Regenerative Endodontic Therapies: Harnessing Stem Cells, Scaffolds, and Growth Factors
Abstract
1. Introduction
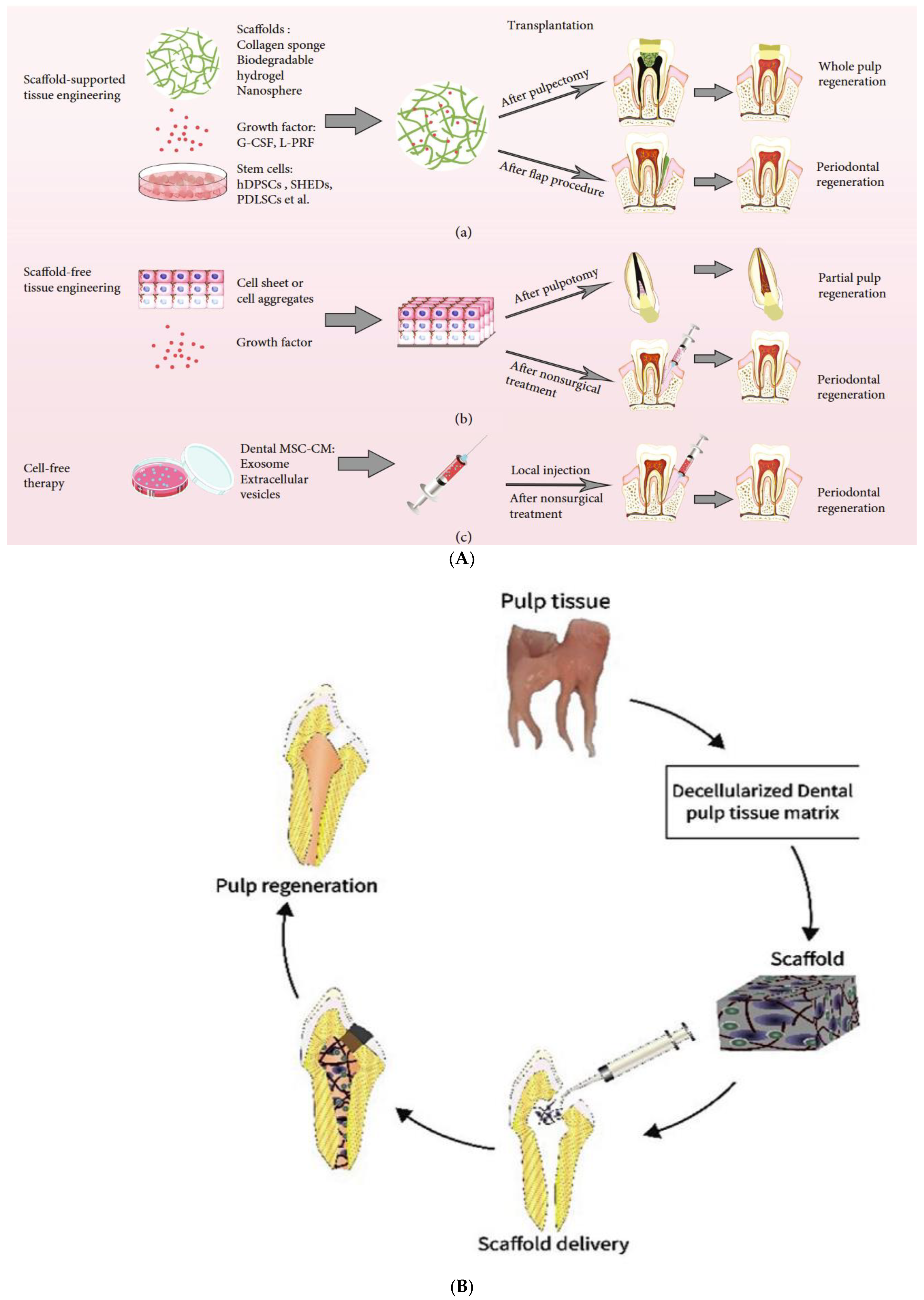
2. Techniques for Tissue Engineering in RETs
2.1. Root Canal Revascularization via Blood Clotting
2.2. Postnatal Stem Cell Therapy
2.3. Replacement Pulp Implantation
2.4. Scaffold Implementation
2.5. Injectable Scaffold Delivery
2.6. Three-Dimensional Cell Printing
| Type of 3D Printer | Materials Used | Role in Endodontics | Ref |
|---|---|---|---|
| SLA | Photosensitive resin | Precise layer-by-layer curing; ideal for creating surgical guides for guided endodontic access and pre-surgical planning (3D) | [14,82] |
| FDM | Thermoplastic filaments (PLA and ABS) | Cost effective for educational models and simulations in endodontic training (3D) | [14,41] |
| PolyJet/MultiJet Printing (MJP) | Photopolymer | Accurate, high-resolution models for surgical planning, such as in autotransplantation or root-end surgery (3D) | [83] |
| Digital Light Processing (DLP) | Photosensitive resin | Used for creating detailed anatomical models for guided surgical procedures (3D) | [84] |
| Selective Laser Sintering (SLS) | Powdered materials (e.g., metal and polymer) | Produces durable and complex structures; useful for creating surgical guides and models involving metallic components (3D) | [79] |
| ColorJet Printing (CJP) | Powder-based materials with binder | Primarily used for educational models and visualization aids in endodontic training, such as replicating anatomical features (3D) | [79] |
2.7. Gene Therapy
3. Stem Cells in RETs: Properties, Sources, and Types
3.1. Fetal Stem Cells
3.2. Umbilical Stem Cells
3.3. Adult Stem Cells
3.4. Mesenchymal Stem Cells (MSCs)
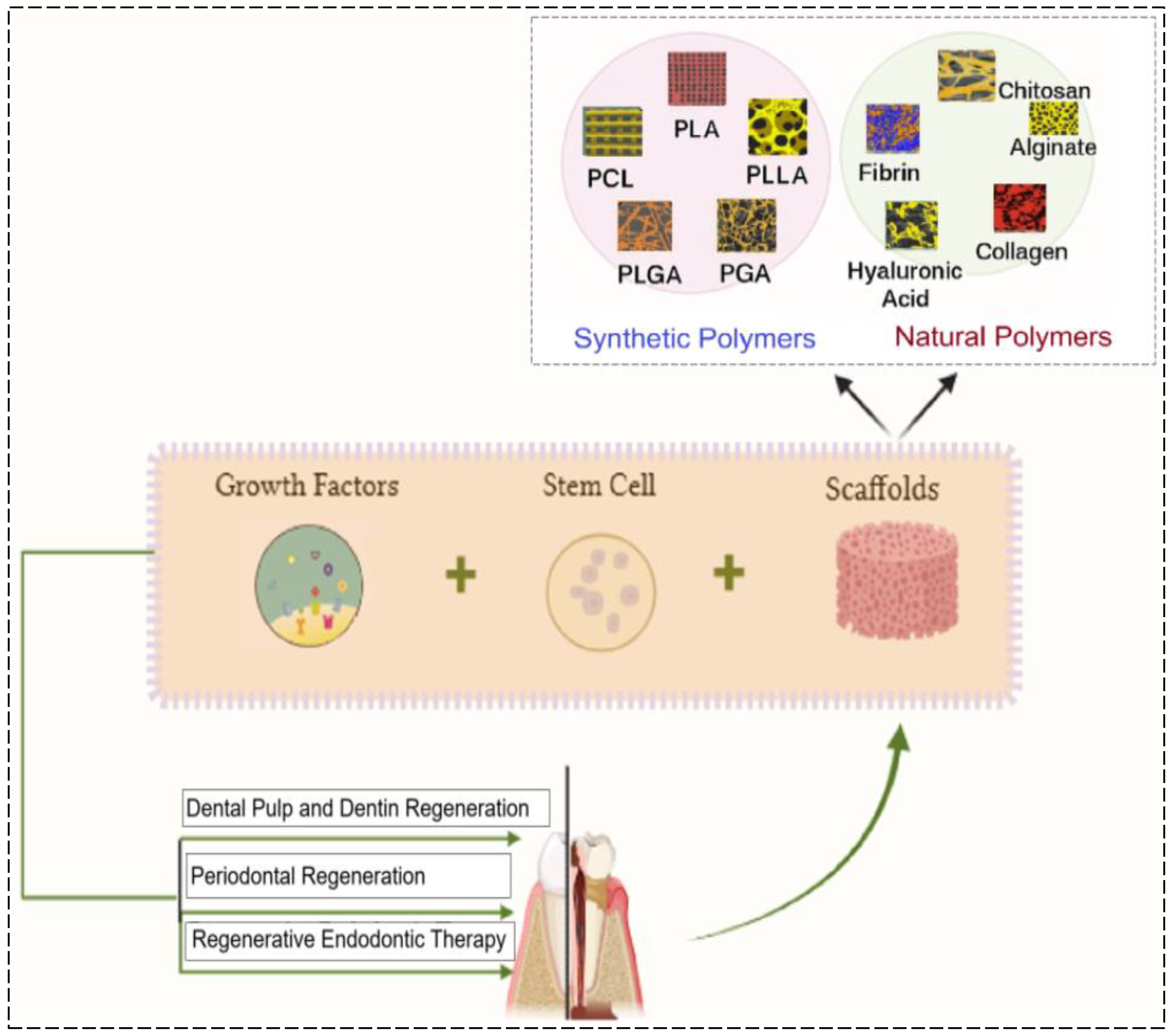
4. Scaffolds in RETs
4.1. Natural Scaffolds
4.1.1. Collagen
4.1.2. Chitosan
4.1.3. Alginate
4.2. Synthetic Scaffolds
4.3. Bioceramic Scaffolds
4.4. Hybrid Scaffolds and Advanced Techniques
| Biomaterial | Source | Key Biochemical Components | Favorable Properties | Limitations | Specific Endodontic Applications | Mechanism/ Function | Target Regeneration | Ref |
|---|---|---|---|---|---|---|---|---|
| Host-derived biomaterial scaffolds | ||||||||
| Blood clot | Host derived | Fibrin |
| Instability, difficulties in invoking bleeding, and hemostasis | Scaffold for RE | Forms fibrin clot and supports cell migration | Pulp tissue regeneration | [3,47,143] |
| Autologous platelet concentrates | Autologous blood | TGF and VEGF |
| Expensive, requires special equipment and reagents | Scaffold for RE | Promotes cell differentiation and tissue healing | Pulp and dentin regeneration | [47,143,144,145] |
| PRP | Autologous blood | Platelets and fibrin |
| Comparatively expensive | Scaffold for pulp regeneration | Promotes stem cell homing and tissue repair | Pulp regeneration | [47,97,144,145,146] |
| PRF | Autologous blood | Platelets and fibrin |
| Time consuming, with special equipment required | Scaffold for pulp and dentin regeneration | Slow release of growth factors; supports tissue repair | Pulp and dentin regeneration | [97,146,147] |
| Decellularized ECM | Human/ animal tissues | ECM proteins And growth factors |
| Time consuming, with difficult preparation | Scaffold for pulp-dentin regeneration | Mimics native ECM and promotes stem cell adhesion | Pulp and dentin regeneration | [147,148,149] |
| Nature-derived biomaterial scaffolds | ||||||||
| Collagen | Bovine/ porcine dermis or tendons | Type I and III collagen proteins | Biocompatible, biodegradable, and viscoelastic | Rapid degradation, weak mechanical strength, and shrinkage | Scaffold for pulp-dentin complex regeneration | Mimics natural ECM and supports cell adhesion and differentiation | Pulp and dentin regeneration | [47,143,150,151] |
| Chitosan | Derived from chitin (shrimp/crab shells) | N-acetyl glucosamine and glucosamine units | Host compatibility; biodegradable; antibacterial properties | Weak mechanical strength and shrinkage | Root canal disinfectant, pulp capping, and scaffold | Forms hydrogels, enhances tissue regeneration, and inhibits bacterial growth | Pulp capping and root regeneration | [47,143,152,153] |
| Alginate | Brown seaweed (Laminaria, Ascophyllum, and Macrocystis) | Sodium alginate (mannuronic and guluronic acids) | Host compatibility; inexpensive and supports nutrient exchange | Weak mechanical strength before cross-linking; shrinkage | Cell delivery, scaffold, and drug delivery system | Forms hydrogels that encapsulate stem cells or growth factors; supports controlled cell release | Pulp regeneration; scaffold for growth factors | [47,143,154,155] |
| Fibrin | Human blood (plasma) | Fibrinogen and thrombin | Host compatibility; creates fibrin clot | Requires clot formation; short-term support | Scaffold for RE | Creating a fibrin clot for cell adhesion and migration | Pulp tissue healing and vascularization | [156,157] |
| Hyaluronic acid | Animal connective tissue (skin and joints) | Hyaluronan polysaccharides | Biocompatible, retains moisture, and promotes tissue healing | Rapid degradation and weak mechanical strength | Pulp tissue hydration, ECM mimic, and scaffold | Retains moisture and promotes cell proliferation and migration | Pulp healing promotes angiogenesis | [158,159] |
| Silk fibroin | Silkworm cocoons (Bombyx mori) | Fibroin and sericin proteins | Biocompatible and promotes cell differentiation; strong scaffold | Limited availability and complicated processing | Scaffold for pulp and periodontal regeneration | Provides mechanical support and promotes differentiation of stem cells | Pulp and periodontal regeneration | [156,157] |
| Gelatin | Hydrolyzed collagen from animal skin/bone | Denatured collagen (Type I) | Biodegradable, forms hydrogels, and enhances cell proliferation | Rapid degradation and weak mechanical strength | Scaffold for cell culture; TE | Forms hydrogels and enhances cell attachment and proliferation | Cell proliferation, scaffold for pulp TE | [160,161,162] |
| Dextran | Produced by bacterial fermentation (Leucistic) | Glucose polymer (α-1,6 glycosidic linkages) | Biocompatible, slowly degrades, and supports prolonged healing | Limited in regenerative capabilities for some tissues | Drug delivery; scaffold for growth factors | Biocompatible; used as a carrier for growth factors or drugs | Controlled drug delivery; tissue repair | [163,164] |
| Synthetic biomaterial scaffolds | ||||||||
| Hydraulic calcium | Synthetic | Tricalcium silicate-based materials | Biocompatible | Tooth discoloration | Scaffold for pulp regeneration | Promotes odontogenic cell differentiation and supports mineralization | Pulp regeneration | [165,166,167] |
| Synthetic polymers | Synthetic |
| Biodegradable, precisely modifiable physicochemical properties | Relatively slow degradation rate and potential host response | Scaffold for pulp regeneration | Provides mechanical support and customizes pore size for stem cell colonization | Pulp regeneration | [168,169] |
| Synthetic hydrogel | Synthetic |
| Biocompatible, injectable, and supports self-assembly | Slow gelation; UV light required for some hydrogels may cause cell death | Scaffold for pulp regeneration | Promotes cell proliferation and mimics ECM | Pulp regeneration | [170,171,172] |
5. Growth Factors in RETs
5.1. Signal Activation
5.2. Stem Cell Modulation
6. Challenges and Future Directions in RETs
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Soares, D.G.; Bordini, E.A.; Swanson, W.B.; de Souza Costa, C.A.; Bottino, M.C. Platform technologies for regenerative endodontics from multifunctional biomaterials to tooth-on-a-chip strategies. Clin. Oral Investig. 2021, 25, 4749–4779. [Google Scholar] [CrossRef] [PubMed]
- Boyd, R.C. Basic endodontic therapy. In Wiggs’s Veterinary Dentistry: Principles and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 311–334. [Google Scholar]
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: Strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Widbiller, M.; Schmalz, G. Endodontic regeneration: Hard shell, soft core. Odontology 2021, 109, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Al-Helou, N. Contemporary Endodontics for Children and Adolescents; Fuks, A.B., Moskovitz, M., Tickotsky, N., Eds.; Springer: Berlin, Germany, 2023. [Google Scholar]
