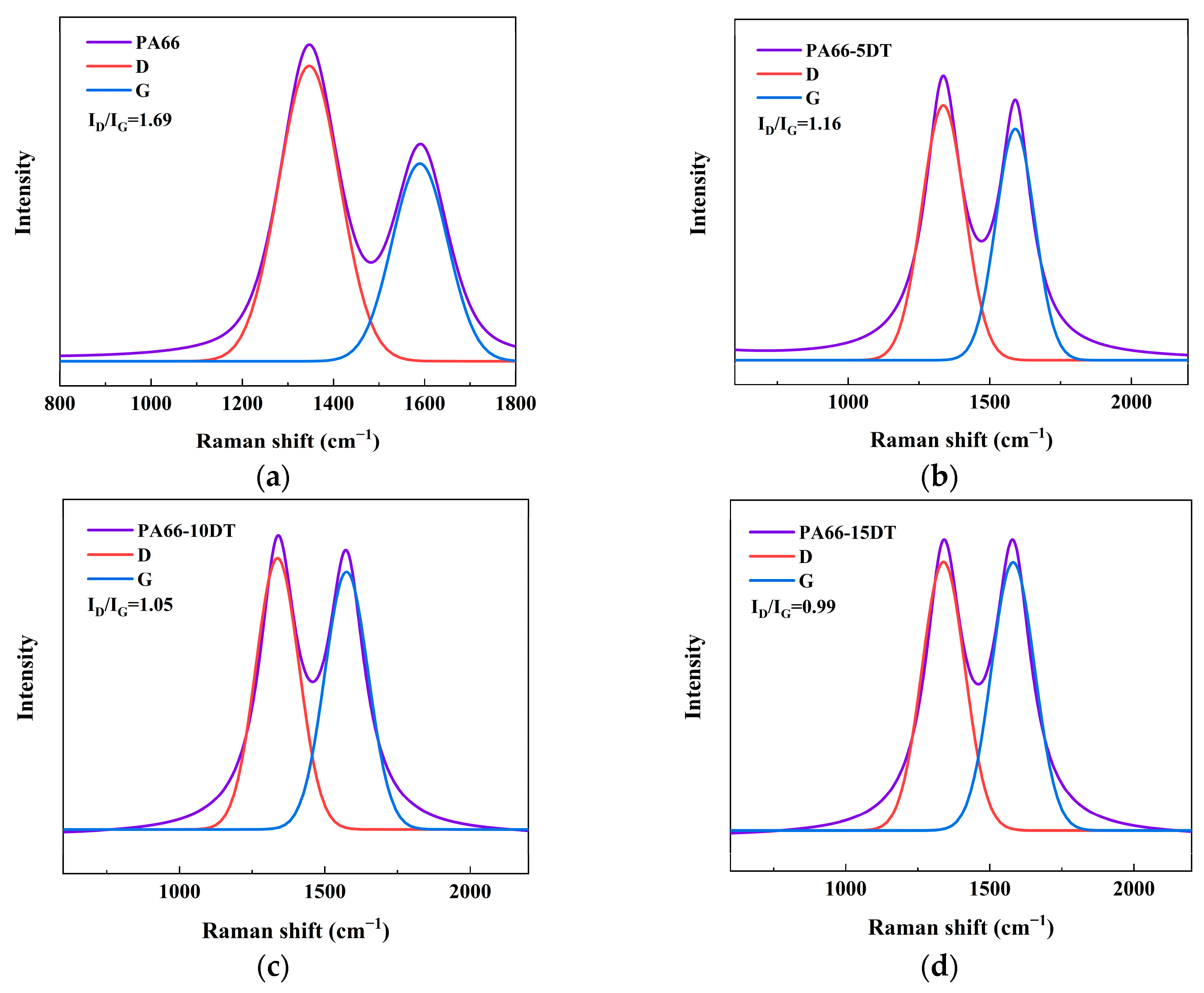
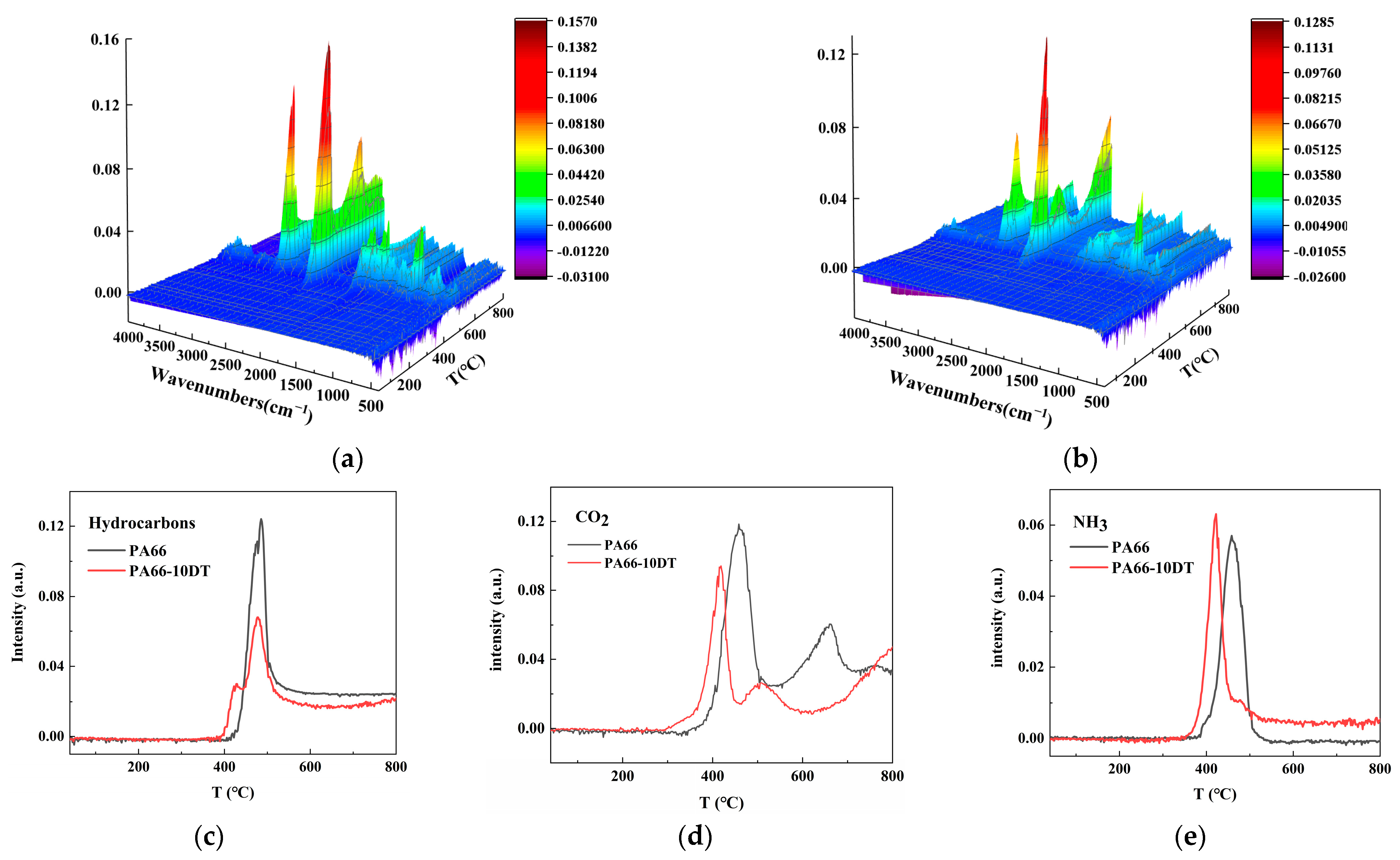
3.6. Analysis of the Flame-Retardant Mechanism
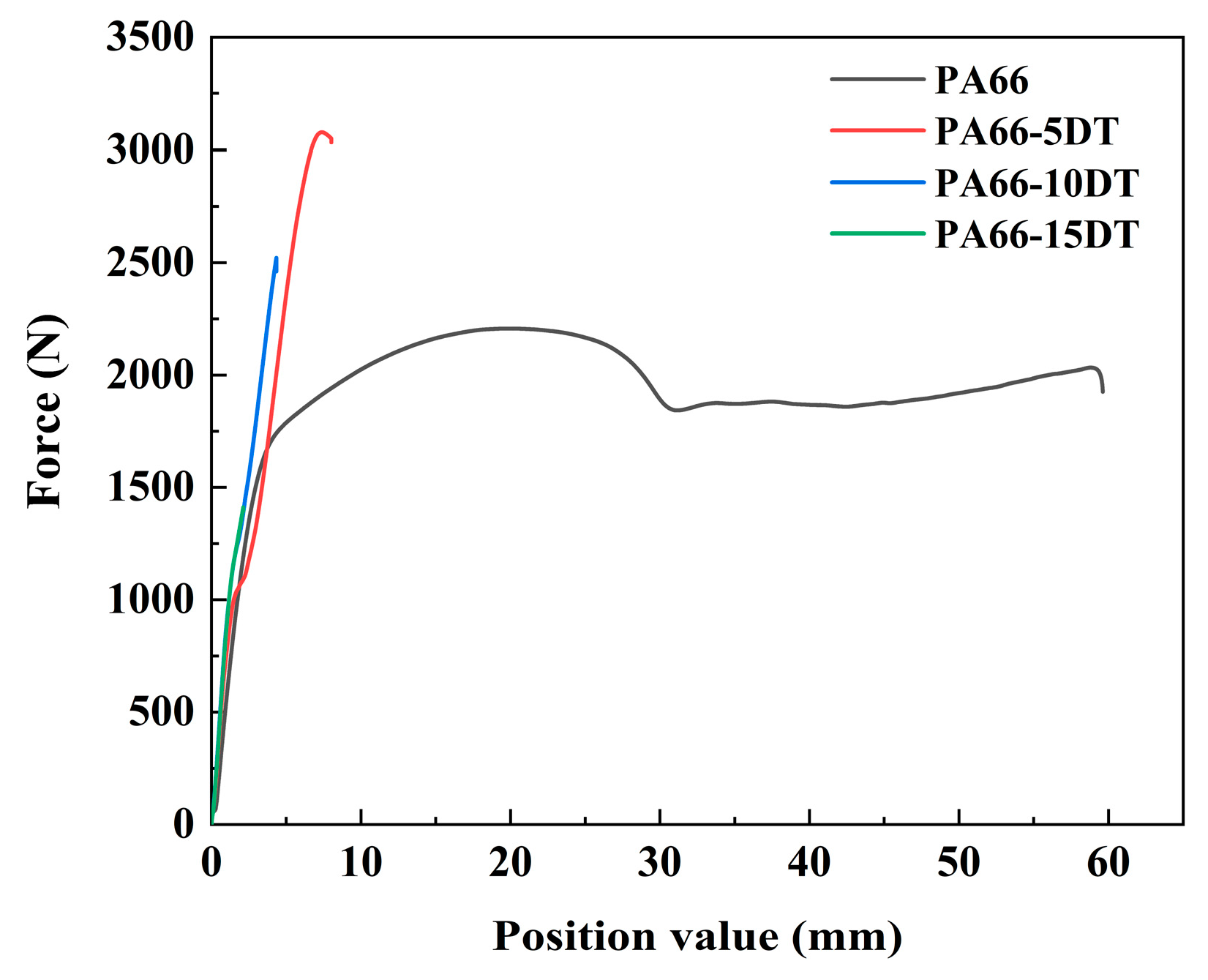
3.7. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chavarria, F.; Paul, D.R. Comparison of nanocomposites based on nylon 6 and nylon 66. Polymer 2004, 45, 8501–8515. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.-Z.; Yang, M. Micro- and nano-scale deformation behavior of nylon 66-based binary and ternary nanocomposites. Compos. Sci. Technol. 2006, 66, 3097–3114. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.; Chen, Z. Mechanical Properties and Flame Retardancy Research of Montmorillonite Intercalate Polyamide 66 Composites. J. Compos. Mater. 2009, 43, 2785–2792. [Google Scholar] [CrossRef]
- Zhan, Z.; Xu, M.; Li, B. Synergistic effects of sepiolite on the flame retardant properties and thermal degradation behaviors of polyamide 66/aluminum diethylphosphinate composites. Polym. Degrad. Stab. 2015, 117, 66–74. [Google Scholar] [CrossRef]
- Jian, Z.; Rong, L.; Shao, F. Flame retardancy and its mechanism of polymers flame retarded by DBDPE/Sb2O2. J. Cent. South Univ. Technol. 2008, 15, 64–68. [Google Scholar]
- Kocaman, M.; Akçay, S.B.; Güler, O. Effect of expandable graphite content on the physical, thermal and mechanical properties of novolac matrix composites: Halogen-free flame-retardant polymer composites. Diam. Relat. Mater. 2024, 150, 111753–111768. [Google Scholar] [CrossRef]
