In Situ 3D Printing of Conformal Bioflexible Electronics via Annealing PEDOT:PSS/PVA Composite Bio-Ink
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Conductive Composite Ink
2.3. In Situ Printing of Strain Sensor Circuits on the Hand
2.4. Strain Sensor Performance Characterization
2.5. Fourier Transform Infrared Spectroscopy Test
2.6. Scanning Electron Microscopy Test
2.7. Tensile Test
2.8. Rheological Research
2.9. Conductivity Measurement
2.10. Biocompatibility Assessment
3. Results
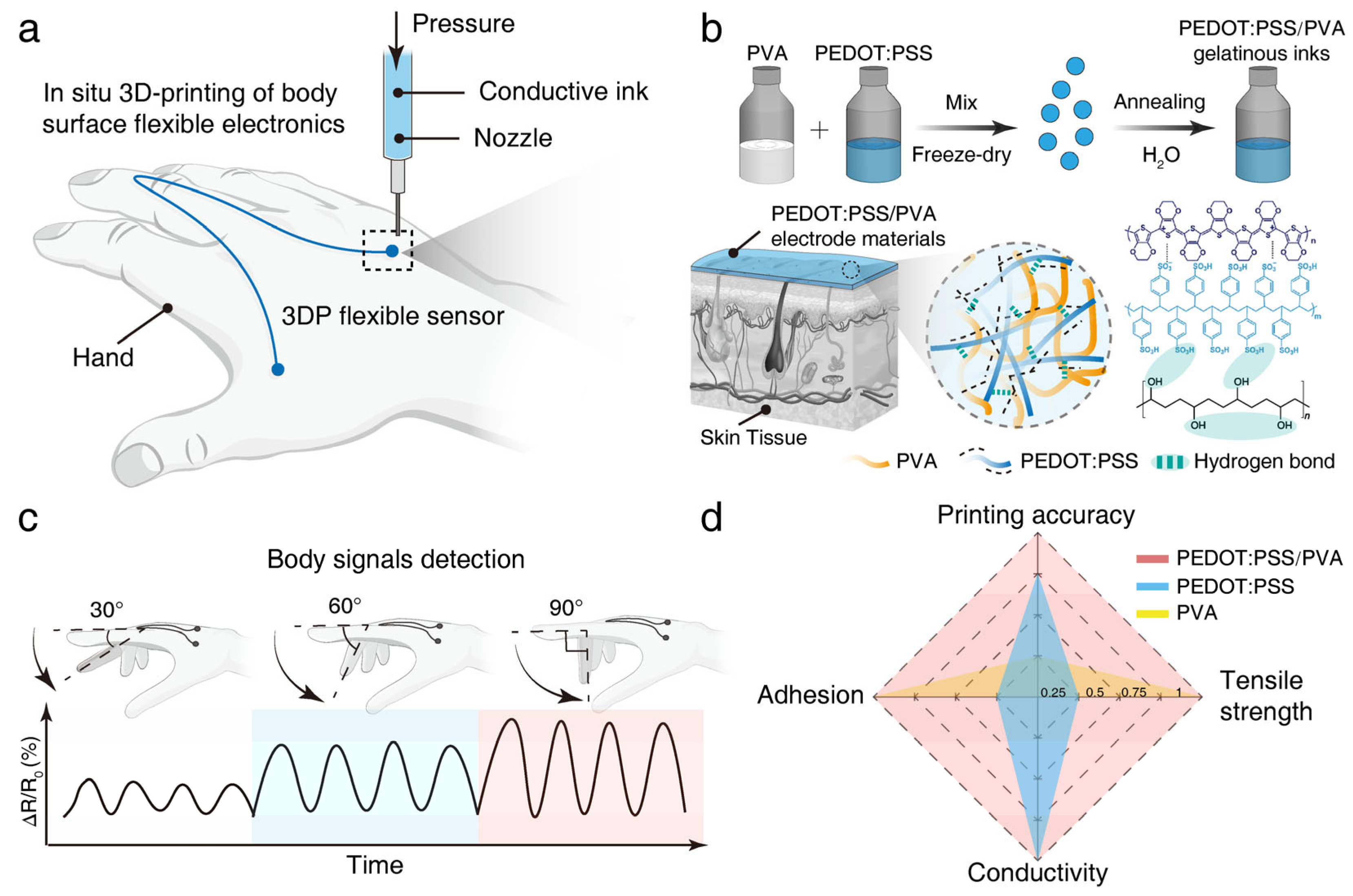
3.1. Design and Characterization of the 3D-Printable Conductive Composite Ink
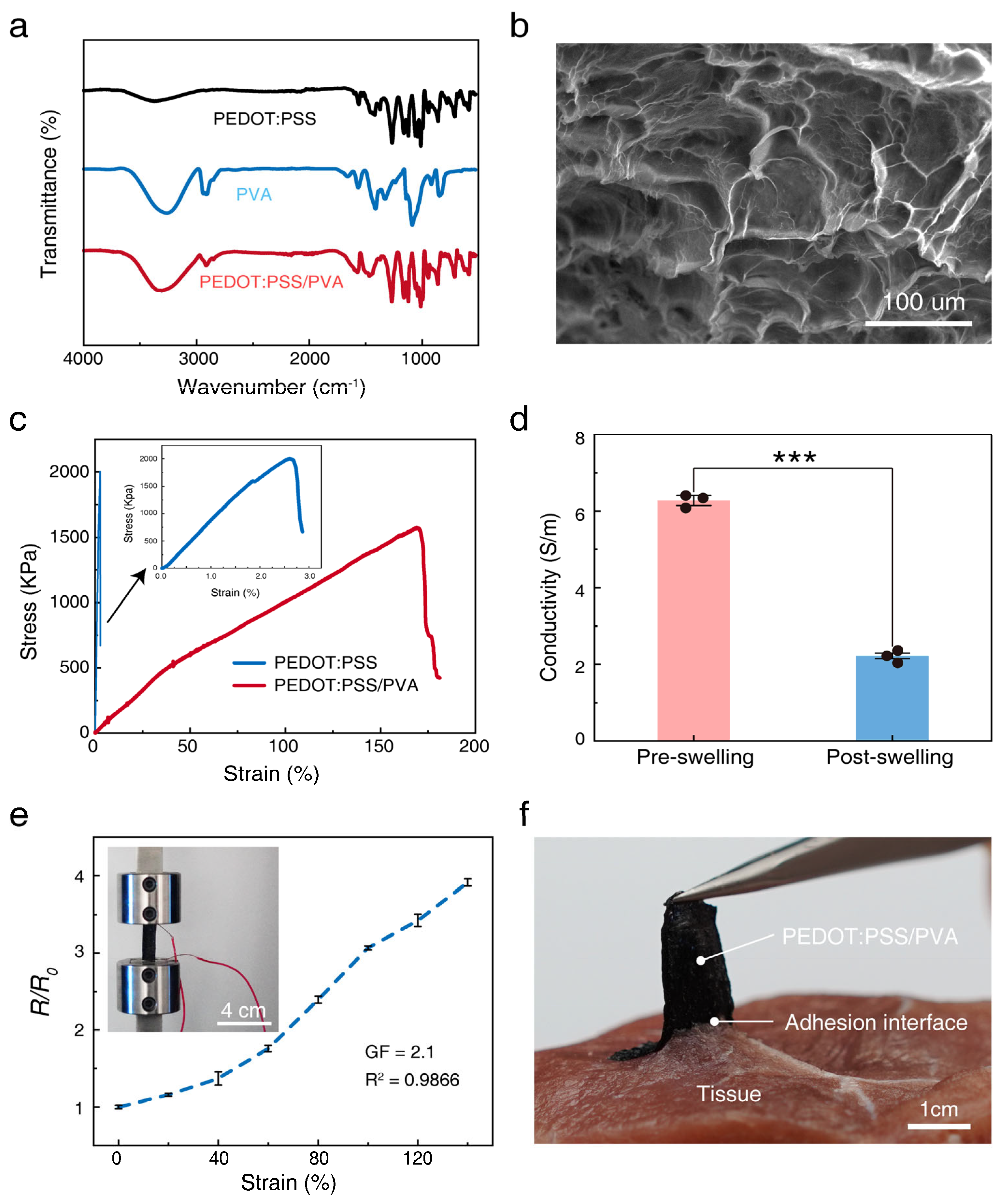
3.2. Properties of the 3D-Printed Conductive Composite Material
3.3. Application of the 3D-Printed Strain Sensor and Electrodes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DIW | Direct Ink Writing |
| PVA | Polyvinyl alcohol |
| DI water | Deionized water |
| 3D | three-dimensional |
| 2D | two-dimensional |
| 3DP | 3D printing |
| G′ | Storage modulus |
| G″ | Loss modulus |
| FTIR | Fourier Transform Infrared Spectroscopy |
| SEM | Scanning Electron Microscopy |
| R/R0 | relative resistance change |
| ΔR/R0 | relative resistance change |
| H&E | Hematoxylin and Eosin |
| σ | Electrical conductivity |
| R | Resistance |
References
- Kim, H.; Kwon, Y.T.; Lim, H.R.; Kim, J.H.; Kim, Y.S.; Yeo, W.H. Recent advances in wearable sensors and integrated functional devices for virtual and augmented reality applications. Adv. Funct. Mater. 2021, 31, 2005692. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, L.; Wang, X. Flexible and wearable healthcare sensors for visual reality health-monitoring. Virtual Real. Intell. Hardw. 2019, 1, 411–427. [Google Scholar] [CrossRef]
- Barfield, W.; Caudell, T. Basic concepts in wearable computers and augmented reality. In Fundamentals of Wearable Computers and Augmented Reality; CRC Press: Boca Raton, FL, USA, 2001; pp. 19–42. [Google Scholar]
- Kong, X.T.; Luo, H.; Huang, G.Q.; Yang, X. Industrial wearable system: The human-centric empowering technology in Industry 4.0. J. Intell. Manuf. 2019, 30, 2853–2869. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J.; et al. Wearable sensors: Modalities, challenges, and prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef]
