Gun–Bullet Model-Based Noncovalent Interactions Boosting Visible Light Photocatalytic Hydrogen Production in Poly Thieno[3,2-b]Thiophene/Graphitic Carbon Nitride Heterojunctions
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis of PTT Nanoparticles
2.2. Preparation of 2D g-C3N4
2.3. Synthesis of PTT/g-C3N4 Heterojunctions
3. Results and Discussion
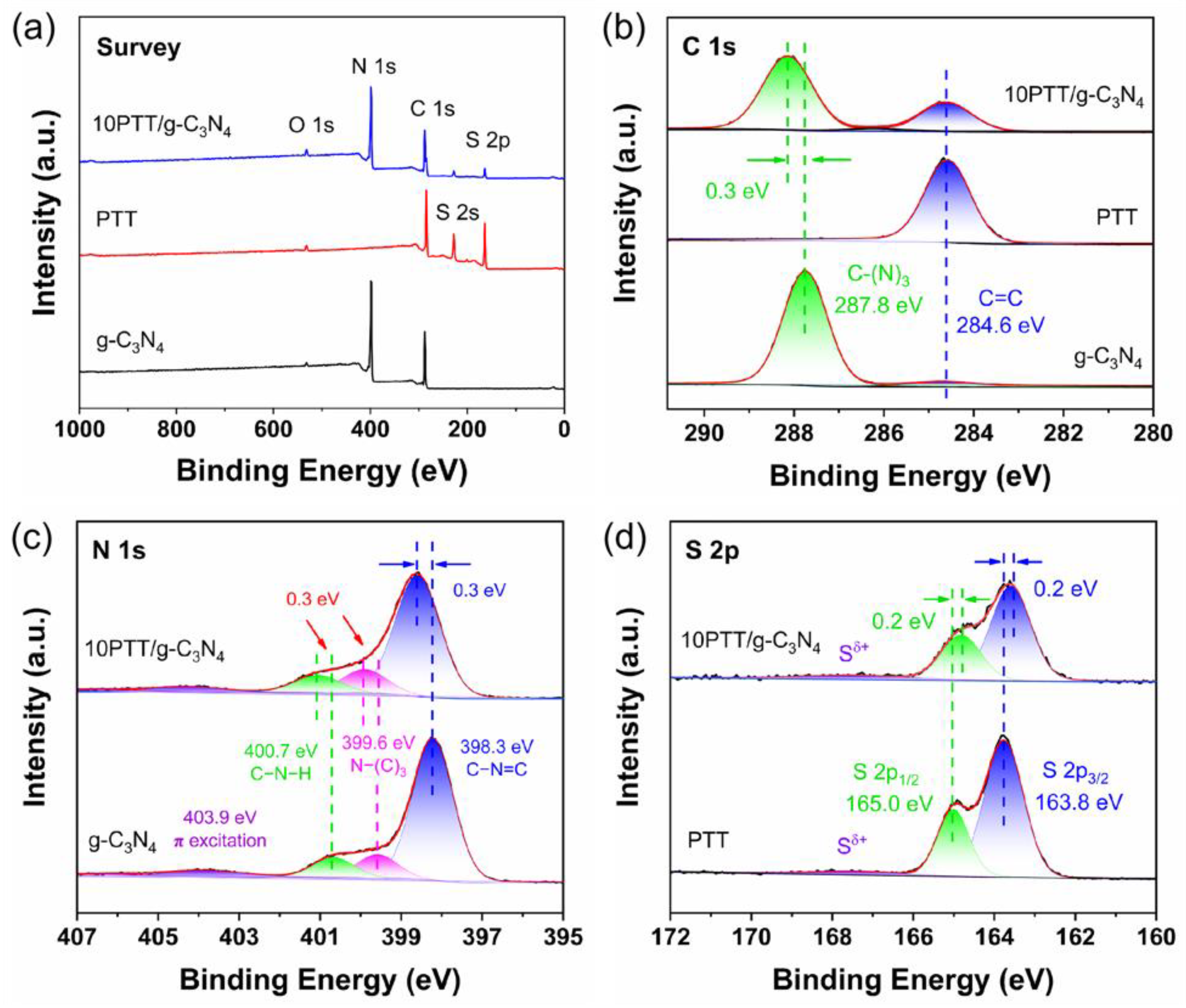
3.1. Structural Identification
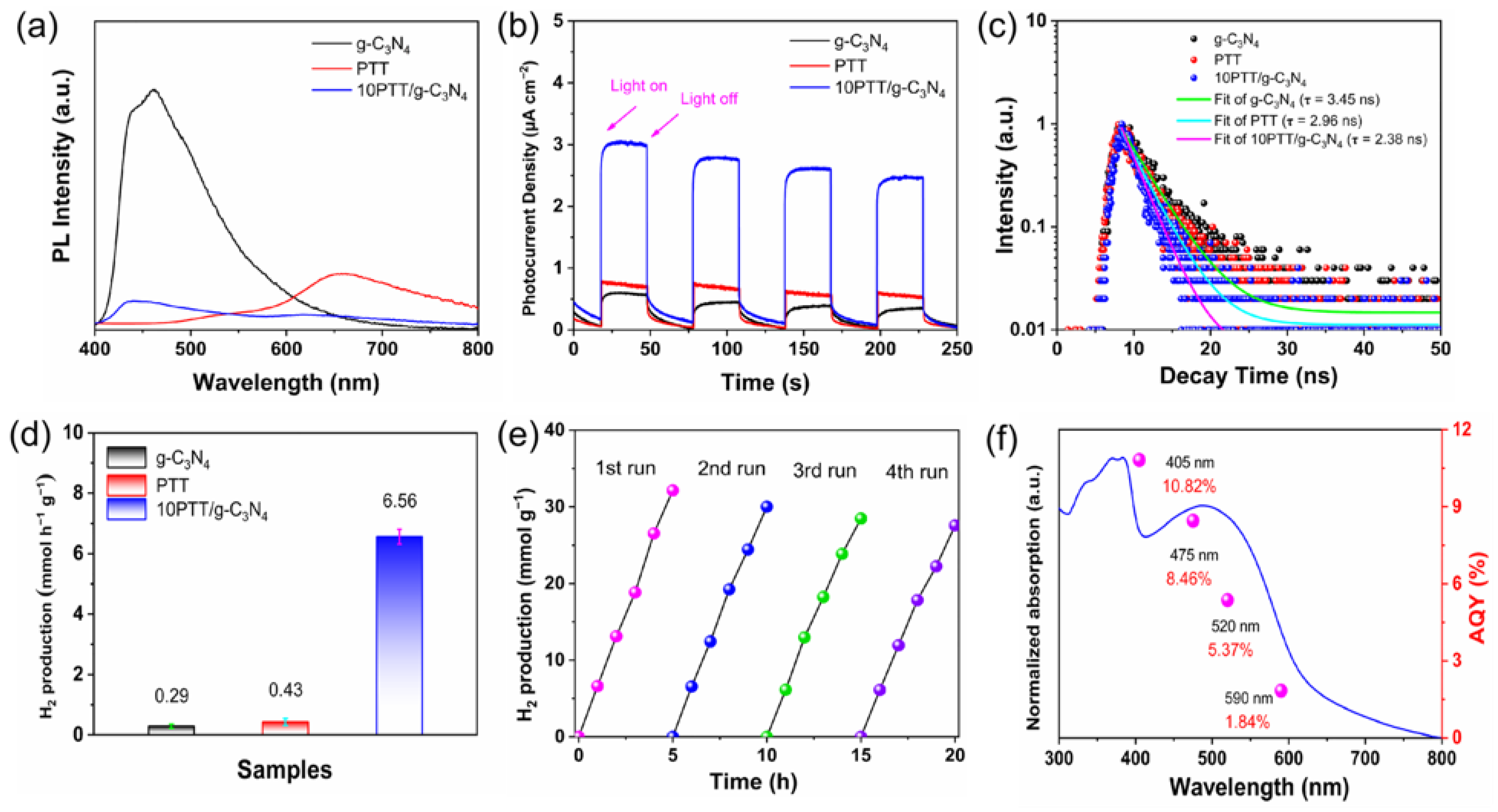
3.2. Charge Separation and Photocatalytic Activities
3.3. Mechanism Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Zhou, H.; Cai, S.; Prabhakaran, D.; Niu, W.; Large, A.; Held, G.; Taylor, R.A.; Wu, X.-P.; Tsang, S.C.E. Electrolyte-assisted polarization leading to enhanced charge separation and solar-to-hydrogen conversion efficiency of seawater splitting. Nat. Catal. 2024, 7, 77–88. [Google Scholar] [CrossRef]
- Wang, X.; An, C.; Zhang, S.; Wang, S.; Li, J.; Zhu, Y. Metal-Free Heterostructured 2D/1D Polymeric Carbon Nitride/Fibrous Phosphorus for Boosted Photocatalytic Hydrogen Production from Pure Water. Sep. Purif. Technol. 2024, 340, 126733. [Google Scholar] [CrossRef]
- Huang, Y.; Jian, Y.; Li, L.; Li, D.; Fang, Z.; Dong, W.; Lu, Y.; Luo, B.; Chen, R.; Yang, Y. A NIR-Responsive Phytic Acid Nickel Biomimetic Complex Anchored on Carbon Nitride for Highly Efficient Solar Hydrogen Production. Angew. Chem. Int. Ed. 2021, 60, 5245–5249. [Google Scholar] [CrossRef]
- Han, C.; Dong, P.; Tang, H.; Zheng, P.; Zhang, C.; Wang, F.; Huang, F.; Jiang, J. Realizing High Hydrogen Evolution Activity under Visible Light using Narrow Band Gap Organic Photocatalysts. Chem. Sci. 2021, 12, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Yu, J.; Xu, D.; Wang, L.; Liang, G.; Zhang, L.; Jaroniec, M. In-situ Formatting Donor-Acceptor Polymer with Giant Dipole Moment and Ultrafast Exciton Separation. Nat. Commun. 2024, 15, 1313. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Sun, F.; Fang, Y.; Wen, Y.; Hong, F.; Shan, B. Molecular Photoelectrodes with Enhanced Photogenerated Charge Transport for Efficient Solar Hydrogen Evolution. J. Am. Chem. Soc. 2025, 147, 7671–7681. [Google Scholar] [CrossRef]