- Cymerman, J.J.; Nosrat, A. Regenerative endodontic treatment as a biologically based approach for non-surgical retreatment of immature teeth. J. Endod. 2020, 46, 44–50. [Google Scholar] [CrossRef]
- Sheehy, E.J.; Kelly, D.J.; O’Brien, F.J. Biomaterial-based endochondral bone regeneration: A shift from traditional tissue engineering paradigms to developmentally inspired strategies. Mater. Today Bio 2019, 3, 100009. [Google Scholar] [CrossRef]
- Ahrendt, G.; Chickering, D.E.; Ranieri, J.P. Angiogenic growth factors: A review for tissue engineering. Tissue Eng. 1998, 4, 117–130. [Google Scholar] [CrossRef]
- Matichescu, A.; Ardelean, L.C.; Rusu, L.C.; Craciun, D.; Bratu, E.A.; Babucea, M.; Leretter, M. Advanced biomaterials and techniques for oral tissue engineering and regeneration—A review. Materials 2020, 13, 5303. [Google Scholar] [CrossRef]
- Doblado, L.R.; Martínez-Ramos, C.; Pradas, M.M. Biomaterials for neural tissue engineering. Front. Nanotechnol. 2021, 3, 643507. [Google Scholar] [CrossRef]
- Khurshid, Z.; Alnaim, A.J.A.; Alhashim, A.A.A.; Imran, E.; Adanir, N. Future of decellularized dental pulp matrix in regenerative endodontics. Eur. J. Dent. 2022, 16, 737–741. [Google Scholar] [CrossRef]
- Sugiaman, V.K.; Jeffrey Naliani, S.; Pranata, N.; Djuanda, R.; Saputri, R.I. Polymeric scaffolds used in dental pulp regeneration by tissue engineering approach. Polymers 2023, 15, 1082. [Google Scholar] [CrossRef]
- Yang, Q.; Zheng, W.; Zhao, Y.; Shi, Y.; Wang, Y.; Sun, H.; Xu, X. Advancing dentin remineralization: Exploring amorphous calcium phosphate and its stabilizers in biomimetic approaches. Dent. Mater. 2024, 40, 1282–1295. [Google Scholar] [CrossRef]
- Huang, T.H.; Chen, J.Y.; Suo, W.H.; Shao, W.R.; Huang, C.Y.; Li, M.T.; Li, Y.Y.; Li, Y.H.; Liang, E.L.; Chen, Y.H.; et al. Unlocking the Future of Periodontal Regeneration: An Interdisciplinary Approach to Tissue Engineering and Advanced Therapeutics. Biomedicines 2024, 12, 1090. [Google Scholar] [CrossRef] [PubMed]
- Farjaminejad, R.; Farjaminejad, S.; Hasani, M.; Garcia-Godoy, F.; Sayahpour, B.; Marya, A.; Jamilian, A. The Role of Tissue Engineering in Orthodontic and Orthognathic Treatment: A Narrative Review. Oral 2025, 5, 21. [Google Scholar] [CrossRef]
- El Gezawi, M.; Wölfle, U.C.; Haridy, R.; Fliefel, R.; Kaisarly, D. Remineralization, regeneration, and repair of natural tooth structure: Influences on the future of restorative dentistry practice. ACS Biomater. Sci. Eng. 2019, 5, 4899–4919. [Google Scholar] [CrossRef] [PubMed]
- Digka, A.; Sakka, D.; Lyroudia, K. Histological assessment of human regenerative endodontic procedures (REP) of immature permanent teeth with necrotic pulp/apical periodontitis: A systematic review. Aust. Endod. J. 2020, 46, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Paryani, K.; Kim, S.G. Regenerative endodontic treatment of permanent teeth after completion of root development: A report of 2 cases. J. Endod. 2013, 39, 929–934. [Google Scholar] [CrossRef]
- Saoud, T.M.; Sigurdsson, A.; Rosenberg, P.A.; Lin, L.M.; Ricucci, D. Treatment of a large cystlike inflam- matory periapical lesion associated with mature necrotic teeth using regenerative endodontic therapy. J. Endod. 2014, 40, 2081–2086. [Google Scholar] [CrossRef]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative endodontics: A review of current status and a call for action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef]
- Krupińska, A.M.; Skośkiewicz-Malinowska, K.; Staniowski, T. Different approaches to the regeneration of dental tissues in regenerative endodontics. Appl. Sci. 2021, 11, 1699. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.; Qin, M.; Ge, L. Pulp revascularization of immature dens invaginatus with periapical periodontitis. J. Endod. 2013, 39, 288–292. [Google Scholar] [CrossRef]
- Jazayeri, H.E.; Lee, S.M.; Kuhn, L.; Fahimipour, F.; Tahriri, M.; Tayebi, L. Polymeric scaffolds for dental pulp tissue engineering: A review. Dent. Mater. 2020, 36, e47–e58. [Google Scholar] [CrossRef]
- Liu, H.; Lu, J.; Jiang, Q.; Haapasalo, M.; Qian, J.; Tay, F.R.; Shen, Y. Biomaterial scaffolds for clinical procedures in endodontic regeneration. Bioact. Mater. 2022, 12, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Kouhi, M.; de Souza Araújo, I.J.; Asa’ad, F.; Zeenat, L.; Bojedla, S.S.R.; Pati, F.; Zolfagharian, A.; Watts, D.C.; Bottino, M.C.; Bodaghi, M. Recent advances in additive manufacturing of patient-specific devices for dental and maxillofacial rehabilitation. Dent. Mater. 2024, 40, 700–715. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Hu, X.; Jiang, N.; Mao, J. Regenerative endodontic therapy: From laboratory bench to clinical practice. J. Adv. Res. 2025, 72, 229–263. [Google Scholar] [CrossRef]
- Srisuwan, T.; Tilkorn, D.J.; Wilson, J.L.; Morrison, W.A.; Messer, H.M.; Thompson, E.W.; Abberton, K.M. Molecular aspects of tissue engineering in the dental field. Periodontology 2000, 2006, 41. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, L.; Yang, B.; Tian, W. Three-dimensional printing biotechnology for the regeneration of the tooth and tooth-supporting tissues. Biotechnol. Bioeng. 2019, 116, 452–468. [Google Scholar] [CrossRef]
- Bansal, R.; Jain, A.; Mittal, S. Current overview on challenges in regenerative endodontics. J. Conserv. Dent. Endod. 2015, 18, 1–6. [Google Scholar]
- Etezadkeyhan, P. Recent Advances in Regenerative Endodontics: Clinical Applications and Challenges. J. Oral Dent. Health Nexus 2024, 1, 29–42. [Google Scholar] [CrossRef]
- Saoud, T.M.; Martin, G.; Chen, Y.H.; Chen, K.L.; Chen, C.A.; Songtrakul, K.; Malek, M.; Sigurdsson, A.; Lin, L.M. Treatment of mature permanent teeth with necrotic pulps and apical periodontitis using regenerative endodontic procedures: A case series. J. Endod. 2016, 42, 57–65. [Google Scholar] [CrossRef]
- Jung, C.; Kim, S.; Sun, T.; Cho, Y.B.; Song, M. Pulp-dentin regeneration: Current approaches and challenges. J. Tissue Eng. 2019, 10, 2041731418819263. [Google Scholar] [CrossRef]
- Duncan, H.F.; Smith, A.J.; Fleming, G.J.P.; Cooper, P.R. Epigenetic modulation of dental pulp stem cells: Implications for regenerative endodontics. Int. Endod. J. 2016, 49, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Cha, S.; Park, Y.S. Regenerative applications using tooth derived stem cells in other than tooth regeneration: A literature review. Stem Cells Int. 2016, 2016, 9305986. [Google Scholar] [CrossRef] [PubMed]
- Farjaminejad, S.; Farjaminejad, R.; Garcia-Godoy, F. Nanoparticles in Bone Regeneration: A Narrative Review of Current Advances and Future Directions in Tissue Engineering. J. Funct. Biomater. 2024, 15, 241. [Google Scholar] [CrossRef]
- Ercal, P.; Pekozer, G.G. A current overview of scaffold-based bone regeneration strategies with dental stem cells. Adv. Exp. Med. Biol. 2020, 1288, 61–85. [Google Scholar]
- Zhang, F.; King, M.W. Biodegradable polymers as the pivotal player in the design of tissue engineering scaffolds. Adv. Healthc. Mater. 2020, 9, 1901358. [Google Scholar] [CrossRef]
- Wang, X.; Krebbers, J.; Charalambous, P.; Machado, V.; Schober, A.; Bosse, F.; Müller, H.W.; Unsicker, K. Growth/differentiation factor-15 and its role in peripheral nervous system lesion and regeneration. Cell Tissue Res. 2015, 362, 317–330. [Google Scholar] [CrossRef]
- Liao, W.; Xu, L.; Wangrao, K.; Du, Y.; Xiong, Q.; Yao, Y. Three-dimensional printing with biomaterials in craniofacial and dental tissue engineering. PeerJ 2019, 7, e7271. [Google Scholar] [CrossRef] [PubMed]
- Farjaminejad, R.; Farjaminejad, S.; Nucci, L.; d’Apuzzo, F.; Grassia, V.; Majidi, K.; Jamilian, A. 3D Printing Approach in Maxillofacial Surgery in Iran: An Evaluation Using the Non-Adoption, Abandonment, Scale-Up, Spread, and Sustainability (NASSS) Framework. Appl. Sci. 2024, 14, 3075. [Google Scholar] [CrossRef]
- Thalakiriyawa, D.S.; Dissanayaka, W.L. Advances in regenerative dentistry approaches: An update. Int. Dent. J. 2024, 74, 25–34. [Google Scholar] [CrossRef]
- Peters, O.A.; Paranjpe, A.; Gaudin, A. Dentine–pulp complex regeneration. In Regenerative Approaches in Dentistry: An Evidence-Based Perspective; Springer Nature: Berlin, Germany, 2021; pp. 35–62. [Google Scholar]
- Gathani, K.M.; Raghavendra, S.S. Scaffolds in regenerative endodontics: A review. Dent. Res. J. 2016, 13, 379–386. [Google Scholar]