- Singh, B.; Kaunert, C.; Vig, K.; Gautam, B.K. Wearable Sensors Assimilated With Internet of Things (IoT) for Advancing Medical Imaging and Digital Healthcare: Real-Time Scenario. In Inclusivity and Accessibility in Digital Health; IGI Global: Hershey, PA, USA, 2024; pp. 275–297. [Google Scholar]
- Chen, Y.; Zhang, Y.; Liang, Z.; Cao, Y.; Han, Z.; Feng, X. Flexible inorganic bioelectronics. npj Flex. Electron. 2020, 4, 2. [Google Scholar] [CrossRef]
- Fallegger, F.; Schiavone, G.; Lacour, S.P. Conformable hybrid systems for implantable bioelectronic interfaces. Adv. Mater. 2020, 32, 1903904. [Google Scholar] [CrossRef]
- Song, E.; Li, J.; Won, S.M.; Bai, W.; Rogers, J.A. Materials for flexible bioelectronic systems as chronic neural interfaces. Nat. Mater. 2020, 19, 590–603. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.W.; Lim, S.; Yi, H.; Wu, Z.; Kwon, I.G.; Yeo, W.H.; Song, E.; Yu, K.J. Conformal fixation strategies and bioadhesives for soft bioelectronics. Adv. Funct. Mater. 2024, 34, 2313728. [Google Scholar] [CrossRef]
- Khan, M.A.; Saibene, M.; Das, R.; Brunner, I.; Puthusserypady, S. Emergence of flexible technology in developing advanced systems for post-stroke rehabilitation: A comprehensive review. J. Neural Eng. 2021, 18, 061003. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Bo, L.; Qi, J.; Zhu, Y. Research Progress on Applying Intelligent Sensors in Sports Science. Sensors 2024, 24, 7338. [Google Scholar] [CrossRef]
- De Pasquale, G.; Ruggeri, V. Sensing strategies in wearable bio-mechanical systems for medicine and sport: A review. J. Micromech. Microeng. 2019, 29, 103001. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Y.; Tang, G.; Ru, J.; Zhu, Z.; Li, B.; Guo, C.F.; Li, L.; Zhu, D. Ionic flexible sensors: Mechanisms, materials, structures, and applications. Adv. Funct. Mater. 2022, 32, 2110417. [Google Scholar] [CrossRef]
- Ochoa, M.; Rahimi, R.; Ziaie, B. Flexible sensors for chronic wound management. IEEE Rev. Biomed. Eng. 2013, 7, 73–86. [Google Scholar] [CrossRef]
- Chen, W.; Lin, J.; Ye, Z.; Wang, X.; Shen, J.; Wang, B. Customized surface adhesive and wettability properties of conformal electronic devices. Mater. Horiz. 2024, 11, 6289–6325. [Google Scholar] [CrossRef]
