Sucrose-Based Screening of a Novel Strain, Limimaricola sp. YI8, and Its Application to Polyhydroxybutyrate Production from Molasses
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Bacterial Strain and Culture Conditions for P(3HB) Synthesis
2.3. Bacterial Isolation and Plate Assay for Identifying P(3HB)-Producing Strain
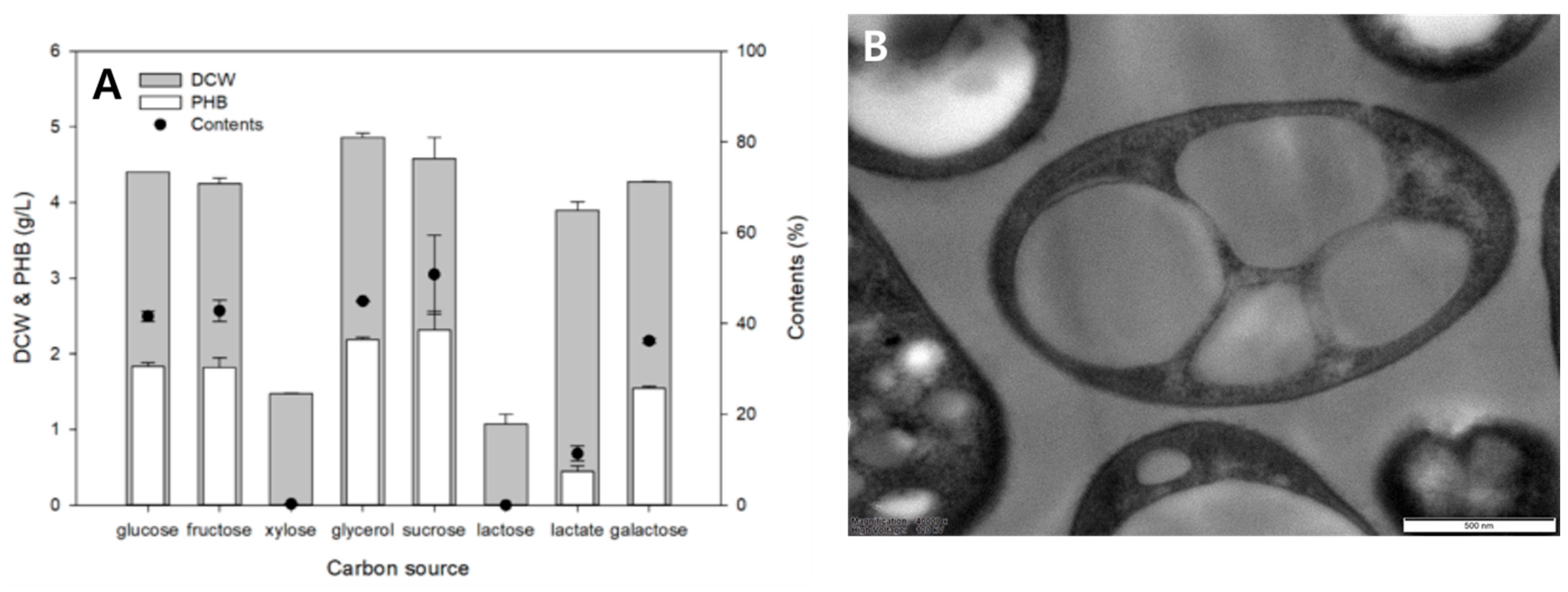
2.4. Evaluation of Carbon Sources Utilization for P(3HB) Production
2.5. Transmission Electron Microscopy Analysis
2.6. Statistical Analysis
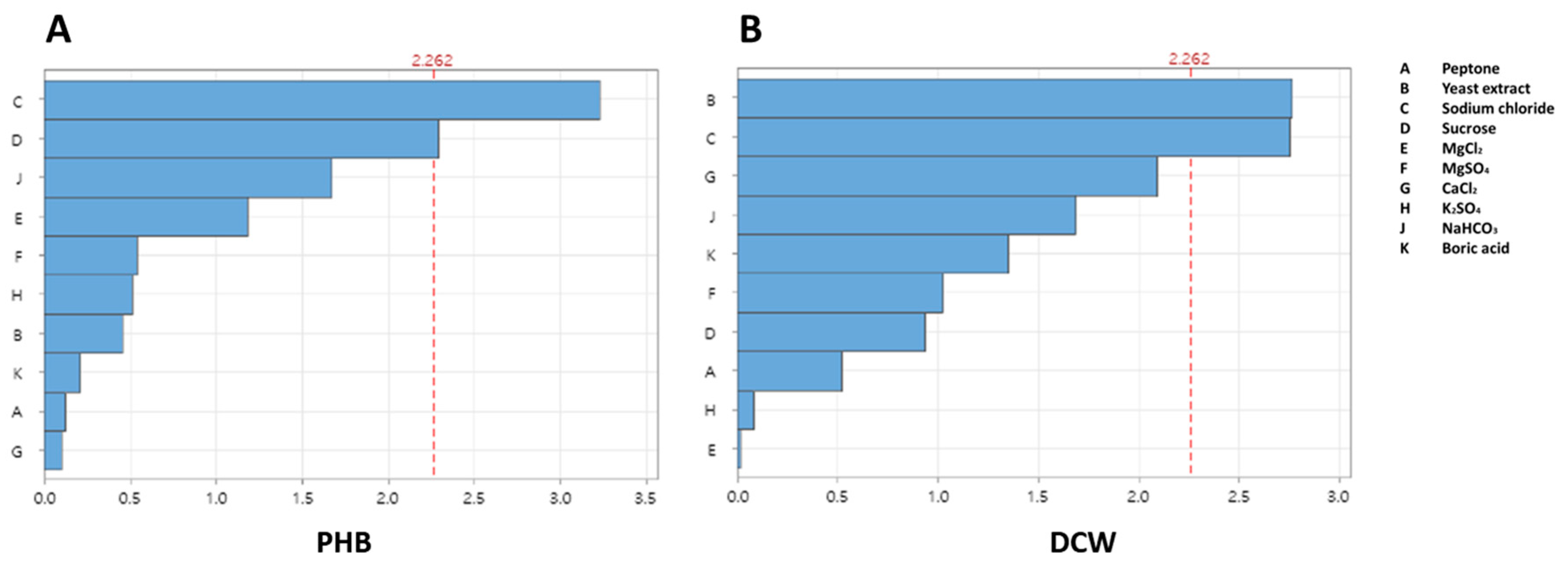
2.7. Screening of Essential Medium Components Using the Plackett–Burman Design
2.8. Box-Behnken Design and Response Surface Methodology
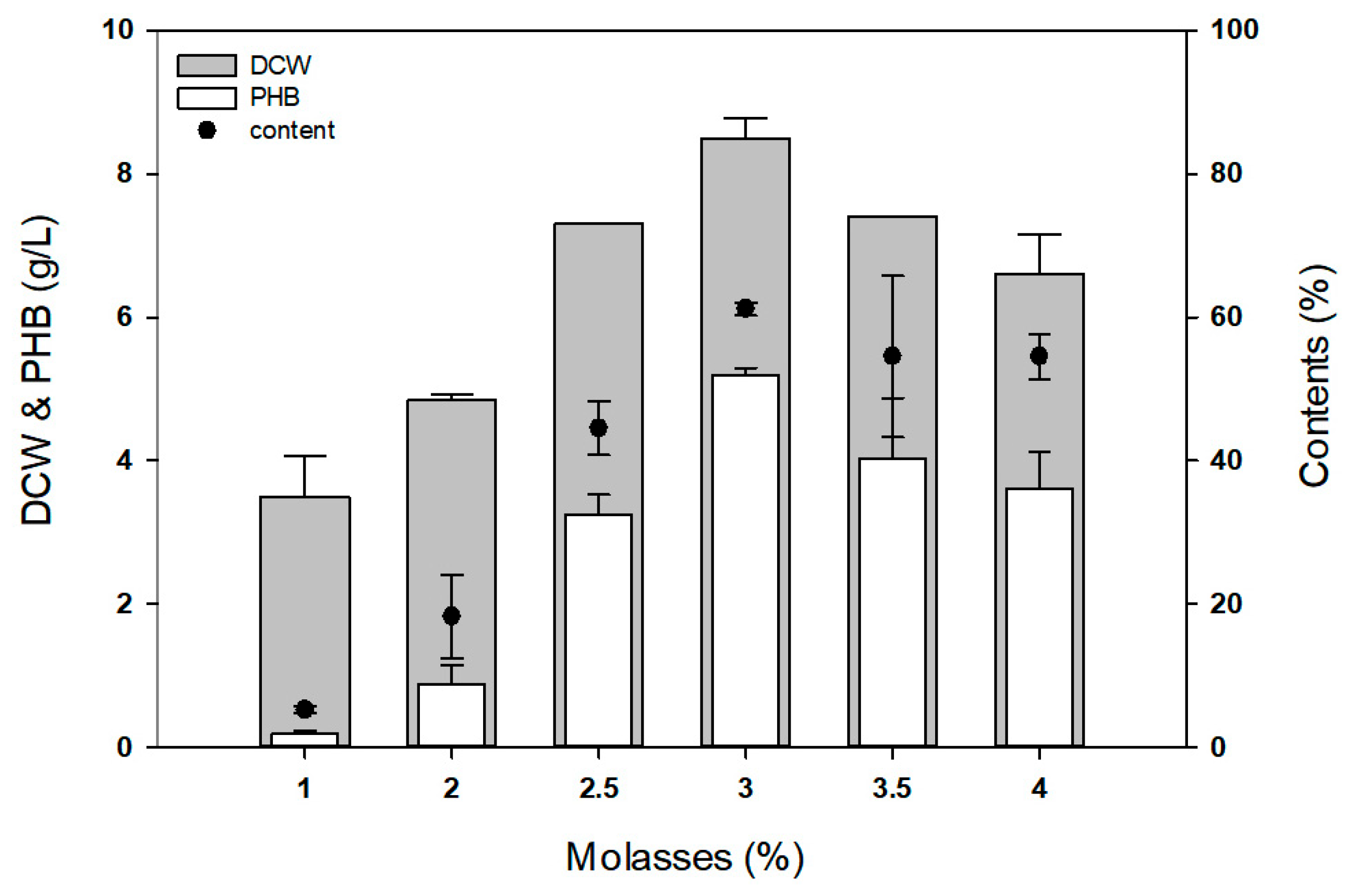
2.9. P(3HB) Production Using Molasses
2.10. Analytical Methods
2.10.1. HPLC
2.10.2. GC
2.11. P(3HB) Extraction and Characterization
2.12. Identification of the Mechanical and Thermal Properties
3. Results and Discussion
3.1. Screening and Isolation of the P(3HB)-Producing Strain Limimaricola sp. YI8
3.2. Screening of Key Medium Components Using the Plackett–Burman Design
3.3. Optimization of Medium Components Using the Box–Behnken Design
3.4. Examination of P(3HB) Production Using Molasses as a Carbon Source
3.5. P(3HB) Film Preparation from Limimaricola sp. YI8 Cultivation
3.6. Mechanical and Physical Properties of P(3HB) Films Obtained from Limimaricola sp. YI8
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| P(3HB) | Poly(3-hydroxybutyrate) |
| DSC | Differential scanning calorimetry |
| UTM | Universal testing machine |
| GC | Gas chromatography |
| GPC | Gel permeation chromatography |
| DCW | Dry cell weight |
| TEM | Transmission electron microscopy |
| PDI | Polydispersity index |
| FID | Flame ionization detector |
References
- Briassoulis, D.; Tserotas, P.; Athanasoulia, I.G. Alternative Optimization Routes for Improving the Performance of Poly(3-Hydroxybutyrate) (PHB) Based Plastics. J. Clean. Prod. 2021, 318, 128555. [Google Scholar] [CrossRef]
- Kim, B.; Oh, S.J.; Hwang, J.H.; Kim, H.J.; Shin, N.; Bhatia, S.K.; Jeon, J.M.; Yoon, J.J.; Yoo, J.; Ahn, J.; et al. Polyhydroxybutyrate Production from Crude Glycerol Using a Highly Robust Bacterial Strain Halomonas sp. YLGW01. Int. J. Biol. Macromol. 2023, 236, 123997. [Google Scholar] [CrossRef] [PubMed]