- Li, M.; Yan, Y.; Zhao, H. A facile and efficient flame-retardant and smoke-suppressant resin coating for expanded polystyrene foams. Compos. Part B Eng. 2020, 185, 117097–117804. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, H.; Chen, L. Eco-friendly synergistic cross-linking flame-retardant strategy with smoke and melt-dripping suppression for condensation polymers. Compos. Part B Eng. 2021, 211, 108664–108676. [Google Scholar] [CrossRef]
- Lv, W.; Lv, J.; Zhu, C. Thermal Stabilities and Flame Retardancy of Polyamide 66 Prepared by In Situ Loading of Amino-Functionalized Polyphosphazene Microspheres. Polymers 2022, 15, 218–229. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, W.; Shi, Y. ADOPO-anchored benzothiadiazole derivative toward efficiently P/N/S synergistic flame retarding of epoxy thermoset. Polym. Adv. Technol. 2022, 33, 3490–3500. [Google Scholar] [CrossRef]
- Ren, X.; Long, S.; Chen, X. Accessible preparation of a novel nitrogen-phosphorus synergetic nanocellulose-based flame retardant with excellent char-forming ability for enhancing the anti-melting droplet property of PA66. Int. J. Biol. Macromol. 2025, 289, 138951–138961. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Z.; Toldy, A. Flame Retardancy via in-Mould Coating and Durability of Flame Retardants After Mechanical Recycling in all-polyamide Composites Prepared by In Situ Polymerisation. Macromol. Mater. Eng. 2024, 76, 311–323. [Google Scholar] [CrossRef]
- Lu, P.; Zhao, Y.; Xu, B. A novel inherently flame-retardant thermoplastic polyamide elastomer. Chem. Eng. J. 2020, 379, 122278–122289. [Google Scholar] [CrossRef]
- Li, Y.; Liu, K.; Zhang, J. Preparation and characterizations of inherent flame retarded polyamide 66 containing the phosphorus linking pendent group. Polym. Adv. Technol. 2018, 29, 951–960. [Google Scholar] [CrossRef]
