Experimental Study on the Coupling of Freeze-Thaw Cycle and Chloride Corrosion of Alkali Slag Cementitious Materials
Abstract
1. Introduction
2. Overview of the Experiment
2.1. Materials
2.1.1. Slag
2.1.2. Coal Ash
2.1.3. Recycled Rubber
2.1.4. Straw Fiber
2.1.5. Standard Sand
2.1.6. Potassium Water Glass
2.1.7. Sodium Hydroxide
2.2. Mix Ratio Design
2.2.1. Adjusting the Modulus of Potassium Silicate to 1.0
2.2.2. Mix Ratio Design
2.3. Pilot Program
2.3.1. Compressive and Flexural Strength Test Methods
2.3.2. Freeze-Thaw Cycle Test Methods
2.3.3. Chloride Ion Corrosion Test Methods
- (1)
- Measure the mass of NaCl solid at 5% concentration and the mass of warm water individually, combine them thoroughly, and gradually introduce the mixture into the immersion tank.
- (2)
- Position the test specimens in a 5% NaCl solution after 28 days of maintenance for a long-term immersion test, ensuring that the liquid level does not exceed 20 mm above the top of the specimens, and cover the immersion container with a film.
- (3)
- Extract a portion of the specimen every 30 days, eliminate surface moisture, and conduct analyses for chloride ion concentration and compressive strength. To maintain a consistent solution concentration, replace the NaCl solution every 30 days.
2.3.4. Freeze-Thaw-Chloride Ion Coupling Test Methods
2.3.5. Microanalysis Test Methods
- (1)