- de Mello, A.F.M.; Vandenberghe, L.P.d.S.; Machado, C.M.B.; Valladares-Diestra, K.K.; de Carvalho, J.C.; Soccol, C.R. Polyhydroxybutyrate Production by Cupriavidus necator in a Corn Biorefinery Concept. Bioresour. Technol. 2023, 370, 128537. [Google Scholar] [CrossRef]
- Khanna, S.; Srivastava, A.K. Statistical Media Optimization Studies for Growth and PHB Production by Ralstonia eutropha. Process. Biochem. 2005, 40, 2173–2182. [Google Scholar] [CrossRef]
- Jung, H.J.; Kim, S.H.; Cho, D.H.; Kim, B.C.; Bhatia, S.K.; Lee, J.; Jeon, J.M.; Yoon, J.J.; Yang, Y.H. Finding of Novel Galactose Utilizing Halomonas sp. YK44 for Polyhydroxybutyrate (PHB) Production. Polymers 2022, 14, 5407. [Google Scholar] [CrossRef] [PubMed]
- Fukui, T.; Chou, K.; Harada, K.; Orita, I.; Nakayama, Y.; Bamba, T.; Nakamura, S.; Fukusaki, E. Metabolite Profiles of Polyhydroxyalkanoate-Producing Ralstonia eutropha H16. Metabolomics 2014, 10, 190–202. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, H.J.; Kim, S.H.; Suh, M.J.; Cho, J.Y.; Ham, S.; Jeon, J.M.; Yoon, J.J.; Bhatia, S.K.; Gurav, R.; et al. Screening of the Strictly Xylose-Utilizing Bacillus sp. SM01 for Polyhydroxybutyrate and Its Co-Culture with Cupriavidus necator NCIMB 11599 for Enhanced Production of PHB. Int. J. Biol. Macromol. 2021, 181, 410–417. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Z.; Tsui, T.H.; Loh, K.C.; Dai, Y.; Tong, Y.W. A Review on Enhancing Cupriavidus necator Fermentation for Poly(3-Hydroxybutyrate) (PHB) Production From Low-Cost Carbon Sources. Front. Bioeng. Biotechnol. 2022, 10, 946085. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Yoon, J.J.; Kim, H.J.; Hong, J.W.; Gi Hong, Y.; Song, H.S.; Moon, Y.M.; Jeon, J.M.; Kim, Y.G.; Yang, Y.H. Engineering of Artificial Microbial Consortia of Ralstonia eutropha and Bacillus subtilis for Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Copolymer Production from Sugarcane Sugar without Precursor Feeding. Bioresour. Technol. 2018, 257, 92–101. [Google Scholar] [CrossRef]