- Cotti, E.; Mereu, M.; Lusso, D. Regenerative treatment of an immature, traumatized tooth with apical periodontitis: Report of a case. J. Endod. 2008, 34, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.M.; Ricucci, D.; Huang, G.J. Regeneration of the dentine–pulp complex with revitalization/revascularization therapy: Challenges and hopes. Int. Endod. J. 2014, 47, 713–724. [Google Scholar] [CrossRef]
- Hargreaves, K.M.; Diogenes, A.; Teixeira, F.B. Treatment options: Biological basis of regenerative endodontic procedures. Pediatr. Dent. 2013, 35, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Ricucci, D.; Gibbs, J.L.; Lin, L.M. Histological findings of revascularized/revitalized immature permanent molar with apical periodontitis using platelet-rich plasma. J. Endod. 2013, 39, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, W.L.; Zhang, C. Scaffold-based and scaffold-free strategies in dental pulp regeneration. J. Endod. 2020, 46, S81–S89. [Google Scholar] [CrossRef]
- Shah, R.; Shimpi, M. Clinical Progresses in Regenerative Dentistry and Dental Tissue Engineering. In Current Advances in Oral and Craniofacial Tissue Engineering; CRC Press: Boca Raton, FL, USA, 2020; pp. 209–228. [Google Scholar]
- Capparè, P.; Tetè, G.; Sberna, M.T.; Panina-Bordignon, P. The emerging role of stem cells in regenerative dentistry. Curr. Gene Ther. 2020, 20, 259–268. [Google Scholar] [CrossRef]
- Soudi, A.; Yazdanian, M.; Ranjbar, R.; Tebyanian, H.; Yazdanian, A.; Tahmasebi, E.; Keshvad, A.; Seifalian, A. Role and application of stem cells in dental regeneration: A comprehensive overview. Excli J. 2021, 20, 454. [Google Scholar]
- Xia, H.; Li, X.; Gao, W.; Fu, X.; Fang, R.H.; Zhang, L.; Zhang, K. Tissue repair and regeneration with endogenous stem cells. Nat. Rev. Mater. 2018, 3, 174–193. [Google Scholar] [CrossRef]
- Huang, G.T.J. A paradigm shift in endodontic management of immature teeth: Conservation of stem cells for regeneration. J. Dent. 2008, 36, 379–386. [Google Scholar] [CrossRef]
- Lee, B.N.; Moon, J.W.; Chang, H.S.; Hwang, I.N.; Oh, W.M.; Hwang, Y.C. A review of the regenerative endodontic treatment procedure. Restor. Dent. Endod. 2015, 40, 179–187. [Google Scholar] [CrossRef]
- Yang, J.; Yuan, G.; Chen, Z. Pulp regeneration: Current approaches and future challenges. Front. Physiol. 2016, 7, 58. [Google Scholar] [CrossRef] [PubMed]
- Alghutaimel, H.A.H. Decellularised Dental Pulp Tissue as a Potential Biological Scaffold for Endodontic Tissue Regeneration. Ph.D. Thesis, University of Leeds, Leeds, UK, 2021. [Google Scholar]
- Woloszyk, A. The Angiogenic Potential of Human Dental Pulp Stem Cells Cultured on 3D Silk Scaffolds. Ph.D. Thesis, University of Zurich, Zurich, Switzerland, 2016. [Google Scholar]
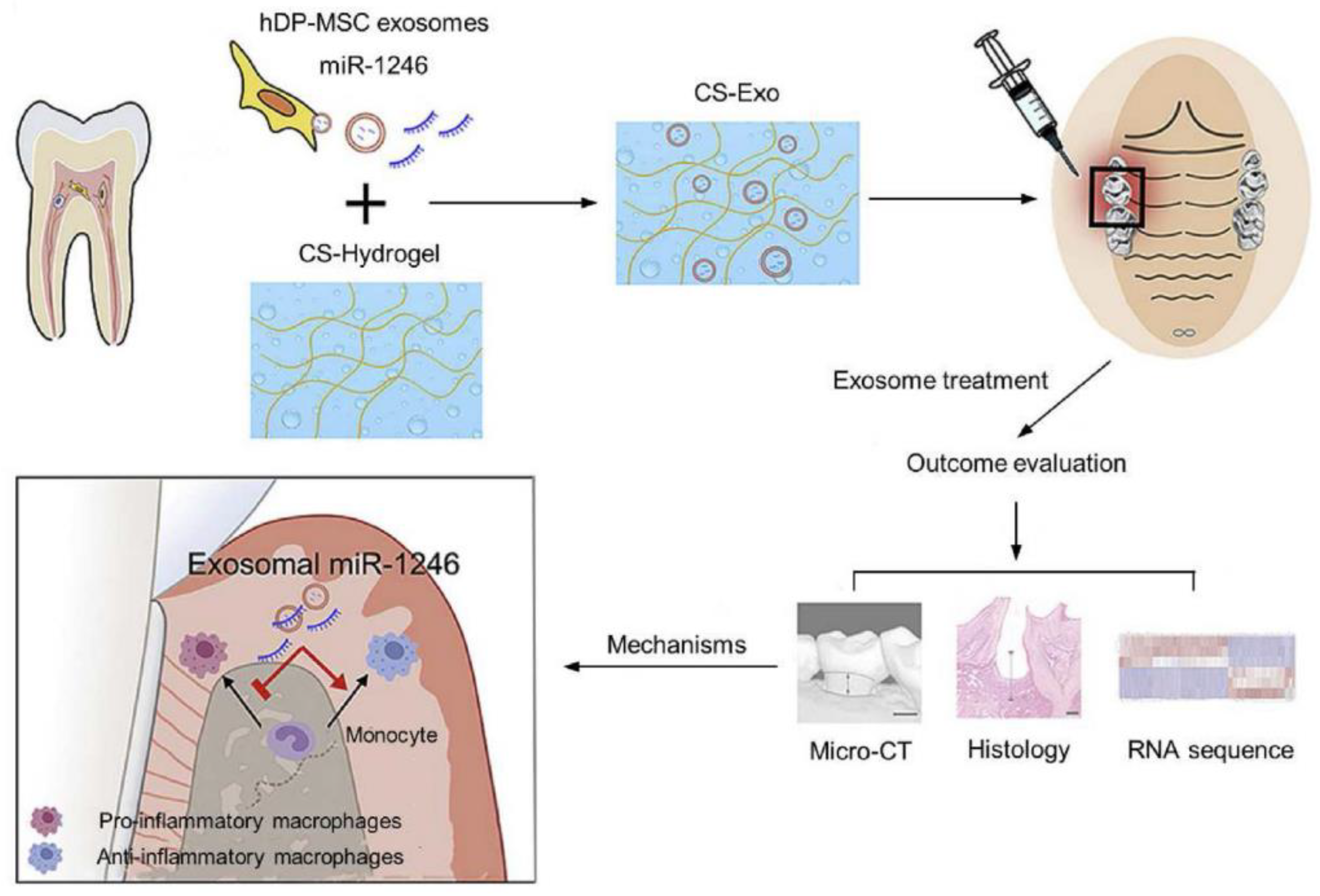
- Lu, H.; Mu, Q.; Ku, W.; Zheng, Y.; Yi, P.; Lin, L.; Li, P.; Wang, B.; Wu, J.; Yu, D.; et al. Functional extracellular vesicles from SHEDs combined with gelatin methacryloyl promote the odontogenic differentiation of DPSCs for pulp regeneration. J. Nanobiotechnol. 2024, 22, 265. [Google Scholar] [CrossRef] [PubMed]
- Luzuriaga, J.; Polo, Y.; Pastor-Alonso, O.; Pardo-Rodríguez, B.; Larrañaga, A.; Unda, F.; Sarasua, J.R.; Pineda, J.R.; Ibarretxe, G. Advances and perspectives in dental pulp stem cell based neuroregeneration therapies. Int. J. Mol. Sci. 2021, 22, 3546. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; De Deus, G.; Kristoffersen, I.M.; Wiig, E.; Reseland, J.E.; Johnsen, G.F.; Silva, E.J.; Haugen, H.J. Regenerative endodontics by cell homing: A review of recent clinical trials. J. Endod. 2023, 49, 4–17. [Google Scholar] [CrossRef]
- Fatehi, F. Natural scaffold materials used in regenerative endodontic: A review. J. Biomater. Tissue Eng. 2013, 3, 597–604. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, H.J.; Yeom, J.H.; Seo, J.S.; Ryu, G.J.; Park, K.H.; Shin, S.I.; Kim, S.Y. Long-term follow-ups of revascularized immature necrotic teeth: Three case reports. Int. J. Oral Sci. 2012, 4, 109–113. [Google Scholar] [CrossRef]
- Siddiqui, Z.; Acevedo-Jake, A.M.; Griffith, A.; Kadincesme, N.; Dabek, K.; Hindi, D.; Kim, K.K.; Kobayashi, Y.; Shimizu, E.; Kumar, V. Cells and material-based strategies for regenerative endodontics. Bioact. Mater. 2022, 14, 234–249. [Google Scholar] [CrossRef]
- Nakashima, M.; Akamine, A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J. Endod. 2005, 31, 711–718. [Google Scholar] [CrossRef]
- Pozos-Guillén, A.; Flores, H. Dentin-pulp complex regeneration. In Current Advances in Oral and Craniofacial Tissue Engineering; CRC Press: Boca Raton, FL, USA, 2020; pp. 159–181. [Google Scholar]
- Albuquerque, M.T.P.; Valera, M.C.; Nakashima, M.; Nör, J.E.; Bottino, M.C. Tissue-engineering-based strategies for regenerative endodontics. J. Dent. Res. 2014, 93, 1222–1231. [Google Scholar] [CrossRef]
- Alkhursani, S.A.; Ghobashy, M.M.; Al-Gahtany, S.A.; Meganid, A.S.; Abd El-Halim, S.M.; Ahmad, Z.; Khan, F.S.; Atia, G.A.N.; Cavalu, S. Application of nano-inspired scaffolds-based biopolymer hydrogel for bone and periodontal tissue regeneration. Polymers 2022, 14, 3791. [Google Scholar] [CrossRef]
- Dubey, N.; Ribeiro, J.S.; Zhang, Z.; Xu, J.; Ferreira, J.A.; Qu, L.; Mei, L.; Fenno, J.C.; Schwendeman, A.; Schwendeman, S.P.; et al. Gelatin methacryloyl hydrogel as an injectable scaffold with multi-therapeutic effects to promote antimicrobial disinfection and angiogenesis for regenerative endodontics. J. Mater. Chem. B 2023, 11, 3823–3835. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.A.; Eiro, N.; Vaca, A.; Vizoso, F.J. Towards a new concept of regenerative endodontics based on mesenchymal stem cell-derived secretomes products. Bioengineering 2022, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, D.B.; Diogo, P.; Gomes, B.P.; Peça, J.; Santos, J.M.M. Scaffolds for Dentin–Pulp Complex Regeneration. Medicina 2023, 60, 7. [Google Scholar] [CrossRef] [PubMed]
- Orti, V.; Collart-Dutilleul, P.Y.; Piglionico, S.; Pall, O.; Cuisinier, F.; Panayotov, I. Pulp regeneration concepts for nonvital teeth: From tissue engineering to clinical approaches. Tissue Eng. Part B Rev. 2018, 24, 419–442. [Google Scholar] [CrossRef]
- Gelman, R.; Park, H. Pulp revascularization in an immature necrotic tooth: A case report. Pediatr. Dent. 2012, 34, 496–499. [Google Scholar]
- Moussa, D.G.; Aparicio, C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 58–75. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Duncan, H.F.; El-Karim, I.A.; Cooper, P.R. Dental Pulp Stem Cells in Endodontics: Advances, Applications, and Challenges. In Handbook of Stem Cell Applications; Springer: Singapore, 2024; pp. 305–344. [Google Scholar]
- Rahman, S.U.; Nagrath, M.; Ponnusamy, S.; Arany, P.R. Nanoscale and macroscale scaffolds with controlled-release polymeric systems for dental craniomaxillofacial tissue engineering. Materials 2018, 11, 1478. [Google Scholar] [CrossRef]
- Iranmanesh, P.; Ehsani, A.; Khademi, A.; Asefnejad, A.; Shahriari, S.; Soleimani, M.; Ghadiri Nejad, M.; Saber-Samandari, S.; Khandan, A. Application of 3D bioprinters for dental pulp regeneration and tissue engineering (porous architecture). Transp. Porous Media 2022, 142, 265–293. [Google Scholar] [CrossRef]
- EzEldeen, M.; Moroni, L.; Nejad, Z.M.; Jacobs, R.; Mota, C. Biofabrication of engineered dento-alveolar tissue. Biomater. Adv. 2023, 148, 213371. [Google Scholar] [CrossRef]
- Choi, D.; Qiu, M.; Hwang, Y.C.; Oh, W.M.; Koh, J.T.; Park, C.; Lee, B.N. The Effects of 3-dimensional bioprinting calcium silicate cement/methacrylated gelatin scaffold on the proliferation and differentiation of human dental pulp stem cells. Materials 2022, 15, 2170. [Google Scholar] [CrossRef]
- Jadhav, G.; Shah, N.; Logani, A. Revascularization with and without platelet-rich plasma in nonvital, immature, anterior teeth: A pilot clinical study. J. Endod. 2012, 38, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Nosrat, A.; Homayounfar, N.; Oloomi, K. Drawbacks and unfavorable outcomes of regenerative endodontic treatments of necrotic immature teeth: A literature review and report of a case. J. Endod. 2012, 38, 1428–1434. [Google Scholar] [CrossRef]
- Anderson, J.; Wealleans, J.; Ray, J. Endodontic applications of 3D printing. Int. Endod. J. 2018, 51, 1005–1018. [Google Scholar] [CrossRef]
- Yang, H.; Fang, H.; Wang, C.; Wang, Y.; Qi, C.; Zhang, Y.; Zhou, Q.; Huang, M.; Wang, M.; Wu, M. 3D printing of customized functional devices for smart biomedical systems. SmartMat 2023, 5, e1244. [Google Scholar] [CrossRef]