- X-ray Diffractometer (XRD): XRD tests were performed on the samples from the specimens using D8-Advance from Bruker Corporation (Billerica, MA, USA). The X-ray diffractometer depicted in Figure 4b was utilized; the test block was crushed and subsequently ground into a powder using a mortar and pestle. The powder, which passed through a 0.075 mm sieve, was then placed in a beaker and dried in a drying oven at 40 °C until a constant weight was achieved. The physical phase was analyzed using X-ray diffraction (XRD) with a measurement precision of ≤0.010 and a scanning range of 10° to 90°.
- (2)
- Scanning Electron Microscope (SEM): SEM analyses were performed on the specimens using SUPERTM 55 from Carl Zeiss AG (Oberkochen, Germany). In scanning electron microscopy, as depicted in Figure 4a, the center of the fractured section of the 28-day compressive test block is immersed in anhydrous ethanol to halt hydration for 7 days. Subsequently, it is removed and dried in a 60 °C oven until a constant weight is achieved. After drying, the sample is affixed to a tray for gold sputtering, followed by examination using scanning electron microscopy.
3. Results and Discussions
3.1. AASCM Freeze-Thaw Cycle Test
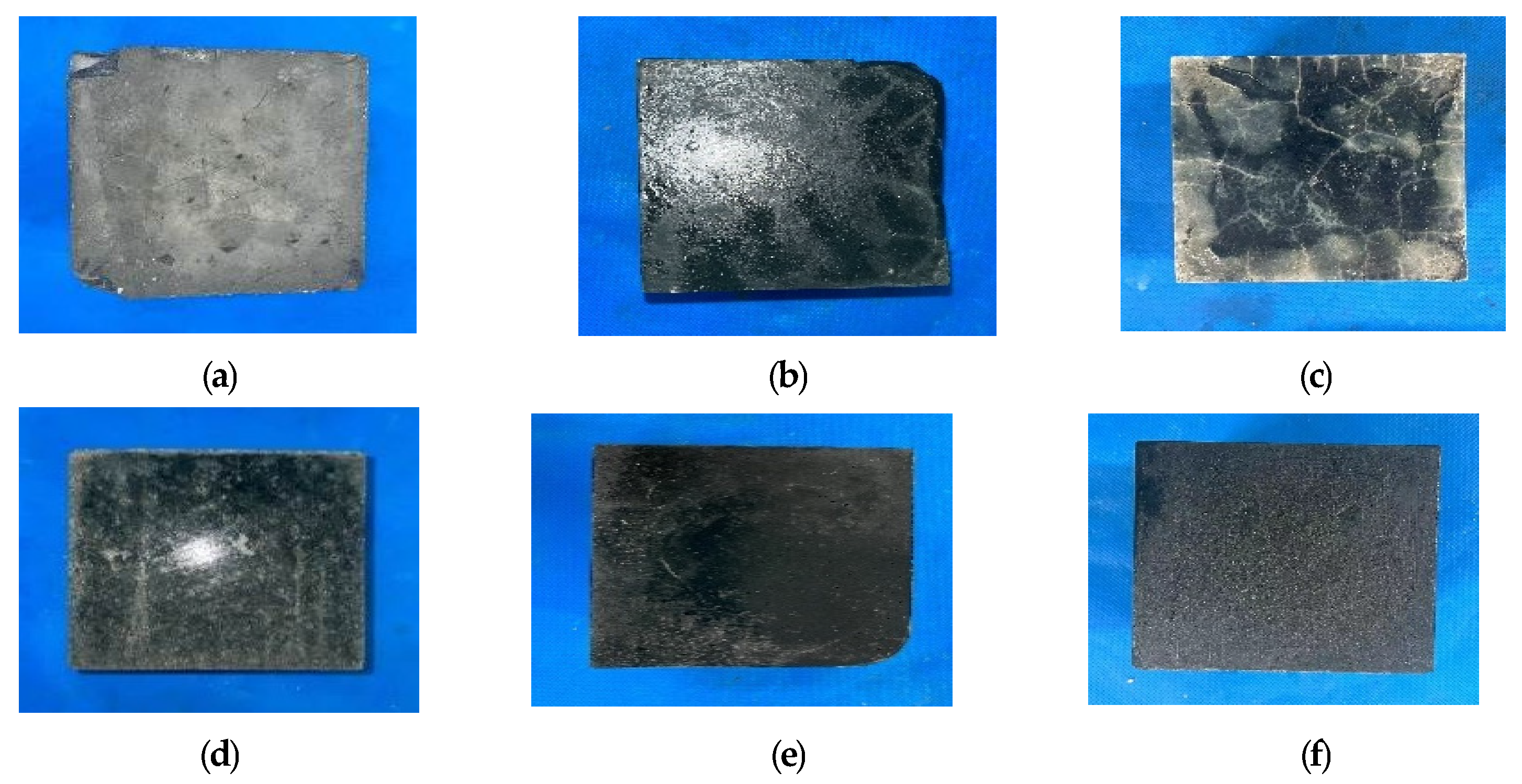
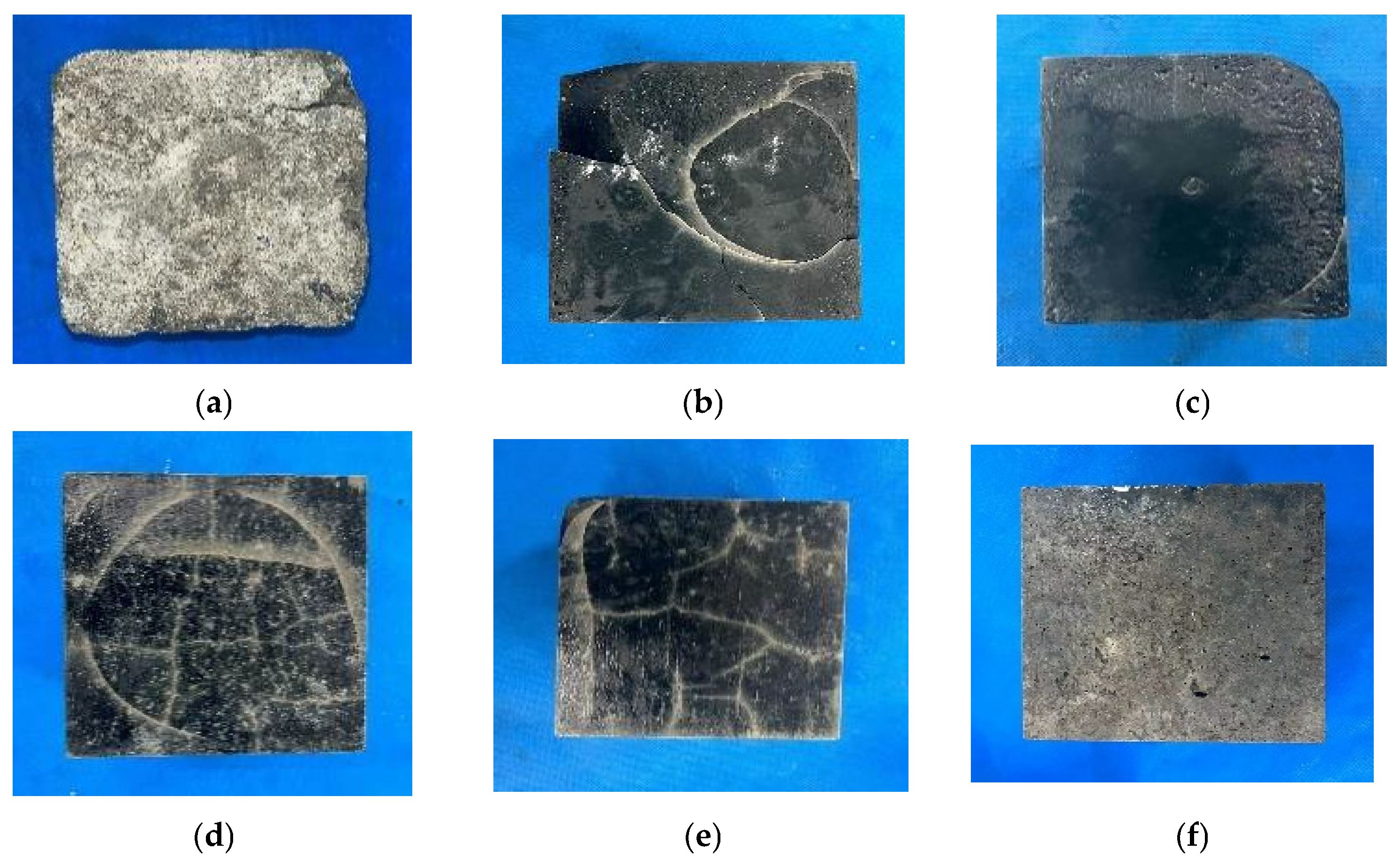
3.1.1. Appearance and Morphological Changes of Specimens After Freeze-Thaw Cycle
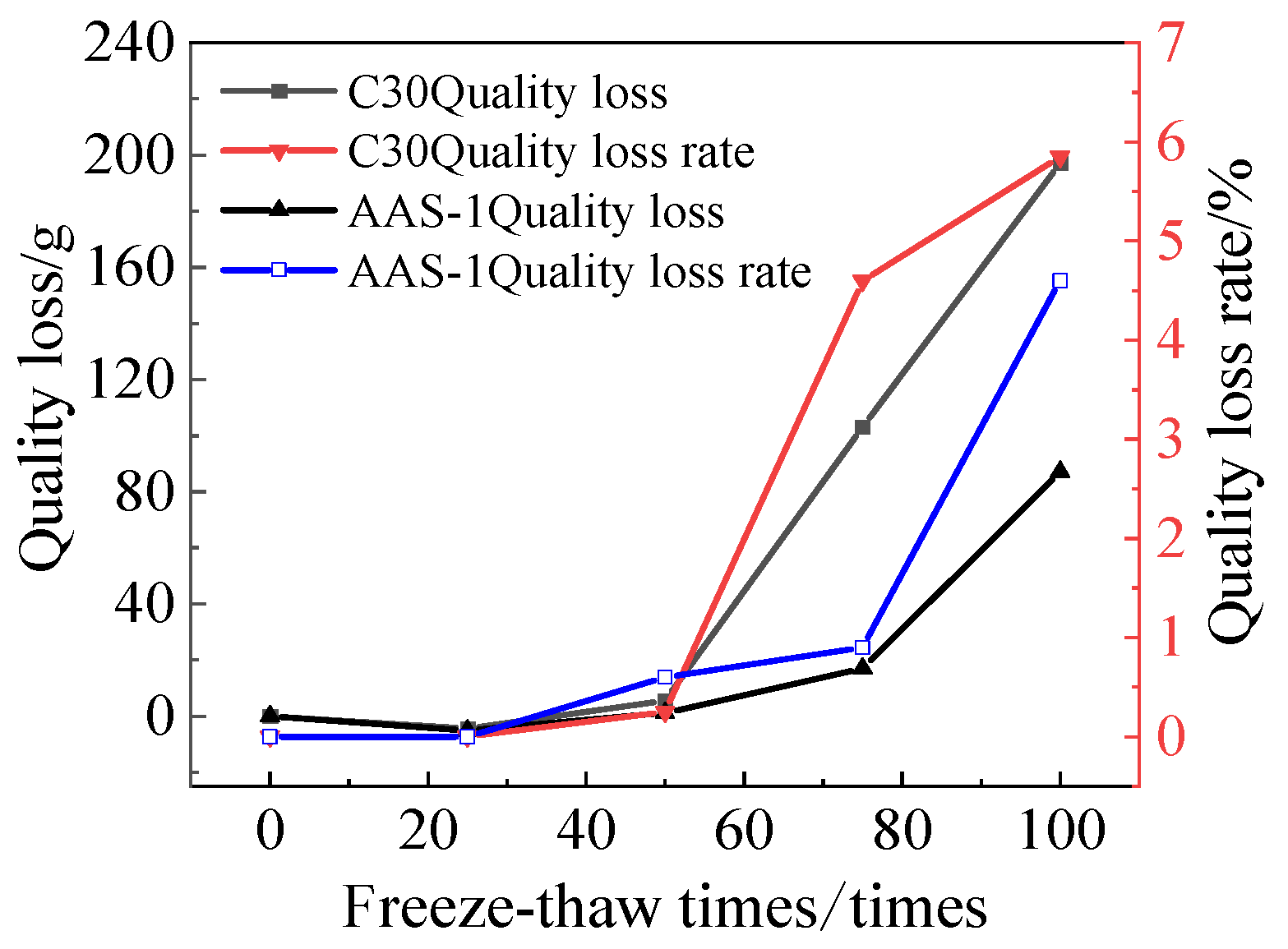
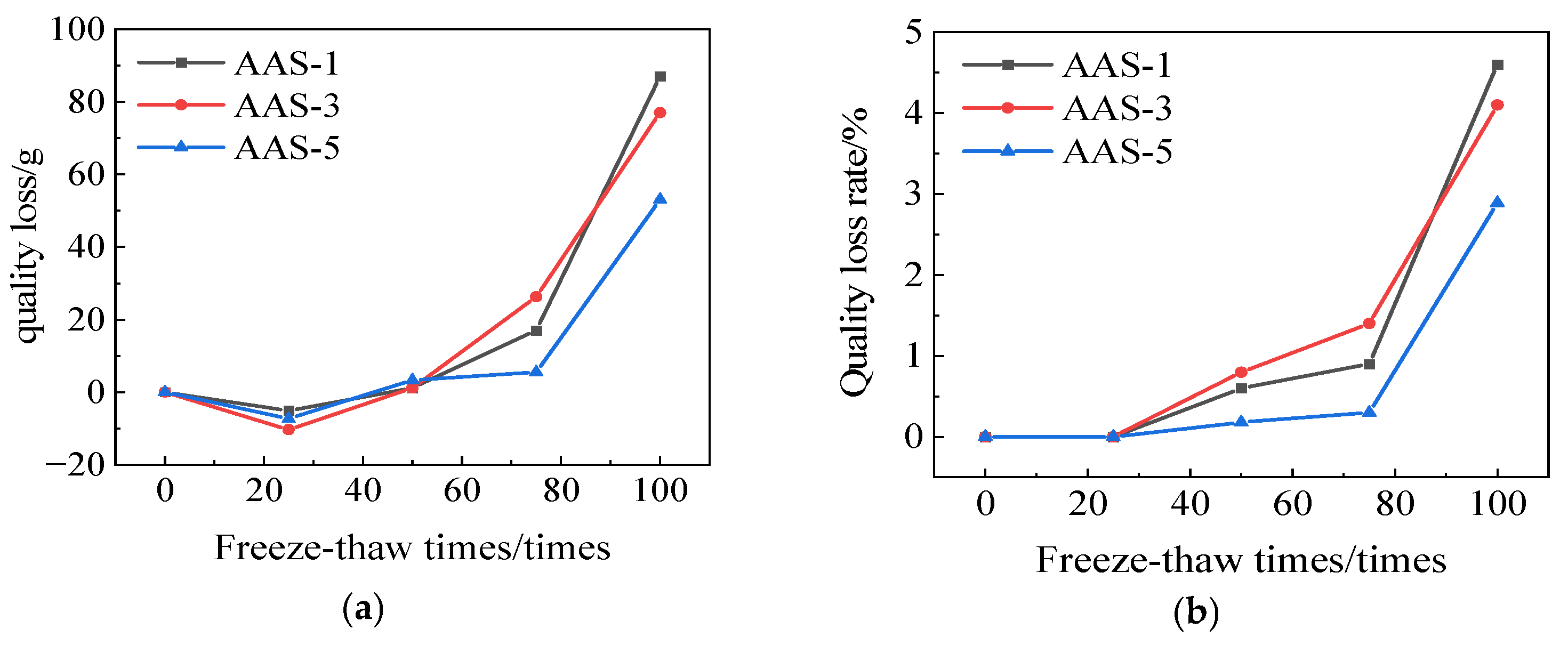
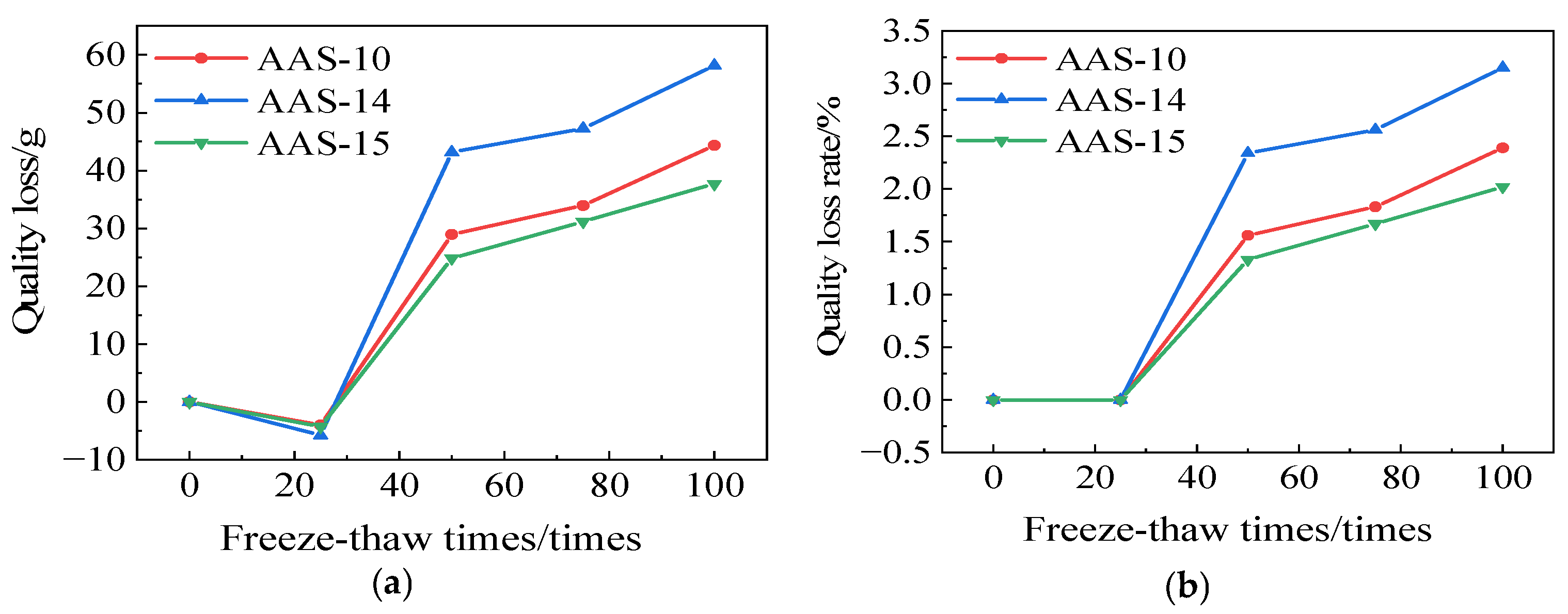
3.1.2. Rate of Quality Loss
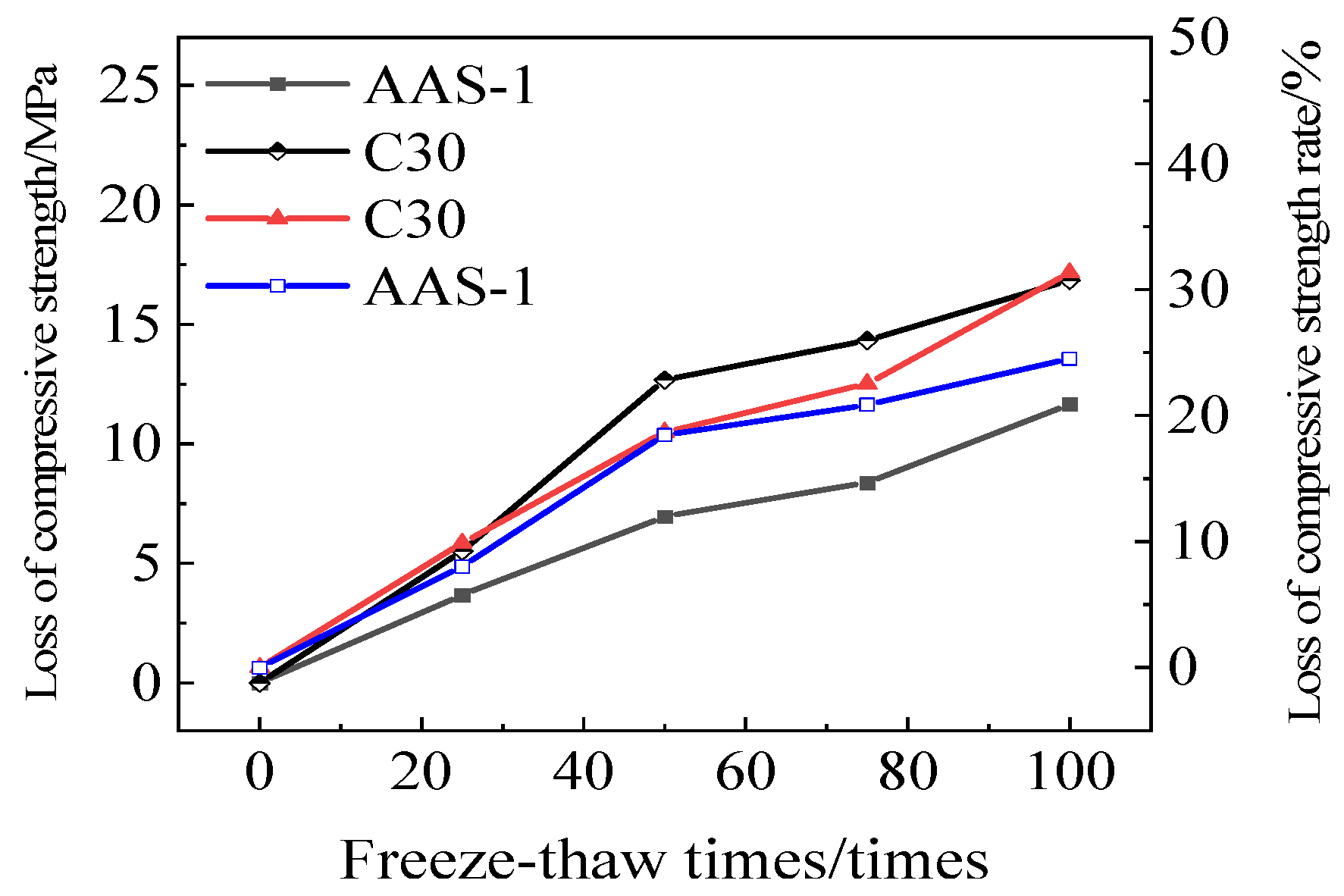
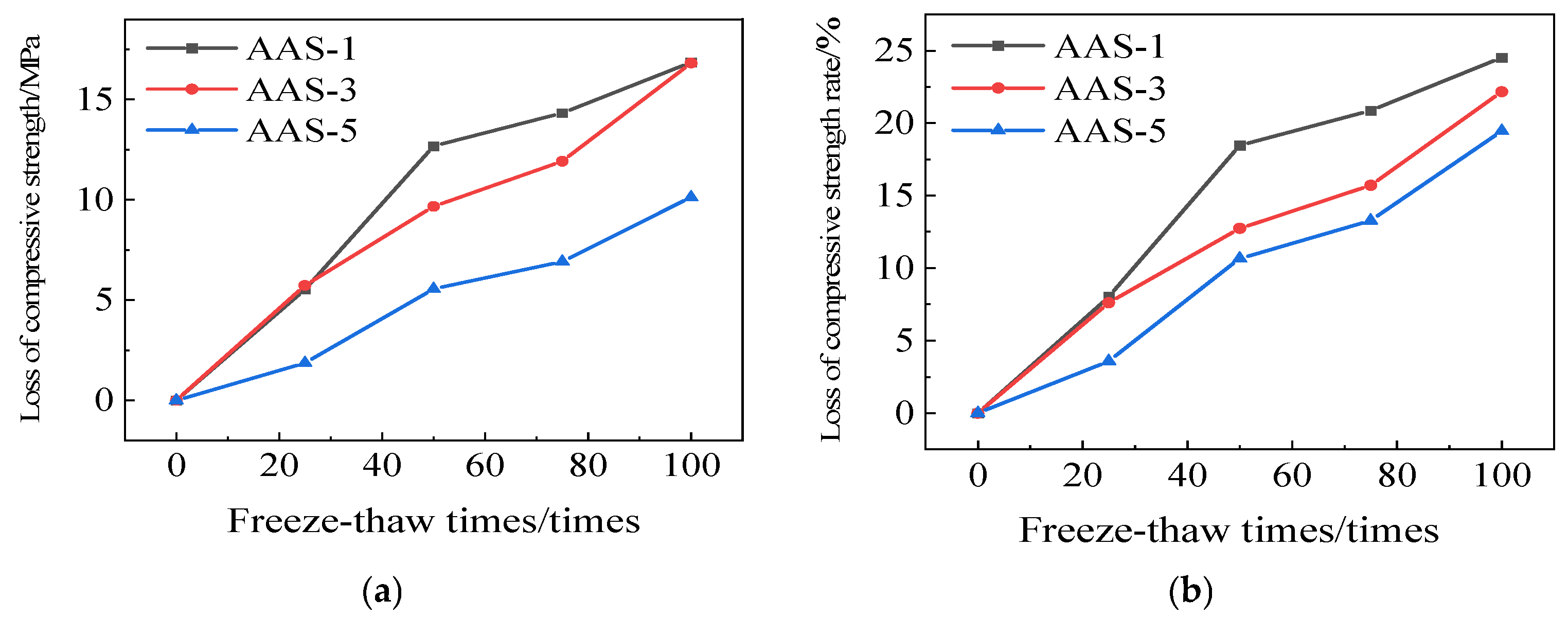