- Liang, X.; Chen, G.; Lin, S.; Zhang, J.; Wang, L.; Zhang, P.; Wang, Z.; Wang, Z.; Lan, Y.; Ge, Q.; et al. Anisotropically Fatigue-Resistant Hydrogels. Adv. Mater. 2021, 33, 2102011. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Chen, G.; Lin, S.; Zhang, J.; Wang, L.; Zhang, P.; Lan, Y.; Liu, J. Bioinspired 2D Isotropically Fatigue-Resistant Hydrogels. Adv. Mater. 2022, 34, 2107106. [Google Scholar] [CrossRef]
- Liang, X.; Chen, G.; Lei, I.; Zhang, P.; Wang, Z.; Chen, X.; Lu, M.; Zhang, J.; Wang, Z.; Sun, T.; et al. Impact-Resistant Hydrogels by Harnessing 2D Hierarchical Structures. Adv. Mater. 2022, 35, 2207587. [Google Scholar] [CrossRef]
- Zhang, Y.H.P.; Sun, J.; Ma, Y. Biomanufacturing: History and perspective. J. Ind. Microbiol. Biotechnol. 2017, 44, 773–784. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, Y.; Ma, L.; Gao, L.; Li, Y.; Xue, Q.; Yang, H.; Cui, Z. 3D bioprinting: An emerging technology full of opportunities and challenges. Bio-Des. Manuf. 2018, 1, 2–13. [Google Scholar] [CrossRef]
- Skardal, A. Perspective: “Universal” bioink technology for advancing extrusion bioprinting-based biomanufacturing. Bioprinting 2018, 10, e00026. [Google Scholar] [CrossRef]
- Arslan-Yildiz, A.; El Assal, R.; Chen, P.; Guven, S.; Inci, F.; Demirci, U. Towards artificial tissue models: Past, present, and future of 3D bioprinting. Biofabrication 2016, 8, 014103. [Google Scholar] [CrossRef] [PubMed]
- Santoni, S.; Gugliandolo, S.G.; Sponchioni, M.; Moscatelli, D.; Colosimo, B. 3D bioprinting: Current status and trends—A guide to the literature and industrial practice. Bio-Des. Manuf. 2022, 5, 14–42. [Google Scholar] [CrossRef]
- He, Y.; Mao, T.; Gu, Y.; Yang, Y.; Ding, J. A simplified yet enhanced and versatile microfluidic platform for cyclic cell stretching on an elastic polymer. Biofabrication 2020, 12, 045032. [Google Scholar] [CrossRef]
- Cui, H.; Nowicki, M.; Fisher, J.; Zhang, L. 3D bioprinting for organ regeneration. Adv. Healthc. Mater. 2017, 6, 1601118. [Google Scholar] [CrossRef]
- Ng, W.L.; Chan, A.; Ong, Y.S.; Chua, C.K. Deep learning for fabrication and maturation of 3D bioprinted tissues and organs. Virtual Phys. Prototyp. 2020, 15, 340–358. [Google Scholar] [CrossRef]
- He, Y.; Yu, Y.; Yang, Y.; Gu, Y.; Mao, T.; Shen, Y.; Liu, Q.; Liu, R.; Ding, J. Design and aligner-assisted fast fabrication of a microfluidic platform for quasi-3D cell studies on an elastic polymer. Bioact. Mater. 2022, 15, 288–304. [Google Scholar] [CrossRef]