- Kustra, P.; Dobroś, K.; Zarzecka, J. Making use of three-dimensional models of teeth, manufactured by stereolithographic technology, in practical teaching of endodontics. Eur. J. Dent. Educ. 2021, 25, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, D.; Zhang, F.; Xie, S.; Wu, G.; Hu, Q.; Yan, F.; Tang, X. Comparison of selective laser melting and stereolithography etching templates for guided endodontics. PeerJ 2024, 12, e17646. [Google Scholar] [CrossRef]
- Alageel, O.; Wazirian, B.; Almufleh, B.; Tamimi, F. Fabrication of dental restorations using digital technologies: Techniques and materials. In Digital Restorative Dentistry: A Guide to Materials, Equipment, and Clinical Procedures; Springer Nature: Berlin, Germany, 2019; pp. 55–91. [Google Scholar]
- Liu, Y.; Liang, L.; Rajan, S.S.; Damade, Y.; Zhang, X.; Mishra, K.; Qu, L.; Dubey, N. Recent advances in additive manufacturing for tooth restorations. Appl. Mater. Today 2024, 39, 102275. [Google Scholar] [CrossRef]
- Ashammakhi, N.; GhavamiNejad, A.; Tutar, R.; Fricker, A.; Roy, I.; Chatzistavrou, X.; Hoque Apu, E.; Nguyen, K.L.; Ahsan, T.; Pountos, I.; et al. Highlights on advancing frontiers in tissue engineering. Tissue Eng. Part B Rev. 2022, 28, 633–664. [Google Scholar] [CrossRef]
- Umapathy, V.R.; Natarajan, P.M.; Swamikannu, B. Regenerative Strategies in Dentistry: Harnessing Stem Cells, Biomaterials and Bioactive Materials for Tissue Repair. Biomolecules 2025, 15, 546. [Google Scholar] [CrossRef]
- Gong, T.; Heng, B.C.; Lo, E.C.M.; Zhang, C. Current advance and future prospects of tissue engineering approach to dentin/pulp regenerative therapy. Stem Cells Int. 2016, 2016, 9204574. [Google Scholar] [CrossRef]
- Makandar, S.D. Regenerative Endodontics-challenges and Future Direction. Int. J. Innov. Eng. Res. Technol. 2021, 8, 314–316. [Google Scholar]
- Chavez-Granados, P.A.; Manisekaran, R.; Acosta-Torres, L.S.; Garcia-Contreras, R. CRISPR/Cas gene-editing technology and its advances in dentistry. Biochimie 2022, 194, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Marvaniya, J.; Agarwal, K.; Mehta, D.N.; Parmar, N.; Shyamal, R.; Patel, J. Minimal invasive endodontics: A comprehensive narrative review. Cureus 2022, 14, e25984. [Google Scholar] [CrossRef]
- Fu, X.; Kim, H.S. Dentin Mechanobiology: Bridging the Gap between Architecture and Function. Int. J. Mol. Sci. 2024, 25, 5642. [Google Scholar] [CrossRef]
- Malhotra, N. Bioreactors design, types, influencing factors and potential application in dentistry. A literature review. Curr. Stem Cell Res. Ther. 2019, 14, 351–366. [Google Scholar] [CrossRef]
- Iviglia, G.; Kargozar, S.; Baino, F. Biomaterials, current strategies, and novel nano-technological approaches for periodontal regeneration. J. Funct. Biomater. 2019, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhuo, L.; Hu, X.; Lu, S.; Dong, C. Unveiling the future of endodontics: An update on dental pulp regeneration strategies. Saudi Dent. J. 2024; in press. [Google Scholar] [CrossRef]
- Alyafei, S.H.; Anil, S. Regenerative Approaches in Gingival Tissue Engineering. In Advances in Gingival Diseases and Conditions; IntechOpen: London, UK, 2024. [Google Scholar]
- Takeuchi, N.; Hayashi, Y.; Murakami, M.; Alvarez, F.J.; Horibe, H.; Iohara, K.; Nakata, K.; Nakamura, H.; Nakashima, M. Similar in vitro effects and pulp regeneration in ectopic tooth transplantation by basic fibroblast growth factor and granulocyte-colony stimulating factor. Oral Dis. 2015, 21, 113–122. [Google Scholar] [CrossRef]
- de Pablo, J.A.; Serrano, L.J.; García-Arranz, M.; Romeu, L.; Liras, A. Gene and Cell Therapy in Dental Tissue Regeneration. In Human Tooth and Developmental Dental Defects—Compositional and Genetic Implications; IntechOpen: London, UK, 2021. [Google Scholar]
- Orduña, J.F.G.; Caviedes-Bucheli, J.; Céspedes, M.C.M.; Jimeno, E.B.; Biedma, B.M.; Segura-Egea, J.J.; López-López, J. Use of platelet-rich plasma in endodontic procedures in adults: Regeneration or repair? A report of 3 cases with 5 years of follow-up. J. Endod. 2017, 43, 1294–1301. [Google Scholar] [CrossRef]
- Cabaña-Muñoz, M.E.; Pelaz Fernández, M.J.; Parmigiani-Cabaña, J.M.; Parmigiani-Izquierdo, J.M.; Merino, J.J. Adult mesenchymal stem cells from oral cavity and surrounding areas: Types and biomedical applications. Pharmaceutics 2023, 15, 2109. [Google Scholar] [CrossRef]
- Hashemi-Beni, B.; Khoroushi, M.; Foroughi, M.R.; Karbasi, S.; Khademi, A.A. Tissue engineering: Dentin–pulp complex regeneration approaches (A review). Tissue Cell 2017, 49, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Kwack, K.H.; Lee, H.W. Clinical potential of dental pulp stem cells in pulp regeneration: Current endodontic progress and future perspectives. Front. Cell Dev. Biol. 2022, 10, 857066. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D. A Roadmap to Nonhematopoietic Stem Cell-Based Therapeutics: From the Bench to the Clinic; Chen, X.D., Ed.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Eramo, S.; Natali, A.; Pinna, R.; Milia, E. Dental pulp regeneration via cell homing. Int. Endod. J. 2018, 51, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry–Part II: Clinical applications. J. Prosthodont. Res. 2012, 56, 229–248. [Google Scholar] [CrossRef]
- Huang, G.J.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Collart-Dutilleul, P.Y.; Chaubron, F.; De Vos, J.; Cuisinier, F.J. Allogenic banking of dental pulp stem cells for innovative therapeutics. World J. Stem Cells 2015, 7, 1010. [Google Scholar]
- Wang, L.H.; Gao, S.Z.; Bai, X.L.; Chen, Z.L.; Yang, F. An up-to-date overview of dental tissue regeneration using dental origin mesenchymal stem cells: Challenges and road ahead. Front. Bioeng. Biotechnol. 2022, 10, 855396. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.; Cavacas, M.A.; Machado, V.; Mendes, J.J. Dental stem cells: Recent progresses in tissue engineering and regenerative medicine. Ann. Med. 2017, 49, 644–651. [Google Scholar] [CrossRef]
- Goldberg, M. Stem cells: Tools for dental tissues regeneration. J. Dent. Health Oral Disord Ther. 2017, 6, 111–116. [Google Scholar] [CrossRef][Green Version]
- Zhang, W.; Yelick, P.C. Tooth repair and regeneration: Potential of dental stem cells. Trends Mol. Med. 2021, 27, 501–511. [Google Scholar] [CrossRef]
- Deepyanti, D.; Srishti, D.; Ghosh, D.M.; Saha, D.A. Regenerative Endodontics; Dentomed Publication House: Amritsar, India, 2021. [Google Scholar]
- Rosa, V. What and where are the stem cells for dentistry? Singap. Dent. J. 2013, 34, 13–18. [Google Scholar] [CrossRef]
- Bansal, R.; Bansal, R. Regenerative endodontics: A state of the art. Indian J. Dent. Res. 2011, 22, 122–131. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Song, M.; Kim, E.; Shon, W.; Chugal, N.; Bogen, G.; Lin, L.; Kim, R.H.; Park, N.H.; Kang, M.K. Pulp-dentin regeneration: Current state and future prospects. J. Dent. Res. 2015, 94, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- KBatsali, A.; Kastrinaki, M.C.; APapadaki, H.; Pontikoglou, C. Mesenchymal stem cells derived from Wharton’s Jelly of the umbilical cord: Biological properties and emerging clinical applications. Curr. Stem Cell Res. Ther. 2013, 8, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Shen, Z.; Zhan, P.; Yang, J.; Huang, Q.; Huang, S.; Chen, L.; Lin, Z. Functional dental pulp regeneration: Basic research and clinical translation. Int. J. Mol. Sci. 2021, 22, 8991. [Google Scholar] [CrossRef]
- Shaikh, M.S.; Shahzad, Z.; Tash, E.A.; Janjua, O.S.; Khan, M.I.; Zafar, M.S. Human umbilical cord mesenchymal stem cells: Current literature and role in periodontal regeneration. Cells 2022, 11, 1168. [Google Scholar] [CrossRef]
- Nada, O.A.; El Backly, R.M. Stem cells from the apical papilla (SCAP) as a tool for endogenous tissue regeneration. Front. Bioeng. Biotechnol. 2018, 6, 103. [Google Scholar] [CrossRef]
- Nakashima, M.; Iohara, K.; Murakami, M. Dental pulp stem cells and regeneration. Endod. Top. 2013, 28, 38–50. [Google Scholar] [CrossRef]
- Galler, K.M.; Weber, M.; Korkmaz, Y.; Widbiller, M.; Feuerer, M. Inflammatory response mechanisms of the dentine–pulp complex and the periapical tissues. Int. J. Mol. Sci. 2021, 22, 1480. [Google Scholar] [CrossRef]