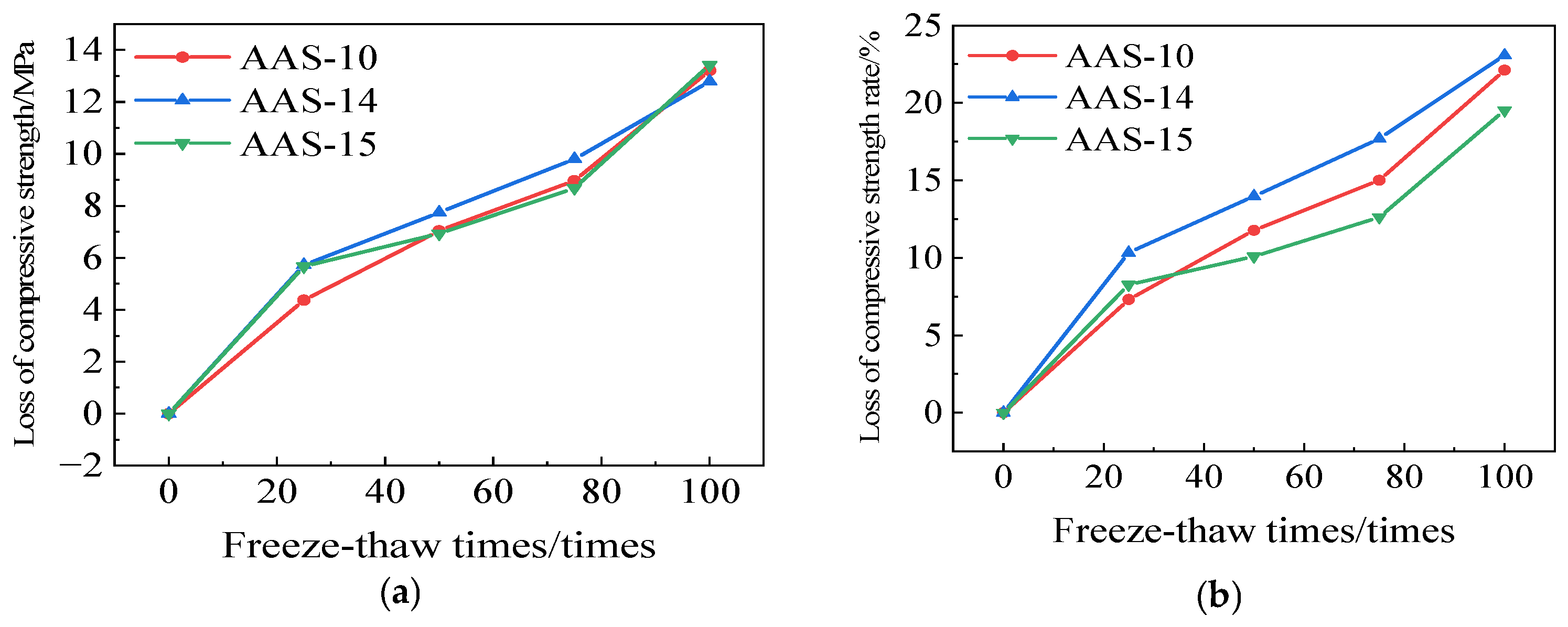
3.1.3. Rate of Loss of Compressive Strength
3.2. AASCM Chloride Corrosion Test
3.3. AASCM Freeze-Thaw-Chloride Corrosion Coupling Test
3.3.1. Appearance Changes of AASCM and C30 Concrete Specimens After Freeze-Thaw Coupling with Chloride Ions
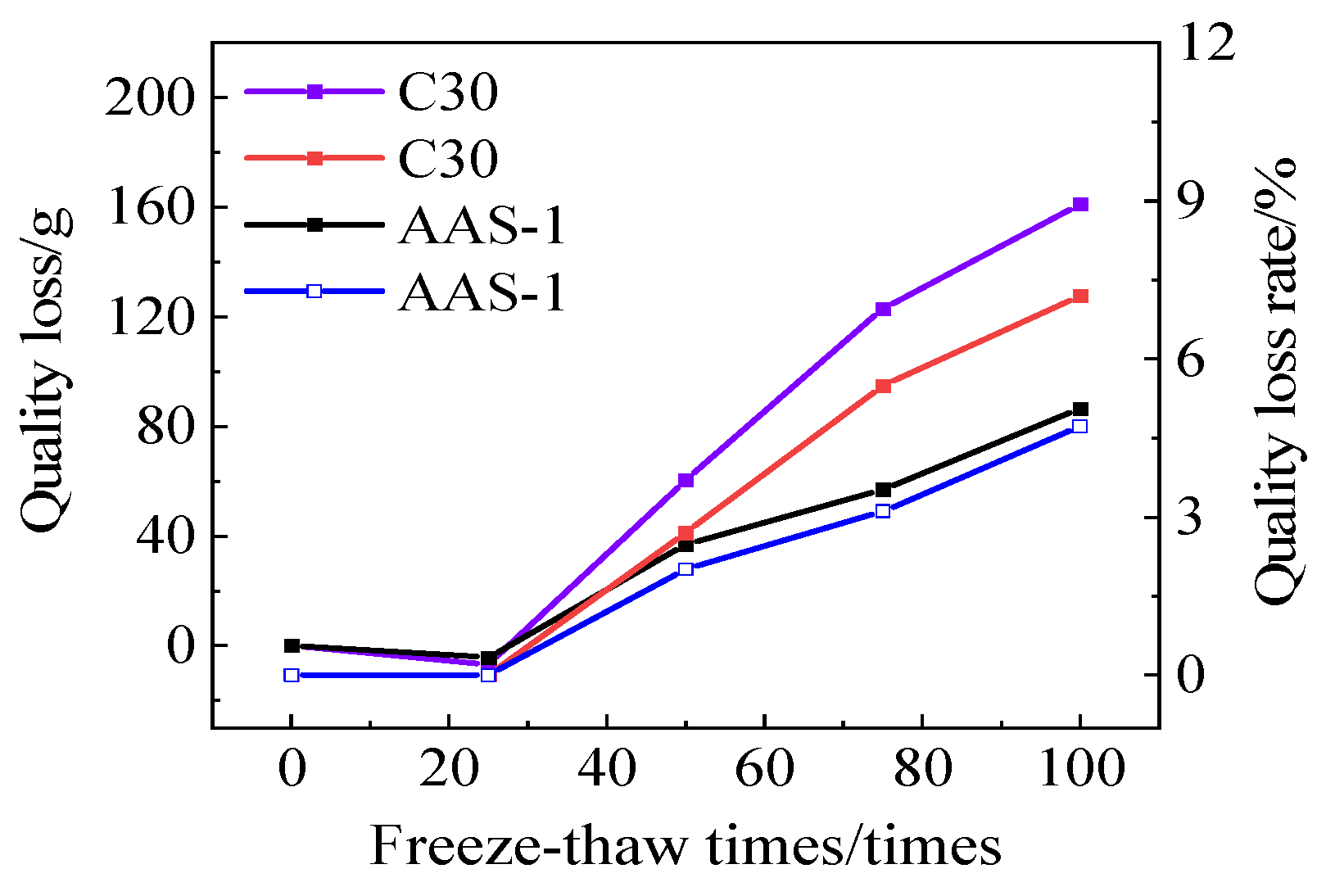
3.3.2. Rate of Quality Loss
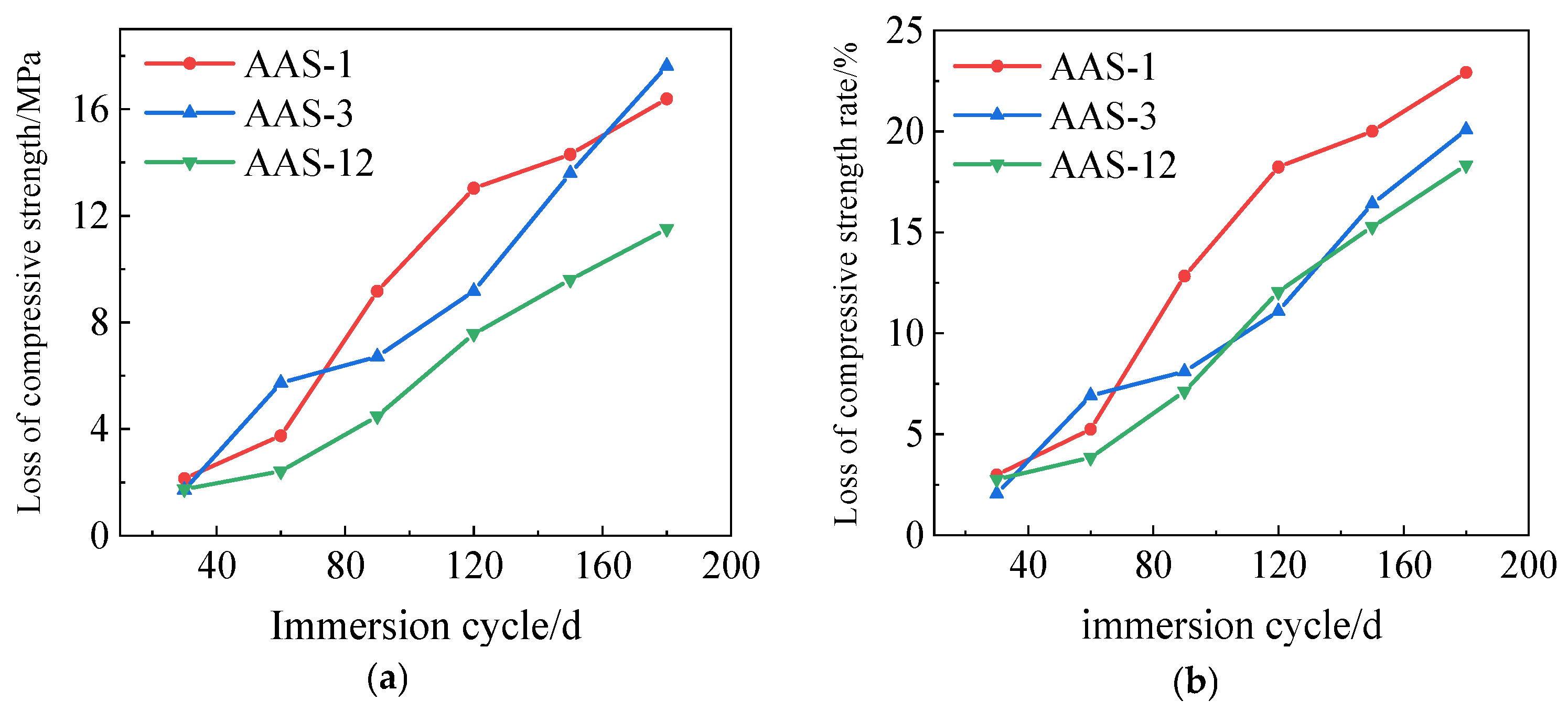
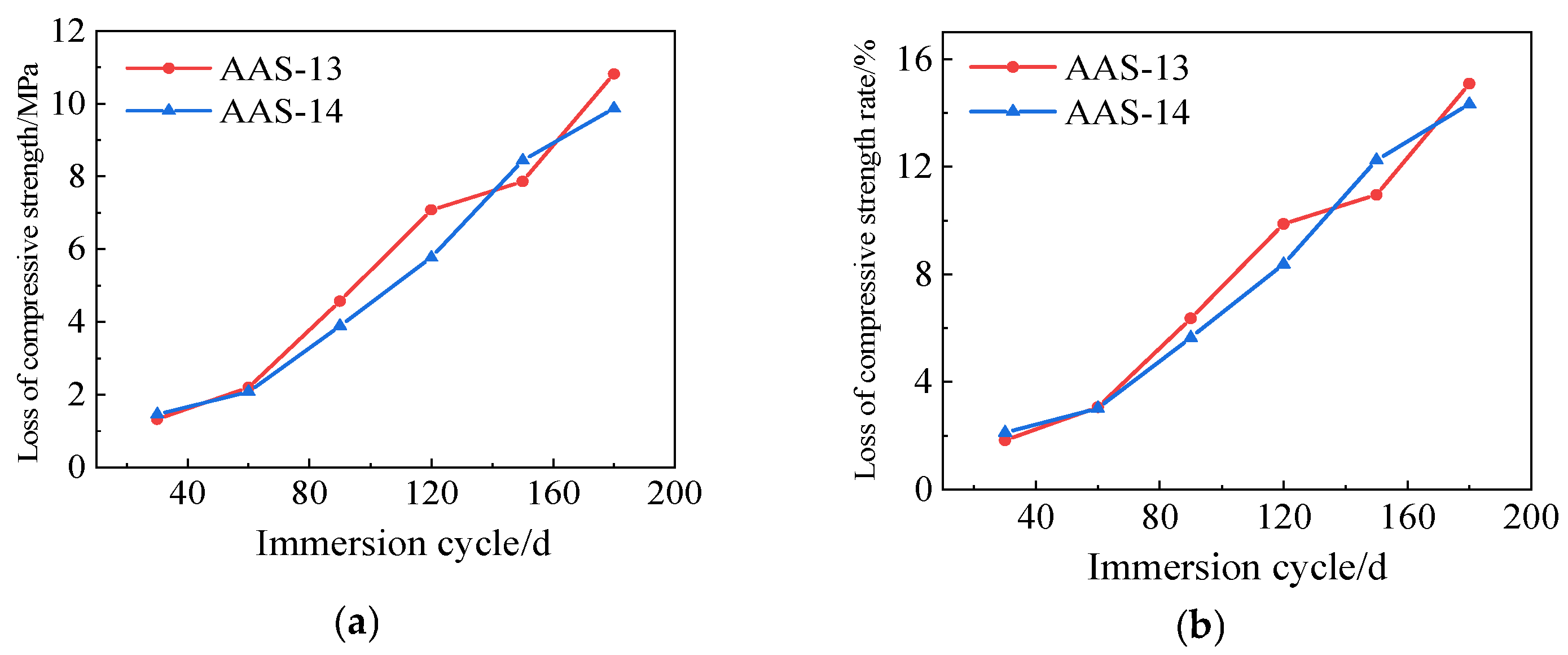
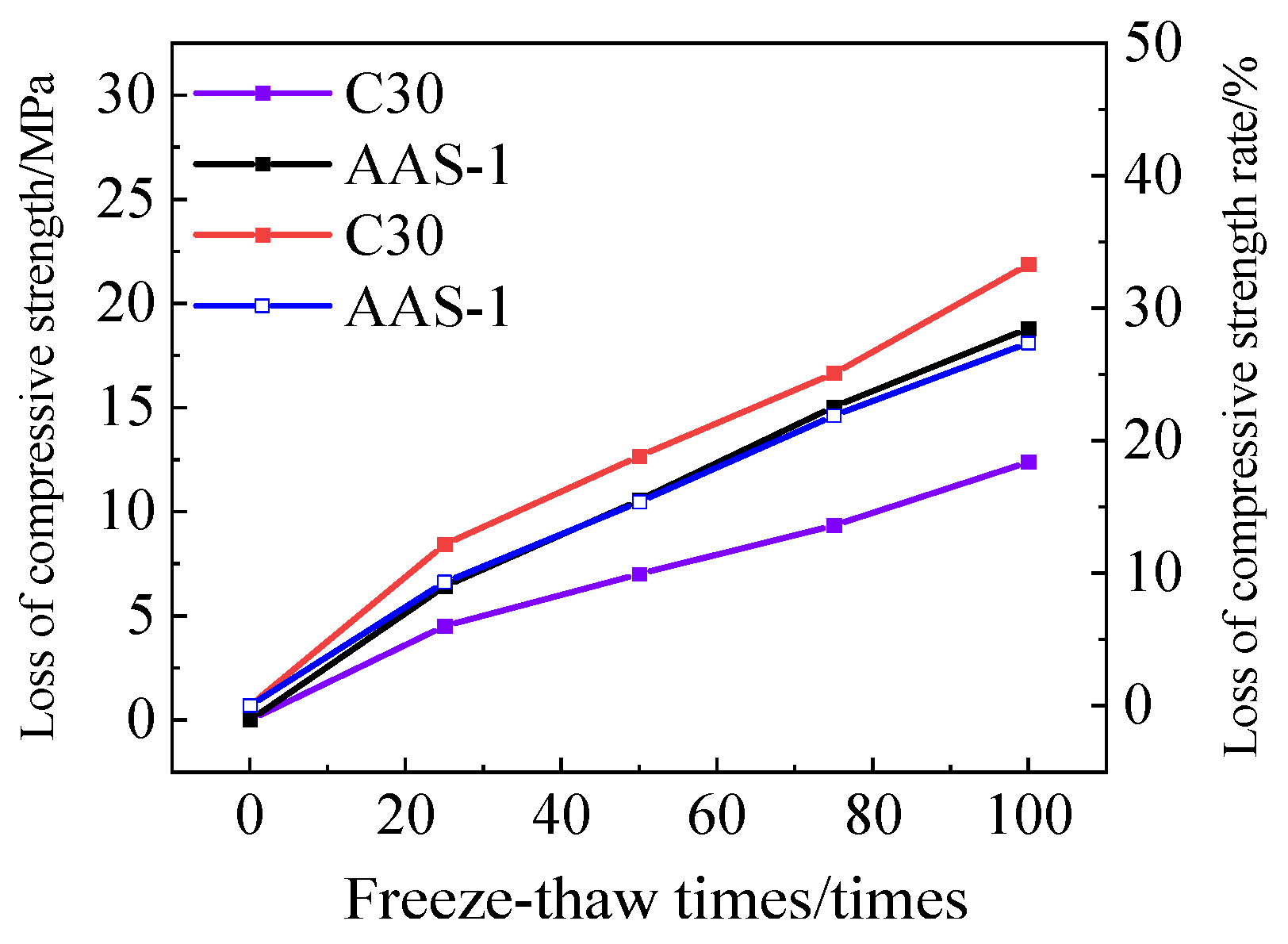
3.3.3. Rate of Loss of Compressive Strength
3.4. Microscopic Analysis
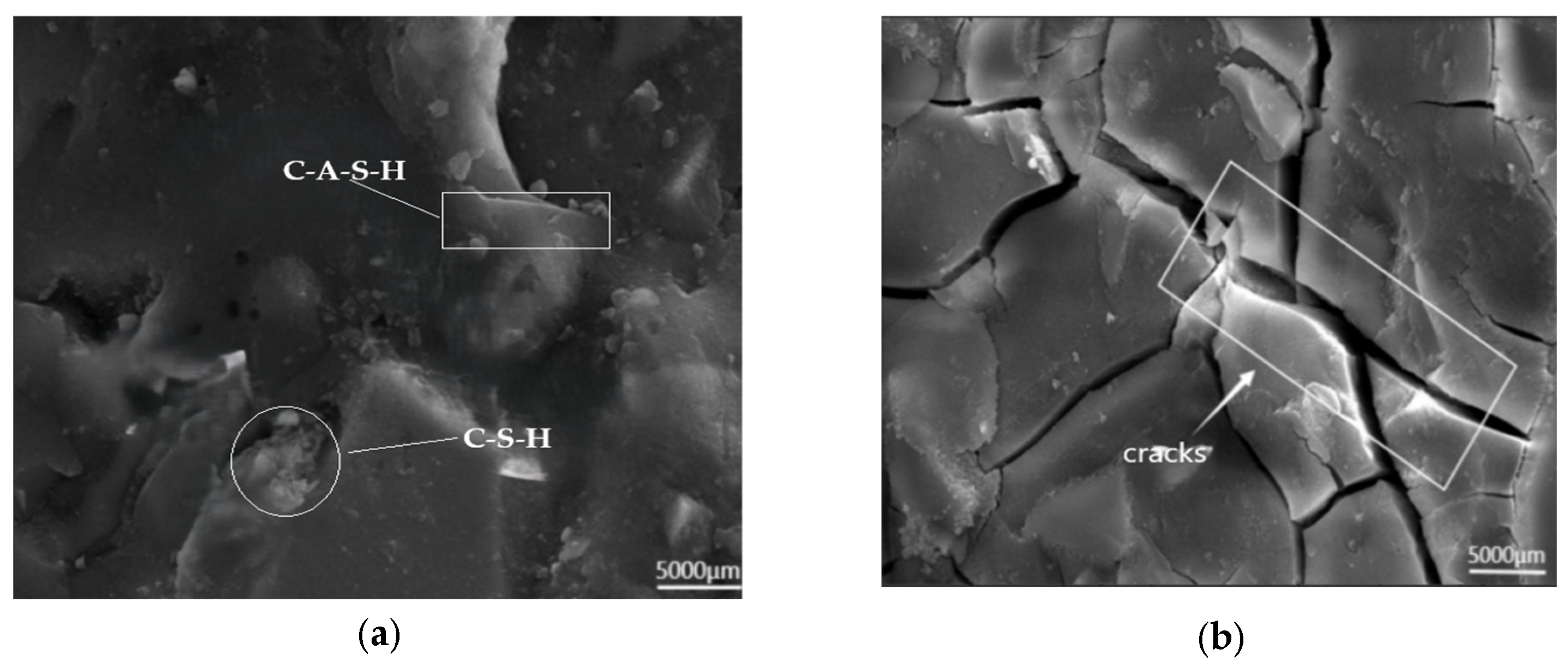
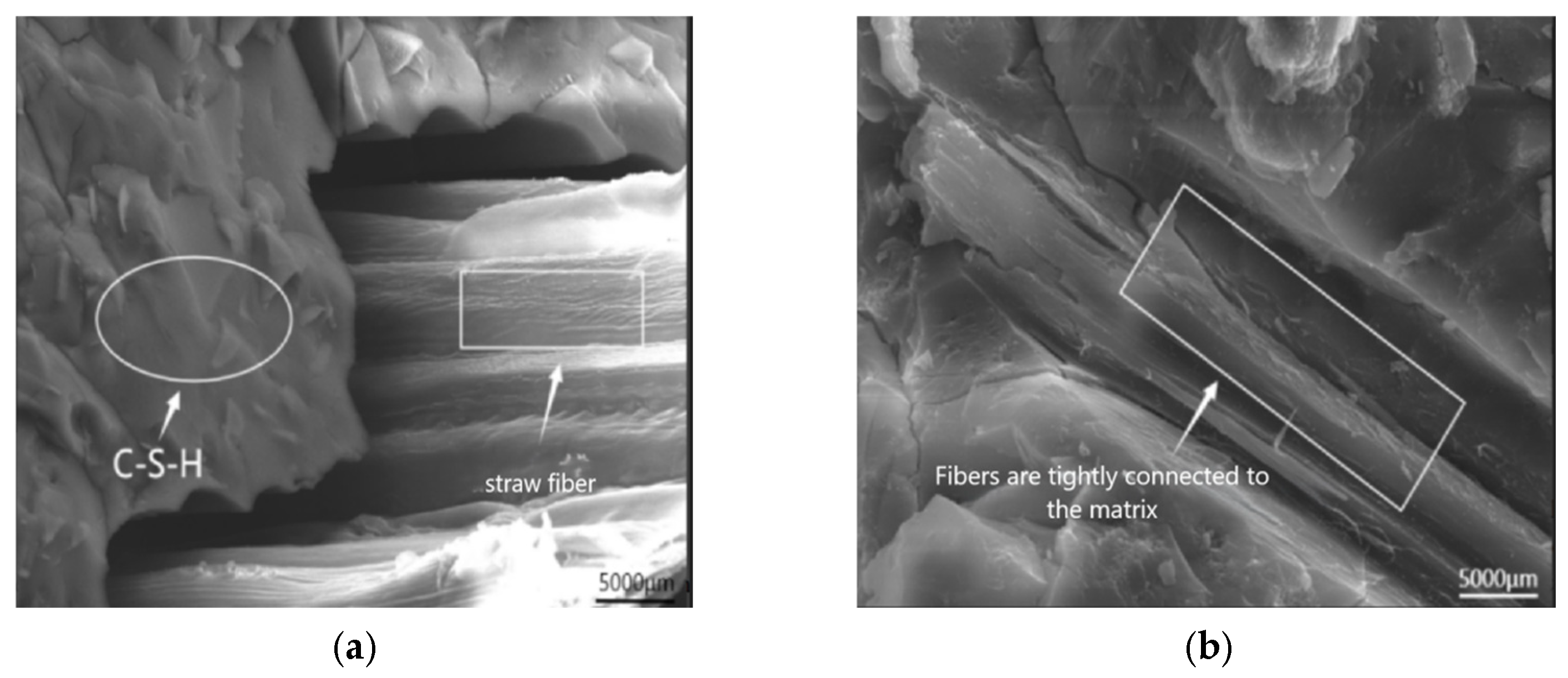
SEM Analysis of Freeze-Thaw-Chloride Coupled Specimens
4. Conclusions
- (1)
- During freeze-thaw cycles, AASCM that had both coal ash and recycled rubber lost more mass and less compressive strength than AASCM that was made of only coal ash. The combination made the AASCM more resistant to frost. The addition of coal ash and straw fiber to the AASCM resulted in a decrease in the ion concentration in the deeper layer. This improved it at absorbing chloride ions after 180 days of immersion. The AASCM that had coal ash and recycled rubber added to it was better at absorbing chloride ions than the AASCM that only had one additive.
- (2)
- AASCM, which incorporates coal ash, recycled rubber, and straw fiber, significantly improves the interaction between freeze-thaw and chloride ions. Its mass loss rate and compressive strength loss rate are less favorable than those of other compositions, thus meeting the durability criteria for cold climates.