- Zhu, D.; Mo, D.; Ma, X.; Zhou, Q.; Liu, H.; Xu, J.; Zhou, W.; Zhao, F. Effect of Polymerization Solvent, Potential, and Temperature on Morphology and Capacitance Properties of Poly (Thieno[3,2-b]Thiophene) Films. Synth. Met. 2016, 220, 155–161. [Google Scholar] [CrossRef]
- Malov, V.V.; Ghosh, T.; Nair, V.C.; Maslov, M.M.; Katin, K.P.; Unni, K.N.N.; Tameev, A.R. Hole Mobility in Thieno[3,2-b]Thiophene Oligomers. Mendeleev Commun. 2019, 29, 218–219. [Google Scholar] [CrossRef]
- Zhou, B.; Dai, T.; Zhou, J.; Chen, Y.; Geng, Y.; Lei, P.; Zheng, G.; Zeng, Q.; Zhou, E. Conjugated D–π–A Photovoltaic Polymers Containing Thieno[3,2-b]Thiophene π-Bridge. Mater. Chem. Front. 2024, 8, 1563–1590. [Google Scholar] [CrossRef]
- Gabrian, L.; Giurgi, G.I.; Stroia, I.; Bogdan, E.; Crian, A.P.; Hdade, N.D.; Grosu, I.; Terec, A. Exploring the Optoelectronic Properties of D-A and A-D-A 2,2′ -Bi[3,2-b]Thienothiophene Derivatives. Molecules 2022, 27, 8463. [Google Scholar] [CrossRef]
- Dai, C.; Liu, B. Conjugated Polymers for Visible-Light-Driven Photocatalysis. Energy Environ. Sci. 2020, 13, 24–52. [Google Scholar] [CrossRef]
- Zhang, G.; Lan, Z.A.; Wang, X. Conjugated Polymers: Catalysts for Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2016, 55, 15712–15727. [Google Scholar] [CrossRef]
- Zhong, D.; Jia, X.; Zhang, X.; Zhao, J.; Wang, D.; Fang, Y.; Zhang, Z.; Rosei, F.; Li, Y. Optimization of g-C3N4 Nanostructures by CH2 Introduction and Relay Modification for Photocatalytic Hydrogen Evolution. ACS Appl. Nano Mater. 2024, 7, 27508–27519. [Google Scholar] [CrossRef]
- Yu, H.; Shi, R.; Zhao, Y.; Bian, T.; Zhao, Y. Alkali-Assisted Synthesis of Nitrogen Deficient Graphitic Carbon Nitride with Tunable Band Structures for Efficient Visible-Light-Driven Hydrogen Evolution. Adv. Mater. 2017, 29, 1605148. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hu, K.; Chu, M.; Li, Y.; Jing, L. Mg-O-Bridged Polypyrrole/g-C3N4 Nanocomposites as Efficient Visible-Light Catalysts for Hydrogen Evolution. ChemSusChem 2020, 13, 3707–3717. [Google Scholar] [CrossRef]
- Wang, Y.; Vogel, A.; Sachs, M.; Sprick, R.S.; Tang, J. Current Understanding and Challenges of Solar-Driven Hydrogen Generation using Polymeric Photocatalysts. Nat. Energy 2019, 4, 746–760. [Google Scholar] [CrossRef]