- Sohn, Y.J.; Kim, H.T.; Baritugo, K.A.; Song, H.M.; Ryu, M.H.; Kang, K.H.; Jo, S.Y.; Kim, H.; Kim, Y.J.; Choi, J.; et al. Biosynthesis of Polyhydroxyalkanoates from Sucrose by Metabolically Engineered Escherichia coli Strains. Int. J. Biol. Macromol. 2020, 149, 593–599. [Google Scholar] [CrossRef]
- Arikawa, H.; Matsumoto, K.; Fujiki, T. Polyhydroxyalkanoate Production from Sucrose by Cupriavidus necator Strains Harboring Csc Genes from Escherichia coli W. Appl. Microbiol. Biotechnol. 2017, 101, 7497–7507. [Google Scholar] [CrossRef] [PubMed]
- Sahu, O. Assessment of Sugarcane Industry: Suitability for Production, Consumption, and Utilization. Ann. Agrar. Sci. 2018, 16, 389–395. [Google Scholar] [CrossRef]
- Teclu, D.; Tivchev, G.; Laing, M.; Wallis, M. Determination of the Elemental Composition of Molasses and Its Suitability as Carbon Source for Growth of Sulphate-Reducing Bacteria. J. Hazard. Mater. 2009, 161, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.; Nazir, Y.; Hameed, A.; Yang, W.; Mustafa, K.; Song, Y. Optimization of Diverse Carbon Sources and Cultivation Conditions for Enhanced Growth and Lipid and Medium-Chain Fatty Acid (MCFA) Production by Mucor circinelloides. Fermentation 2019, 5, 35. [Google Scholar] [CrossRef]
- El-Kadi, S.M.; Elbagory, M.; EL-Zawawy, H.A.H.; EL-Shaer, H.F.A.; Shoukry, A.A.; El-Nahrawy, S.; Omara, A.E.-D.; Ali, D.F.I. Biosynthesis of Poly-ß-Hydroxybutyrate (PHB) from Different Bacterial Strains Grown on Alternative Cheap Carbon Sources. Polymers 2021, 13, 3801. [Google Scholar] [CrossRef]
- Nascimento, V.M.; Silva, L.F.; Gomez, J.G.C.; Fonseca, G.G. Growth of Burkholderia sacchari LFM 101 Cultivated in Glucose, Sucrose and Glycerol at Different Temperatures. Sci. Agric. 2016, 73, 429–433. [Google Scholar] [CrossRef]
- Quillaguamán, J.; Muñoz, M.; Mattiasson, B.; Hatti-Kaul, R. Optimizing Conditions for Poly(β-Hydroxybutyrate) Production by Halomonas boliviensis LC1 in Batch Culture with Sucrose as Carbon, Source. Appl. Microbiol. Biotechnol. 2007, 74, 981–986. [Google Scholar] [CrossRef]
- Park, S.J.; Jang, Y.-A.; Noh, W.; Oh, Y.H.; Lee, H.; David, Y.; Baylon, M.G.; Shin, J.; Yang, J.E.; Choi, S.Y.; et al. Metabolic Engineering of Ralstonia eutropha for the Production of Polyhydroxyalkanoates from Sucrose. Biotechnol. Bioeng. 2015, 112, 638–643. [Google Scholar] [CrossRef]
