The Effect of Tertiary Amines as Catalysts on the Ring-Opening Polymerization of Benzoxazines
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
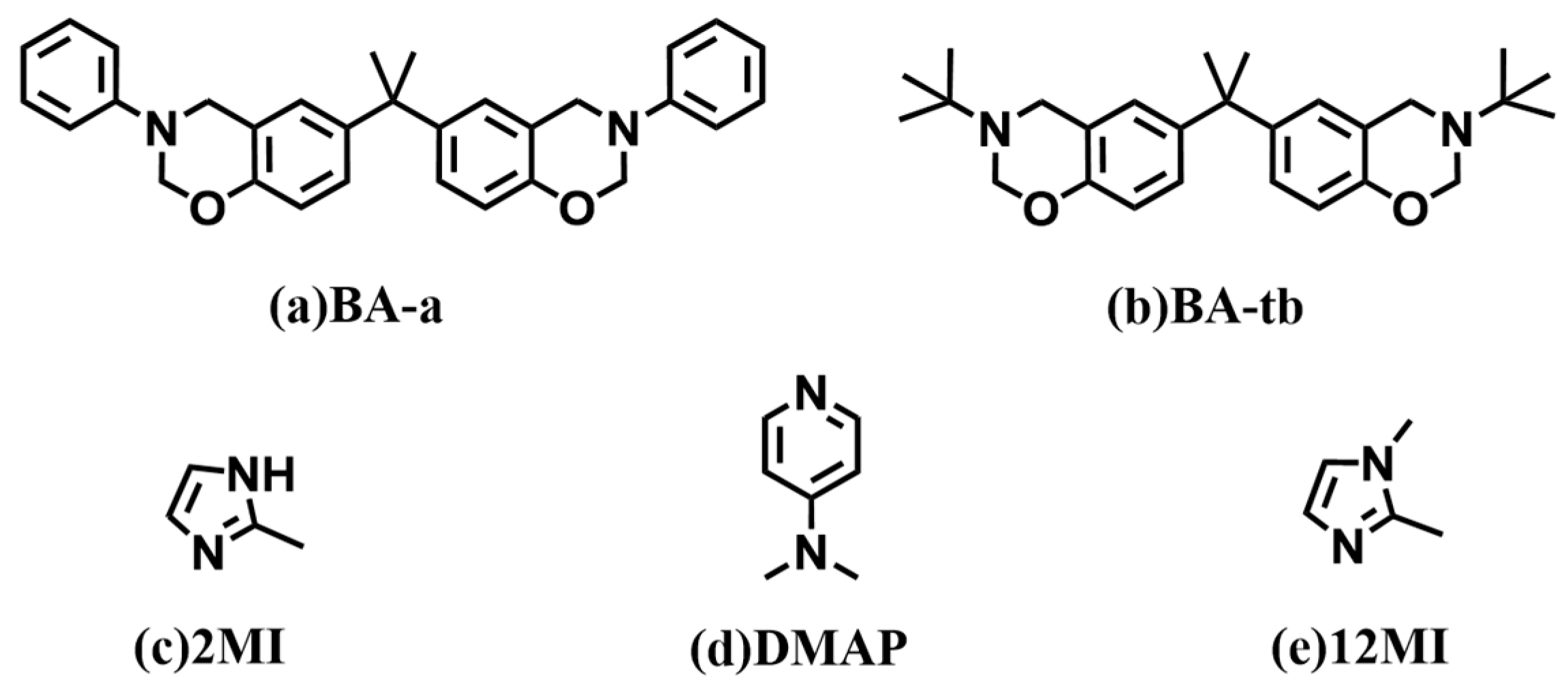
2.2. Synthesis of Benzoxazine Monomers
2.3. Preparation of Benzoxazine/Amine Systems
2.4. Measurements
3. Results and Discussion
3.1. Curing Behaviors of Benzoxazine/Amine Systems
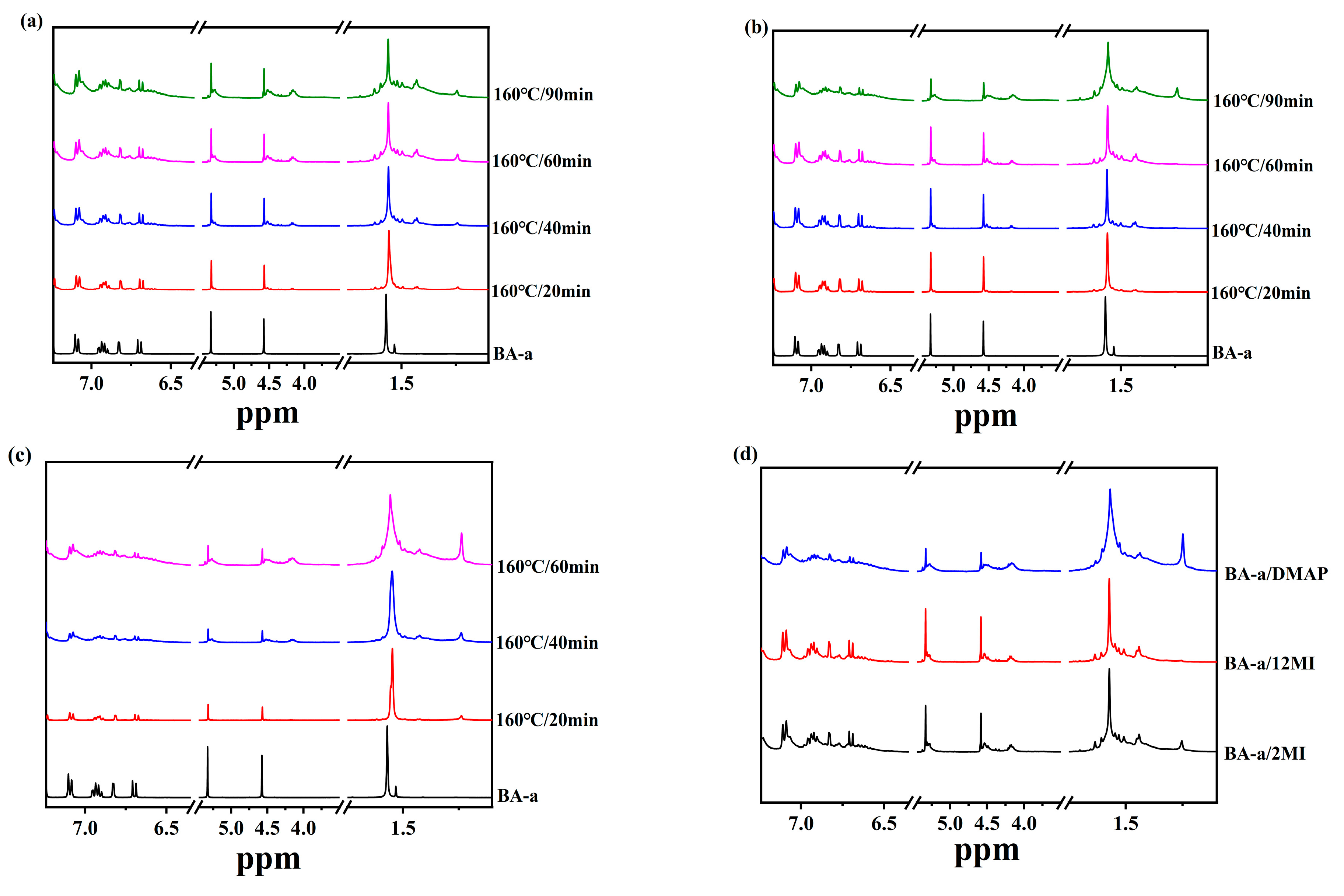
3.2. Structural Changes in the Benzoxazine/Amine Systems During Polymerization
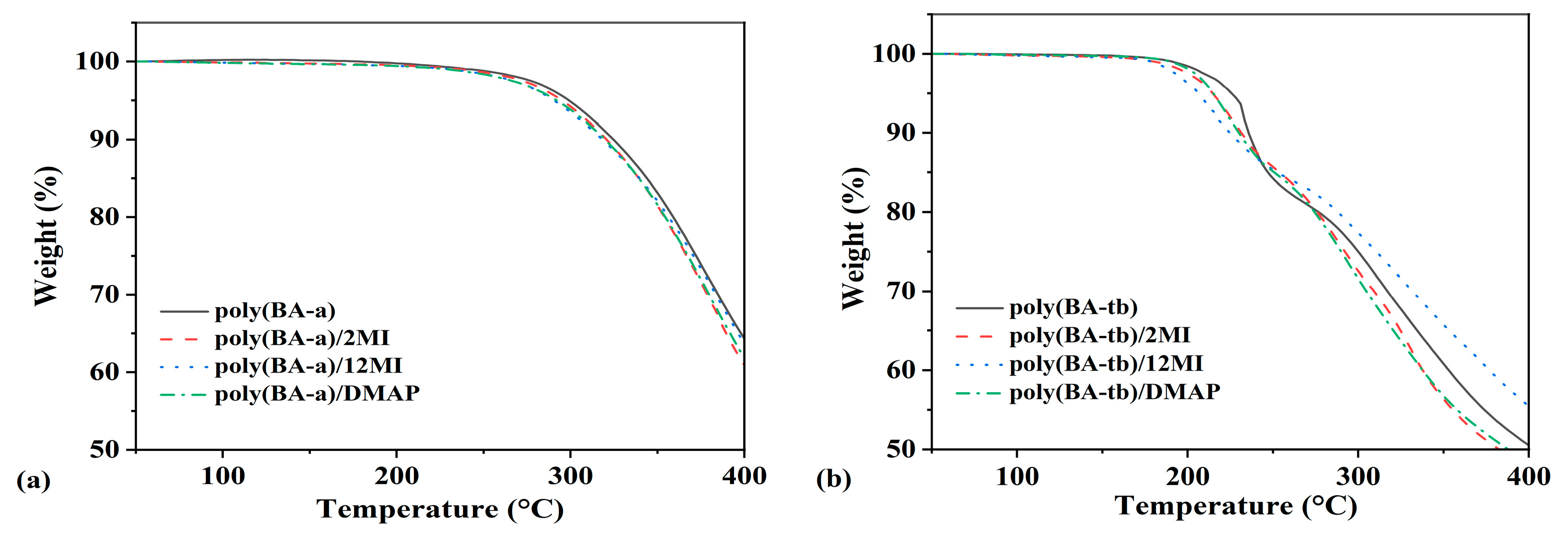
3.3. Thermal Stability of Cured Benzoxazine/Amine Systems
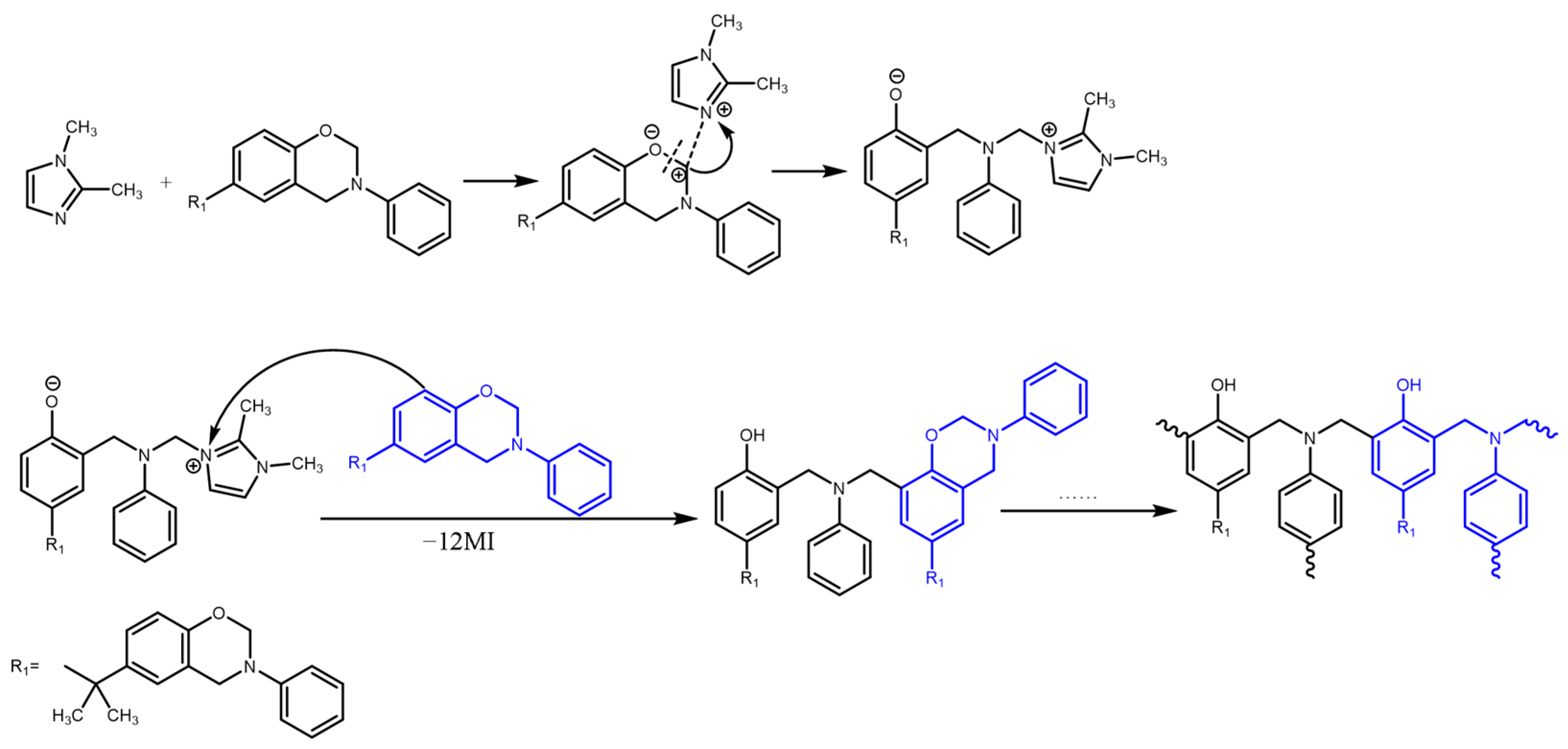
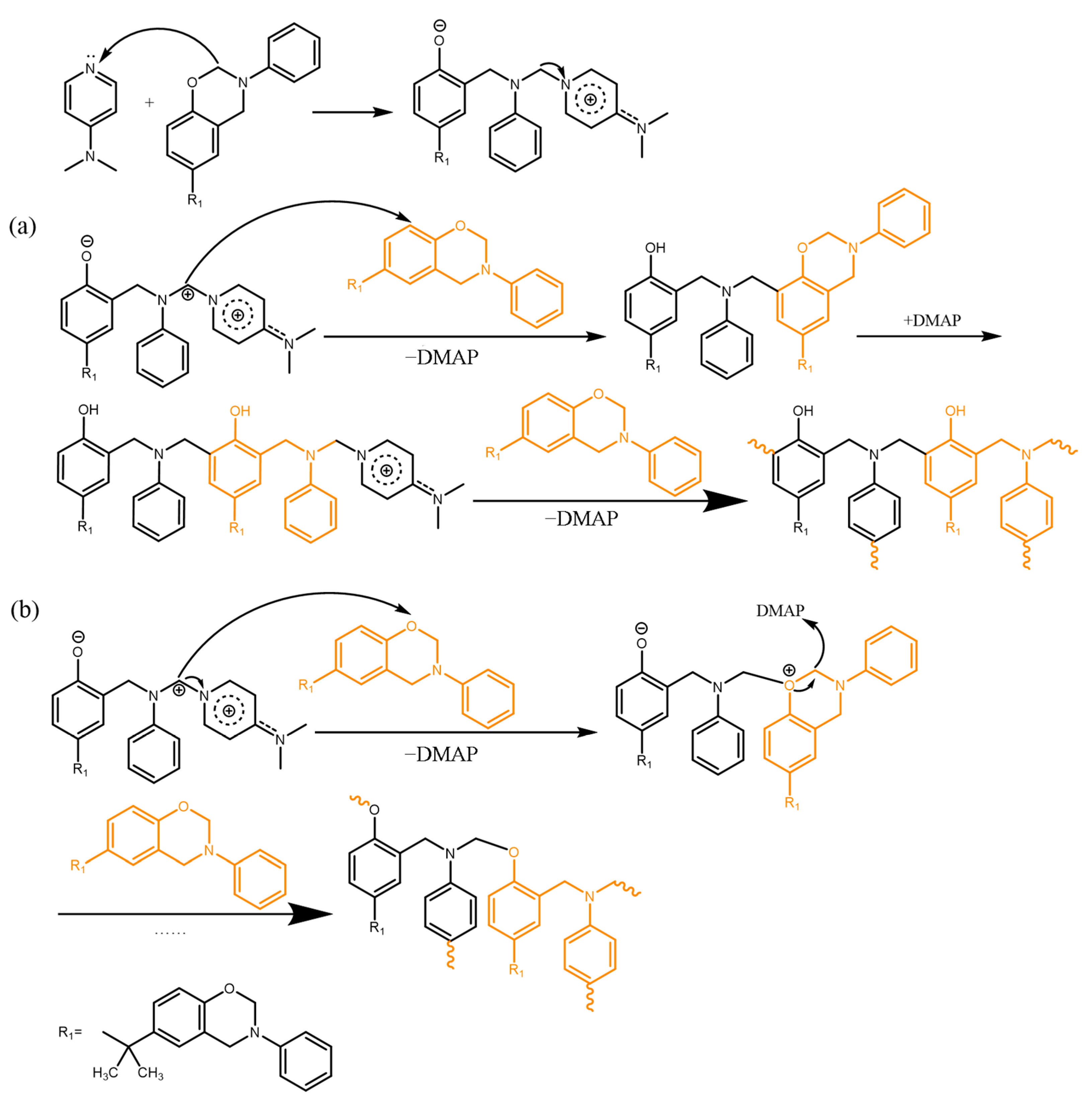
3.4. Curing Mechanisms of the Benzoxazine/Amine Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yoon, J.; Choi, J.; Park, C.; Kim, Y. Experimental study on thermal and mechanical characteristics of two resin composites using the co-curing process. J. Korea Inst. Mil. Sci. Technol. 2020, 23, 475–484. [Google Scholar] [CrossRef]
- Zhao, C.; Qin, Y.; Wang, X.; Xiao, H. Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites. e-Polymers 2022, 22, 379–388. [Google Scholar] [CrossRef]