- (3)
- SEM analysis indicates that the C-A-S-H gel exhibit a comparatively dense and stable structure. The incorporation of recycled rubber into the AASCM matrix can postpone crack formation, thus augmenting the material’s brittleness, while simultaneously improving its ductility and frost resistance. Additionally, the straw fiber in the AASCM matrix acts as a bridging agent, and the C-S-H gel mixes with it in a big way, which helps stop cracks from forming and spreading and also has some ability to absorb chloride ions. After XRD analysis, it was found that AASCM with 20% coal ash makes more C-A-S-H gels, which improves the material’s mechanical properties.
- (4)
- At present, there are still some limitations in the research on the freeze-thaw cycle and chloride corrosion of AASCM, mainly reflected in the lack of systematic comparison of coupled damage behaviors, the lack of in-depth comparative analysis with existing theoretical models, and the limited application of quantitative indicators. In the future, by establishing more comprehensive benchmark tests, including key chloride thresholds, freeze-thaw damage indices, and other parameters, combined with quantitative indicators such as percentage differences and effect sizes, it is expected to more accurately evaluate the durability of AASCM and verify its improvements and innovations in practical applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.; Xu, Y.; Huang, H.; Dai, J. Structural behavior of FRP connector enabled precast geopolymer concrete sandwich panels subjected to one-side fire exposure. Fire Saf. J. 2022, 128, 103524. [Google Scholar] [CrossRef]