- Schmid, M.; Raschbauer, M.; Song, H.; Bauer, C.; Neureiter, M. Effects of Nutrient and Oxygen Limitation, Salinity and Type of Salt on the Accumulation of Poly(3-Hydroxybutyrate) in Bacillus megaterium Uyuni S29 with Sucrose as a Carbon Source. N. Biotechnol. 2021, 61, 137–144. [Google Scholar] [CrossRef]
- Kulpreecha, S.; Boonruangthavorn, A.; Meksiriporn, B.; Thongchul, N. Inexpensive Fed-Batch Cultivation for High Poly(3-Hydroxybutyrate) Production by a New Isolate of Bacillus megaterium. J. Biosci. Bioeng. 2009, 107, 240–245. [Google Scholar] [CrossRef]
- Geethu, M.; Chandrashekar, H.R.; Divyashree, M.S. Statistical Optimisation of Polyhydroxyalkanoate Production in Bacillus Endophyticus Using Sucrose as Sole Source of Carbon. Arch. Microbiol. 2021, 203, 5993–6005. [Google Scholar] [CrossRef] [PubMed]
- Miranda De Sousa Dias, M.; Koller, M.; Puppi, D.; Morelli, A.; Chiellini, F.; Braunegg, G. Fed-Batch Synthesis of Poly(3-Hydroxybutyrate) and Poly(3-Hydroxybutyrate-Co-4-Hydroxybutyrate) from Sucrose and 4-Hydroxybutyrate Precursors by Burkholderia sacchari Strain DSM 17165. Bioengineering 2017, 4, 36. [Google Scholar] [CrossRef]
- Wang, T.; Wirth, J.S.; Whitman, W.B. Limimaricola. In Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2021; pp. 1–10. [Google Scholar]
- Ding, W.; Li, Y.; Chen, M.; Chen, R.; Tian, X.; Yin, H.; Zhang, S. Structures and Antitumor Activities of Ten New and Twenty Known Surfactins from the Deep-Sea Bacterium Limimaricola sp. SCSIO 53532. Bioorg. Chem. 2022, 120, 105589. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Brigham, C.J.; Song, E.; Jeon, J.-M.; Rha, C.K.; Sinskey, A.J. Biosynthesis of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Containing a Predominant Amount of 3-hydroxyvalerate by Engineered Escherichia coli Expressing Propionate-CoA Transferase. J. Appl. Microbiol. 