- Pang, X.; Li, Y.; Wu, X.; Zhang, B.; Hao, M.; Zhu, Y.; Zhang, Y.; Qin, C.; Zhan, H.; Qin, C. Phosphate Ester Functionalized Fluorene-Benzothiadiazole Alternating Copolymer/Hydroxylated g-C3N4 Heterojunctions for Efficient Hydrogen Evolution under Visible-Light Irradiation. J. Colloid Interface Sci. 2023, 652, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, K.; Hong, W.; Zong, R.; Zhu, Y. Visible Light Photoactivity Enhancement via CuTCPP Hybridized g-C3N4 Nanocomposite. Appl. Catal. B Environ. 2015, 166, 366–373. [Google Scholar] [CrossRef]
- Lin, L.; Hou, C.; Zhang, X.; Wang, Y.; Chen, Y.; He, T. Highly Efficient Visible-Light Driven Photocatalytic Reduction of CO2 over g-C3N4 Nanosheets/Tetra(4-carboxyphenyl) Porphyrin Iron(III) Chloride Heterogeneous Catalysts. Appl. Catal. B Environ. 2018, 221, 312–319. [Google Scholar] [CrossRef]
- Ju, H.; Wang, B.; Li, M.; Hao, J.; Si, W.; Song, S.; Mei, K.; Sue, C.H.; Wang, J.; Jia, C.; et al. Tracking Noncovalent Interactions of π, π-Hole, and Ion in Molecular Complexes at the Single-Molecule Level. J. Am. Chem. Soc. 2024, 146, 25290–25298. [Google Scholar] [CrossRef]
- Cui, Y.; Ding, Z.; Liu, P.; Antonietti, M.; Fu, X.; Wang, X. Metal-Free Activation of H2O2 by g-C3N4 under Visible Light Irradiation for the Degradation of Organic Pollutants. Phys. Chem. Chem. Phys. 2012, 14, 1455–1462. [Google Scholar] [CrossRef]
- Li, Y.; Pang, X.; Zhao, Q.; Zhang, B.; Guo, X.; Zhang, Y.; Xie, Y.; Qin, C.; Jing, L. Controlled Synthesis of Nitro-Terminated Oligothiophene/Crystallinity-Improved g-C3N4 Heterojunctions for Enhanced Visible-Light Catalytic H2 Production. ACS Appl. Mater. Interfaces 2023, 15, 5365–5377. [Google Scholar] [CrossRef]
- Zhang, Y.; Pang, X.; Li, Y.; Qu, Y.; Zhang, B.; Li, Z.; Hao, M.; Zhu, Y.; Qin, C. Construction of an All-Organic S-scheme Heterostructure Based on PEDOT Immobilized g-C3N4 Nanosheets by Electrostatic Self-Assembly with Enhanced Visible-Light Photocatalytic Hydrogen Production. Sep. Purif. Technol. 2024, 345, 127378. [Google Scholar] [CrossRef]