- Wu, H.; Krifa, M.; Koo, J.H. Inherently Flame Retardant Nylon 6 Nanocomposite Fibers. Fibers Polym. 2018, 19, 1500–1512. [Google Scholar] [CrossRef]
- Spieß, B.; Metzsch-Zilligen, E.; Pfaendner, R. A New Class of Oxyimides: Oxyimide Ethers and their Use as Flame Retardants. Macromol. Mater. Eng. 2021, 306, 52–60. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Y.; Zuo, C. Improving the flame retardant and anti-dripping performance of polyamide 66 inspired by vegetable tanning. Polym. Degrad. Stab. 2023, 217, 110517–110528. [Google Scholar] [CrossRef]
- Fu, H.; Cui, Y.; Lv, J. Fire retardant mechanism of PA66 modified by a “trinity” reactive flame retardant. J. Appl. Polym. Sci. 2019, 137, 47488–47495. [Google Scholar] [CrossRef]
- Liu, T.; Tao, Y.; Ni, X. The synthesis and properties of an intumescent zinc-chelated organometallic flame retardant. Polym. Degrad. Stab. 2025, 232, 111142–111153. [Google Scholar] [CrossRef]
- Zang, L.; Wagner, S.; Ciesielski, M. Novel star-shaped and hyperbranched phosphorus-containing flame retardants in epoxy resins. Polym. Adv. Technol. 2011, 22, 1182–1191. [Google Scholar] [CrossRef]
- Jia, D.; He, J.; Yang, R. The synthesis and characterization of a DOPO derivative bearing an active terminal epoxy group: E-DOPO and its application in rigid polyurethane foam. J. Fire Sci. 2018, 37, 47–66. [Google Scholar] [CrossRef]
- Jiao, D.; Zhao, H.; Sima, H. Engineering flame retardant epoxy resins with strengthened mechanical property by using reactive catechol functionalized DOPO compounds. Chem. Eng. J. 2024, 485, 149910–149926. [Google Scholar] [CrossRef]