- Hernández-Monjaraz, B.; Santiago-Osorio, E.; Monroy-García, A.; Ledesma-Martínez, E.; Mendoza-Núñez, V.M. Mesenchymal stem cells of dental origin for inducing tissue regeneration in periodontitis: A mini-review. Int. J. Mol. Sci. 2018, 19, 944. [Google Scholar] [CrossRef] [PubMed]
- Nikoloudaki, G. Functions of matricellular proteins in dental tissues and their emerging roles in orofacial tissue development, maintenance, and disease. Int. J. Mol. Sci. 2021, 22, 6626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Song, J.; Zhang, H.; Huang, E.; Song, D.; Tollemar, V.; Wang, J.; Wang, J.; Mohammed, M.; Wei, Q.; et al. Wnt and BMP signaling crosstalk in regulating dental stem cells: Implications in dental tissue engineering. Genes Dis. 2016, 3, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, W.; Li, Y.; Ren, L.; Deng, H.; Yin, X.; Gao, X.; Pan, S.; Niu, Y. Human umbilical cord mesenchymal stem cell differentiation into odontoblast-like cells and endothelial cells: A potential cell source for dental pulp tissue engineering. Front. Physiol. 2020, 11, 593. [Google Scholar] [CrossRef]
- Kiarashi, M.; Bayat, H.; Shahrtash, S.A.; Etajuri, E.A.; Khah, M.M.; Al-Shaheri, N.A.; Nasiri, K.; Esfahaniani, M.; Yasamineh, S. Mesenchymal stem cell-based scaffolds in regenerative medicine of dental diseases. Stem Cell Rev. Rep. 2024, 20, 688–721. [Google Scholar] [CrossRef] [PubMed]
- Mantesso, A.; Sharpe, P. Dental stem cells for tooth regeneration and repair. Expert Opin. Biol. Ther. 2009, 9, 1143–1154. [Google Scholar] [CrossRef]
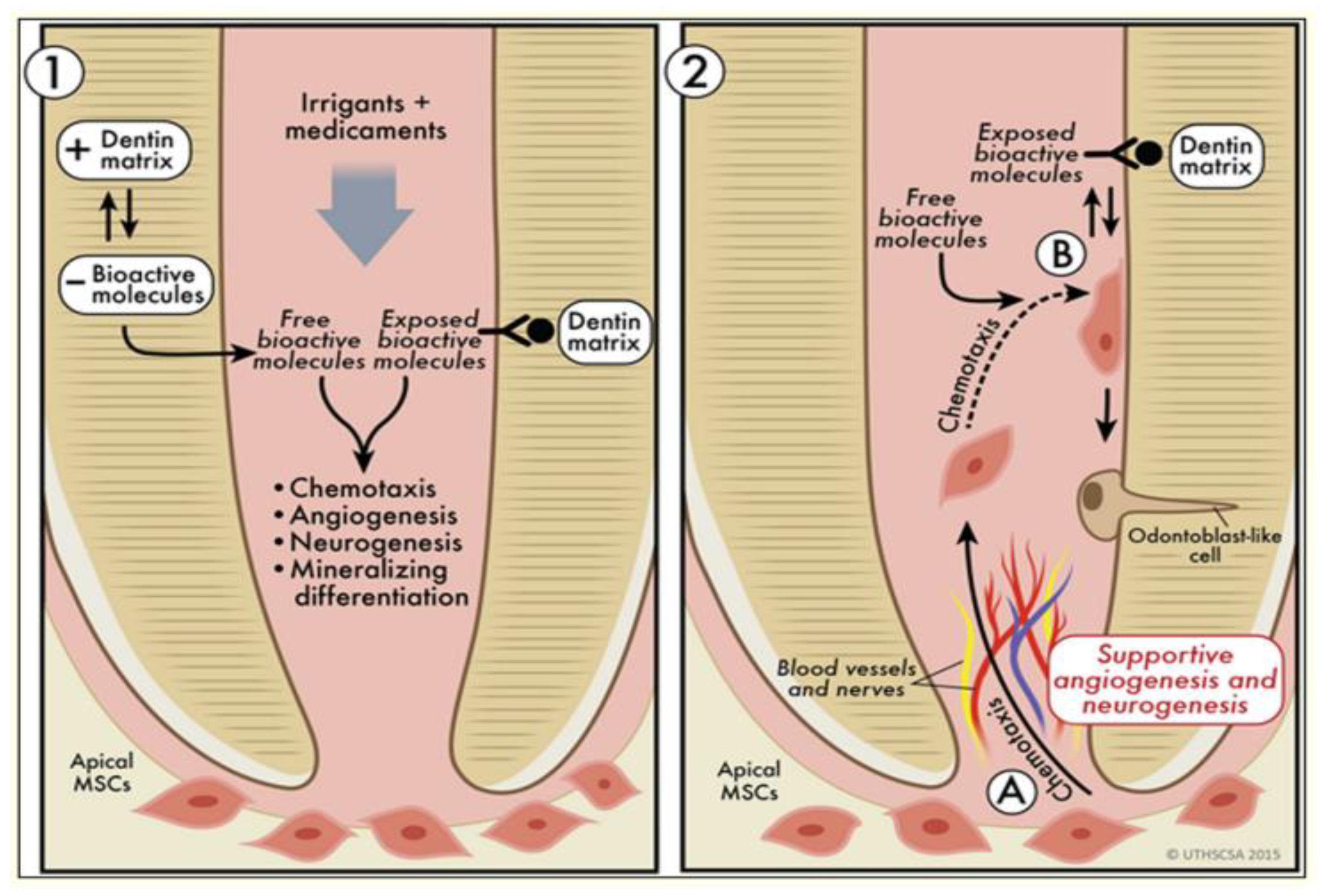
- Smith, A.J.; Duncan, H.F.; Diogenes, A.; Simon, S.; Cooper, P.R. Exploiting the bioactive properties of the dentin-pulp complex in regenerative endodontics. J. Endod. 2016, 42, 47–56. [Google Scholar] [CrossRef]
- Donnaloja, F.; Jacchetti, E.; Soncini, M.; Raimondi, M.T. Natural and synthetic polymers for bone scaffolds optimization. Polymers 2020, 12, 905. [Google Scholar] [CrossRef]
- Watcharadulyarat, N.; Rattanatayarom, M.; Ruangsawasdi, N.; Hongdilokkul, N.; Patikarnmonthon, N. Dextran-Based Nanoparticles for Encapsulation of Ciprofloxacin. In Proceedings of the 21st International Union of Materials Research Societies, Chiang Mai, Thailand, 23–26 February 2021; IOP Publishing: Bristol, UK, 2022; Volume 2175, p. 012006. [Google Scholar]
- Paul, M.; Pramanik, S.D.; Sahoo, R.N.; Dey, Y.N.; Nayak, A.K. Dental delivery systems of antimicrobial drugs using chitosan, alginate, dextran, cellulose and other polysaccharides: A review. Int. J. Biol. Macromol. 2023, 247, 125808. [Google Scholar] [CrossRef]
- Murad, A.H. Immunomodulatory Biomaterials in Dental Pulp Regenera-tion: Towards Enhancing Endodontic Treatment Outcomes. Future Dent. Res. 2023, 1, 1–11. [Google Scholar] [CrossRef]
- Marei, M. Regenerative Dentistry; Morgan & Claypool Publishers: Rafael, CA, USA, 2010. [Google Scholar]
- Rad, R.M.; Atila, D.; Akgün, E.E.; Evis, Z.; Keskin, D.; Tezcaner, A. Evaluation of human dental pulp stem cells behavior on a novel nanobiocomposite scaffold prepared for regenerative endodontics. Mater. Sci. Eng. C 2019, 100, 928–948. [Google Scholar]
- Rohban, R.; Pieber, T.R. Mesenchymal stem and progenitor cells in regeneration: Tissue specificity and regenerative potential. Stem Cells Int. 2017, 2017, 5173732. [Google Scholar] [CrossRef] [PubMed]
- Al-Sharabi, N.; Xue, Y.; Fujio, M.; Ueda, M.; Gjerde, C.; Mustafa, K.; Fristad, I. Bone marrow stromal cell paracrine factors direct osteo/odontogenic differentiation of dental pulp cells. Tissue Eng. Part A 2014, 20, 3063–3072. [Google Scholar] [CrossRef]
- Noohi, P.; Abdekhodaie, M.J.; Nekoofar, M.H.; Galler, K.M.; Dummer, P.M. Advances in scaffolds used for pulp–dentine complex tissue engineering: A narrative review. Int. Endod. J. 2022, 55, 1277–1316. [Google Scholar] [CrossRef]
- Farjaminejad, S.; Shojaei, S.; Goodarzi, V.; Khonakdar, H.A.; Abdouss, M. Tuning properties of bio-rubbers and its nanocomposites with addition of succinic acid and ε-caprolactone monomers to poly (glycerol sebacic acid) as main platform for application in tissue engineering. Eur. Polym. J. 2021, 159, 110711. [Google Scholar] [CrossRef]
- Huang, X.; Li, Z.; Liu, A.; Liu, X.; Guo, H.; Wu, M.; Yang, X.; Han, B.; Xuan, K. Microenvironment influences odontogenic mesenchymal stem cells mediated dental pulp regeneration. Front. Physiol. 2021, 12, 656588. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.G.; Moore, E.; Thomas, A.; Johnson, J.A. Graphene-Based Materials in Dental Applications: Antibacterial, Biocompatible, and Bone Regenerative Properties. Int. J. Biomater. 2023, 2023, 8803283. [Google Scholar] [CrossRef]
- Mkhabela, V.J. Novel Bio-Nanocomposite Scaffolds for Tissue Engineering Application. Ph.D. Thesis, University of Johannesburg, Johannesburg, South Africa, 2015. [Google Scholar]
- Kim, D.S.; Kim, Y.S.; Bae, W.J.; Lee, H.J.; Chang, S.W.; Kim, W.S.; Kim, E.C. The role of SDF-1 and CXCR 4 on odontoblastic differentiation in human dental pulp cells. Int. Endod. J. 2014, 47, 534–541. [Google Scholar] [CrossRef]
- Farjaminejad, S.; Farjaminejad, R.; Hasani, M.; Garcia-Godoy, F.; Abdouss, M.; Marya, A.; Harsoputranto, A.; Jamilian, A. Advances and Challenges in Polymer-Based Scaffolds for Bone Tissue Engineering: A Path Towards Personalized Regenerative Medicine. Polymers 2024, 16, 3303. [Google Scholar] [CrossRef]
- Riaz, A.; Shah, F.A. Regenerating the pulp–dentine complex using autologous platelet concentrates: A critical appraisal of the current histological evidence. Tissue Eng. Regen. Med. 2021, 18, 37–48. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Shi, S. Interplay between mesenchymal stem cells and lymphocytes: Implications for immunotherapy and tissue regeneration. J. Dent. Res. 2012, 91, 1003–1010. [Google Scholar] [CrossRef]
- Ulusoy, A.T.; Turedi, I.; Cimen, M.; Cehreli, Z.C. Evaluation of blood clot, platelet-rich plasma, platelet-rich fibrin, and platelet pellet as scaffolds in regenerative endodontic treatment: A prospective randomized trial. J. Endod. 2019, 45, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiar, H.; Esmaeili, S.; Tabatabayi, S.F.; Ellini, M.R.; Nekoofar, M.H.; Dummer, P.M. Second-generation platelet concentrate (platelet-rich fibrin) as a scaffold in regenerative endodontics: A case series. J. Endod. 2017, 43, 401–408. [Google Scholar] [CrossRef]
- Song, J.S.; Takimoto, K.; Jeon, M.; Vadakekalam, J.; Ruparel, N.B.; Diogenes, A. Decellularized human dental pulp as a scaffold for regenerative endodontics. J. Dent. Res. 2017, 96, 640–646. [Google Scholar] [CrossRef]
- Bakhtiar, H.; Rajabi, S.; Pezeshki-Modaress, M.; Ellini, M.R.; Panahinia, M.; Alijani, S.; Mazidi, A.; Kamali, A.; Azarpazhooh, A.; Kishen, A. Optimizing methods for bovine dental pulp decellularization. J. Endod. 2021, 47, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Sumita, Y.; Honda, M.J.; Ohara, T.; Tsuchiya, S.; Sagara, H.; Kagami, H.; Ueda, M. Performance of collagen sponge as a 3-D scaffold for tooth-tissue engineering. Biomaterials 2006, 27, 3238–3248. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Liu, H.; Peng, C. Clinical and radiographic assessment of the efficacy of a collagen membrane in regenerative endodontics: A randomized, controlled clinical trial. J. Endod. 2017, 43, 1465–1471. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef]
- Bakopoulou, A.; Georgopoulou, A.; Grivas, I.; Bekiari, C.; Prymak, O.; Loza, Κ.; Epple, M.; Papadopoulos, G.C.; Koidis, P.; Chatzinikolaidou, Μ. Dental pulp stem cells in chitosan/gelatin scaffolds for enhanced orofacial bone regeneration. Dent. Mater. 2019, 35, 310–327. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Lambricht, L.; De Berdt, P.; Vanacker, J.; Leprince, J.; Diogenes, A.; Goldansaz, H.; Bouzin, C.; Préat, V.; Dupont-Gillain, C.; Des Rieux, A. The type and composition of alginate and hyaluronic-based hydrogels influence the viability of stem cells of the apical papilla. Dent. Mater. 2014, 30, e349–e361. [Google Scholar] [CrossRef] [PubMed]
- Virlan, M.J.R.; Calenic, B.; Zaharia, C.; Greabu, M. Silk fibroin and potential uses in regenerative dentistry—A systematic review. Stomatol. Edu J. 2015, 1, 108–114. [Google Scholar] [CrossRef]
- Narayanam, R.; Cardoso, L.M.; dos Reis-Prado, A.H.; de Carvalho, A.B.G.; Anselmi, C.; Mahmoud, A.H.; Fenno, J.C.; Dal-Fabbro, R.; Bottino, M.C. Antimicrobial Silk Fibroin Methacrylated Scaffolds for Regenerative Endodontics. J. Endod. 2024, 50, 1752–1760.e2. [Google Scholar] [CrossRef]