- Shim, G.; Kim, S.; Eom, H.; Ahn, D.; Park, J.; Choi, S. Improvement in ballistic impact resistance of a transparent bulletproof material laminated with strengthened soda-lime silicate glass. Compos. Part B Eng. 2015, 77, 169–178. [Google Scholar] [CrossRef]
- Peng, W.; Chen, X.; Wang, J. Study on the curing behavior of polythiol/phenolic/epoxy resin and the mechanical and thermal properties of the com-posites. Mater. Res. Express 2021, 8, 55302. [Google Scholar] [CrossRef]
- Liu, X.; Gu, Y. Study on the volumetric change during ring-opening polymerization of benzoxazines. Acta Polym. Sin. 2000, 8, 612–619. [Google Scholar]
- Zhang, K.; Wang, F.; Yang, B.; Li, L.; Gao, L.; Sun, Y.; Guo, F. Mechanical response and failure mechanisms of natural bamboo fiber reinforced poly-benzoxazine composite subjected to split-hopkinson tensile bar loading. Polymers 2022, 14, 1450. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, A.; Prabunathan, P.; Kumaravel, A.; Manoj, M.; Alagar, M. Bio-based polybenzoxazine composites for oil-water separation, sound absorption and corrosion resistance applications. Polym. Test. 2020, 86, 106443. [Google Scholar] [CrossRef]
- Chen, D.; Liu, B.; Wang, X.; Li, X.; Xu, X.; He, J.; Yang, R. High flame retardant and heat-resistance, low dielectric benzoxazine resin with phthalimide structure. Polym. Degrad. Stab. 2022, 205, 110150. [Google Scholar] [CrossRef]