- Zong, X.; Miao, X.; Hua, S.; An, L.; Gao, X.; Jiang, W.; Qu, D.; Zhou, Z.; Liu, X.; Sun, Z. Structure Defects Assisted Photocatalytic H2 Production for Polythiophene Nanofibers. Appl. Catal. B Environ. 2017, 211, 98–105. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Guo, P.; Lv, J.; Wang, X.; Tong, J.; Xia, Y. Impact of Linker Positions for Thieno[3,2-b]Thiophene in Wide Band Gap Benzo[1,2-b:4,5-6′]Dithiophene-Based Photovoltaic Polymers. J. Mater. Res. 2019, 34, 2057–2066. [Google Scholar] [CrossRef]
- Liu, B.; Chen, X.; He, Y.; Li, Y.; Xu, X.; Xiao, L.; Li, L.; Zou, Y. New Alkylthienyl Substituted Benzo[1,2-b:4,5-b′]Dithiophene-Based Polymers for High Performance Solar Cells. J. Mater. Chem. A 2013, 1, 570–577. [Google Scholar] [CrossRef]
- Chen, J.; Dong, C.L.; Zhao, D.; Huang, Y.C.; Wang, X.; Samad, L.; Dang, L.; Shearer, M.; Shen, S.; Guo, L. Molecular Design of Polymer Heterojunctions for Efficient Solar–Hydrogen Conversion. Adv. Mater. 2017, 29, 1606198. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Fu, Q.; Zhang, G. Dimension-Matching Crystallized Linear Conjugated Polymer/Graphitic Carbon Nitride Heterojunctions for Boosting Visible/Near-Infrared Light Photocatalytic Hydrogen Production. Appl. Surf. Sci. 2025, 679, 161198. [Google Scholar] [CrossRef]
- Dong, H.; Jiang, S.; Jiang, L.; Liu, Y.; Li, H.; Hu, W.; Wang, E.; Yan, S.; Wei, Z.; Xu, W. Nanowire Crystals of a Rigid Rod Conjugated Polymer. J. Am. Chem. Soc. 2009, 131, 17315–17320. [Google Scholar] [CrossRef]
- Jiang, K.B.; Huang, W.; Song, T.T.; Wu, P.X.; Wang, W.F.; Chen, Q.S.; Wang, M.S.; Guo, G.C. Photobreeding Heterojunction on Semiconductor Materials for Enhanced Photocatalysis. Adv. Funct. Mater. 2023, 33, 2304351. [Google Scholar] [CrossRef]
- Yang, R.; Shi, H.; Zhao, J.; Zhang, H.; Zhong, M.; Yang, P. Novel Asymmetric Aggregation Strategy to Boost Charge Separation in Carbon Nitride Polymers for High-Performance Hydrogen Photosynthesis. ACS Catal. 2024, 14, 9607–9617. [Google Scholar] [CrossRef]
- Mao, L.; Zhai, B.; Shi, J.; Kang, X.; Lu, B.; Liu, Y.; Cheng, C.; Jin, H.; Lichtfouse, E.; Guo, L. Supercritical CH3OH-Triggered Isotype Heterojunction and Groups in g-C3N4 for Enhanced Photocatalytic H2 Evolution. ACS Nano 2024, 18, 13939–13949. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Zheng, J.; Xiao, T.; Fu, Q.; Yang, C.; Wang, D.; Zhang, G. Anti-Z-Scheme Polymer/Polymer Heterojunctions Achieving Synchronous Enhancement of Light Absorption and Directly Spatial Charge Separation. Chem. Eng. J. 2024, 497, 154816. [Google Scholar] [CrossRef]