- AlHowaish, N.A.; AlSudani, D.I.; AlMuraikhi, N.A. Evaluation of a hyaluronic acid hydrogel (Restylane Lyft) as a scaffold for dental pulp regeneration in a regenerative endodontic organotype model. Odontology 2022, 110, 726–734. [Google Scholar] [CrossRef]
- Casale, M.; Moffa, A.; Vella, P.; Sabatino, L.; Capuano, F.; Salvinelli, B.; Lopez, M.A.; Carinci, F.; Salvinelli, F. Hyaluronic acid: Perspectives in dentistry. A systematic review. Int. J. Immunopathol. Pharmacol. 2016, 29, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Flores, H.; Nabeshima, C.K.; Sarra, G.; Moreira, M.S.; Arana-Chavez, V.E.; Marques, M.M.; de Lima Machado, M.E. Development and characterization of a new chitosan-based scaffold associated with gelatin, microparticulate dentin and genipin for endodontic regeneration. Dent. Mater. 2021, 37, e414–e425. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Sanz, C.K.; Münchow, E.A.; Kalra, N.; Dubey, N.; Suárez, C.E.C.; Fenno, J.C.; Lund, R.G.; Bottino, M.C. Photocrosslinkable methacrylated gelatin hydrogel as a cell-friendly injectable delivery system for chlorhexidine in regenerative endodontics. Dent. Mater. 2022, 38, 1507–1517. [Google Scholar] [CrossRef]
- Alghofaily, M.; Almana, A.; Alrayes, J.; Lambarte, R.; Weir, M.D.; Alsalleeh, F. Chitosan–Gelatin Scaffolds Loaded with Different Antibiotic Formulations for Regenerative Endodontic Procedures Promote Biocompatibility and Antibacterial Activity. J. Funct. Biomater. 2024, 15, 186. [Google Scholar] [CrossRef]
- Heggendorn, F.L.; Nascimento, M.B.D.; Lima, A.M.; Ribeiro, A.A. Demineralized dentin matrix technique-a comparison of different demineralizing solutions. Braz. Dent. J. 2023, 34, 72–84. [Google Scholar] [CrossRef]
- Gao, X.; Qin, W.; Wang, P.; Wang, L.; Weir, M.D.; Reynolds, M.A.; Zhao, L.; Lin, Z.; Xu, H.H. Nano-structured demineralized human dentin matrix to enhance bone and dental repair and regeneration. Appl. Sci. 2019, 9, 1013. [Google Scholar] [CrossRef]
- Sanz, J.L.; Rodriguez-Lozano, F.J.; Lopez-Gines, C.; Monleon, D.; Llena, C.; Forner, L. Dental stem cell signaling pathway activation in response to hydraulic calcium silicate-based endodontic cements: A systematic review of in vitro studies. Dent. Mater. 2021, 37, e256–e268. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Atmeh, A.; Li, X.; Meschi, N. Present status and future directions: Hydraulic materials for endodontic use. Int. Endod. J. 2022, 55, 710–777. [Google Scholar] [CrossRef] [PubMed]
- Demirkaya, K.; Can Demirdöğen, B.; Öncel Torun, Z.; Erdem, O.; Tunca, Y.M. The effects of hydraulic calcium silicate containing endodontic materials on oxidative stress in erythrocytes and liver. Turk. J. Biochem. 2018, 43, 333–341. [Google Scholar] [CrossRef]
- Dobrzańska, J.; Gołombek, K.; Dobrzański, L.B. Polymer materials used in endodontic treatment-in vitro testing. Arch. Mater. Sci. Eng. 2012, 58, 110–115. [Google Scholar]
- Zein, N.; Harmouch, E.; Lutz, J.C.; Fernandez De Grado, G.; Kuchler-Bopp, S.; Clauss, F.; Offner, D.; Hua, G.; Benkirane-Jessel, N.; Fioretti, F. Polymer-based instructive scaffolds for endodontic regeneration. Materials 2019, 12, 2347. [Google Scholar] [CrossRef]
- Aksel, H.; Mahjour, F.; Bosaid, F.; Calamak, S.; Azim, A.A. Antimicrobial activity and biocompatibility of antibiotic-loaded chitosan hydrogels as a potential scaffold in regenerative endodontic treatment. J. Endod. 2020, 46, 1867–1875. [Google Scholar] [CrossRef]
- Duncan, H.F.; Cooper, P.R. The bioactive properties of dentine and molecular advances in pulp regeneration. In Endodontic Advances and Evidence-Based Clinical Guidelines; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 51–73. [Google Scholar]
- Brizuela, C.; Huang, G.T.J.; Diogenes, A.; Botero, T.; Khoury, M. The four pillars for successful regenerative therapy in endodontics: Stem cells, biomaterials, growth factors, and their synergistic interactions. Stem Cells Int. 2022, 2022, 1580842. [Google Scholar] [CrossRef]
- Zeng, Q.; Nguyen, S.; Zhang, H.; Chebrolu, H.P.; Alzebdeh, D.; Badi, M.A.; Kim, J.R.; Ling, J.; Yang, M. Release of growth factors into root canal by irrigations in regenerative endodontics. J. Endod. 2016, 42, 1760–1766. [Google Scholar] [CrossRef]
- Mandracci, P.; Mussano, F.; Rivolo, P.; Carossa, S. Surface treatments and functional coatings for biocompatibility improvement and bacterial adhesion reduction in dental implantology. Coatings 2016, 6, 7. [Google Scholar] [CrossRef]
- Nakashima, M. Bone morphogenetic proteins in dentin regeneration for potential use in endodontic therapy. Cytokine Growth Factor Rev. 2005, 16, 369–376. [Google Scholar] [CrossRef]
- Huang, G.J.; Garcia-Godoy, F. Missing concepts in de novo pulp regeneration. J. Dent. Res. 2014, 93, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, N.; Fahey, M.; Prime, S.S.; Smith, A.J. Comparative analysis of transforming growth factor-beta isoforms 1-3 in human and rabbit dentine matrices. Arch. Oral Biol. 1997, 42, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Buchalla, W.; Hiller, K.A.; Federlin, M.; Eidt, A.; Schiefersteiner, M.; Schmalz, G. Influence of root canal disinfectants on growth factor release from dentin. J. Endod. 2015, 41, 363–368. [Google Scholar] [CrossRef]
- Smith, A.J.; Lesot, H. Induction and regulation of crown dentinogenesis: Embryonic events as a template for dental tissue repair? Crit. Rev. Oral Biol. Med. 2001, 12, 425–437. [Google Scholar] [CrossRef]
- Thesleff, I.; Vaahtokari, A.; Partanen, A.M. Regulation of organogenesis: Common molecular mechanisms regulating the development of teeth and other organs. Int. J. Dev. Biol. 1995, 39, 35–50. [Google Scholar] [PubMed]
- Liu, J.; Jin, T.; Chang, S.; Ritchie, H.H.; Smith, A.J.; Clarkson, B.H. Matrix and TGF-beta-related gene expression during human dental pulp stem cell (DPSC) mineralization. Vitr. Cell. Dev. Biol.-Anim. 2007, 43, 120–128. [Google Scholar] [CrossRef]
- Huojia, M.; Muraoka, N.; Yoshizaki, K.; Fukumoto, S.; Nakashima, M.; Akamine, A.; Nonaka, K.; Ohishi, M. TGF-beta3 induces ectopic mineralization in fetal mouse dental pulp during tooth germ development. Dev. Growth Differ. 2005, 47, 141–152. [Google Scholar] [CrossRef]
- Sloan, A.J.; Smith, A.J. Stimulation of the dentine-pulp complex of rat incisor teeth by transforming growth factor-beta isoforms 1–3 in vitro. Arch. Oral Biol. 1999, 44, 149–156. [Google Scholar] [CrossRef]
- Thomadakis, G.; Ramoshebi, L.N.; Crooks, J.; Rueger, D.C.; Ripamonti, U. Immunolocalization of bone morphogenetic protein-2 and -3 and osteogenic protein-1 during murine tooth root morphogenesis and in other craniofacial structures. Eur. J. Oral Sci. 1999, 107, 368–377. [Google Scholar] [CrossRef]
- Iohara, K.; Nakashima, M.; Ito, M.; Ishikawa, M.; Nakasima, A.; Akamine, A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J. Dent. Res. 2004, 83, 590–595. [Google Scholar] [CrossRef]
- Chen, S.; Gluhak-Heinrich, J.; Martinez, M.; Li, T.; Wu, Y.; Chuang, H.H.; Chen, L.; Dong, J.; Gay, I.; MacDougall, M. Bone morphogenetic protein 2 mediates dentin sialophosphoprotein expression and odontoblast differentiation via NF-Y signaling. J. Biol. Chem. 2008, 283, 19359–19370. [Google Scholar] [CrossRef] [PubMed]
- About, I.; Laurent-Maquin, D.; Lendahl, U.; Mitsiadis, T.A. Nestin expression in embryonic and adult human teeth under normal and pathological conditions. Am. J. Pathol. 2000, 157, 287–295. [Google Scholar] [CrossRef]
- Helder, M.N.; Karg, H.; Bervoets, T.J.M.; Vukicevic, S.; Burger, E.H.; D’souza, R.N.; Wöltgens, J.H.M.; Karsenty, G.; Bronckers, A.L.J.J. Bone morphogenetic protein-7 (osteogenic protein-1, OP-1) and tooth development. J. Dent. Res. 1998, 77, 545–554. [Google Scholar] [CrossRef]
- Suzuki, T.; Lee, C.H.; Chen, M.; Zhao, W.; Fu, S.Y.; Qi, J.J.; Chotkowski, G.; Eisig, S.B.; Wong, A.; Mao, J.J. Induced migration of dental pulp stem cells for in vivo pulp regeneration. J. Dent. Res. 2011, 90, 1013–1018. [Google Scholar] [CrossRef]
- Kim, K.; Lee, C.H.; Kim, B.K.; Mao, J.J. Anatomically shaped tooth and periodontal regeneration by cell homing. J. Dent. Res. 2010, 89, 842–847. [Google Scholar] [CrossRef]
