Emerging Trends in Silane-Modified Nanomaterial–Polymer Nanocomposites for Energy Harvesting Applications
Abstract
1. Introduction
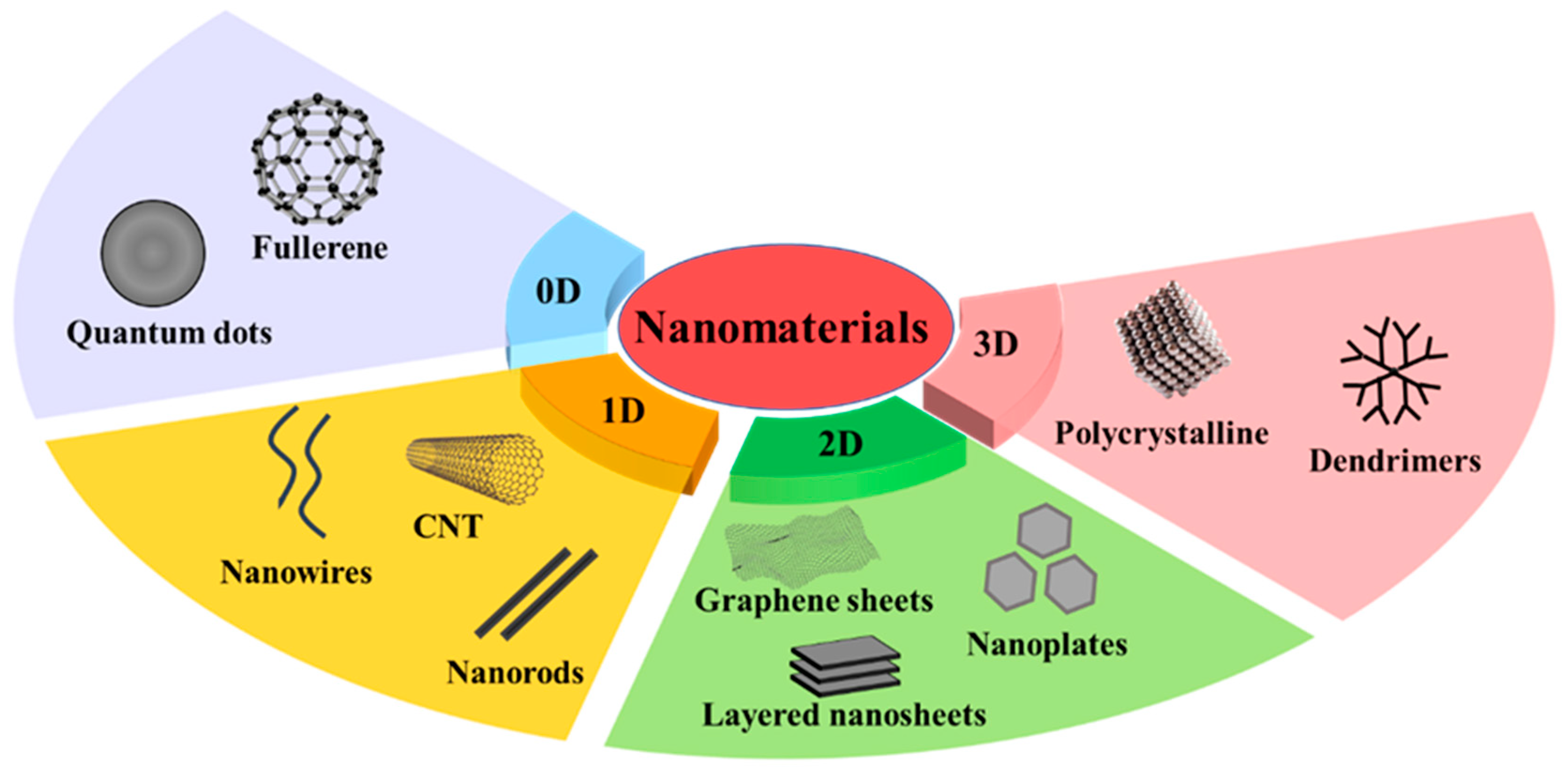
2. Classification of NMs
3. Synthesis of NMs
3.1. Physical Methods
3.1.1. Ball Milling
3.1.2. Chemical Vapor Deposition (CVD)
3.1.3. Laser Ablation
3.1.4. Arc Discharge
3.2. Chemical Methods
3.2.1. Chemical Reduction
3.2.2. Sol–Gel Process
3.2.3. Hydrothermal/Solvothermal Synthesis
3.2.4. Emulsion Techniques
3.3. Biological Methods
3.3.1. Plant-Mediated Synthesis
3.3.2. Bacteria in Synthesis of NPs
3.3.3. Fungi Mediated Synthesis
3.3.4. Yeast in the Synthesis of NPs
4. Surface Modification Techniques of NMs
4.1. Physical Techniques
4.2. Chemical Techniques
| Modification Method | Modifying Agent | NM | References |
|---|---|---|---|
| Physical technique | SDS | TiO2 | [51] |
| CTAB | Fe3O4 | [52] | |
| Octylamine | CNT | [53] | |
| CTAB | CNT | [54] | |
| SDS | GO | [55] | |
| PVP | GO | [56] | |
| Chemical technique | n-octadecyl thiol | FeO, CoO, NiO, CuO | [58] |
| Phosphonic acid | SnO2 | [59] | |
| Aminophenyl, nitrophenyl, benzoic acid | CNT | [60] | |
| MPS | CNT | [61] | |
| TA | GO | [62] | |
| VTES | GO | [63] |
5. Chemistry of Silane
5.1. Classification of Silanes
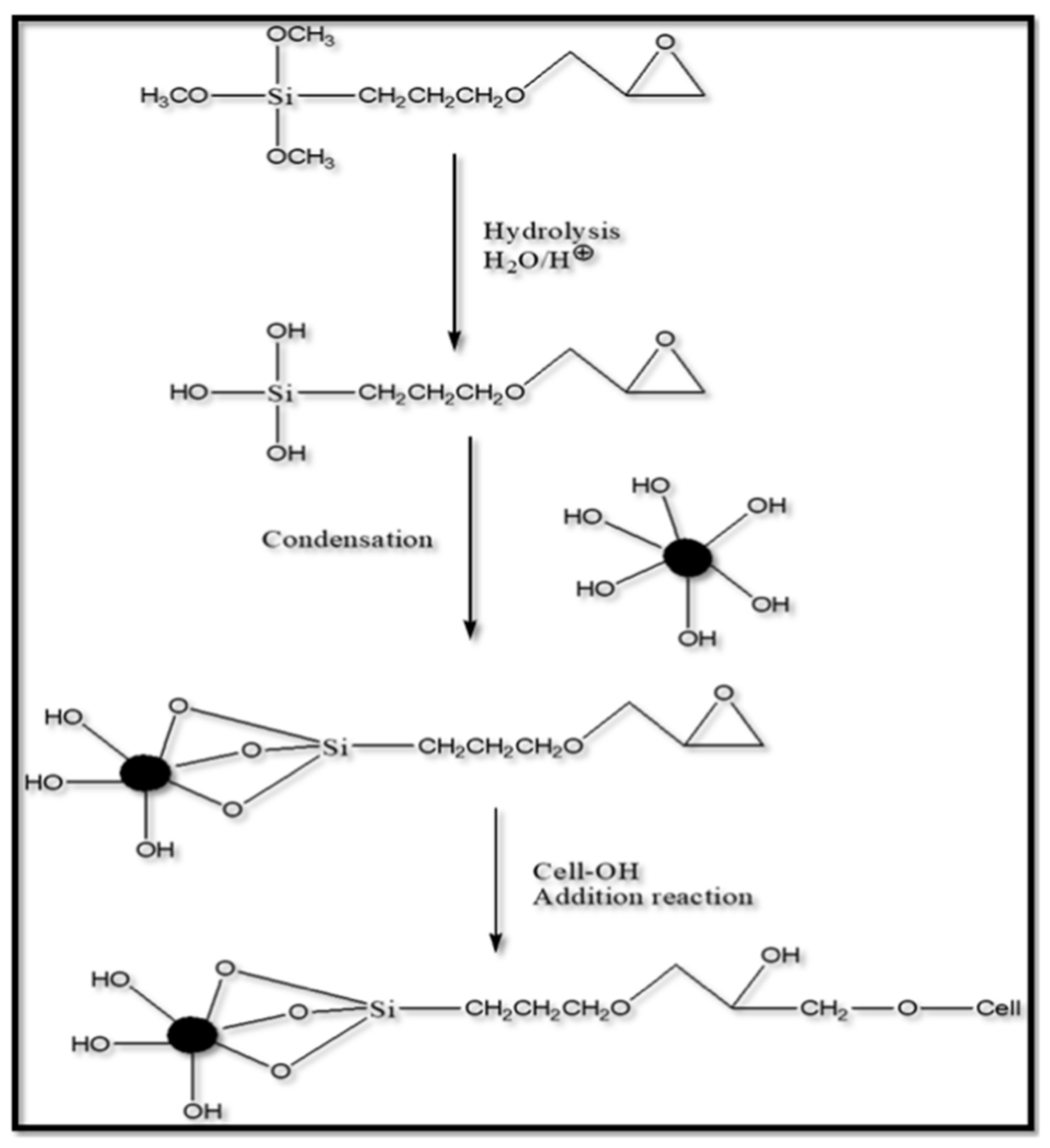
5.2. Mechanism of Silane Modification
5.3. Factors Affecting Silane Modification
5.4. Silanes Used for the Modification of NMs
6. Silane Modification of NMs
6.1. Fullerene
6.2. CNT
6.3. Graphene
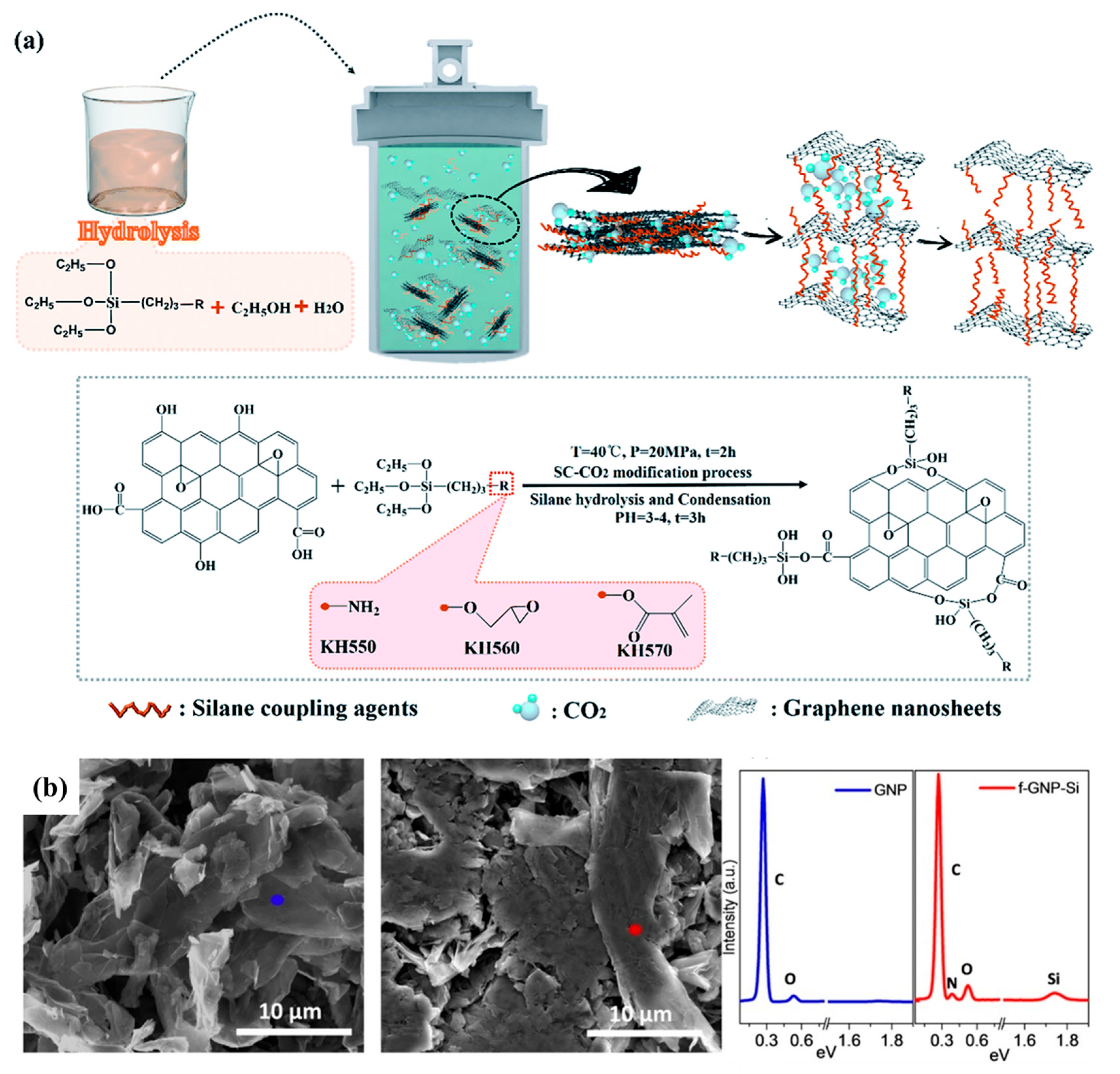
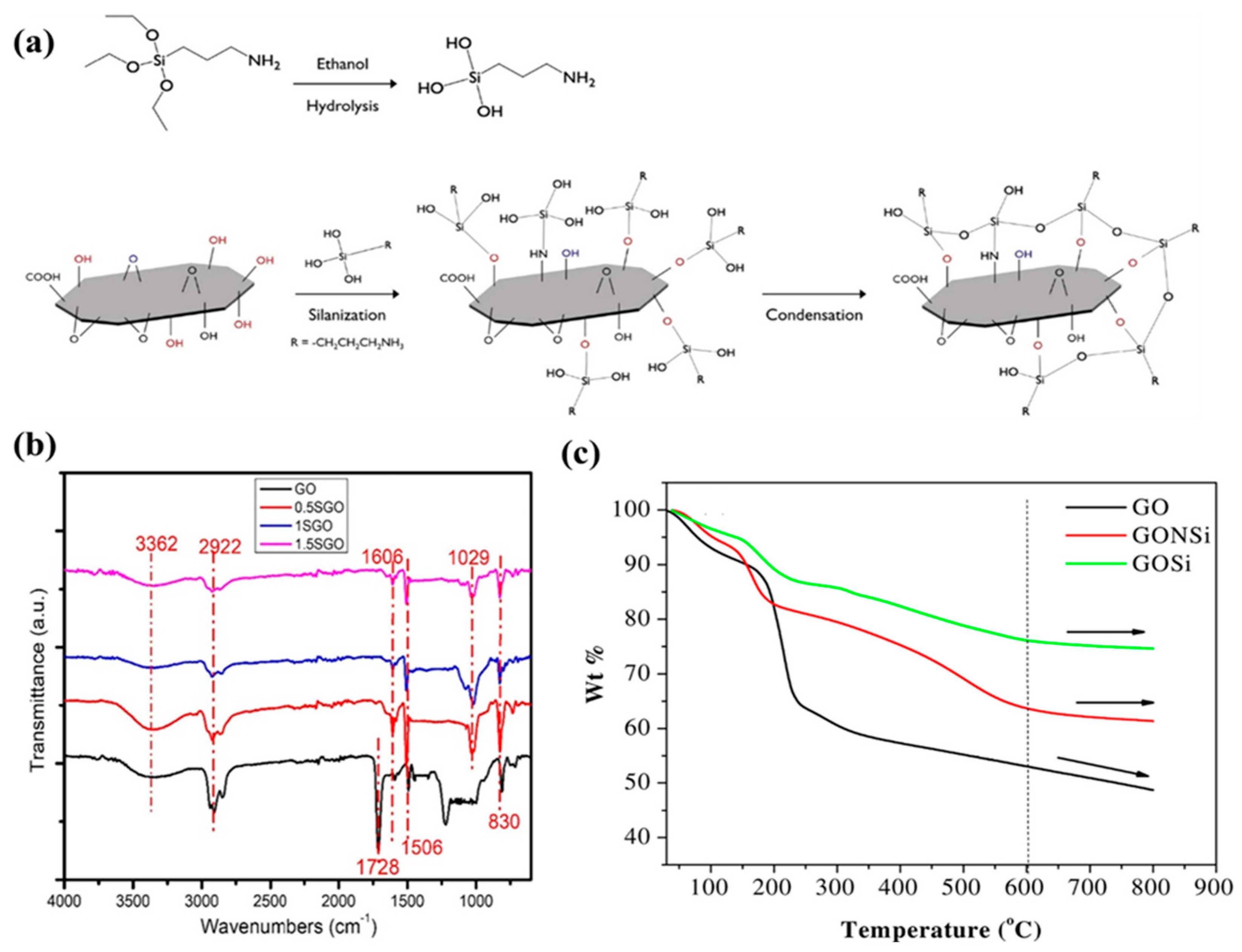
6.4. Graphene Oxide
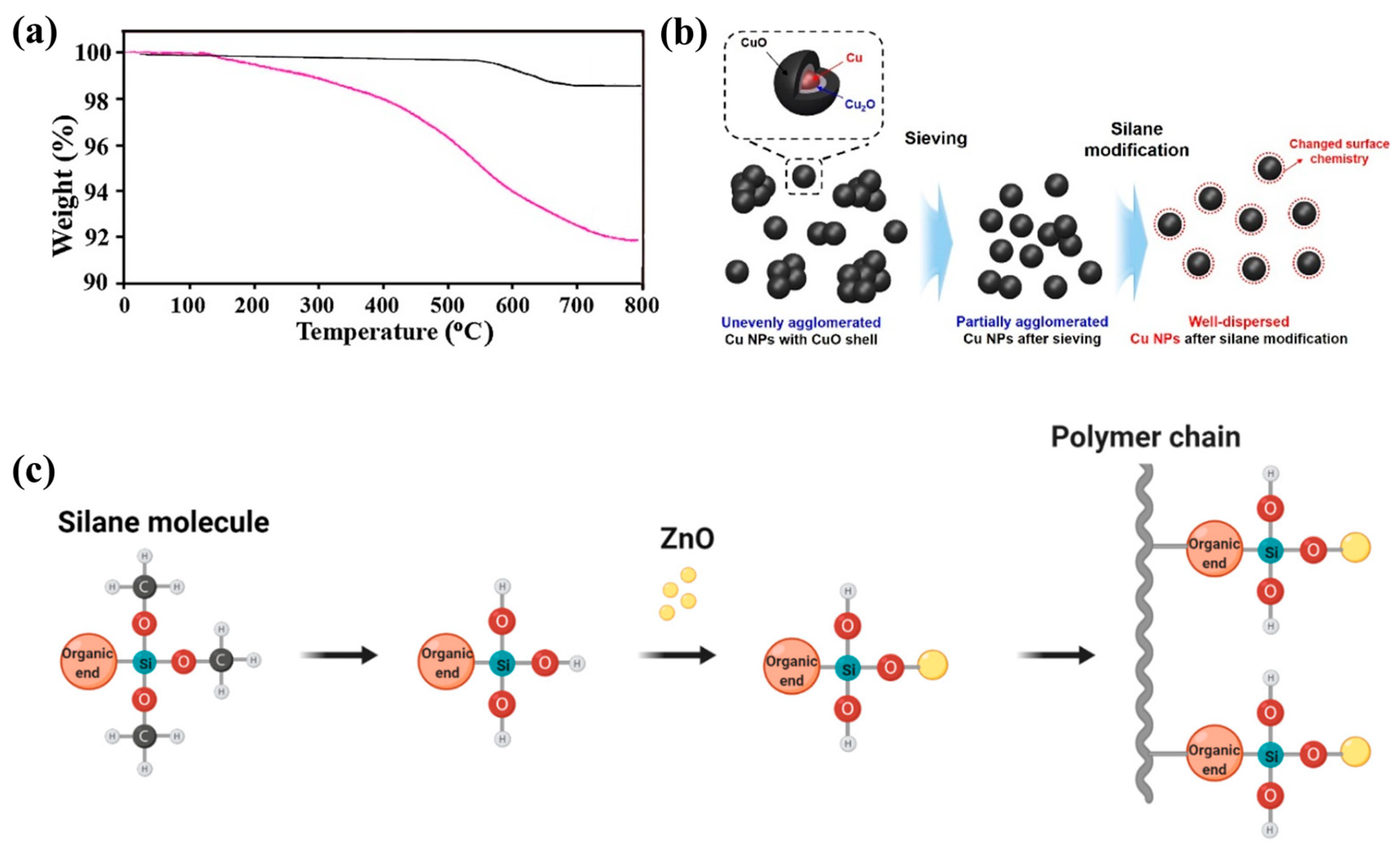
6.5. Metal Oxides
6.6. Other NMs
| S. No | Types of NM | Silane Modifier | Substrates Integrated with Silane Modified NM | References |
|---|---|---|---|---|
| 1 | Fullerene | APTES | TPU | [92] |
| APTES | Montmorillonite | [94] | ||
| APTES | Epoxy resin | [95,96] | ||
| 2 | CNT | GPTMS | Epoxy resin | [76] |
| APTES | Cementitious matrix | [77] | ||
| APTMS | Epoxy resin | [83] | ||
| APTES | PP, PVC | [104] | ||
| APTES | TPU | [10] | ||
| VTES | LLDPE | [105] | ||
| TESPT | NR matrix | [106] | ||
| p-CMPTMS | Glass | [108] | ||
| APTES | POM | [109] | ||
| APTES | SiO2 NPs | [110] | ||
| APTES | PA66 | [111] | ||
| AEAPTS | Epoxy resin | [112] | ||
| HDTMS | Ethyl cellulose | [113] | ||
| APTES | UD Kenaf | [114] | ||
| BTESPA | Epoxy resin | [115] | ||
| 3 | Graphene | APTES | PVDF | [78] |
| MPS | N-isopropylacrylamide | [123] | ||
| APTES, GPTMS, MPS | PET | [124] | ||
| APTMS | SR matrix | [126] | ||
| APTES | Epoxy resin | [127] | ||
| 4 | GO | VTES | Vinyl-ester resin | [86] |
| APTES | Cement | [159] | ||
| APTES | PU | [131] | ||
| APTES | Nylon 66 | [132] | ||
| AAPS | PA6 | [133] | ||
| ETEOS | PI | [134] | ||
| APTES | PAN | [135] | ||
| APTMS | Geopolymer | [136] | ||
| APTES, TEOS | Epoxy resin | [137] | ||
| TMSPED | Epoxy resin | [138] | ||
| GPTMS | Epoxy resin | [85,139] | ||
| TESPIC | PUU | [160] | ||
| TESPIC | Cerium marix | [161] | ||
| APTES | LDH | [162] | ||
| AEAPTS | Glutaraldehyde | [163] | ||
| 5 | MO NP | |||
| ZnO | TEOS, APTES | PVC | [145] | |
| TiO2 | APTES | PA | [146] | |
| CeO2 | TESPT | NBR | [147] | |
| CuO | APTMS | Solar cell electrode | [148] | |
| ZnO | APTES | Epoxy resin | [149] | |
| MgO | GPTMS | Epoxy resin | [150] | |
| ZnO | MPS | PAN | [151] | |
| Al2O3 | APTES | Epoxy resin | [84] | |
| SiO2 | VTMO | Polyurethane | [153] | |
| ZnO | APTMS | Polybenzoxazine | [154] | |
| 6 | Other NMs | |||
| Ag NPs | MPS | Epoxy resin | [155] | |
| Au NPs | MPS | MOF | [156] | |
| CB | MPS | Solvent | [157] | |
| CB | DTS | Pt NPs | [158] |
7. Applications
7.1. Textile Industries
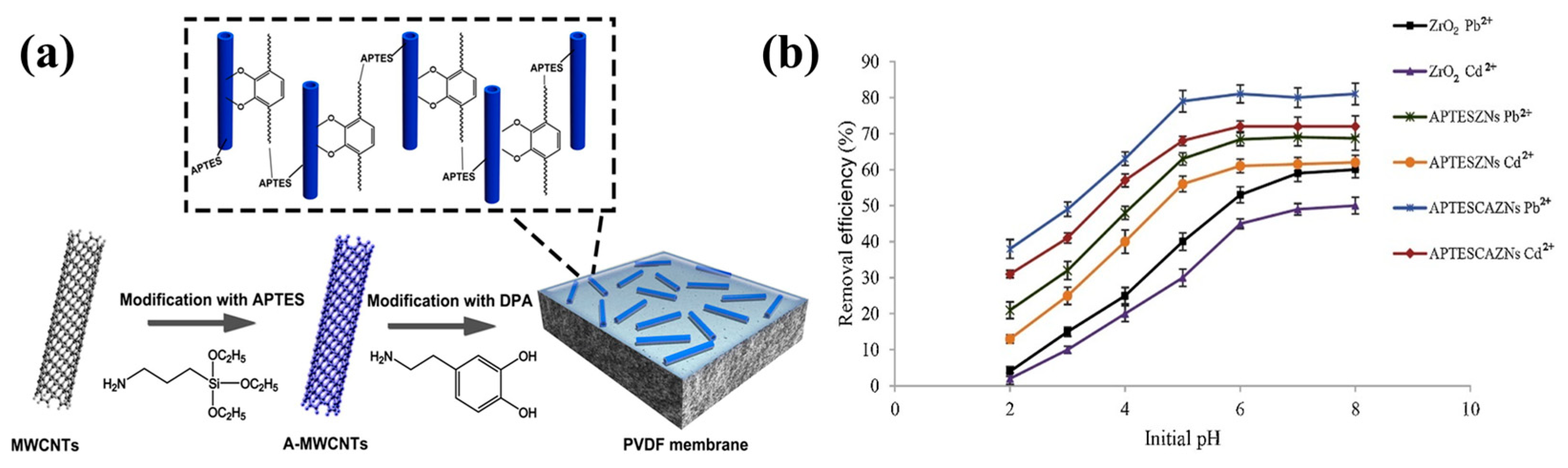
7.2. Water Treatment
7.3. Painting and Coatings
7.4. Catalysis
7.5. Biomedical Applications
7.6. Membrane Applications
7.7. Energy Harvesting Application
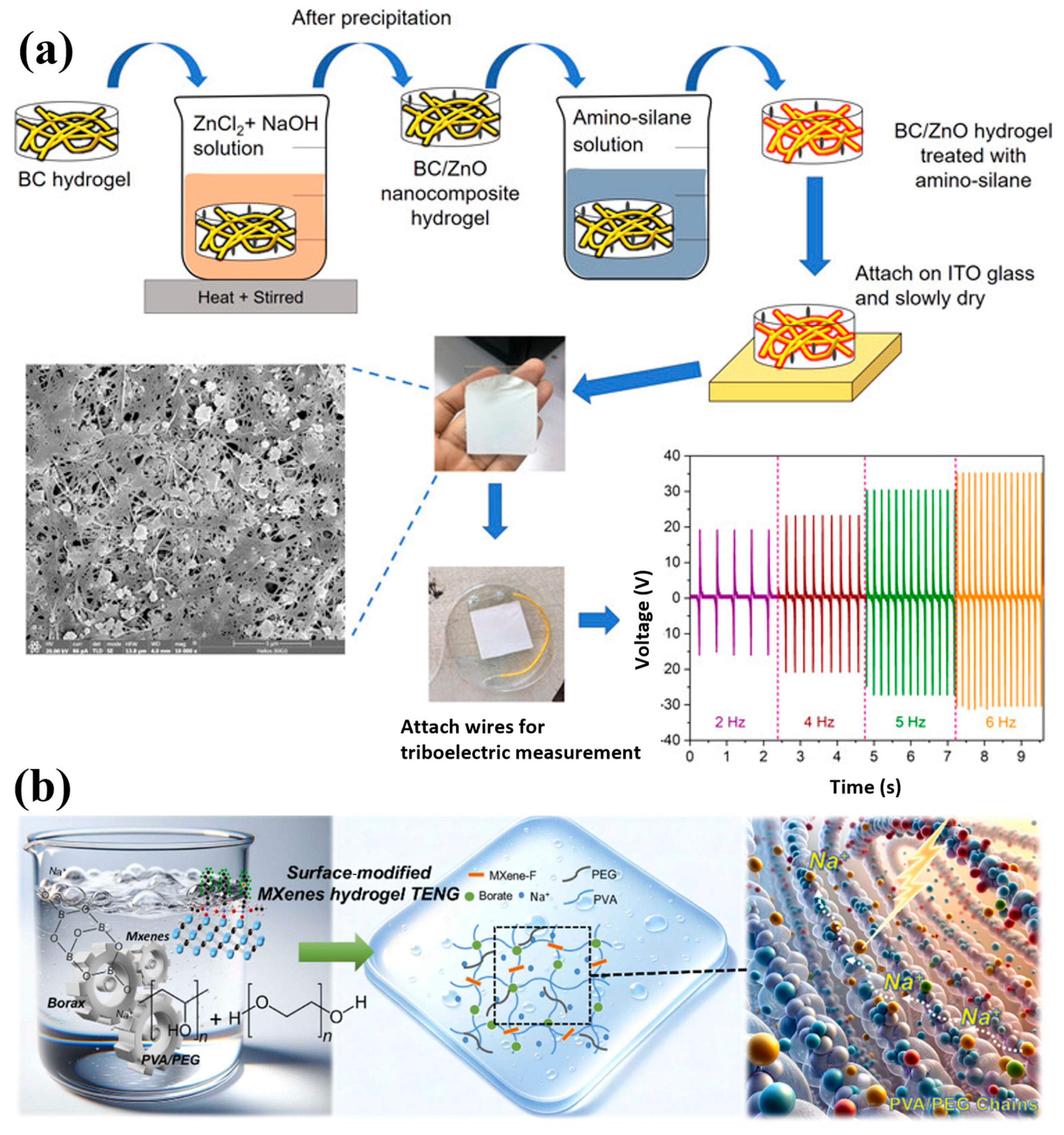
7.7.1. Triboelectric Nanogenerator (TENG) Application
7.7.2. Piezoelectric Nanogenerator (PENG) Application
7.7.3. Photovoltaic Application
| S. No | Type of NM | Silane Modifier | Application | References |
|---|---|---|---|---|
| 1 | ZnO NP | GPTMS | Textile industry | [170] |
| 2 | CuO NP | AEAPTMS, APTES, MPS | Textile industry | [168] |
| 3 | CNT | APTES | Water treatment | [172] |
| 4 | Fe3O4 | APTES | Water treatment | [174] |
| 5 | ZrO2 | APTES | Water treatment | [175] |
| 6 | Fe3O4 | APTES | Water treatment | [173] |
| 7 | CNT | APTES | Paintings and coatings | [182] |
| 8 | GO | APTES | Paintings and coatings | [180] |
| 9 | ZrO2 | APTES | Paintings and coatings | [183] |
| 10 | ZnO | AEAPTMS | Paintings and coatings | [184] |
| 11 | ZnO | HDTMS | Paintings and coatings | [179] |
| 12 | GO | VTES | Paintings and coatings | [185] |
| 13 | GO | APTES | Catalysis | [186] |
| 14 | Fe3O4 | APTES | Catalysis | [187] |
| 15 | Fe3O4 | GPTMS | Catalysis | [188] |
| 16 | GO | MPS | Biomedical application | [191] |
| 17 | CNT | MPS | Biomedical application | [192] |
| 18 | Fe3O4 | APTES | Biomedical application | [193] |
| 19 | Fe3O4 | APTES | Biomedical application | [194] |
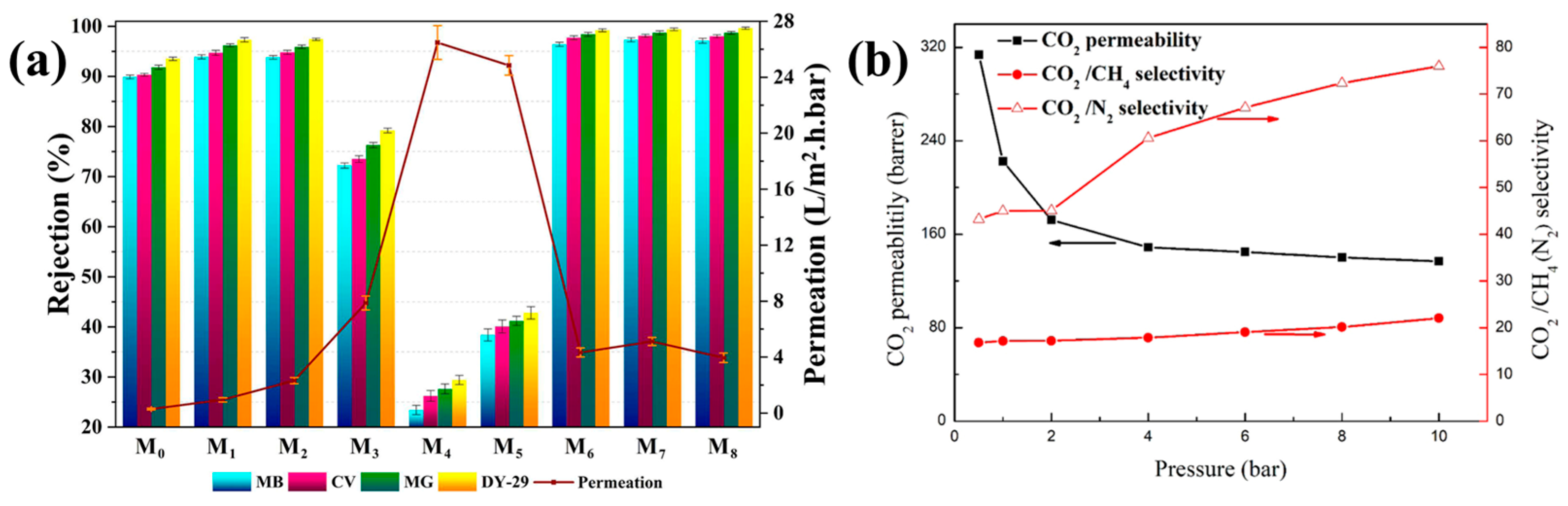
| 20 | GO | APTES | Nanofiltration membrane | [196] |
| 21 | GO | APTES | Mixed matrix membranes | [197] |
| 22 | Graphene | VTES | Nanofiltration membranes | [198] |
| 23 | CB | MPS | Microporous layer | [199] |
| 24 | ZnO | APTES | TENG | [210] |
| 25 | MXene nanosheets | CPTMS | TENG | [205] |
| 26 | Ag nanowires | FAS | TENG | [211] |
| 27 | Cellulose nanofibrils | PFOTES | TENG | [212] |
| 28 | LTA zeolite | GPTMS | TENG | [209] |
| 29 | SiO2 | HDTMS | TENG | [213] |
| 30 | ZnO | APTES | PENG | [217] |
| 31 | KNN nanorod | APTMS | PENG | [218] |
| 32 | KNN nanorod | APTMS | PENG | [219] |
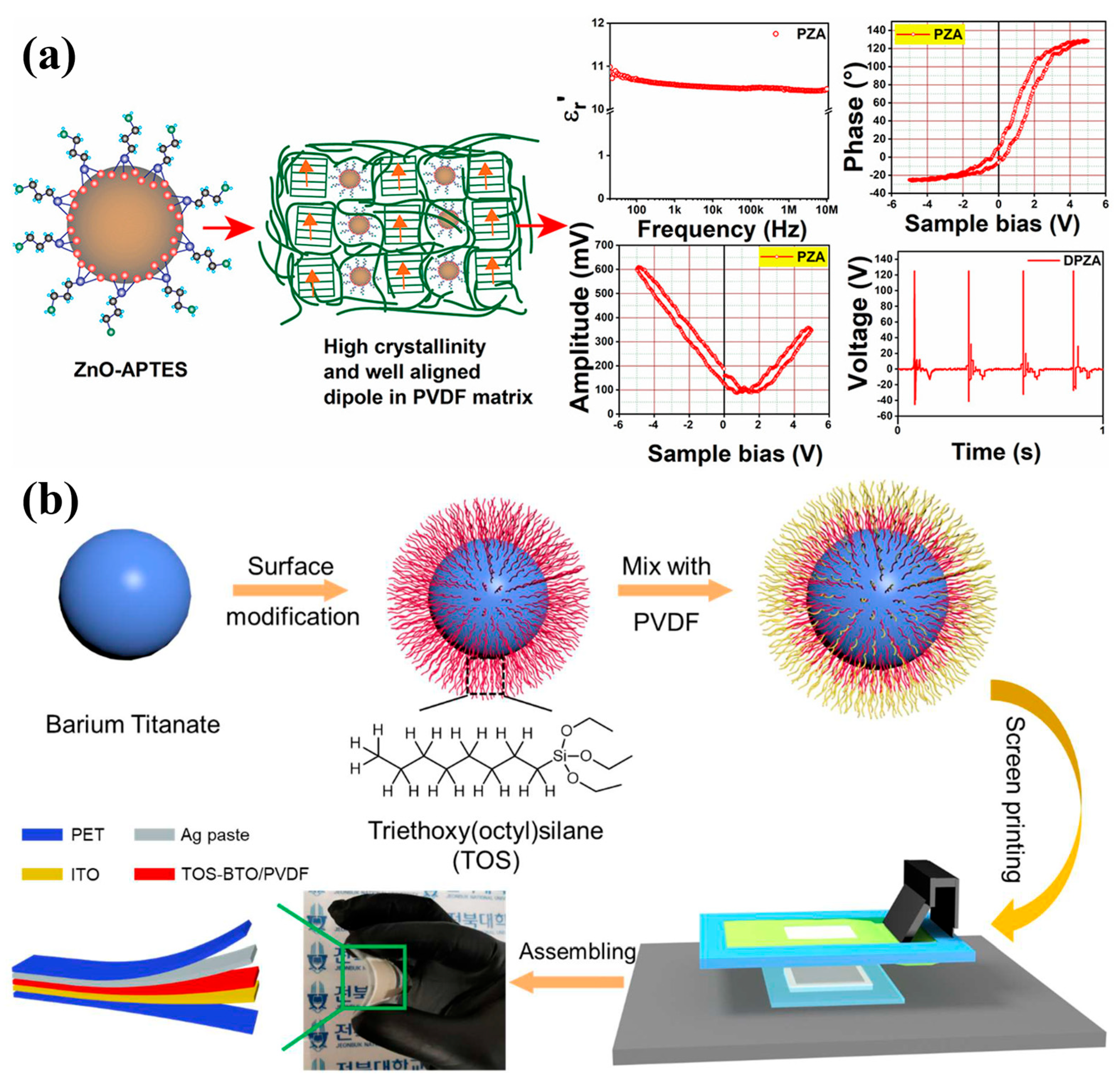
| 33 | BTO | TEVS | PENG | [220] |
| 34 | BTO | TOS | PENG | [221] |
| 35 | TiO2 | APTMS | Photovoltaics | [224] |
| 36 | TiO2 | APTMS | Photovoltaics | [225] |