- Dunkers, J.; Ishida, H. Reaction of benzoxazine-based phenolic resins with strong and weak carboxylic acids and phenols as catalysts. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 1913–1921. [Google Scholar] [CrossRef]
- Lee, H.; Liu, Y. Thermally stable, flame retardant, low-dielectric constants, and flexible thermosetting resins based on a tetra-functional benzoxazine compound possessing a cyclic siloxane core. J. Appl. Polym. Sci. 2022, 139, e52605. [Google Scholar]
- Chaisuwan, T.; Ishida, H. High-performance maleimide and nitrile-functionalized benzoxazines with good processibility for advanced composites applications. J. Appl. Polym. Sci. 2006, 101, 548–558. [Google Scholar] [CrossRef]
- Wang, Y.X.; Ishida, H. Cationic ring-opening polymerization of benzoxazines. Polymer 1999, 40, 4563–4570. [Google Scholar] [CrossRef]
- Wang, Y.X.; Ishida, H. Synthesis and properties of new thermoplastic polymers from substituted 3,4-dihydro-2h-1,3-benzoxazines. Macromolecules 2000, 33, 2839–2847. [Google Scholar] [CrossRef]
- Ghosh, N.N.; Kiskan, B.; Yagci, Y. Polybenzoxazines—New high performance thermosetting resins: Synthesis and properties. Prog. Polym. Sci. 2007, 32, 1344–1391. [Google Scholar] [CrossRef]