- Gogoi, D.; Kumar, M.; Singh, J. A comprehensive review on hydrogel-based bio-ink development for tissue engineering scaffolds using 3D printing. Ann. 3D Print. Med. 2024, 15, 100159. [Google Scholar] [CrossRef]
- Yao, Z.; Feng, X.; Wang, Z.; Zhan, Y.; Wu, X.; Xie, W.; Wang, Z.; Zhang, G. Techniques and applications in 3D bioprinting with chitosan bio-inks for drug delivery: A review. Int. J. Biol. Macromol. 2024, 270, 134752. [Google Scholar] [CrossRef]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J.; Shavandi, A. Natural hydrogel-based bio-inks for 3D bioprinting in tissue engineering: A review. Gels 2022, 8, 179. [Google Scholar] [CrossRef]
- Saunders, R.E.; Derby, B. Inkjet printing biomaterials for tissue engineering: Bioprinting. Int. Mater. Rev. 2014, 59, 430–448. [Google Scholar] [CrossRef]
- Tang, H.; Li, Y.; Chen, B.; Chen, X.; Han, Y.; Guo, M.; Xia, H.; Song, R.; Zhang, X.; Zhou, J. In situ forming epidermal bioelectronics for daily monitoring and comprehensive exercise. ACS Nano 2022, 16, 17931–17947. [Google Scholar] [CrossRef] [PubMed]
- Ershad, F.; Patel, S.; Yu, C. Wearable bioelectronics fabricated in situ on skins. npj Flex. Electron. 2023, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.; Jiang, S.; Liu, S.; Zhang, L.; Miao, S.; Zhou, F.; Zheng, B. Fish gelatin and gellan gum mixture as edible ink for 3D printing. J. Food Eng. 2024, 362, 111762. [Google Scholar] [CrossRef]
- Yu, H.; Chi, S.; Li, D.; Wang, L.; Wang, Y. Effect of gums on the multi-scale characteristics and 3D printing performance of potato starch gel. Innov. Food Sci. Emerg. Technol. 2022, 80, 103102. [Google Scholar] [CrossRef]
- Amorim, P.A.; d’Ávila, M.A.; Anand, R.; Moldenaers, P.; Van Puyvelde, P.; Bloemen, V. Insights on shear rheology of inks for extrusion-based 3D bioprinting. Bioprinting 2021, 22, e00129. [Google Scholar] [CrossRef]
- Wang, F.; Xue, Y.; Chen, X.; Zhang, P.; Shan, L.; Duan, Q.; Xing, J.; Lan, Y.; Lu, B.; Liu, J.; et al. 3D printed implantable hydrogel bioelectronics for electrophysiological monitoring and electrical modulation. Adv. Funct. Mater. 2024, 34, 2314471. [Google Scholar] [CrossRef]
- Wang, X.; Feng, G.; Li, M.; Ge, M. Effect of PEDOT: PSS content on structure and properties of PEDOT: PSS/poly (vinyl alcohol) composite fiber. Polym. Bull. 2019, 76, 2097–2111. [Google Scholar] [CrossRef]