| 37 | TiO2 | APTMS | Photovoltaics | [204] |
| 38 | ZnO | APTMS | Photovoltaics | [226] |
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Han, J.; Zhao, D.; Li, D.; Wang, X.; Jin, Z.; Zhao, K. Polymer-based nanomaterials and applications for vaccines and drugs. Polymers 2018, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.-Y.; Baek, J.-B. Nanocomposites derived from polymers and inorganic nanoparticles. Materials 2010, 3, 3654–3674. [Google Scholar] [CrossRef]
- Moradi, F.; Ghaedi, A.; Fooladfar, Z.; Bazrgar, A. Recent advance on nanoparticles or nanomaterials with anti-multidrug resistant bacteria and anti-bacterial biofilm properties: A systematic review. Heliyon 2023, 9, e22105. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Peng, W.; Zare, Y.; Rhee, K.Y. Effects of Size and Aggregation/Agglomeration of Nanoparticles on the Interfacial/Interphase Properties and Tensile Strength of Polymer Nanocomposites. Nanoscale Res. Lett. 2018, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Atif, R.; Inam, F. Reasons and remedies for the agglomeration of multilayered graphene and carbon nanotubes in polymers. Beilstein J. Nanotechnol. 2016, 7, 1174–1196. [Google Scholar] [CrossRef] [PubMed]
- Ngouangna, E.N.; Jaafar, M.Z.; Norddin, M.M.; Agi, A.; Oseh, J.O.; Mamah, S. Surface modification of nanoparticles to improve oil recovery Mechanisms: A critical review of the methods, influencing Parameters, advances and prospects. J. Mol. Liq. 2022, 360, 119502. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Vengatesan, M.R.; Mittal, V. Surface modification of nanomaterials for application in polymer nanocomposites: An overview. In Surface Modification of Nanoparticle and Natural Fiber Fillers; Wiley Online Library: Hoboken, NJ, USA, 2015; pp. 1–28. [Google Scholar]
- Aziz, T.; Ullah, A.; Fan, H.; Jamil, M.I.; Khan, F.U.; Ullah, R.; Iqbal, M.; Ali, A.; Ullah, B. Recent progress in silane coupling agent with its emerging applications. J. Polym. Environ. 2021, 29, 3427–3443. [Google Scholar] [CrossRef]
- Wondu, E.; Lule, Z.C.; Kim, J. Fabrication of high dielectric properties and higher thermal conductivity thermoplastic polyurethane composites with CNT-covered SrTiO3. Polym. Test. 2022, 110, 107576. [Google Scholar] [CrossRef]
- Ganguly, S.; Mondal, S.; Das, P.; Bhawal, P.; Das, T.K.; Ghosh, S.; Remanan, S.; Das, N.C. An insight into the physico-mechanical signatures of silylated graphene oxide in poly(ethylene methyl acrylate) copolymeric thermoplastic matrix. Macromol. Res. 2019, 27, 268–281. [Google Scholar] [CrossRef]
- Liu, W.T. Nanoparticles and their biological and environmental applications. J. Biosci. Bioeng. 2006, 102, 1–7. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Recent Advancements in Nanobiosensors: Current Trends, Challenges, Applications, and Future Scope. Biosensors 2022, 12, 892. [Google Scholar] [CrossRef] [PubMed]
- Barhoum, A.; García-Betancourt, M.L.; Jeevanandam, J.; Hussien, E.A.; Mekkawy, S.A.; Mostafa, M.; Omran, M.M.; S Abdalla, M.; Bechelany, M. Review on natural, incidental, bioinspired, and engineered nanomaterials: History, definitions, classifications, synthesis, properties, market, toxicities, risks, and regulations. Nanomaterials 2022, 12, 177. [Google Scholar] [CrossRef] [PubMed]
- Gabris, M.A.; Ping, J. Carbon nanomaterial-based nanogenerators for harvesting energy from environment. Nano Energy 2021, 90, 106494. [Google Scholar] [CrossRef]
- Pan, K.; Zhong, Q. Organic nanoparticles in foods: Fabrication, characterization, and utilization. Annu. Rev. Food Sci. Technol. 2016, 7, 245–266. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Yadav, T.P.; Yadav, R.M.; Singh, D.P. Mechanical milling: A top down approach for the synthesis of nanomaterials and nanocomposites. Nanosci. Nanotechnol. 2012, 2, 22–48. [Google Scholar] [CrossRef]
- Rajput, N. Methods of preparation of nanoparticles–A review. Int. J. Adv. Eng. Technol. 2015, 7, 1806–1811. [Google Scholar]
- Ghorbani, H.R. A review of methods for synthesis of Al nanoparticles. Orient. J. Chem 2014, 30, 1941–1949. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, C.; Chen, H.; Ji, H.; Zhu, Q.; Yang, W.; Chen, L.; Chen, Z.; Zhu, W. Scalable and facile synthesis of V2O5 nanoparticles via ball milling for improved aerobic oxidative desulfurization. Green Energy Environ. 2021, 6, 169–175. [Google Scholar] [CrossRef]
- Ghaemi, F.; Ali, M.; Yunus, R.; Othman, R.N. Synthesis of carbon nanomaterials using catalytic chemical vapor deposition technique. In Synthesis, Technology and Applications of Carbon Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–27. [Google Scholar]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef]
- Vallejos, S.; Selina, S.; Annanouch, F.E.; Gràcia, I.; Llobet, E.; Blackman, C. Aerosol assisted chemical vapour deposition of gas sensitive SnO2 and Au-functionalised SnO2 nanorods via a non-catalysed vapour solid (VS) mechanism. Sci. Rep. 2016, 6, 28464. [Google Scholar] [CrossRef]
- Lim, J.Y.; Mubarak, N.M.; Abdullah, E.C.; Nizamuddin, S.; Khalid, M.; Inamuddin. Recent trends in the synthesis of graphene and graphene oxide based nanomaterials for removal of heavy metals—A review. J. Ind. Eng. Chem. 2018, 66, 29–44. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Semaltianos, N. Nanoparticles by laser ablation. Crit. Rev. Solid State Mater. Sci. 2010, 35, 105–124. [Google Scholar] [CrossRef]
- Rivera-Chaverra, M.J.; Restrepo-Parra, E.; Acosta-Medina, C.D.; Mello, A.; Ospina, R. Synthesis of oxide iron nanoparticles using laser ablation for possible hyperthermia applications. Nanomaterials 2020, 10, 2099. [Google Scholar] [CrossRef]
- Fang, F.; Kennedy, J.; Manikandan, E.; Futter, J.; Markwitz, A. Morphology and characterization of TiO2 nanoparticles synthesized by arc discharge. Chem. Phys. Lett. 2012, 521, 86–90. [Google Scholar] [CrossRef]