- Gaibor, N.; Mateus, R.; Leitao, D.; Cristelo, N.; Miranda, T.; Pereira, E.N.; Cunha, V.M. Sustainability assessment of half-sandwich panels based on alkali-activated ceramic/slag wastes cement versus conventional building solutions. J. Clean. Prod. 2023, 389, 136108. [Google Scholar] [CrossRef]
- Kumar, S.; Das, C.S.; Lao, J.; Alrefaei, Y.; Dai, J.G. Effect of sand content on bond performance of engineered geopolymer composites (EGC) repair material. Constr. Build. Mater. 2022, 328, 127080. [Google Scholar] [CrossRef]
- Kato, E.; Kato, Y.; Uomoto, T. Development of Simulation Model of Chloride Ion Transportation in Cracked Concrete. J. Adv. Concr. Technol. 2005, 3, 85–94. [Google Scholar] [CrossRef]
- Fang, Y.H.; Gu, Y.M.; Kang, Q.B. Effect of Fly Ash, MgO and Curing Solution on the Chemical Shrinkage of Alkali-activated Slag Cement. Adv. Mater. Res. 2011, 168, 2008–2012. [Google Scholar] [CrossRef]
- Yu, Q.; Ye, W. Experimental Study on the Compressive Strength And Frost Resistance of Rubber Concrete. Appl. Mech. Mater. 2013, 433, 2016–2019. [Google Scholar] [CrossRef]
- Zhao, Y. Preparation and Performance Study of Alkali-Excited Slag-Coal Ash-Based Shield Slag Brick; Beijing Architecture University: Beijing, China, 2023. [Google Scholar]