2012, 113, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Shim, Y.H.; Jeon, J.M.; Brigham, C.J.; Kim, Y.H.; Kim, H.J.; Seo, H.M.; Lee, J.H.; Kim, J.H.; Yi, D.H.; et al. Starch Based Polyhydroxybutyrate Production in Engineered Escherichia coli. Bioprocess Biosyst. Eng. 2015, 38, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Cho, D.H.; Jung, H.J.; Kim, B.; Kim, S.H.; Bhatia, S.K.; Gurav, R.; Jeon, J.M.; Yoon, J.J.; Kim, W.; et al. Finding of Novel Polyhydroxybutyrate Producer Loktanella sp. SM43 Capable of Balanced Utilization of Glucose and Xylose from Lignocellulosic Biomass. Int. J. Biol. Macromol. 2022, 208, 809–818. [Google Scholar] [CrossRef]
- Jung, H.J.; Shin, Y.; Hwang, J.H.; Shin, N.; Kim, H.J.; Oh, S.J.; Choi, T.R.; Park, H.J.; Jung, J.H.; Bhatia, S.K.; et al. Establishment of an Optimized Electroporation Method for Halomonas sp. YK44 and Its Application in the Coproduction of PHB and Isobutanol. Biotechnol. Bioprocess Eng. 2024, 29, 339–351. [Google Scholar] [CrossRef]
- Jung, H.R.; Choi, T.R.; Han, Y.H.; Park, Y.L.; Park, J.Y.; Song, H.S.; Yang, S.Y.; Bhatia, S.K.; Gurav, R.; Park, H.A.; et al. Production of Blue-Colored Polyhydroxybutyrate (PHB) by One-Pot Production and Coextraction of Indigo and PHB from Recombinant Escherichia coli. Dye. Pigment. 2020, 173, 107889. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, S.H.; Jung, H.J.; Cho, D.H.; Kim, B.C.; Bhatia, S.K.; Ahn, J.; Jeon, J.M.; Yoon, J.J.; Lee, J.; et al. Finding a Benign Plasticizer to Enhance the Microbial Degradation of Polyhydroxybutyrate (PHB) Evaluated by PHB Degrader Microbulbifer sp. SOL66. Polymers 2022, 14, 3625. [Google Scholar] [CrossRef]
- Valappil, S.P.; Misra, S.K.; Boccaccini, A.R.; Keshavarz, T.; Bucke, C.; Roy, I. Large-Scale Production and Efficient Recovery of PHB with Desirable Material Properties, from the Newly Characterised Bacillus Cereus SPV. J. Biotechnol. 2007, 132, 251–258. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, T.R.; Jung, H.R.; Yang, S.Y.; Moon, Y.M.; Song, H.S.; Jeon, J.M.; Choi, K.Y.; Yang, Y.H. Bioconversion of Plant Biomass Hydrolysate into Bioplastic (Polyhydroxyalkanoates) Using Ralstonia eutropha 5119. Bioresour. Technol. 2019, 271, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Li, J.; Chen, Y.; Mai, Z.; Wang, L.; Li, Q.; Zhang, S. Degradation Potential of Alkanes by Diverse Oil-Degrading Bacteria from Deep-Sea Sediments of Haima Cold Seep Areas, South China Sea. Front. Microbiol. 2022, 13, 920067. [Google Scholar] [CrossRef]