- Zhang, D.; Ye, K.; Yao, Y.; Liang, F.; Qu, T.; Ma, W.; Yang, B.; Dai, Y.; Watanabe, T. Controllable synthesis of carbon nanomaterials by direct current arc discharge from the inner wall of the chamber. Carbon 2019, 142, 278–284. [Google Scholar] [CrossRef]
- Landage, S.; Wasif, A.; Dhuppe, P. Synthesis of nanosilver using chemical reduction methods. Int. J. Adv. Res. Eng. Appl. Sci. 2014, 3, 14–22. [Google Scholar]
- Zhang, Q.-L.; Yang, Z.-M.; Ding, B.-J.; Lan, X.-Z.; Guo, Y.-J. Preparation of copper nanoparticles by chemical reduction method using potassium borohydride. Trans. Nonferrous Met. Soc. China 2010, 20, s240–s244. [Google Scholar] [CrossRef]
- Mackenzie, J.D.; Bescher, E.P. Chemical routes in the synthesis of nanomaterials using the sol–gel process. Acc. Chem. Res. 2007, 40, 810–818. [Google Scholar] [CrossRef]
- Hasnidawani, J.; Azlina, H.; Norita, H.; Bonnia, N.; Ratim, S.; Ali, E. Synthesis of ZnO nanostructures using sol-gel method. Procedia Chem. 2016, 19, 211–216. [Google Scholar] [CrossRef]
- Li, J.; Wu, Q.; Wu, J. Synthesis of Nanoparticles via Solvothermal and Hydrothermal Methods; Springer: Cham, Switzerland, 2016; Volume 12. [Google Scholar]
- Yang, Y.; Matsubara, S.; Xiong, L.; Hayakawa, T.; Nogami, M. Solvothermal synthesis of multiple shapes of silver nanoparticles and their SERS properties. J. Phys. Chem. C 2007, 111, 9095–9104. [Google Scholar] [CrossRef]
- Srivastava, R. Synthesis and characterization techniques of nanomaterials. Int. J. Green Nanotechnol. 2012, 4, 17–27. [Google Scholar] [CrossRef]
- Li, L.; Huang, M.; Liu, J.; Guo, Y. PtxSn/C electrocatalysts synthesized by improved microemulsion method and their catalytic activity for ethanol oxidation. J. Power Sources 2011, 196, 1090–1096. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Naturae 2014, 6, 35–44. [Google Scholar] [CrossRef]
- Ansari, M.A.; Khan, H.M.; Alzohairy, M.A.; Jalal, M.; Ali, S.G.; Pal, R.; Musarrat, J. Green synthesis of Al2O3 nanoparticles and their bactericidal potential against clinical isolates of multi-drug resistant Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2015, 31, 153–164. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface Sci. 2010, 156, 1–13. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanopart. Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, B.S.; Surinder, K.; Verma, M. Green approach for nanoparticle biosynthesis by fungi: Current trends and applications. Crit. Rev. Biotechnol. 2012, 32, 49–73. [Google Scholar] [CrossRef]
- Rai, M.; Bonde, S.; Golinska, P.; Trzcińska-Wencel, J.; Gade, A.; Abd-Elsalam, K.A.; Shende, S.; Gaikwad, S.; Ingle, A.P. Fusarium as a Novel Fungus for the Synthesis of Nanoparticles: Mechanism and Applications. J. Fungi. 2021, 7, 139. [Google Scholar] [CrossRef]
- Mukherjee, P.; Senapati, S.; Mandal, D.; Ahmad, A.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular Synthesis of Gold Nanoparticles by the Fungus Fusarium oxysporum. ChemBioChem 2002, 3, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Mourato, A.; Gadanho, M.; Lino, A.R.; Tenreiro, R. Biosynthesis of Crystalline Silver and Gold Nanoparticles by Extremophilic Yeasts. Bioinorg. Chem. Appl. 2011, 2011, 546074. [Google Scholar] [CrossRef] [PubMed]
- Rong, M.; Zhang, M.; Ruan, W. Surface modification of nanoscale fillers for improving properties of polymer nanocomposites: A review. Mater. Sci. Technol. 2006, 22, 787–796. [Google Scholar] [CrossRef]
- Ahangaran, F.; Navarchian, A.H. Recent advances in chemical surface modification of metal oxide nanoparticles with silane coupling agents: A review. Adv. Colloid Interface Sci. 2020, 286, 102298. [Google Scholar] [CrossRef]
- Li, X.; Yoneda, M.; Shimada, Y.; Matsui, Y. Effect of surfactants on the aggregation and stability of TiO2 nanomaterial in environmental aqueous matrices. Sci. Total Environ. 2017, 574, 176–182. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Meng, W.; Wang, P.; Wu, F.; Tang, Z.; Han, X.; Giesy, J.P. Cetyltrimethylammonium bromide-coated Fe3O4 magnetic nanoparticles for analysis of 15 trace polycyclic aromatic hydrocarbons in aquatic environments by ultraperformance, liquid chromatography with fluorescence detection. Anal. Chem. 2015, 87, 7667–7675. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J. Improvement of thermal conductivity and latent heat of cellulose film using surfactant and surface-treated CNT with stearic acid. Compos. Part A Appl. Sci. Manuf. 2022, 156, 106897. [Google Scholar] [CrossRef]
- Heltina, D.; Fisli, A.; Wulan, P.P. The Influence Surface Modification of CNT Using Surfactant to Formation of Composite. J. Phys. Conf. Ser. 2019, 1351, 012090. [Google Scholar] [CrossRef]
- Salihi, E.Ç.; Wang, J.; Coleman, D.J.L.; Šiller, L. Enhanced removal of nickel(II) ions from aqueous solutions by SDS-functionalized graphene oxide. Sep. Sci. Technol. 2016, 51, 1317–1327. [Google Scholar] [CrossRef]
- Wu, X.; Field, R.W.; Wu, J.J.; Zhang, K. Polyvinylpyrrolidone modified graphene oxide as a modifier for thin film composite forward osmosis membranes. J. Membr. Sci. 2017, 540, 251–260. [Google Scholar] [CrossRef]
- Mallakpour, S.; Madani, M. A review of current coupling agents for modification of metal oxide nanoparticles. Prog. Org. Coat. 2015, 86, 194–207. [Google Scholar] [CrossRef]
- Wang, B.; Li, J.; Wang, G.; Liang, W.; Zhang, Y.; Shi, L.; Guo, Z.; Liu, W. Methodology for robust superhydrophobic fabrics and sponges from in situ growth of transition metal/metal oxide nanocrystals with thiol modification and their applications in oil/water separation. ACS Appl. Mater. Interfaces 2013, 5, 1827–1839. [Google Scholar] [CrossRef] [PubMed]
- Gheonea, R.; Mak, C.; Crasmareanu, E.C.; Simulescu, V.; Plesu, N.; Ilia, G. Surface modification of SnO2 with phosphonic acids. J. Chem. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Cheng, H.K.F.; Bao, H.; Pan, Y.; Li, L.; Chan, S.H. Covalent functionalization of carbon nanotubes for ultimate interfacial adhesion to liquid crystalline polymer. Soft Matter 2011, 7, 9505–9514. [Google Scholar] [CrossRef]