- Choi, J.I.; Park, S.E.; Kim, Y.Y.; Lee, B.Y. Flexural behavior of composite beams of Kagome truss and fiber-reinforced cementitious composites. Constr. Build. Mater. 2022, 361, 129653. [Google Scholar] [CrossRef]
- Cui, Y.; Hao, H.; Li, J.; Chen, W.; Zhang, X. Structural behavior and vibration characteristics of geopolymer composite lightweight sandwich panels for prefabricated buildings. J. Build. Eng. 2022, 57, 104872. [Google Scholar] [CrossRef]
- Mavroulidou, M.; Sanam, I.; Mengasini, L. Mechanical and durability performance of alkali-activated slag cement concretes with carbonate and silicate activators. Sustain. Chem. Pharm. 2023, 31, 100896. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Yang, T.; Wu, Z. The Shear Behavior of Insulated Precast Concrete Sandwich Panels Reinforced with BFRP. Buildings 2022, 12, 1326. [Google Scholar] [CrossRef]
- Ren, X.; Liu, Z.; Will, H. An analysis of the freeze-thaw damage mechanism of concrete. J. Concr. 2024, 6, 12–18. [Google Scholar]
- Alchaar, A.; Abed, F. Finite element analysis of a thin-shell concrete sandwich panel under eccentric loading. J. Build. Eng. 2020, 32, 101804. [Google Scholar] [CrossRef]
- Zhu, Q. Study on the Corrosion Characteristics of Steel Reinforcement in Foam Concrete Under Chloride Ion Erosion; Anhui University of Technology: Maanshan, China, 2021. [Google Scholar]
- Luo, D.; Weiss, J.; Bu, Y. Using Fick’s Second Law and Nernst-Planck Approach in Prediction of Chloride Ingress in Concrete Materials. Adv. Civ. Eng. Mater. 2014, 3, 153–159. [Google Scholar]