- Hou, J.; Tan, Z.A.; Yan, Y.; He, Y.; Yang, C.; Li, Y. Synthesis and Photovoltaic Properties of Two-Dimensional Conjugated Polythiophenes with Bi (thienylenevinylene) Side Chains. J. Am. Chem. Soc. 2006, 128, 4911–4916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yu, M.; Liu, T.; Cong, M.; Liu, X.; Yang, H.; Bai, Y.; Zhu, Q.; Zhang, S.; Gu, H.; et al. Accelerated Discovery of Molecular Nanojunction Photocatalysts for Hydrogen Evolution by Using Automated Screening and Flow Synthesis. Nat. Synth. 2024, 3, 595–605. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Z.; Zhang, S.; Ye, H.; Kong, L.; Gong, X.; Hua, J.; Tian, H. Molecular Engineering of Donor–Acceptor Conjugated Polymer/g-C3N4 Heterostructures for Significantly Enhanced Hydrogen Evolution Under Visible-Light Irradiation. Adv. Funct. Mater. 2018, 28, 1804512. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, B.M.; Pang, X.L.; Li, Z.J.; Zhang, Y.; Hao, M.; Zhu, Y.; Qin, C.L.; Jing, L.Q. Improved Visible-Light Photocatalytic H2 Evolution of G-C3N4 Nanosheets by Constructing Heterojunctions with Nano-Sized Poly (3-Thiophenecarboxylic Acid) and Coordinating Fe(III). Nanomaterials 2023, 13, 1338. [Google Scholar] [CrossRef]
- Xu, L.P.; Tian, B.N.; Wang, T.Y.; Yu, Y.; Wu, Y.C.; Cui, J.W.; Cao, Z.N.; Wu, J.H.; Zhang, W.K.; Zhang, Q.; et al. Direct Z-Scheme Polymeric Heterojunction Boosts Photocatalytic Hydrogen Production via a Rebuilt Extended π-Delocalized Network. Energy Environ. Sci. 2022, 15, 5059–5068. [Google Scholar] [CrossRef]
- An, C.; Sikandaier, A.; Guo, X.; Zhu, Y.; Tang, H.; Yang, D. Hierarchical S-Scheme Heterojunction of Red Phosphorus Nanoparticles Embedded Flower-like CeO2 Triggering Efficient Photocatalytic Hydrogen Production. Acta Phys.-Chim. Sin. 2024, 40, 2405019. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, Y.; Chen, Z.; Zhang, Z.; Fang, X. Constructing a Novel Ternary Fe(III)/Graphene/g-C3N4 Composite Photocatalyst with Enhanced Visible-Light Driven Photocatalytic Activity via Interfacial Charge Transfer Effect. Appl. Catal. B Environ. 2016, 183, 231–241. [Google Scholar] [CrossRef]