- Wu, G.; Liu, S.; Wu, X.; Ding, X. Core-shell structure of carbon nanotube nanocapsules reinforced poly(lactic acid) composites. J. Appl. Polym. Sci. 2017, 134, 44919. [Google Scholar] [CrossRef]
- Sainz-Urruela, C.; Vera-López, S.; Paz San Andrés, M.; Díez-Pascual, A.M. Surface functionalization of graphene oxide with tannic acid: Covalent vs non-covalent approaches. J. Mol. Liq. 2022, 357, 119104. [Google Scholar] [CrossRef]
- Lyu, Y.; Gu, C.; Tao, J.; Yao, X.; Zhao, G.; Dai, C. Thermal-resistant, shear-stable and salt-tolerant polyacrylamide/surface-modified graphene oxide composite. J. Mater. Sci. 2019, 54, 14752–14762. [Google Scholar] [CrossRef]
- Matinlinna, J.P.; Lung, C.Y.K.; Tsoi, J.K.H. Silane adhesion mechanism in dental applications and surface treatments: A review. Dent. Mater. 2018, 34, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Indumathy, B.; Sathiyanathan, P.; Prasad, G.; Reza, M.S.; Prabu, A.A.; Kim, H. A Comprehensive Review on Processing, Development and Applications of Organofunctional Silanes and Silane-Based Hyperbranched Polymers. Polymers 2023, 15, 2517. [Google Scholar] [CrossRef] [PubMed]
- Cotton, F.A.; Wilkinson, G.; Murillo, C.A.; Bochmann, M. Advanced Inorganic Chemistry; John Wiley and Sons, Inc.: New York, NY, USA, 1999. [Google Scholar]
- Witucki, G.L. A silane primer: Chemistry and applications of alkoxy silanes. J. Coat. Technol. 1993, 65, 57. [Google Scholar]
- Indumathy, B.; Gunasekhar, R.; Sathiyanathan, P.; Arun, A.P. Chemistry and Applications of Organosilanes—An Overview. ECS Trans. 2022, 107, 14539. [Google Scholar]
- Antonucci, J.M.; Dickens, S.H.; Fowler, B.O.; Xu, H.H.; McDonough, W.G. Chemistry of silanes: Interfaces in dental polymers and composites. J. Res. Natl. Inst. Stand. Technol. 2005, 110, 541–548. [Google Scholar] [CrossRef]
- Goyal, S. Silanes: Chemistry and applications. J. Indian Prosthodont. Soc. 2006, 6, 14–18. [Google Scholar] [CrossRef]
- Gellman, A.; Naasz, B.; Schmidt, R.; Chaudhury, M.; Gentle, T. Secondary neutral mass spectrometry studies of germanium-silane coupling agent-polymer interphases. J. Adhes. Sci. Technol. 1990, 4, 597–601. [Google Scholar] [CrossRef]
- Zhu, D.; Hu, N.; Schaefer, D.W. Water-based sol–gel coatings for military coating applications. Handb. Waterborne Coat. 2020, 1–27. [Google Scholar]
- Somasundaram, S. Silane coatings of metallic biomaterials for biomedical implants: A preliminary review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2901–2918. [Google Scholar] [CrossRef]
- Al-Saadi, S.; Singh Raman, R. Silane Coatings for Corrosion and Microbiologically Influenced Corrosion Resistance of Mild Steel: A Review. Materials 2022, 15, 7809. [Google Scholar] [CrossRef]
- Janamphansang, L.; Wootthikanokkhan, J.; Nawalertpanya, S. Preparation of VO2 Nanoparticles with Surface Functionalization for Thermochromic Application. Eng. J. 2019, 23, 205–215. [Google Scholar] [CrossRef]
- Nylander, A.; Fu, Y.; Huang, M.; Liu, J. Covalent anchoring of carbon nanotube-based thermal interface materials using epoxy-silane monolayers. IEEE Trans. Compon. Packag. Manuf. Technol. 2018, 9, 427–433. [Google Scholar] [CrossRef]
- Silvestro, L.; Ruviaro, A.S.; de Matos, P.R.; Pelisser, F.; Mezalira, D.Z.; Gleize, P.J.P. Functionalization of multi-walled carbon nanotubes with 3-aminopropyltriethoxysilane for application in cementitious matrix. Constr. Build. Mater. 2021, 311, 125358. [Google Scholar] [CrossRef]
- Lin, B.; Li, Z.-T.; Yang, Y.; Li, Y.; Lin, J.-C.; Zheng, X.-M.; He, F.-A.; Lam, K.-H. Enhanced dielectric permittivity in surface-modified graphene/PVDF composites prepared by an electrospinning-hot pressing method. Compos. Sci. Technol. 2019, 172, 58–65. [Google Scholar] [CrossRef]
- Chambers, R.C.; Jones Jr, W.E.; Haruvy, Y.; Webber, S.E.; Fox, M.A. Influence of steric effects on the kinetics of ethyltrimethoxysilane hydrolysis in a fast sol-gel system. Chem. Mater. 1993, 5, 1481–1486. [Google Scholar] [CrossRef]
- Jiang, H.; Zheng, Z.; Li, Z.; Wang, X. Effects of Temperature and Solvent on the Hydrolysis of Alkoxysilane under Alkaline Conditions. Ind. Eng. Chem. Res. 2006, 45, 8617–8622. [Google Scholar] [CrossRef]
- Jiang, H.; Zheng, Z.; Wang, X. Kinetic study of methyltriethoxysilane (MTES) hydrolysis by FTIR spectroscopy under different temperatures and solvents. Vib. Spectrosc. 2008, 46, 1–7. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Ge, X. Construction of Amperometric Glucose Sensor using Carbon Nanotubes and IO4-oxidized GOx. DEStech Trans. Eng. Technol. Res. 2017. [Google Scholar] [CrossRef]
- Mishra, K.; Singh, R.P. Effect of APTMS modification on multiwall carbon nanotube reinforced epoxy nanocomposites. Compos. Part B Eng. 2019, 162, 425–432. [Google Scholar] [CrossRef]
- Huang, J.-q.; Liu, K.; Song, X.; Zheng, G.; Chen, Q.; Sun, J.; Jin, H.; Jiang, L.; Jiang, Y.; Zhang, Y. Incorporation of Al2O3, GO, and Al2O3@ GO nanoparticles into water-borne epoxy coatings: Abrasion and corrosion resistance. RSC Adv. 2022, 12, 24804–24820. [Google Scholar] [CrossRef]
- Jiang, H.; Ji, Y.; Gan, J.; Wang, L. Enhancement of thermal and mechanical properties of bismaleimide using a graphene oxide modified by epoxy silane. Materials 2020, 13, 3836. [Google Scholar] [CrossRef] [PubMed]
- Gholiha, H.M.; Ghadami, A.; Monajjemi, M.; Ehsani, M. Enhanced Physical and Mechanical Properties of Flake–Shape/Vinyl-ester Nanocomposites Through Surface Modification of Graphene and Glass Flake: A Comparison with Simulated Data. Biointerface Res. Appl. Chem. 2020, 11, 11316–11337. [Google Scholar]
- Mishra, R.; Militky, J. Carbon-based nanomaterials. In Nanotechnology in Textiles: Theory and Application; Woodhead Publishing: Cambridge, UK, 2018; p. 163. [Google Scholar]
- Kroto, H.W.; Fischer, J.E.; Cox, D.E. The Fullerenes; Newnes: London, UK, 2012. [Google Scholar]
- Klupp, G.; Margadonna, S.; Prassides, K. Fullerenes, in Reference Module in Materials Science and Materials Engineering; Elsevier Inc.: Amsterdam, The Nederland, 2016. [Google Scholar]