- Finkelman, R.D.; Mohan, S.; Jennings, J.C.; Taylor, A.K.; Jepsen, S.; Baylink, D.J. Quantitation of growth factors IGF-I, SGF/IGF-II, and TGF-beta in human dentin. J. Bone Miner. Res. 1990, 5, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Duncan, H.F.; Smith, A.J.; Fleming, G.J.P.; Reid, C.; Smith, G.; Cooper, P.R. Release of bio-active dentine extracellular matrix components by histone deacetylase inhibitors (HDACi). Int. Endod. J. 2015; in press. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Huang, D.; Lu, X.; Feng, G.; Xing, J.; Lu, J.; Xu, K.; Xia, W.; Meng, Y.; Tao, T.; et al. Insulin-like growth factor 1 can promote proliferation and osteogenic differentiation of human dental pulp stem cells via mTOR pathway. Dev. Growth Differ. 2014, 56, 615–624. [Google Scholar] [CrossRef]
- Wang, S.; Mu, J.; Fan, Z.; Yu, Y.; Yan, M.; Lei, G.; Tang, C.; Wang, Z.; Zheng, Y.; Yu, J.; et al. Insulin-like growth factor 1 can promote the osteogenic differentiation and osteogenesis of stem cells from apical papilla. Stem Cell Res. 2012, 8, 346–356. [Google Scholar] [CrossRef]
- Tomson, P.L.; Lumley, P.J.; Alexander, M.Y.; Smith, A.J.; Cooper, P.R. Hepatocyte growth factor is sequestered in dentine matrix and promotes regeneration-associated events in dental pulp cells. Cytokine 2013, 61, 622–629. [Google Scholar] [CrossRef]
- Forte, G.; Minieri, M.; Cossa, P.; Antenucci, D.; Sala, M.; Gnocchi, V.; Fiaccavento, R.; Carotenuto, F.; De Vito, P.; Baldini, P.M.; et al. Hepatocyte growth factor effects on mesenchymal stem cells: Proliferation, migration, and differentiation. Stem Cells 2006, 24, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Roberts-Clark, D.J.; Smith, A.J. Angiogenic growth factors in human dentine matrix. Arch. Oral Biol. 2000, 45, 1013–1016. [Google Scholar] [CrossRef]
- Musson, D.S.; McLachlan, J.L.; Sloan, A.J.; Smith, A.J.; Cooper, P.R. Adrenomedullin is expressed during rodent dental tissue development and promotes cell growth and mineralization. Biol. Cell 2010, 102, 145–157. [Google Scholar] [CrossRef]
- Tomson, P.L.; Grover, L.M.; Lumley, P.J.; Sloan, A.J.; Smith, A.J.; Cooper, P.R. Dissolution of bio-active dentine matrix components by mineral trioxide aggregate. J. Dent. 2007, 35, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Smith, A.J.; Berdal, A.; Lumley, P.J.; Cooper, P.R. The MAP kinase pathway is involved in odontoblast stimulation via p38 phosphorylation. J. Endod. 2010, 36, 256–259. [Google Scholar] [CrossRef]
- Morelli, T.; Neiva, R.; Nevins, M.L.; McGuire, M.K.; Scheyer, E.T.; Oh, T.J.; Braun, T.M.; Nör, J.E.; Bates, D.; Giannobile, W.V. Angiogenic biomarkers and healing of living cellular constructs. J. Dent. Res. 2011, 90, 456–462. [Google Scholar] [CrossRef]
- Fiedler, J.; Roderer, G.; Gunther, K.P.; Brenner, R.E. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J. Cell Biochem. 2002, 87, 305–312. [Google Scholar] [CrossRef]
- Yokose, S.; Kadokura, H.; Tajima, N.; Hasegawa, A.; Sakagami, H.; Fujieda, K.; Katayama, T. Platelet-derived growth factor exerts disparate effects on odontoblast differentiation depending on the dimers in rat dental pulp cells. Cell Tissue Res. 2004, 315, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Xin, X.; Moioli, E.K.; Chung, J.; Lee, C.H.; Chen, M.; Fu, S.Y.; Koch, P.D.; Mao, J.J. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng. Part A 2010, 16, 3023–3031. [Google Scholar] [CrossRef]
- Arthur, A.; Rychkov, G.; Shi, S.; Koblar, S.A.; Gronthos, S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells 2008, 26, 1787–1795. [Google Scholar] [CrossRef]
- Vanacker, J.; Viswanath, A.; De Berdt, P.; Everard, A.; Cani, P.D.; Bouzin, C.; Feron, O.; Diogenes, A.; Leprince, J.G.; des Rieux, A. Hypoxia modulates the differentiation potential of stem cells of the apical papilla. J. Endod. 2014, 40, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Kinnaird, T.; Stabile, E.; Burnett, M.S.; Shou, M.; Lee, C.W.; Barr, S.; Fuchs, S.; Epstein, S.E. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 2004, 109, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- McCoy, R.J.; Widaa, A.; Watters, K.M.; Wuerstle, M.; Stallings, R.L.; Duffy, G.P.; O’Brien, F.J. Orchestrating osteogenic differentiation of mesenchymal stem cells: Identification of placental growth factor as a mechano sensitive gene with a pro-osteogenic role. Stem Cells 2013, 31, 2420–2431. [Google Scholar] [CrossRef]
- de Almeida, J.F.; Chen, P.; Henry, M.A.; Diogenes, A. Stem cells of the apical papilla regulate trigeminal neurite outgrowth and targeting through a BDNF-dependent mechanism. Tissue Eng. Part A 2014, 20, 3089–3100. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, L.M.; Ee, X.; Iyer, N.; Hunter, D.; Mackinnon, S.E.; Wood, M.D.; Sakiyama-Elbert, S.E. Finely tuned temporal and spatial delivery of GDNF promotes enhanced nerve regeneration in a long nerve defect model. Tissue Eng. Part A 2015, 21, 2852–2864. [Google Scholar] [CrossRef]
- Gale, Z.; Cooper, P.R.; Scheven, B.A. Effects of glial cell line-derived neurotrophic factor on dental pulp cells. J. Dent. Res. 2011, 90, 1240–1245. [Google Scholar] [CrossRef]
- Müller, A.S.; Artner, M.; Janjić, K.; Edelmayer, M.; Kurzmann, C.; Moritz, A.; Agis, H. Synthetic Clay–based Hypoxia Mimetic Hydrogel for Pulp Regeneration: The Impact on Cell Activity and Release Kinetics Based on Dental Pulp–derived Cells In Vitro. J. Endod. 2018, 44, 1263–1269. [Google Scholar] [CrossRef]
- Zhang, R.; Xie, L.; Wu, H.; Yang, T.; Zhang, Q.; Tian, Y.; Liu, Y.; Han, X.; Guo, W.; He, M.; et al. Alginate/laponite hydrogel microspheres co-encapsulating dental pulp stem cells and VEGF for endodontic regeneration. Acta Biomater. 2020, 113, 305–316. [Google Scholar] [CrossRef]
- Wei, Y.; Lyu, P.; Bi, R.; Chen, X.; Yu, Y.; Li, Z.; Fan, Y. Neural regeneration in regenerative endodontic treatment: An overview and current trends. Int. J. Mol. Sci. 2022, 23, 15492. [Google Scholar] [CrossRef]




| Case Study | Patient Demographics | Tooth Condition | Procedure | Follow-Up and Outcomes | Ref |
|---|---|---|---|---|---|
| Case 1 | 11-year-old girl | Tooth #8, necrotic with closed apex | Apical revascularization with sodium hypochlorite, calcium hydroxide, MTA, and collagen membrane | 18-month follow-up; asymptomatic, with resolution of periapical radiolucency | [17] |
| Case 2 | 14-year-old female | Tooth #9, necrotic with closed apex | Apical revascularization with sodium hypochlorite, calcium hydroxide, MTA, and collagen membrane | 22-month follow-up; asymptomatic, with resolved periapical radiolucency | [17] |
| Case 3 | 23-year-old female | Teeth #7 and #8, necrotic with periapical lesions | Chemo-mechanical debridement, triple-antibiotic paste, and MTA over blood clot | 12-month follow-up; symptom reduction, with decreased periapical radiolucency size | [18] |
| Case 4 | 21-year-old male | Necrotic mature teeth, symptomatic apical periodontitis | Sodium hypochlorite irrigation, calcium hydroxide, MTA on collagen membrane, and evoked bleeding | 10-month follow-up; radiographic healing, asymptomatic | [19] |
| Case 5 | 9-year-old girl | Necrotic immature permanent central incisor with sinus tract | Regenerative treatment with Ca (OH)2, induced bleeding, and MTA over blood clot | 2.5-year follow-up; continued root development, root wall thickening, and apical closure | [20] |
| Case 6 | 11-year-old boy | Immature dens invaginatus with periapical periodontitis | Pulp revascularization with NaOCl irrigation, triple-antibiotic paste, and glass ionomer cement | Complete healing, apex closure, and root wall thickening | [21] |
| Case 7 | 9-year-old boy | Immature mandibular molar with apical periodontitis | Revascularization with platelet-rich plasma and blood clot | Successful apical healing and tissue regeneration | [22] |
| Case 8 | 12-year-old boy | Immature necrotic tooth with periapical radiolucency | Revascularization with triple-antibiotic paste and MTA over blood clot | 24-month follow-up; apex closure and root wall thickening | [23] |
| Case 9 | 8-year-old boy | Necrotic immature permanent tooth with apical abscess | Revascularization with triple-antibiotic paste, induced bleeding, and MTA seal | 11-month follow-up; complete apexogenesis and healing | [24] |
| Case 10 | 15-year-old boy | Nonvital immature anterior tooth with periapical lesion | Revascularization with PRP and collagen sponge | 12-month follow-up; apical closure and root elongation | [25] |
| Case 11 | 14-year-old female | Immature maxillary central incisors, necrotic | Regenerative endodontic treatment with triple-antibiotic paste, induced bleeding, and MTA seal | 6-year follow-up; root functionality, healed apices, and discoloration issues | [26] |