- Park, Y.L.; Bhatia, S.K.; Gurav, R.; Choi, T.R.; Kim, H.J.; Song, H.S.; Park, J.Y.; Han, Y.H.; Lee, S.M.; Park, S.L.; et al. Fructose Based Hyper Production of Poly-3-Hydroxybutyrate from Halomonas sp. YLGW01 and Impact of Carbon Sources on Bacteria Morphologies. Int. J. Biol. Macromol. 2020, 154, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Râpă, M.; Popa, E.E.; Râpă, M.; Darie-Niţă, R.N.; Grosu, E.; Tănase, E.E.; Trifoi, A.R.; Pap, T.; Vasile, C. Effect of Plasticizers on Melt Processability and Properties of PHB. J. Optoelectron. Adv. Mater. 2015, 17, 1778–1784. [Google Scholar]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, Structure and Properties of Polyhydroxyalkanoates: Biological. Polyesters 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Melo, J.D.D.; Carvalho, L.F.M.; Medeiros, A.M.; Souto, C.R.O.; Paskocimas, C.A. A Biodegradable Composite Material Based on Polyhydroxybutyrate (PHB) and Carnauba Fibers. Compos. B Eng. 2012, 43, 2827–2835. [Google Scholar] [CrossRef]
- Saratale, G.D.; Oh, M.K. Characterization of Poly-3-Hydroxybutyrate (PHB) Produced from Ralstonia eutropha Using an Alkali-Pretreated Biomass Feedstock. Int. J. Biol. Macromol. 2015, 80, 627–635. [Google Scholar] [CrossRef]



| Strain | Culture | DCW (g/L) | P(3HB) (g/L) | P(3HB) Content (%) | Ref. |
|---|---|---|---|---|---|
| Reunions naejangsanensis BIO-TAS2-2 | Batch | 3.47 | 0.93 | 26.80 | [15] |
| Burkholderia sacchari LFM 101 | Batch | 2.6540.076 | 0.879 | 33.125.989 | [16] |
| Burkholderia sacchari DSM 17165 | Fed Batch | 70.0 | 36.8 | 53.0 | [17] |
| Halomonas boliviensis | Batch | 14 | 7.7 | 54.0 | [18] |
| Ralstonia eutropha 437-540 (pKM212-SacCReAB) | Batch | 0.590.01 | 0.340.01 | 56.00.7 | [19] |
| Bacillus megaterium uyuni S29 | Batch | 12.350.34 | − | 50 | [20] |
| Bacillus megaterium BA-019 | Batch | 7.05 | 3.8 | 55.46 | [21] |
| Bacillus endophyticus | Batch | 1.72 | 0.8 | 49.47 | [22] |
| Limimaricola sp. YI8 | Batch | 11.1 | 6.2 | 55.86 | This study |
| Strain or Plasmid | Description | Ref. |
|---|---|---|
| Bacterial strain | ||
| Limimaricola sp. YI8 | This study | |
| Escherichia coli KSYH(DE3) | ΔaraBAD, ΔrhaBAD, BW25113 (DE3) derivative | [25] |