- Feldman, A.; Wachtel, E.; Vaughan, G. The brill transition in transcrystalline nylon-66. Macromolecules 2006, 39, 4455–4459. [Google Scholar] [CrossRef]
- R, B. Behavior of polyamides on heating. J. Parkt. Chem. 1942, 161, 49–63. [Google Scholar]
- Liu, X.; Wu, Q.; Zhang, Q. Phase transition in polyamide-66/montmorillonite nanocomposites on annealing. J. Polym. Sci. Part B Polym. Phys. 2002, 41, 63–67. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, X.; Zhou, Y. Molecular design of reactive flame retardant for preparing biobased flame retardant polyamide 56. Polym. Degrad. Stab. 2023, 207, 110212–110224. [Google Scholar] [CrossRef]
- Gao, J.; Huang, W.; He, W. Superior flame retardancy of glass fiber-reinforced polyamide 6T composites by synergism between DOPO-based derivative and carbon nanotube. J. Therm. Anal. Calorim. 2021, 147, 1265–1274. [Google Scholar] [CrossRef]
- Negrell, C.; Frénéhard, O.; Sonnier, R. Self-extinguishing bio-based polyamides. Polym. Degrad. Stab. 2016, 134, 10–18. [Google Scholar] [CrossRef]
- Huang, G.; Gao, J.; Wang, X. How can graphene reduce the flammability of polymer nanocomposites. Mater. Lett. 2012, 66, 187–189. [Google Scholar] [CrossRef]
- Qi, Y.; Wu, W.; Liu, X. Preparation and characterization of aluminum hypophosphite/reduced graphene oxide hybrid material as a flame retardant additive for PBT. Fire Mater. 2017, 41, 195–208. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Yuan, B.; Fan, A.; Yang, M. The effects of graphene on the flammability and fire behavior of intumescent flame retardant polypropylene composites at different flame scenarios. Polym. Degrad. Stab. 2017, 143, 42–56. [Google Scholar] [CrossRef]