- Zhang, B.M.; Li, Y.; Pang, X.L.; Qu, Y.; Li, Z.J.; Zhao, Q.; Zhang, Y.; Zhu, Y.; Zhang, P.X.; Qin, C.L. Tightly Connected Poly(3-Thiophene Boronic Acid)/g-C3N4 Heterojunctions for Enhanced Visible-Light Photocatalytic Hydrogen Production. ChemPhotoChem 2023, 7, e202300117. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, Y.; Hu, K.; Guo, X.; Qu, Y.; Li, Z.; Yang, F.; Liu, H.; Qin, C.; Jing, L. Controlled Synthesis of Nitro-Terminated Poly [2-(3-thienyl)-ethanol]/g-C3N4 Nanosheet Heterojunctions for Efficient Visible-Light Photocatalytic Hydrogen Evolution. ACS Sustain. Chem. Eng. 2021, 9, 7306–7317. [Google Scholar] [CrossRef]
- Liu, B.; Rocca, D.; Yan, H.; Pan, D. Beyond Conformational Control: Effects of Noncovalent Interactions on Electronic Properties of Conjugated Polymers. JACS Au 2021, 1, 2182–2187. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Yang, J.; Li, W.; Wu, Z.; Zhu, Y. Construction of Interfacial Electric Field via Dual-Porphyrin Heterostructure Boosting Photocatalytic Hydrogen Evolution. Adv. Mater. 2021, 34, 2106807. [Google Scholar] [CrossRef] [PubMed]
- Lyons, R.J.; Yang, Y.; Mcqueen, E.; Luo, L.; Cooper, A.I.; Zwijnenburg, M.A.; Sprick, R.S. Polymer Photocatalysts with Side Chain Induced Planarity for Increased Activity for Sacrificial Hydrogen Production from Water. Adv. Energy Mater. 2024, 14, 2303680. [Google Scholar] [CrossRef]
- Liu, A.; Gedda, L.; Axelsson, M.; Pavliuk, M.; Tian, H. Panchromatic Ternary Polymer Dots Involving Sub-Picosecond Energy and Charge Transfer for Efficient and Stable Photocatalytic Hydrogen Evolution. J. Am. Chem. Soc. 2021, 143, 2875–2885. [Google Scholar] [CrossRef]
- Xing, Z.; Chen, Z.; Zong, X.; Wang, L. A New Type of Carbon Nitride-based Polymer Composite for Enhanced Photocatalytic Hydrogen Production. Chem. Commun. 2014, 50, 6762–6764. [Google Scholar] [CrossRef]
- He, F.; Chen, G.; Yu, Y.; Hao, S.; Zheng, Y. Facile Approach to Synthesize g-PAN/g-C3N4 Composites with Enhanced Photocatalytic H2 Evolution Activity. ACS Appl. Mater. Interfaces 2014, 6, 7171–7179. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, C.; Jiang, N.; Zhao, Z.; Xu, A. g-C3N4 Hydrogen-Bonding Viologen for Significantly Enhanced Visible-Light Photocatalytic H2 Evolution. ACS Catal. 2017, 7, 8228–8234. [Google Scholar] [CrossRef]