- Xiao, H. Study on the Durability of Rubberized Concrete Under the Coupling of Freeze-Thaw Cycle and Chloride Ion Erosion; Shenyang University: Shenyang, China, 2020. [Google Scholar]
- Kai-Mo, S. Research on the Durability of Polymer Fast-Hardening Cement Mortar Based on Coupled Conditions of Freeze-Thaw Cycle and Chloride-Salt Erosion; Lanzhou University of Science and Technology: Lanzhou, China, 2016. [Google Scholar]
- Korniejenko, K.; Figiela, B.; Miernik, K.; Ziejewska, C.; Marczyk, J.; Hebda, M.; Cheng, A.; Lin, W.-T. Mechanical and Fracture Properties of Long Fiber Reinforced Geopolymer Composites. Materials 2021, 14, 5183. [Google Scholar] [CrossRef]
- Behfarnia, K.; Shahbaz, M. The effect of elevated temperature on the residual tensile strength and physical properties of the alkali-activated slag concrete. J. Build. Eng. 2018, 20, 442–454. [Google Scholar] [CrossRef]
- Mikhailova, O.; Šimonová, H.; Topolář, L.; Rovnaník, P. Influence of Polymer Additives on Mechanical Fracture Properties and on Shrinkage of Alkali Activated Slag Mortars. Key Eng. Mater. 2018, 761, 39–44. [Google Scholar] [CrossRef]
- Aydın, S. A ternary optimisation of mineral additives of alkali activated cement mortars. Constr. Build. Mater. 2013, 43, 131–138. [Google Scholar] [CrossRef]
- GB/T18046-2017; Granulated Blast Furnace Slag Powder for Use in Cement, Mortar and Concrete. Industry Standard—Construction Industry: Beijing, China, 2017.