- Liu, Q.; Qiu, J.; Yang, C.; Zang, L.; Zhang, G.; Sakai, E. High-performance PVA/PEDOT: PSS hydrogel electrode for all-gel-state flexible supercapacitors. Adv. Mater. Technol. 2021, 6, 2000919. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Wang, Y.; Guo, X.; Zhang, Y.; Liu, P.; Liu, C.; Zhang, Y. Highly stretchable strain sensor based on polyurethane substrate using hydrogen bond-assisted laminated structure for monitoring of tiny human motions. Smart Mater. Struct. 2018, 27, 035013. [Google Scholar] [CrossRef]
- Zhou, Z.; He, Z.; Yin, S.; Xie, X.; Yuan, W. Adhesive, stretchable and antibacterial hydrogel with external/self-power for flexible sensitive sensor used as human motion detection. Compos. Part B Eng. 2021, 220, 108984. [Google Scholar] [CrossRef]
- Zhao, R.; Zhao, Z.; Song, S.; Wang, Y. Multifunctional conductive double-network hydrogel sensors for multiscale motion detection and temperature monitoring. ACS Appl. Mater. Interfaces 2023, 15, 59854–59865. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, D.; Wang, Z.; Zhang, H.; Xia, H.; Mao, R.; Cai, H.; Luan, H. Self-adhesive, anti-freezing MXene-based hydrogel strain sensor for motion monitoring and handwriting recognition with deep learning. ACS Appl. Mater. Interfaces 2023, 15, 29413–29424. [Google Scholar] [CrossRef] [PubMed]
- Entifar, S.A.N.; Entifar, N.A.E.; Wibowo, A.F.; Kim, J.H.; Sembiring, Y.S.; Saputro, J.M.W.; Kim, H.-G.; Kim, J.-O.; Xie, G.; Oh, J.; et al. Extremely-low electrical-hysteresis hydrogels for multifunctional wearable sensors and osmotic power generators. Chem. Eng. J. 2025, 509, 160971. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Wang, X.; Yin, M.; Wang, K.; Zhou, D.; Zheng, H.; Yu, S.; Li, S.; Chen, K.; et al. Mechanical robust, adhesive, self-healable and biodegradable protein-based electronic skin sensors for smart elderly care. Chem. Eng. J. 2024, 482, 148785. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, L.; Yeo, J.C.; Lim, C.T. Flexible hybrid sensors for health monitoring: Materials and mechanisms to render wearability. Adv. Mater. 2020, 32, 1902133. [Google Scholar] [CrossRef]
- Sembiring, Y.S.; Vo, T.T.T.; Entifar, S.A.N.; Wibowo, A.F.; Kim, J.H.; Entifar, N.A.E.; Kim, J.H.; Baek, S.W.; Lee, S.I.; Kim, M.S.; et al. Recyclable, Conductive Alginate-Based Hydrogels with High Stretchability and Low Electrical Hysteresis for Wireless Wearable Sensors. J. Mater. Chem. A 2025, 13, 15200–15212. [Google Scholar] [CrossRef]