- Samoilova, N.A.; Krayukhina, M.A.; Klemenkova, Z.S.; Naumkin, A.V.; Buzin, M.I.; Mezhuev, Y.O.; Turetsky, E.A.; Andreev, S.M.; Anuchina, N.M.; Popov, D.A. Hydrophilization and Functionalization of Fullerene C60 with Maleic Acid Copolymers by Forming a Non-Covalent Complex. Polymers 2024, 16, 1736. [Google Scholar] [CrossRef] [PubMed]
- De La Puente, F.L.; Nierengarten, J.-F. Fullerenes: Principles and Applications; Royal Society of Chemistry: London, UK, 2011. [Google Scholar]
- Tayfun, U.; Kanbur, Y.; Abaci, U.; Guney, H.Y.; Bayramli, E. Mechanical, flow and electrical properties of thermoplastic polyurethane/fullerene composites: Effect of surface modification of fullerene. Compos. Part B Eng. 2015, 80, 101–107. [Google Scholar] [CrossRef]
- Wang, C.; Guo, Z.-X.; Fu, S.; Wu, W.; Zhu, D. Polymers containing fullerene or carbon nanotube structures. Prog. Polym. Sci. 2004, 29, 1079–1141. [Google Scholar] [CrossRef]
- Chen, Q.; Sai, T.; Fang, Z.; Guo, Z. Thermal stability and oxygen resistance of polypropylene composites with fullerene/montmorillonite hybrid fillers. J. Therm. Anal. Calorim. 2021, 146, 1383–1392. [Google Scholar] [CrossRef]
- Das, S.; Halder, S.; Kumar, K. A comprehensive study on step-wise surface modification of C60: Effect of oxidation and silanization on dynamic mechanical and thermal stability of epoxy nanocomposite. Mater. Chem. Phys. 2016, 179, 120–128. [Google Scholar] [CrossRef]
- Das, S.; Halder, S.; Sinha, A.; Imam, M.A.; Khan, N.I. Assessing Nanoscratch Behavior of Epoxy Nanocomposite Toughened with Silanized Fullerene. ACS Appl. Nano Mater. 2018, 1, 3653–3662. [Google Scholar] [CrossRef]
- Erkmen, B. Polystyrene-Based and Carbon Fabric-Reinforced Polymer Composites Containing Carbon Nanotubes: Preparation, Modification and Characterization. Ph.D. Thesis, Middle East Technical University, Ankara, Turkije, 2020. [Google Scholar]
- Yim, Y.-J.; Yoon, Y.-H.; Kim, S.-H.; Lee, J.-H.; Chung, D.-C.; Kim, B.-J. Carbon Nanotube/Polymer Composites for Functional Applications. Polymers 2025, 17, 119. [Google Scholar] [CrossRef]
- Mohd Nurazzi, N.; Asyraf, M.M.; Khalina, A.; Abdullah, N.; Sabaruddin, F.A.; Kamarudin, S.H.; Ahmad, S.b.; Mahat, A.M.; Lee, C.L.; Aisyah, H. Fabrication, functionalization, and application of carbon nanotube-reinforced polymer composite: An overview. Polymers 2021, 13, 1047. [Google Scholar] [CrossRef]
- de la Calle, I.; Romero-Rivas, V. The role of nanomaterials in analytical chemistry: Trace metal analysis. In Applications of Nanomaterials; Elsevier: Amsterdam, The Nederland, 2018; pp. 251–301. [Google Scholar]
- Mallakpour, S.; Soltanian, S. Surface functionalization of carbon nanotubes: Fabrication and applications. RSC Adv. 2016, 6, 109916–109935. [Google Scholar] [CrossRef]
- Gulati, P.; Kaur, P.; Rajam, M.V.; Srivastava, T.; Ali, M.A.; Mishra, P.; Islam, S.S. Leukemia biomarker detection by using photoconductive response of CNT electrode: Analysis of sensing mechanism based on charge transfer induced Fermi level fluctuation. Sens. Actuators B Chem. 2018, 270, 45–55. [Google Scholar] [CrossRef]
- Kalyani, M. Mechanical Behavior and surface modification of CNTs that are functionalized with Polymer Composites. Int. J. Res. Appl. Sci. Eng. Technol. 2020, 8, 1453–1459. [Google Scholar] [CrossRef]
- Mashhadzadeh, A.H.; Fereidoon, A.; Ahangari, M.G. Surface modification of carbon nanotubes using 3-aminopropyltriethoxysilane to improve mechanical properties of nanocomposite based polymer matrix: Experimental and density functional theory study. Appl. Surf. Sci. 2017, 420, 167–179. [Google Scholar] [CrossRef]
- Azizi, H.; Fallahi, H.; Ghasemi, I.; Karrabi, M.; Nazemian, M. Silane Modification of Carbon Nanotubes and Preparation of Silane Cross-Linked LLDPE/MWCNT Nanocomposites. J. Vinyl Addit. Technol. 2020, 26, 113–126. [Google Scholar] [CrossRef]
- Ding, Y.; Cao, X.; Weng, G.; Yin, Q.; Wang, L.; Chen, Z. Crack growth behavior of natural rubber influenced by functionalized carbon nanotubes. J. Appl. Polym. Sci. 2017, 134, 44527. [Google Scholar] [CrossRef]
- Hong, T.; Jeong, S.-M.; Choi, Y.K.; Lim, T.; Ju, S. Superhydrophobic, elastic, and conducting polyurethane-carbon nanotube–silane–aerogel composite microfiber. Polymers 2020, 12, 1772. [Google Scholar] [CrossRef]
- Takada, T.; Kitamura, Y.; Takakuwa, S. Fabrication of infrared-responsive carbon nanotube coating on glass surface through covalent bond formation using photoreactive silane coupling agent. J. Ceram. Soc. Jpn. 2020, 128, 1066–1071. [Google Scholar] [CrossRef]
- Goriparthi, B.K.; Naveen, P.; Ravi Sankar, H.; Ghosh, S. Effect of functionalization and concentration of carbon nanotubes on mechanical, wear and fatigue behaviours of polyoxymethylene/carbon nanotube nanocomposites. Bull. Mater. Sci. 2019, 42, 98. [Google Scholar] [CrossRef]
- Peng, B.; Takai, C.; Razavi-khosroshahi, H.; Fuji, M. Effect of silane modification on CNTs/silica composites fabricated by a non-firing process to enhance interfacial property and dispersibility. Adv. Powder Technol. 2018, 29, 2091–2096. [Google Scholar] [CrossRef]
- Kang, S.; Kim, J.; Park, J.H.; Jung, I.; Park, M. Multiwalled carbon nanotube pretreatment to enhance tensile properties, process stability, and filler dispersion of polyamide 66 nanocomposites. Compos. Part B Eng. 2020, 198, 108204. [Google Scholar] [CrossRef]
- Jamshaid, F.; Khan, R.U.; Islam, A.; Ahmad, A.; Adrees, M.; Dilshad, R. Tactical tuning of mechanical and thermo-mechanical properties of glass fiber/epoxy multi-scale composites by incorporating N-(2-aminoethyl)-3-aminopropyl trimethoxysilane functionalized carbon nanotubes. Iran. Polym. J. 2020, 29, 875–889. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, W. Superhydrophobic/superoleophilic and reinforced ethyl cellulose sponges for oil/water separation: Synergistic strategies of cross-linking, carbon nanotube composite, and nanosilica modification. ACS Appl. Mater. Interfaces 2017, 9, 29167–29176. [Google Scholar] [CrossRef]