- GB/T1596-2017; Fly Ash Used in Cement and Concrete. Industry Standard—Construction Industry: Beijing, China, 2017.
- Zhu, J.; Song, L.; Qu, Z.; Wang, X.; Wen, Z.; Liu, X.; Wang, H. Mechanical Strengths of Alkali-Activated Blast Furnace Slag Powder with Different Alkali Activators and Plant Fibers. Coatings 2023, 13, 664. [Google Scholar] [CrossRef]
- Feng, S.; Zhu, J.; Wang, R.; Qu, Z.; Song, L.; Wang, H. The Influence of CaO and MgO on the Mechanical Properties of Alkali-Activated Blast Furnace Slag Powder. Materials 2022, 15, 6128. [Google Scholar] [CrossRef]
- Li, Y.; Li, B.; Zang, L.; Ma, C.; Zhu, J. C60 Corrosion law and mathematical model of chloride ion in high strength concrete after freeze-thaw cycle. Adv. Civ. Eng. 2021, 10, 101155. [Google Scholar]
- Wu, H.; Jin, F.; Zhou, A.; Du, Y. The engineering properties and reaction mechanism of MgO-activated slag cement-clayey sand-bentonite (MSB) cutoff wall backfills. Constr. Build. Mater. 2021, 271, 121890. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, S.; Song, L.; Qu, Z.; Wang, H. Influence of Carbon Dioxide Curing on the Corrosion Resistance of Reinforced Cement Mortar under the External Erosion of NaCl Freeze–Thaw Cycle. Appl. Sci. 2022, 12, 5061. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, B.; Wang, B.; Lin, X.; Zhu, J.; Huang, P.; Ji, T. Fluidity, mechanical properties, shrinkage of alkali-activated slag/stainless steel slag mortars with composite activators. J. Build. Eng. 2023, 75, 106877. [Google Scholar] [CrossRef]
- JGJ/T70-2009; Standard for Test Method of Performance on Building Mortar. Industry Standard—Construction Industry: Beijing, China, 2009.
- GB/T 50082-2009; Standard for Long-term Performance and Durability Test Methods of Ordinary Concrete. Industry Standard—Construction Industry: Beijing, China, 2009.
- He, J.; Zheng, W.; Bai, W.; Hu, T.; He, J.; Song, X. Effect of reactive MgO on hydration and properties of alkali-activated slag pastes with different activators. Constr. Build. Mater. 2021, 271, 121608. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Candamano, S.; Crea, F.; Gazzaniga, G.; Pastore, T. The combined use of admixtures for shrinkage reduction in one-part alkali activated slag-based mortars and pastes. Constr. Build. Mater. 2020, 248, 118682. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, S.; Feng, J. Early hydration properties and microstructure evolutions of MgO-activated slag materials at different curing temperatures. Ceram. Int. 2022, 48, 17104–17115. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, S.; Guo, H.; Chen, X.; Huang, Z.; Jiang, S.; Li, M. Multi-omics analysis of tumor angiogenesis characteristics and potential epigenetic regulation mechanisms in renal clear cell carcinoma. Cell Commun. Signal. 2021, 19, 39. [Google Scholar] [CrossRef]
- Zheng, W.; He, J.; Tong, Y.; He, J.; Song, X.; Sang, G. Investigation of effects of reactive MgO on autogenous and drying shrinkage of near-neutral salt activated slag cement. Ceram. Int. 2022, 48, 5518–5526. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, W.; Lu, Z.; Chen, H. Shrinkage compensation of alkali-activated slag concrete and microstructural analysis. Constr. Build. Mater. 2014, 66, 422–428. [Google Scholar] [CrossRef]
- Singh, R.J.; Raut, A.; Murmu, A.L.; Jameel, M. Influence of glass powder incorporated foamed geopolymer blocks on thermal and energy analysis of building envelope. J. Build. Eng. 2021, 43, 102520. [Google Scholar] [CrossRef]












| Component | SiO2 | Al2O3 | CaO | MgO | Fe2O3 | Else |
|---|---|---|---|---|---|---|
| Content (%) | 36.9 | 15.66 | 37.57 | 9.3 | 0.36 | 0.57 |
| Project/Technical Requirements | Grade I | Grade II | Grade III |
|---|---|---|---|
| Fineness (45 μm square hole sieve) | 12.0 | 25.0 | 45.0 |
| Water demand ratio | 95 | 105 | 115 |
| Heat loss | 5.0 | 8.0 | 15.0 |
| Number | Slag (kg) | Coal Ash (kg) | Recycled Rubber (kg) | Standard Sand (kg) | Straw Fiber(kg) | Water Gel Ratio |
|---|---|---|---|---|---|---|
| AAS-1 | 1200 | / | / | / | / | 0.21 |
| AAS-2 | 1080 | 120 | / | / | / | 0.21 |
| AAS-3 | 960 | 240 | / | / | / | 0.21 |
| AAS-4 | 840 | 360 | / | / | / | 0.21 |
| AAS-5 | 1200 | / | 60 | / | / | 0.21 |
| AAS-6 | 1200 | / | 120 | / | / | 0.21 |
| AAS-7 | 1200 | / | 180 | / | / | 0.21 |
| AAS-8 | 960 | 240 | 60 | / | / | 0.21 |
| AAS-9 | 900 | 240 | 60 | 60 | / | 0.21 |
| AAS-10 | 840 | 240 | 60 | 120 | / | 0.21 |
| AAS-11 | 780 | 240 | 60 | 240 | / | 0.21 |
| AAS-12 | 1200 | / | / | / | 12 | 0.21 |
| AAS-13 | 960 | 240 | / | / | 12 | 0.21 |
| AAS-14 | 960 | 240 | / | 120 | 12 | 0.21 |
| AAS-15 | 960 | 240 | 60 | 120 | 12 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Li, Z.; Huang, Y.; Li, Y.; Huang, Y.; Min, H. Experimental Study on the Coupling of Freeze-Thaw Cycle and Chloride Corrosion of Alkali Slag Cementitious Materials. Polymers 2025, 17, 1474. https://doi.org/10.3390/polym17111474
Zhu J, Li Z, Huang Y, Li Y, Huang Y, Min H. Experimental Study on the Coupling of Freeze-Thaw Cycle and Chloride Corrosion of Alkali Slag Cementitious Materials. Polymers. 2025; 17(11):1474. https://doi.org/10.3390/polym17111474
Chicago/Turabian StyleZhu, Jing, Zhiming Li, Ying Huang, Yuankai Li, Yapu Huang, and Hao Min. 2025. "Experimental Study on the Coupling of Freeze-Thaw Cycle and Chloride Corrosion of Alkali Slag Cementitious Materials" Polymers 17, no. 11: 1474. https://doi.org/10.3390/polym17111474
APA StyleZhu, J., Li, Z., Huang, Y., Li, Y., Huang, Y., & Min, H. (2025). Experimental Study on the Coupling of Freeze-Thaw Cycle and Chloride Corrosion of Alkali Slag Cementitious Materials. Polymers, 17(11), 1474. https://doi.org/10.3390/polym17111474