- Hayat, A.; Raziq, F.; Khan, M.; Ullah, I.; Khan, W. Visible-light Enhanced Photocatalytic Performance of Polypyrrole/g-C3N4 Composites for Water Splitting to Evolve H2 and Pollutants Degradation. J. Photochem. Photobiol. A 2019, 379, 88–98. [Google Scholar] [CrossRef]
- Miao, H.; Yang, J.; Sheng, Y.; Li, W.; Zhu, Y. Controlled Synthesis of Higher Interfacial Electron Transfer Graphite-Like Carbon Nitride/Perylenetetracarboxylic Diimide Heterogeneous for Enhanced Photocatalytic Activity. Sol. RRL 2021, 5, 2000453. [Google Scholar] [CrossRef]
- Yan, H.; Huang, Y. Polymer Composites of Carbon Nitride and Poly (3-hexylthiophene) to Achieve Enhanced Hydrogen Production from Water under Visible Light. Chem. Commun. 2011, 47, 4168–4170. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, L.; Chen, Z.; Yang, X.; Yu, Y.; Zhang, W.; Wang, Y.; Shi, Y.; Loh, K.; Xu, Q. Photocatalytic Hydrogen Evolution under Ambient Conditions on Polymeric Carbon Nitride/Donor-π-Acceptor Organic Molecule Heterostructures. Adv. Funct. Mater. 2020, 30, 2005106. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.M. Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Bian, J.; Zhang, Z.Q.; Feng, J.N.; Thangamuthu, M.; Yang, F.; Sun, L.; Li, Z.J.; Qu, Y.; Tang, D.Y.; Lin, Z.W.; et al. Energy Platform for Directed Charge Transfer in the Cascade Z-Scheme Heterojunction: CO2 Photoreduction without a Cocatalyst. Angew. Chem. Int. Ed. 2021, 60, 20906–20914. [Google Scholar] [CrossRef]



| Heterojunctions | Mass (mg) | Experimental Conditions | Light Source | Activity (mmol/g/h) | AQY (%) | Ref. |
|---|---|---|---|---|---|---|
| C3N4-2 wt%PEDOT | 100 | 10 vol % triethanolamine (TEOA), 1 wt% Pt | λ > 400 nm | 0.082 | — | [47] |
| PAN/g-C3N4 | 100 | 10 vol % TEOA, 1.5 wt% Pt | λ > 400 nm | 0.37 | — | [48] |
| PCzF/g-C3N4 | 100 | 10 vol % TEOA, 1 wt% Pt | λ > 420 nm | 0.628 | 27 @ 440 nm | [27] |
| 0.5Fe-2PTA/g-C3N4 | 100 | 10 vol % TEOA, 1 wt% Pt | λ > 420 nm | 0.687 | 0.27@ 520 nm | [37] |
| g-C3N4/CBV2+ | 50 | 10 vol % TEOA, 1 wt% Pt | λ > 420 nm | 0.831 | 3.8 @ 420 nm | [49] |