- Chutayothin, P.; Ishida, H. Cationic ring-opening polymerization of 1,3-benzoxazines: Mechanistic study using model compounds. Macromolecules 2010, 43, 4562–4572. [Google Scholar] [CrossRef]
- Chen, J.; Jian, R.; Yang, K.; Bai, W.; Huang, C.; Lin, Y.; Zheng, B.; Wei, F.; Lin, Q.; Xu, Y. Urushiol-based benzoxazine copper polymer with low surface energy, strong substrate adhesion and antibacterial for marine antifouling application. J. Clean. Prod. 2021, 318, 128527. [Google Scholar] [CrossRef]
- Liu, C.; Shen, D.; Maria Sebastian, R.; Marquet, J.; Schoenfeld, R. Mechanistic studies on ring-opening polymerization of benzoxazines: A mechanistically based catalyst design. Macromolecules 2011, 44, 4616–4622. [Google Scholar] [CrossRef]
- Arslan, M.; Kiskan, B.; Yagci, Y. Ring-opening polymerization of 1,3-benzoxazines via borane catalyst. Polymers 2018, 10, 239. [Google Scholar] [CrossRef]
- Zhang, S.; Ran, Q.; Fu, Q.; Gu, Y. Preparation of transparent and flexible shape memory polybenzoxazine film through chemical structure manipulation and hydrogen bonding control. Macromolecules 2018, 51, 6561–6570. [Google Scholar] [CrossRef]