- Sapiai, N.; Jumahat, A.; Jawaid, M.; Khan, A. Effect of MWCNT surface functionalisation and distribution on compressive properties of kenaf and hybrid kenaf/glass fibres reinforced polymer composites. Polymers 2020, 12, 2522. [Google Scholar] [CrossRef]
- Mozaffarinasab, H.; Jamshidi, M. Surface modification of carbon nanotubes by a bifunctional amine silane; effects on physical/mechanical/thermal properties of epoxy nanocomposite. Prog. Org. Coat. 2023, 179, 107521. [Google Scholar] [CrossRef]
- Chakraborthy, A.; Nuthalapati, S.; Nag, A.; Altinsoy, M.E.; He, S. Graphene-based triboelectric nanogenerators for energy-harvesting applications. Sens. Actuators A Phys. 2024, 380, 116046. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.-e.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Hummers Jr, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Li, F.; Tang, M.; Li, T.; Zhang, L.; Hu, C. Two-dimensional graphene/g-C3N4 in-plane hybrid heterostructure for enhanced photocatalytic activity with surface-adsorbed pollutants assistant. Appl. Catal. B Environ. 2020, 268, 118397. [Google Scholar] [CrossRef]
- Li, J.; Zheng, H.; Liu, L.; Meng, F.; Cui, Y.; Wang, F. Modification of graphene and graphene oxide and their applications in anticorrosive coatings. J. Coat. Technol. Res. 2021, 18, 311–331. [Google Scholar] [CrossRef]
- Innes, J.R.; Young, R.J.; Papageorgiou, D.G. Graphene nanoplatelets as a replacement for carbon black in rubber compounds. Polymers 2022, 14, 1204. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Development of graphene-based polymeric nanocomposites: A brief overview. Polymers 2021, 13, 2978. [Google Scholar] [CrossRef]
- Kang, W.; Liang, J.; Liu, T.; Long, H.; Huang, L.; Shi, Q.; Zhang, J.; Deng, S.; Tan, S. Preparation of silane-dispersed graphene crosslinked vinyl carboxymethyl chitosan temperature-responsive hydrogel with antibacterial properties. Int. J. Biol. Macromol. 2022, 200, 99–109. [Google Scholar] [CrossRef]
- Wei, J.; Peng, S.; Xue, B.; Yang, Z.; Qin, S.; Yu, J.; Xu, G. Effect of silane functionalized graphene prepared by a supercritical carbon dioxide process on the barrier properties of polyethylene terephthalate composite films. RSC Adv. 2019, 9, 21903–21910. [Google Scholar] [CrossRef]
- Guo, X.; Li, H.; Wang, C. Improvement on freeze-thaw durability of municipal solid waste incineration fly ash-based autoclaved wall blocks by triethoxy(octyl)silane/nano-Al2O3 and triethoxy(octyl)silane/graphene nanoplatelets coatings. J. Clean. Prod. 2023, 392, 136188. [Google Scholar] [CrossRef]
- Ren, H.; Cunha, E.; Li, Z.; Wang, L.; Kinloch, I.A.; Yi, D.; Kretinin, A.; Sun, Q.; Fan, Z.; Young, R.J. Silane-functionalized graphene nanoplatelets for silicone rubber nanocomposites. J. Mater. Sci. 2022, 57, 2683–2696. [Google Scholar] [CrossRef]
- Huang, Z.; Li, F.; Liu, C.; Yang, M.; Pan, B.; Rao, W.; Lei, Y.; Yu, C. Bifunctional modification of graphene by phosphorus/nitrogen-containing silane compounds for smoke suppression and flame retardancy of epoxy resins. J. Appl. Polym. Sci. 2023, 140, e54285. [Google Scholar] [CrossRef]
- Md Said, N.H.; Liu, W.W.; Khe, C.S.; Lai, C.W.; Zulkepli, N.N.; Aziz, A. Review of the past and recent developments in functionalization of graphene derivatives for reinforcement of polypropylene nanocomposites. Polym. Compos. 2021, 42, 1075–1108. [Google Scholar] [CrossRef]
- Babak, F.; Abolfazl, H.; Alimorad, R.; Parviz, G. Preparation and mechanical properties of graphene oxide: Cement nanocomposites. Sci. World J. 2014, 2014, 276323. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Luceño Sánchez, J.A.; Peña Capilla, R.; García Díaz, P. Recent developments in graphene/polymer nanocomposites for application in polymer solar cells. Polymers 2018, 10, 217. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.H. Fabrication of silane-grafted graphene oxide and its effect on the structural, thermal, mechanical, and hysteretic behavior of polyurethane. Sci. Rep. 2020, 10, 19152. [Google Scholar] [CrossRef]
- Torres-Castillo, C.S.; Fuentes-Agustin, J.E.; García-Reyes, E.M.; Zamudio-Aguilar, M.A.; Morales-Zamudio, L.; Lozano, T.; Navarro-Pardo, F.; Sanchez-Valdez, S.; Martinez-Colunga, G.; Karami, S. Effect of non-functionalized and functionalized graphene oxide with a silane agent on the thermal and rheological properties of nylon 6, 6. Iran. Polym. J. 2023, 32, 139–149. [Google Scholar] [CrossRef]
- Chen, Y.; Li, D.; Yang, W.; Xiao, C. Enhancement of mechanical, thermal and tribological properties of AAPS-modified graphene oxide/polyamide 6 nanocomposites. Compos. Part B Eng. 2018, 138, 55–65. [Google Scholar] [CrossRef]
- Fazil, S.; Bangesh, M.; Rehman, W.; Liaqat, K.; Saeed, S.; Sajid, M.; Waseem, M.; Shakeel, M.; Bibi, I.; Guo, C.-Y. Mechanical, thermal, and dielectric properties of functionalized graphene oxide/polyimide nanocomposite films. Nanomater. Nanotechnol. 2019, 9, 1847980418821037. [Google Scholar] [CrossRef]
- Manu, M.; Roy, K.R.; Hassan, S.B.A.; Masihadas, A. Effect of EPD coated silanized graphene oxide on carbon fiber reinforced plastic: An emphasis on mechanical properties at cryogenic temperatures. Surf. Coat. Technol. 2022, 451, 129043. [Google Scholar]
- Tang, Z.Q.; Sui, H.; de Souza, F.B.; Sagoe-Crentsil, K.; Duan, W. Silane-modified graphene oxide in geopolymer: Reaction kinetics, microstructure, and mechanical performance. Cem. Concr. Compos. 2023, 139, 104997. [Google Scholar] [CrossRef]
- Abdel Rehim, M.; Turky, G. Silane-functionalized graphene oxide/epoxy resin nanocomposites: Dielectric and thermal studies. J. Appl. Polym. Sci. 2019, 136, 48253. [Google Scholar] [CrossRef]
- Jamali, N.; Khosravi, H.; Rezvani, A.; Tohidlou, E. Mechanical properties of multiscale graphene oxide/basalt fiber/epoxy composites. Fibers 2019, 20, 138–146. [Google Scholar] [CrossRef]