- Chen, X.; Ma, C.; Jiao, C. Synergistic effects between iron-graphene and melamine salt of pentaerythritol phosphate on flame retardant thermoplastic polyurethane. Polym. Adv. Technol. 2016, 27, 1508–1516. [Google Scholar] [CrossRef]
- Duan, R.; Wu, H.; Li, J. Phosphor nitrile functionalized UiO-66-NH2/graphene hybrid flame retardants for fire safety of epoxy. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 128093–128114. [Google Scholar] [CrossRef]
- Meng, D.; Guo, J.; Wang, A. The fire performance of polyamide66 fabric coated with soybean protein isolation. Prog. Org. Coat. 2020, 148, 105835–105842. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, X.; Xu, C. An inherently flame-retardant polyamide 6 containing a phosphorus group prepared by transesterification polymerization. Polymer 2020, 207, 122890–122902. [Google Scholar] [CrossRef]
| Samples | PA66 (g) | DT (g) | Tns-(2.4-di-tert-butyl)-Phosphite | Antioxidant 1010 |
|---|---|---|---|---|
| PA66 | 1000 | 0 | 1 | 2 |
| PA66-5DT | 950 | 50 | 1 | 2 |
| PA66-10DT | 900 | 100 | 1 | 2 |
| PA66-15DT | 850 | 150 | 1 | 2 |
| Sample | T5% (°C) | TMax1 (°C) | TMax2 (°C) | Char Residue at 800 °C (%) |
|---|---|---|---|---|
| DT | 317.6 | 444.6 | - | |
| PA66 | 403.4 | 446.1 | - | 0.79 |
| PA66-5DT | 374.8 | 426.7 | 461.1 | 0.89 |
| PA66-10DT | 373.4 | 413.8 | 459.4 | 1.32 |
| PA66-15DT | 356.2 | 407.4 | 462.7 | 1.56 |
| Sample | LOI | UL-94 | ||||
|---|---|---|---|---|---|---|
| t1/s | t2/s | Dripping | Ignition | Rating | ||
| PA66 | 22.6% | >30 | >30 | Yes | Yes | NR |
| PA66-5DT | 23.4% | 5.7 | 2.5 | Yes | Yes | V-2 |
| PA66-10DT | 25.6% | 3.8 | 1.1 | Yes | Yes | V-2 |