- Zhang, S.; Ran, Q.; Fu, Q.; Gu, Y. Controlled polymerization of 3,4-dihydro-2h-1,3-benzoxazine and its properties tailored by lewis acids. React. Funct. Polym. 2019, 139, 75–84. [Google Scholar] [CrossRef]
- Yang, W.; Xie, Y.; Chen, J.; Huang, C.; Xu, Y.; Lin, Y. Metal ion-catalyzed low-temperature curing of urushiol-based polybenzoxazine. Front. Chem. 2022, 10, 879605. [Google Scholar] [CrossRef]
- Zeng, M.; Wang, J.; Li, R.; Liu, J.; Chen, W.; Xu, Q.; Gu, Y. The curing behavior and thermal property of graphene oxide/benzoxazine nanocomposites. Polymer 2013, 54, 3107–3116. [Google Scholar] [CrossRef]
- Devaraju, S.; Eswar, P.; Gangadhar Reddy, T.; Ravi Kumar, K. Metal salts used as an efficient catalyst to reduce the ring opening polymerization temperature of benzoxazines. J. Macromol. Sci. Part A Pure Appl. Chem. 2022, 59, 567–573. [Google Scholar] [CrossRef]
- Wang, G.; Li, Y.; Mei, Z.; Xin, J.; Pan, W.; Wang, Q. Curing mechanism and kinetics of benzoxazine co-catalyzed by transition metal salt and phenolic resin. Thermochim. Acta 2022, 710, 179182. [Google Scholar]
- Sun, J.; Wei, W.; Xu, Y.; Qu, J.; Liu, X.; Endo, T. A curing system of benzoxazine with amine: Reactivity, reaction mechanism and material properties. RSC Adv. 2015, 5, 19048–19057. [Google Scholar] [CrossRef]