- Jamali, N.; Khosravi, H.; Rezvani, A.; Tohidlou, E.; Poulis, J. Viscoelastic and dry-sliding wear properties of basalt fiber-reinforced composites based on a surface-modified graphene oxide/epoxy matrix. J. Ind. Text. 2021, 50, 939–953. [Google Scholar] [CrossRef]
- Amrutha, B.; Prasad, G.; Sathiyanathan, P.; Reza, M.S.; Kim, H.; Pathak, M.; Prabu, A.A. Fabrication of CuO-NP-doped PVDF composites based Electrospun triboelectric nanogenerators for wearable and biomedical applications. Polymers 2023, 15, 2442. [Google Scholar] [CrossRef]
- Koshy, J.T.; Vasudevan, D.; Sangeetha, D.; Prabu, A.A. Biopolymer based multifunctional films loaded with anthocyanin rich floral extract and ZnO nano particles for smart packaging and wound healing applications. Polymers 2023, 15, 2372. [Google Scholar] [CrossRef]
- Zhang, W.; Lai, E.P. Chemical Functionalities of 3-aminopropyltriethoxy-silane for Surface Modification of Metal Oxide Nanoparticles. Silicon 2022, 14, 6535–6545. [Google Scholar] [CrossRef]
- Soytaş, S.H.; Oğuz, O.; Menceloğlu, Y.Z. Polymer nanocomposites with decorated metal oxides. In Polymer Composites with Functionalized Nanoparticles; Elsevier: Amsterdam, The Nederland, 2019; pp. 287–323. [Google Scholar]
- Grasset, F.; Saito, N.; Li, D.; Park, D.; Sakaguchi, I.; Ohashi, N.; Haneda, H.; Roisnel, T.; Mornet, S.; Duguet, E. Surface modification of zinc oxide nanoparticles by aminopropyltriethoxysilane. J. Alloys Compd. 2003, 360, 298–311. [Google Scholar] [CrossRef]
- Ma, X.; Dong, Z.; Wang, B.; Liu, L.; Ye, R. Preparation of ZnO nanoparticles modified with silane coupling-agents to fabricateanti-UV Poly(vinyl chloride) films. Turk. J. Chem. 2022, 46, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Dinari, M.; Haghighi, A. Surface modification of TiO2 nanoparticle by three dimensional silane coupling agent and preparation of polyamide/modified-TiO2 nanocomposites for removal of Cr(VI) from aqueous solutions. Prog. Org. Coat. 2017, 110, 24–34. [Google Scholar] [CrossRef]
- Biuk Afshari, B.; Jamshidi, M.; Rostami, M.; Ghamarpoor, R. Improving the Mechanical/Anticorrosive Properties of a Nitrile Rubber-Based Adhesive Filled with Cerium Oxide Nanoparticles Using a Two-Step Surface Modification Method. ACS Omega 2022, 7, 44912–44927. [Google Scholar] [CrossRef]
- Jang, Y.-R.; Ryu, C.-H.; Chu, J.-H.; Nam, J.-B.; Kim, H.-S. Multiple intense pulsed light sintering of silane surface modified Cu oxide nanoparticle paste on Si wafer substrate for solar cell electrode. Thin Solid Film. 2021, 722, 138577. [Google Scholar] [CrossRef]
- Jaramillo, A.F.; Montoya, L.F.; Prabhakar, J.M.; Sanhueza, J.P.; Fernández, K.; Rohwerder, M.; Rojas, D.; Montalba, C.; Melendrez, M.F. Formulation of a multifunctional coating based on polyphenols extracted from the Pine radiata bark and functionalized zinc oxide nanoparticles: Evaluation of hydrophobic and anticorrosive properties. Prog. Org. Coat. 2019, 135, 191–204. [Google Scholar] [CrossRef]
- Hornak, J.; Trnka, P.; Kadlec, P.; Michal, O.; Mentlík, V.; Šutta, P.; Csányi, G.M.; Tamus, Z.Á. Magnesium oxide nanoparticles: Dielectric properties, surface functionalization and improvement of epoxy-based composites insulating properties. Nanomaterials 2018, 8, 381. [Google Scholar] [CrossRef]
- Aamer, H.; Kim, S.-B.; Oh, J.-M.; Park, H.; Jo, Y.-M. ZnO-Impregnated Polyacrylonitrile Nanofiber Filters against Various Phases of Air Pollutants. Nanomaterials 2021, 11, 2313. [Google Scholar] [CrossRef]
- da Silva, B.L.; Caetano, B.L.; Chiari-Andréo, B.G.; Pietro, R.C.L.R.; Chiavacci, L.A. Increased antibacterial activity of ZnO nanoparticles: Influence of size and surface modification. Colloids Surf. B. Biointerfaces 2019, 177, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Güdümcüoğlu, N.; Sevim Ünlütürk, S.; Çelik, İ.M.; Yener, H.B.; Helvaci, Ş.Ş. Development of surface modified SiO2 nanoparticles incorporated clearcoats for automotive industry. Surf. Interfaces 2025, 56, 105491. [Google Scholar] [CrossRef]
- Habes, A.; Derradji, M.; Mehelli, O.; Benaliouche, F.; Abdous, S.; Medjaouri, Y.; Abderrahim, N.C.; Fodil, H.; Kadi, M.E.A. Effective attenuation of electromagnetic waves via silane surface modified zinc oxide/polybenzoxazine nanocomposites for EMI shielding application. Mater. Today Commun. 2024, 38, 107608. [Google Scholar] [CrossRef]
- Xianxue, L.; Bingyun, Z.; Limei, X.; Dandan, W.; Zonglin, L.; Huichai, Z. Study on properties of conductive adhesive prepared with silver nanoparticles modified by silane coupling agent. Rare Met. Mater. Eng. 2012, 41, 24–27. [Google Scholar] [CrossRef]
- Rehacek, V.; Hotovy, I.; Predanocy, M.; Vojs, M.; Marton, M. Surface modification of metal oxide films by gold nanoparticles. J. Phys. Conf. Ser. 2019, 1319, 012005. [Google Scholar] [CrossRef]
- Atif, M.; Bongiovanni, R.; Giorcelli, M.; Celasco, E.; Tagliaferro, A. Modification and characterization of carbon black with mercaptopropyltrimethoxysilane. Appl. Surf. Sci. 2013, 286, 142–148. [Google Scholar] [CrossRef]
- Lee, W.H.; Seo, J.; Lee, T.; Kim, H. Preparation of a self-assembled organosilane coating on carbon black as a catalyst support in polymer electrolyte membrane fuel cells. J. Power Sources 2015, 274, 1140–1146. [Google Scholar] [CrossRef]
- Guo, L.; Wu, J.; Wang, H. Mechanical and perceptual characterization of ultra-high-performance cement-based composites with silane-treated graphene nano-platelets. Constr. Build. Mater. 2020, 240, 117926. [Google Scholar] [CrossRef]
- Ahmadian-Alam, L.; Teymoori, M.; Mahdavi, H. Graphene oxide-anchored reactive sulfonated copolymer via simple one pot condensation polymerization: Proton-conducting solid electrolytes. J. Polym. Res. 2018, 25, 13. [Google Scholar] [CrossRef]
- Parhizkar, N.; Shahrabi, T.; Ramezanzadeh, B. Steel surface pre-treated by an advance and eco-friendly cerium oxide nanofilm modified by graphene oxide nanosheets; electrochemical and adhesion measurements. J. Alloys Compd. 2018, 747, 109–123. [Google Scholar] [CrossRef]