| Case 12 | 12-year-old boy | Teeth #8 and #9, necrotic pulp, apical periodontitis | PRF-based regenerative endodontic procedure; triple-antibiotic paste, Bio dentine, glass ionomer, and PRF scaffold | 30-month follow-up; arrested external root resorption (ERR), apical closure, and asymptomatic state | [27] |
| Case 13 | 12-year-old girl | Mandibular left second premolar with chronic abscess and incomplete root development | Sodium hypochlorite irrigation, tri-antibiotic paste, blood clot scaffold, MTA, and glass ionomer cement | 18-month follow-up; resolution of periapical radiolucency, root maturation, and asymptomatic tooth | [28] |
| Case 14 | 9-year-old girl | Immature, traumatized maxillary central incisor with sinus tract | Minimal instrumentation, sodium hypochlorite irrigation, calcium hydroxide paste, induced bleeding, and MTA placement | 2.5-year follow-up; progressive thickening of root walls, apical closure, asymptomatic condition, and sinus tract healing | [29] |
| Case 15 | 12-year-old boy | Tooth #8, post-trauma, symptomatic apical periodontitis with ERR | Triple-antibiotic paste, induced bleeding, PRF scaffold, glass ionomer, and Biodentine | 24-month follow-up; significant healing, apical closure, arrested ERR, and asymptomatic condition | [30] |
| Technique | Description | Advantages | Disadvantages | Ref |
|---|---|---|---|---|
| Root canal revascularization | Opening the tooth apex up to 1 mm to allow blood flow into the root canals | Low chance of immune system rejection; reduced risk of transmitting pathogens. | Few documented cases; risk of tissue death if reinfection occurs. | [32] |
| Stem cell therapy | Injecting autologous or allogenic stem cells or cell mixtures into the tooth via a matrix | Quick; Straightforward delivery with minimal discomfort; cells are easy to obtain. | Low survival rate for the cells; does not generate fully functional pulp; high complication risk. | [33,34] |
| Pulp implant | Cultivating pulp tissue in sheets and surgically implanting it | Cell sheets are relatively easy to grow in the lab; more stable than injecting individual cells. | Limited size due to lack of blood flow; requires precise adaptation to root canal shape. | [35,36] |
| Scaffold implant | Seeding pulp cells on a 3D scaffold for surgical implantation | Provides a framework for cell structure Some materials may support new blood vessel formation | Low cell viability post-implantation must fit accurately within the root canal | [37,38] |
| 3D cell printing | Using inkjet-style devices to place cell layers in a hydrogel for surgical placement | Enables precise positioning of different types of cells | Needs exact fit for the root canal; effectiveness in living organisms remains unproven in early research | [39,40] |
| Injectable scaffolds | Delivery of hydrogels or cell-laden hydrogels via injection | Simple to apply; may act as a scaffold substitute to support tissue regeneration | Limited control over the tissue development process; low cell viability; effectiveness not yet validated in early trials | [41,42] |
| Gene therapy | Transferring mineralizing genes to vital pulp cells of necrotic or symptomatic teeth | Potentially eliminates the need for traditional root canal procedures and might reduce the need for stem cell transplants | Many cells in damaged teeth are nonviable; challenging to control; potential health risks; lacks FDA approval | [32,43] |
| Type of Cells | Benefits/ Properties | Considerations/ Challenges | Sources | Potential Applications | Immune Response | Ref |
|---|---|---|---|---|---|---|
| Autologous cells (host’s own cells) |
|
|
|
|
| [31,103,104] |
| Allogenic cells (donor cells) |
|
|
|
|
| [104,105,106] |
| Xenogenic cells (cells from different species) |
|
|
|
|
| [31,67,104] |
| Growth Factor | Primary Source | Regenerative Function | Ref |
|---|---|---|---|
| TGF-β1 | Dentin matrix-activated TH1 cells NK cells | Promotes the initial differentiation of odontoblasts and supports the formation of tertiary dentin. | [31,176,177,178,179,180] |
| TGF-β2 | Platelets Macrophages Bone | Enhances the differentiation of DPSCs into cells capable of mineralizing dentin. | [31,176,177,181] |
| TGF-β3 | Platelets Macrophages Bone | Stimulates the differentiation of odontoblasts, aiding in dentin formation. | [31,176,182,183] |
| BMP-2 | Bone Cartilage | Stimulates odontoblast differentiation in both laboratory and animal models and enhances alkaline phosphatase activity and DSPP induction. | [176,184,185,186] |
| BMP-4 | Bone Cartilage | Promotes odontoblast differentiation and dentin matrix formation. | [176,184,187] |
| BMP-7 | Bone tissue Kidneys | Encourages the mineralization of DPSCs, enhancing their ability to form hard tissue. | [176,188,189,190] |
| IGF-1 | Liver Local tissues | Promotes the growth and mineralizing differentiation of DPSCs and SCAP. | [176,191,192,193,194] |
| Hepatocyte Growth Factor | Liver Released during tissue injury | Facilitates the migration, proliferation, and survival of MSCs in the dental pulp. | [176,195,196] |
| VEGF | Cells in hypoxic conditions | Induces the formation of new blood vessels, promoting healing and tissue regeneration in dental tissues. | [176,178,197] |
| Adrenomedullin | Bone marrow Injured tissues | Supports odontoblastic differentiation through signaling pathways that activate p38. | [176,198,199,200] |
| FGF-2 | Pituitary Adrenal glands | Promotes the migration and growth of stem cells, as well as the formation of blood vessels. | [176,197,201] |
| Platelet-Derived Growth Factor | Platelets Endothelial cells Placenta | Stimulates angiogenesis, enhances MSC migration, and modulates the process of odontoblastic differentiation. | [176,177,180,202,203,204] |
| Epidermal Growth Factor | Submaxillary glands Keratinocytes | Enhances the neurogenic differentiation of DPSCs and promotes healing of damaged tissues. | [31,176,197,205,206] |
| Placenta Growth Factor | Placenta | Facilitates the growth of blood vessels and supports the differentiation of MSCs into osteogenic cells. | [176,197,207,208] |
| Brain-Derived Neurotrophic Factor | Brain tissue Neurons | Promotes the survival and growth of neurons, encouraging their regeneration and axonal growth. | [192,209] |
| Glial Cell Line-Derived Neurotrophic Factor | Skeletal muscle Central nervous system | Stimulates nerve regeneration and supports the survival and proliferation of pulp cells during tissue repair. | [192,210,211,212] |
| Growth/Differentiation Factor 15 | Nerve tissue Various cell types | Supports the regeneration and maintenance of neuronal cells, playing a key role in post-injury recovery. | [192,213] |
| NGF | Secreted by neurons Target tissue | Essential for the survival and regeneration of neurons, promoting recovery after nerve injury. | [31] |
| CSF | A wide range of cells | Stimulates the proliferation of specific stem cells, supporting tissue repair and regeneration. | [31] |
| EGF | Submaxillary glands | Promotes the proliferation of various cell types, including epithelial, glial, and mesenchymal cells, aiding wound healing. | [31] |
| FGF | A wide range of cells | Encourages the proliferation of a variety of cell types, supporting tissue repair and regeneration. | [31] |
| IGF | Liver Variety of cells | Promotes cell growth and differentiation across various tissues, supporting overall tissue regeneration. | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farjaminejad, R.; Farjaminejad, S.; Garcia-Godoy, F. Regenerative Endodontic Therapies: Harnessing Stem Cells, Scaffolds, and Growth Factors. Polymers 2025, 17, 1475. https://doi.org/10.3390/polym17111475
Farjaminejad R, Farjaminejad S, Garcia-Godoy F. Regenerative Endodontic Therapies: Harnessing Stem Cells, Scaffolds, and Growth Factors. Polymers. 2025; 17(11):1475. https://doi.org/10.3390/polym17111475
Chicago/Turabian StyleFarjaminejad, Rosana, Samira Farjaminejad, and Franklin Garcia-Godoy. 2025. "Regenerative Endodontic Therapies: Harnessing Stem Cells, Scaffolds, and Growth Factors" Polymers 17, no. 11: 1475. https://doi.org/10.3390/polym17111475
APA StyleFarjaminejad, R., Farjaminejad, S., & Garcia-Godoy, F. (2025). Regenerative Endodontic Therapies: Harnessing Stem Cells, Scaffolds, and Growth Factors. Polymers, 17(11), 1475. https://doi.org/10.3390/polym17111475