- Zong, J.; Ran, Q. Ring opening reaction of 3,4-dihydro-2h-1,3-benzoxazine with amines at room temperature. ChemistrySelect 2019, 4, 6687–6696. [Google Scholar] [CrossRef]
- Zhang, S.; Zong, J.; Ran, Q.; Gu, Y. Facile preparation of lightweight and robust polybenzoxazine foams. Ind. Eng. Chem. Res. 2020, 59, 7575–7583. [Google Scholar] [CrossRef]
- Boswell, P.G. On the calculation of activation energies using a modified kissinger method. J. Therm. Anal. Calorim. 2005, 18, 353–358. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, P.; Criado, J.; Pérez-Maqueda, L. Kissinger kinetic analysis of data obtained under different heating schedules. J. Therm. Anal. Calorim. 2008, 94, 427–432. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetic analysis of derivative curves in thermal analysis. J. Therm. Anal. 1970, 2, 301–324. [Google Scholar] [CrossRef]
- Varfolomeev, M.A.; Abaidullina, D.I.; Gainutdinova, A.Z.; Solomonov, B.N. FTIR study of h-bonds cooperativity in complexes of 1,2-dihydroxybenzene with proton acceptors in aprotic solvents: Influence of the intramolecular hydrogen bond. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 77, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Ishida, H. Model compounds study on the network structure of polybenzoxazines. Macromolecules 2003, 36, 8320–8329. [Google Scholar] [CrossRef]