| PA66-15DT | 27.2% | 2.3 | 1.1 | Yes | No | V-0 |
| Sample | pHRR/kW∙m−2 | THR/MJ∙m−2 | av-EHC /MJ∙kg−1 | TTI/s | TSP/m2∙kg−1 | Char Residue/% |
|---|---|---|---|---|---|---|
| PA66 | 1107.7 ± 9.1 | 116.4 ± 2.0 | 32.5 ± 0.8 | 12 ± 0.3 | 5.43 ± 0.1 | 0.4 ± 0.0 |
| PA66-5DT | 741.8 ± 5.2 | 98.8 ± 2.2 | 29.1 ± 0.7 | 58 ± 1.2 | 13.75 ± 0.4 | 1.3 ± 0.0 |
| PA66-10DT | 728.4 ± 2.3 | 95.8 ± 1.9 | 28.4 ± 0.4 | 37 ± 0.9 | 15.47 ± 0.4 | 1.7 ± 0.0 |
| PA66-15DT | 608.1 ± 4.0 | 94.3 ± 1.7 | 26.7 ± 0.4 | 39 ± 0.7 | 15.95 ± 0.5 | 2.1 ± 0.0 |
| Sample | Tensile Strength (MPa) | Tensile Modulus (GPa) | Elongation at Break (%) |
|---|---|---|---|
| PA66 | 53.0 ± 1.0 | 2.26 ± 0.1 | 37 ± 1.3 |
| PA66-5DT | 76.9 ± 1.7 | 3.89 ± 0.2 | 8.8 ± 0.2 |
| PA66-10DT | 63.0 ± 1.1 | 3.49 ± 0.2 | 2.8 ± 0.0 |
| PA66-15DT | 36.2 ± 0.7 | 2.45 ± 0.1 | 2.4 ± 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; He, J.; Li, X. Effect of Multiple Phosphorus-Nitrogen Flame Retardant on the Properties of PA66. Polymers 2025, 17, 1537. https://doi.org/10.3390/polym17111537
Zhang H, He J, Li X. Effect of Multiple Phosphorus-Nitrogen Flame Retardant on the Properties of PA66. Polymers. 2025; 17(11):1537. https://doi.org/10.3390/polym17111537
Chicago/Turabian StyleZhang, Haoyang, Jiyu He, and Xiangmei Li. 2025. "Effect of Multiple Phosphorus-Nitrogen Flame Retardant on the Properties of PA66" Polymers 17, no. 11: 1537. https://doi.org/10.3390/polym17111537
APA StyleZhang, H., He, J., & Li, X. (2025). Effect of Multiple Phosphorus-Nitrogen Flame Retardant on the Properties of PA66. Polymers, 17(11), 1537. https://doi.org/10.3390/polym17111537