| 1 wt%PPy/g-C3N4 | 50 | 20 vol % TEOA, 3 wt% Pt | λ > 420 nm | 1.1 | 3.8 @ 420 nm | [50] |
| 1NP-3Mg-CN | 50 | 10 vol % TEOA, 1 wt% Pt | λ > 420 nm | 1.496 | 8.7 @ 405 nm | [15] |
| g-C3N4/PDI | 10 | 0.2 M AA, 1 wt% Pt | λ > 420 nm | 1.65 | — | [51] |
| 3PTBA/CN | 50 | 10 vol % TEOA, 1 wt% Pt | λ > 420 nm | 1.74 | 0.95@ 520 nm | [41] |
| g-C3N4-P3HT | 300 | 0.25 M Na2S-0.25 M Na2SO3 1 wt% Pt | λ > 400 nm | 1.867 | 2.9 @ 420 nm | [52] |
| 5N-PTEtOH/g-C3N4 | 100 | 10 vol % TEOA, 1 wt% Pt | λ > 420 nm | 2.424 | 7.8 @ 405 nm | [42] |
| PPFBT/CN-OH | 50 | 10 vol % TEOA, 1 wt% Pt | λ > 420 nm | 2.66 | 7.7 @ 420 nm | [17] |
| PEDOT/g-C3N4 | 50 | 0.1 M AA, 1 wt% Pt | λ > 420 nm | 3.15 | 10.56@ 405 nm | [23] |
| 20OTh5/g-C3N4 | 20 | 0.1 M AA, 1 wt% Pt | λ > 420 nm | 3.63 | 7.22 @ 520 nm | [22] |
| PCN/TBT | 10 | 10 vol % TEOA, 3 wt% Pt | λ > 420 nm | 4.63 | 3.0 @ 450 nm | [53] |
| 10PTT/g-C3N4 | 20 | 0.1 M AA, 2 wt% Pt | λ > 420 nm | 6.56 | 10.82 @ 405 nm 5.37 @ 520 nm | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Tong, J.; Chai, Z.; Wu, Y.; Wang, D.; Li, H. Gun–Bullet Model-Based Noncovalent Interactions Boosting Visible Light Photocatalytic Hydrogen Production in Poly Thieno[3,2-b]Thiophene/Graphitic Carbon Nitride Heterojunctions. Polymers 2025, 17, 1417. https://doi.org/10.3390/polym17101417
Li Y, Tong J, Chai Z, Wu Y, Wang D, Li H. Gun–Bullet Model-Based Noncovalent Interactions Boosting Visible Light Photocatalytic Hydrogen Production in Poly Thieno[3,2-b]Thiophene/Graphitic Carbon Nitride Heterojunctions. Polymers. 2025; 17(10):1417. https://doi.org/10.3390/polym17101417
Chicago/Turabian StyleLi, Yong, Jialu Tong, Zihao Chai, Yuanyuan Wu, Dongting Wang, and Hongbin Li. 2025. "Gun–Bullet Model-Based Noncovalent Interactions Boosting Visible Light Photocatalytic Hydrogen Production in Poly Thieno[3,2-b]Thiophene/Graphitic Carbon Nitride Heterojunctions" Polymers 17, no. 10: 1417. https://doi.org/10.3390/polym17101417
APA StyleLi, Y., Tong, J., Chai, Z., Wu, Y., Wang, D., & Li, H. (2025). Gun–Bullet Model-Based Noncovalent Interactions Boosting Visible Light Photocatalytic Hydrogen Production in Poly Thieno[3,2-b]Thiophene/Graphitic Carbon Nitride Heterojunctions. Polymers, 17(10), 1417. https://doi.org/10.3390/polym17101417