- Han, L.; Laura Salum, M.; Zhang, K.; Froimowicz, P.; Ishida, H. Intrinsic self-initiating thermal ring-opening polymerization of 1,3-benzoxazines without the influence of impurities using very high purity crystals. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3434–3445. [Google Scholar] [CrossRef]
- Hofle, G.; Steglich, W.; Vorbruggen, H. 4-dialkylaminopyridines as acylation catalysts. 4. 4-dialkylaminopyridines as highly active acylation catalysts. Angew. Chem. Int. Ed. Engl. 1978, 17, 569. [Google Scholar] [CrossRef]
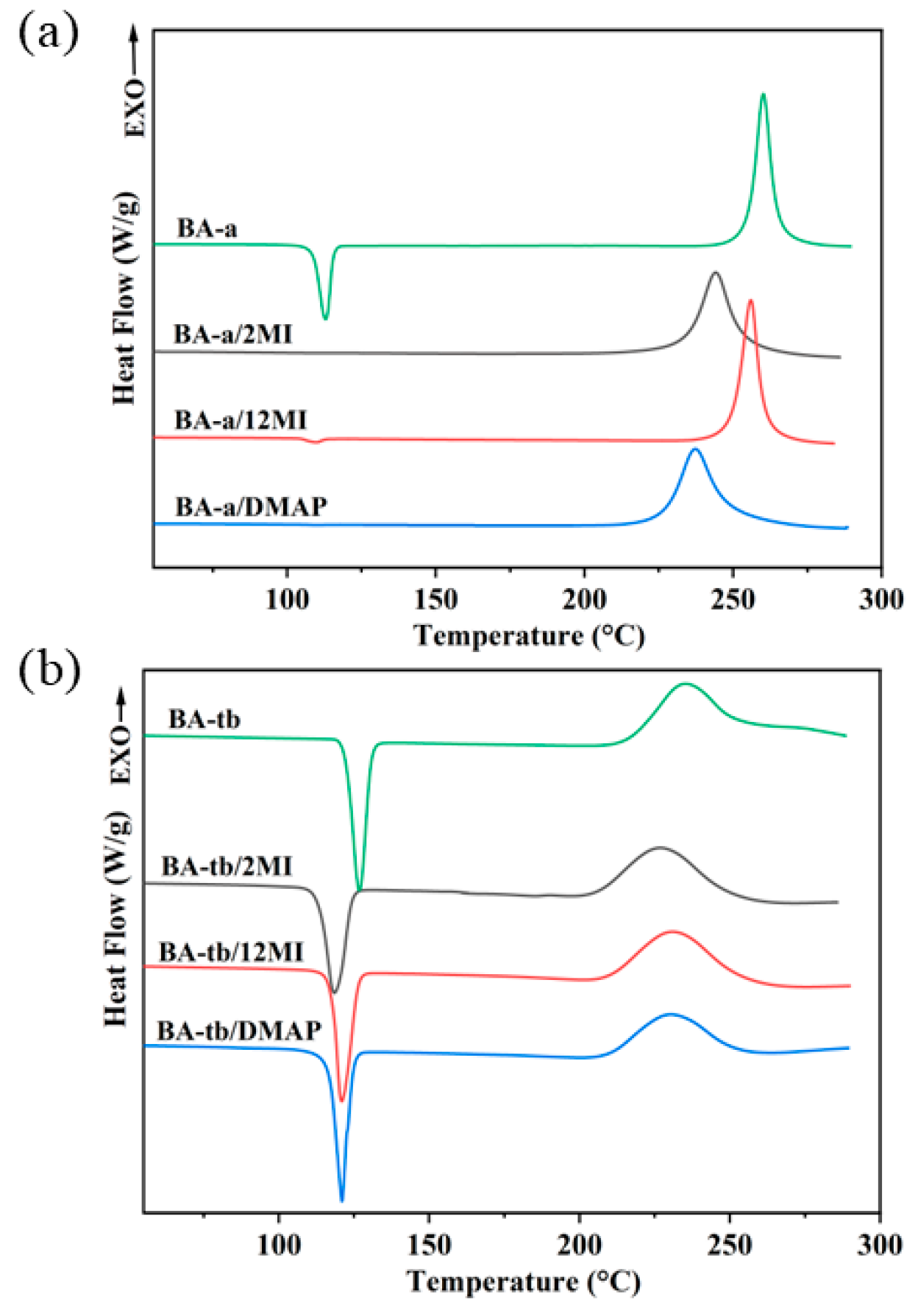




| Sample | Tonset/°C | Tp/°C |
|---|---|---|
| BA-a | 248 | 260 |
| BA-a\2MI | 228 | 244 |
| BA-a\12MI | 245 | 256 |
| BA-a\DMAP | 220 | 237 |
| BA-tb | 214 | 236 |
| BA-tb\2MI | 195 | 227 |
| BA-tb\12MI | 209 | 231 |
| BA-tb\DMAP | 207 | 231 |
| Sample | Ea/kJ mol−1 | |
|---|---|---|
| Kissinger | Ozawa | |
| BA-a | 96.5 | 100.3 |
| BA-a/2MI | 80.0 | 84.4 |
| BA-a/12MI | 92.1 | 96.1 |
| BA-a/DMAP | 73.0 | 77.6 |
| BA-tb | 93.6 | 97.2 |
| BA-tb/2MI | 69.1 | 73.8 |
| BA-tb/12MI | 84.9 | 88.9 |