- Wibowo, A.F.; Nagappan, S.; Entifar, S.A.N.; Kim, J.H.; Sembiring, Y.S.; Han, J.; Oh, J.; Xie, G.; Lee, J.; Kim, J.; et al. Recyclable, ultralow-hysteresis, multifunctional wearable sensors based on water-permeable, stretchable, and conductive cellulose/PEDOT: PSS hybrid films. J. Mater. Chem. A 2024, 12, 19403–19413. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; He, P.; Wei, Y.; Song, J.; Peng, B.; Li, Y. Construction of dual conductive networks based on material jetting for high-performance flexible strain sensors. Addit. Manuf. 2025, 100, 104698. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Li, Z.; Lu, T.J.; Lin, H.; Zhu, Y.; Ahadian, S.; Emaminejad, S.; Dokmeci, M.R.; Xu, F.; et al. Harnessing the wide-range strain sensitivity of bilayered PEDOT: PSS films for wearable health monitoring. Matter 2021, 4, 2886–2901. [Google Scholar] [CrossRef]


| Materials | Strain Range (%) | Electrical Conductivity (S/m) | Gauge Factor | Ref. |
|---|---|---|---|---|
| ALG/PEDOT:PSS hydrogel | 138 | Not Reported | 0.58 | [48] |
| PEDOT:PSS/MWCNT | 80 | Not Reported | 31.2 | [50] |
| CMC-PVA-PEDOT:PSS | 303.8 | 2.04 | 1.034 | [45] |
| HEC/L-PEDOT:PSS | 190 | Not Reported | 1.2 | [49] |
| PEDOT:PSS | 100 | Not Reported | gauge factors of 2.61, 1.87, and 1.59 for the 30%, 50%, and 70% pre-strain groups | [51] |
| This work | 170 | 2 | 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Lu, C.; Zhang, Y.; Cai, Z.; He, Y.; Liang, X. In Situ 3D Printing of Conformal Bioflexible Electronics via Annealing PEDOT:PSS/PVA Composite Bio-Ink. Polymers 2025, 17, 1479. https://doi.org/10.3390/polym17111479
Zhang X, Lu C, Zhang Y, Cai Z, He Y, Liang X. In Situ 3D Printing of Conformal Bioflexible Electronics via Annealing PEDOT:PSS/PVA Composite Bio-Ink. Polymers. 2025; 17(11):1479. https://doi.org/10.3390/polym17111479
Chicago/Turabian StyleZhang, Xuegui, Chengbang Lu, Yunxiang Zhang, Zixi Cai, Yingning He, and Xiangyu Liang. 2025. "In Situ 3D Printing of Conformal Bioflexible Electronics via Annealing PEDOT:PSS/PVA Composite Bio-Ink" Polymers 17, no. 11: 1479. https://doi.org/10.3390/polym17111479
APA StyleZhang, X., Lu, C., Zhang, Y., Cai, Z., He, Y., & Liang, X. (2025). In Situ 3D Printing of Conformal Bioflexible Electronics via Annealing PEDOT:PSS/PVA Composite Bio-Ink. Polymers, 17(11), 1479. https://doi.org/10.3390/polym17111479