| Cupriavidus necator H16 | Gram-negative, Widely used as a model organism for PHA biosynthesis studies | Laboratory stock |
| Plasmids | ||
| pLW487 | pEP2-based plasmid (SpecR) carrying bktB, phaB, and phaC from R. eutropha under the trc promoter. | [26] |
| Limimaricola sp. YI8 | E. coli KSYH::pLW487 | C. necator H16 | ||
|---|---|---|---|---|
| GPC | Mn (×106) | 1.09 ± 0.06 | 1.37 ± 0.02 | 1.27 ± 0.03 |
| Mw (×106) | 1.29 ± 0.05 | 1.58 ± 0.07 | 1.6 ± 0.03 | |
| PDI (Mw/Mn) | 1.24 ± 0.03 | 1.15 ± 0.04 | 1.28 ± 0.01 | |
| UTM | Tensile strength (MPa) | 32.5 | 36.4 | 22.1 |
| Elongation at break (%) | 3 | 7.2 | 12.9 | |
| Young’s modulus (MPa) | 1931.9 | 1337.6 | 701.7 |
| Strain | Tc (°C) | Tm (°C) |
|---|---|---|
| Limimaricola sp. YI8 | 106.2 | 172.2 |
| Escherichia coli KSYH::pLW487 | 112.4 | 175.9 |
| Cupriavidus necator H16 | 84.7 | 172.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Cho, D.; Shin, Y.; Han, Y.; Lim, G.; Jeon, J.; Yoon, J.; Joo, J.; Jeong, H.; Ahn, J.; et al. Sucrose-Based Screening of a Novel Strain, Limimaricola sp. YI8, and Its Application to Polyhydroxybutyrate Production from Molasses. Polymers 2025, 17, 1471. https://doi.org/10.3390/polym17111471
Lee Y, Cho D, Shin Y, Han Y, Lim G, Jeon J, Yoon J, Joo J, Jeong H, Ahn J, et al. Sucrose-Based Screening of a Novel Strain, Limimaricola sp. YI8, and Its Application to Polyhydroxybutyrate Production from Molasses. Polymers. 2025; 17(11):1471. https://doi.org/10.3390/polym17111471
Chicago/Turabian StyleLee, Yeda, Dohyun Cho, Yuni Shin, Yebin Han, Gaeun Lim, Jongmin Jeon, Jeongjun Yoon, Jeongchan Joo, Hwabong Jeong, Jungoh Ahn, and et al. 2025. "Sucrose-Based Screening of a Novel Strain, Limimaricola sp. YI8, and Its Application to Polyhydroxybutyrate Production from Molasses" Polymers 17, no. 11: 1471. https://doi.org/10.3390/polym17111471
APA StyleLee, Y., Cho, D., Shin, Y., Han, Y., Lim, G., Jeon, J., Yoon, J., Joo, J., Jeong, H., Ahn, J., Bhatia, S. K., & Yang, Y. (2025). Sucrose-Based Screening of a Novel Strain, Limimaricola sp. YI8, and Its Application to Polyhydroxybutyrate Production from Molasses. Polymers, 17(11), 1471. https://doi.org/10.3390/polym17111471