| BA-tb/DMAP | 80.2 | 84.4 |
| Sample | T5%/°C | T10%/°C |
|---|---|---|
| Poly(BA-a) | 298 | 325 |
| Poly(BA-a)/2MI | 295 | 320 |
| poly(BA-a)/12MI | 291 | 318 |
| poly(BA-a)/DMAP | 284 | 319 |
| Poly(BA-tb) | 226 | 236 |
| poly(BA-tb)/2MI | 216 | 231 |
| poly(BA-tb)/12MI | 205 | 223 |
| poly(BA-tb)/DMAP | 213 | 231 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Wu, X.; Chen, K.; Yao, J.; Ran, Q. The Effect of Tertiary Amines as Catalysts on the Ring-Opening Polymerization of Benzoxazines. Polymers 2025, 17, 1431. https://doi.org/10.3390/polym17111431
Liu F, Wu X, Chen K, Yao J, Ran Q. The Effect of Tertiary Amines as Catalysts on the Ring-Opening Polymerization of Benzoxazines. Polymers. 2025; 17(11):1431. https://doi.org/10.3390/polym17111431
Chicago/Turabian StyleLiu, Fanghui, Ximeng Wu, Kun Chen, Junbo Yao, and Qichao Ran. 2025. "The Effect of Tertiary Amines as Catalysts on the Ring-Opening Polymerization of Benzoxazines" Polymers 17, no. 11: 1431. https://doi.org/10.3390/polym17111431
APA StyleLiu, F., Wu, X., Chen, K., Yao, J., & Ran, Q. (2025). The Effect of Tertiary Amines as Catalysts on the Ring-Opening Polymerization of Benzoxazines. Polymers, 17(11), 1431. https://doi.org/10.3390/polym17111431