- Gunasekhar, R.; Reza, M.S.; Kim, K.J.; Prabu, A.A.; Kim, H. Electrospun PVDF/aromatic HBP of 4th gen based flexible and self-powered TENG for wearable energy harvesting and health monitoring. Sci. Rep. 2023, 13, 22645. [Google Scholar] [CrossRef] [PubMed]
- Kahyaoglu, L.N.; Rickus, J.L. Robust covalent coupling scheme for the development of fret aptasensor based on amino-silane-modified graphene oxide. Langmuir 2018, 34, 14586–14596. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wen, W.; Wu, J.-M. Titania nanowires functionalized polyester fabrics with enhanced photocatalytic and antibacterial performances. J. Hazard. Mater. 2018, 343, 285–297. [Google Scholar] [CrossRef]
- Gao, S.; Dong, X.; Huang, J.; Li, S.; Li, Y.; Chen, Z.; Lai, Y. Rational construction of highly transparent superhydrophobic coatings based on a non-particle, fluorine-free and water-rich system for versatile oil-water separation. Chem. Eng. J. 2018, 333, 621–629. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Li, B.; Sun, P.; Yang, J.; Xu, H.; Liu, Y. Superhydrophobic and ultraviolet-blocking cotton textiles. ACS Appl. Mater. Interfaces 2011, 3, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- El-Shafei, A.; ElShemy, M.; Abou-Okeil, A. Eco-friendly finishing agent for cotton fabrics to improve flame retardant and antibacterial properties. Carbohydr. Polym. 2015, 118, 83–90. [Google Scholar] [CrossRef]
- Agrawal, N.; Low, P.S.; Tan, J.S.J.; Fong, E.W.M.; Lai, Y.; Chen, Z. Durable easy-cleaning and antibacterial cotton fabrics using fluorine-free silane coupling agents and CuO nanoparticles. Nano Mater. Sci. 2020, 2, 281–291. [Google Scholar] [CrossRef]
- Chruściel, J.J. Modifications of textile materials with functional silanes, liquid silicone softeners, and silicone rubbers—A review. Polymers 2022, 14, 4382. [Google Scholar] [CrossRef]
- Afzal, F.; Ashraf, M.; Manzoor, S.; Aziz, H.; Nosheen, A.; Riaz, S. Development of novel antiviral nanofinishes for bioactive textiles. Polym. Bull. 2022, 80, 8447–8466. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, S.; Singhal, A. Magnetic nanoparticle-based nanocontainers for water treatment. In Smart Nanocontainers; Elsevier: Amsterdam, The Nederland, 2020; pp. 487–498. [Google Scholar]
- Yang, X.; He, Y.; Zeng, G.; Chen, X.; Shi, H.; Qing, D.; Li, F.; Chen, Q. Bio-inspired method for preparation of multiwall carbon nanotubes decorated superhydrophilic poly(vinylidene fluoride) membrane for oil/water emulsion separation. Chem. Eng. J. 2017, 321, 245–256. [Google Scholar] [CrossRef]
- Tamaddoni Moghaddam, S.; Naimi-Jamal, M.R.; Rohlwing, A.; Hussein, F.B.; Abu-Zahra, N. High Removal Capacity of Arsenic from Drinking Water Using Modified Magnetic Polyurethane Foam Nanocomposites. J. Polym. Environ. 2019, 27, 1497–1504. [Google Scholar] [CrossRef]
- Naeimi Bagheini, A.; Saeidi, M.; Boroomand, N. Removal of diazinon pesticide using amino-silane modified magnetite nanoparticles from contaminated water. Int. J. Nanosci. Nanotechnol. 2018, 14, 19–32. [Google Scholar]
- Majedi, A.; Davar, F.; Abbasi, A. Citric acid-silane modified zirconia nanoparticles: Preparation, characterization and adsorbent efficiency. J. Environ. Chem. Eng. 2018, 6, 701–709. [Google Scholar] [CrossRef]
- Pan, T.; Su, Z.; Yan, Y.; Zhu, X.; Qi, F.; Wu, L. The Synergistic Effects of Aminosilane Coupling Agent on the Adhesion Performance of Silane Primer for Silicone Resin Thermal Protection Coating. Polymers 2023, 15, 2361. [Google Scholar] [CrossRef]
- Mounayer, N.; Margel, S. Engineering of Silane–Pyrrolidone Nano/Microparticles and Anti-Fogging Thin Coatings. Polymers 2024, 16, 2013. [Google Scholar] [CrossRef]
- Xavier, J.R. Investigation on the anticorrosion, adhesion and mechanical performance of epoxy nanocomposite coatings containing epoxy-silane treated nano-MoO3 on mild steel. J. Adhes. Sci. Technol. 2020, 34, 115–134. [Google Scholar] [CrossRef]
- Li, C.; Wang, C.; Li, Z.; Cao, Z.; Xie, Y.; Xue, M.; Zhao, J. Preparation of ZnO Nanoparticle/Acrylic Resin Superhydrophobic Coating via Blending Method and Its Wear Resistance and Antibacterial Properties. Materials 2021, 14, 3775. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Wu, L.; Liu, J.; Liu, Y.; Hu, Y.; Xu, J.; Wang, L. Synthesis and corrosion Resistance of silane coupling agent modified graphene oxide/waterborne polyurethane. IOP Conf. Ser. Mater. Sci. Eng. 2019, 631, 022058. [Google Scholar] [CrossRef]
- Hadavand, B.S.; Ataeefard, M.; Bafghi, H.F. Preparation of modified nano ZnO/polyester/TGIC powder coating nanocomposite and evaluation of its antibacterial activity. Compos. Part B Eng. 2015, 82, 190–195. [Google Scholar] [CrossRef]
- Wang, W.; Xian, G.; Li, H. Surface modification of ramie fibers with silanized CNTs through a simple spray-coating method. Cellulose 2019, 26, 8165–8178. [Google Scholar] [CrossRef]
- Wu, J.; Ji, G.; Wu, Q.J.R.a. Preparation of epoxy/ZrO2 composite coating on the Q235 surface by electrostatic spraying and its corrosion resistance in 3.5% NaCl solution. RSC Adv. 2022, 12, 10625–10633. [Google Scholar] [CrossRef]
- Javadi, E.; Ghaffari, M.; Bahlakeh, G.; Taheri, P. Photocatalytic, corrosion protection and adhesion properties of acrylic nanocomposite coating containing silane treated nano zinc oxide: A combined experimental and simulation study. Prog. Org. Coat. 2019, 135, 496–509. [Google Scholar] [CrossRef]
- Chen, C.; Wei, S.; Xiang, B.; Wang, B.; Wang, Y.; Liang, Y.; Yuan, Y. Synthesis of silane functionalized graphene oxide and its application in anti-corrosion waterborne polyurethane composite coatings. Coatings 2019, 9, 587. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Y.; Zhou, Y.; Huang, Y.; Li, S.; Chen, Y.; Wang, R.; Tang, J.; Wu, P.; Zhao, X. Enhanced methanol oxidation on PtNi nanoparticles supported on silane-modified reduced graphene oxide. Int. J. Hydrogen Energy 2022, 47, 6638–6649. [Google Scholar] [CrossRef]
- Safari, J.; Zarnegar, Z. Ultrasonic activated efficient synthesis of chromenes using amino-silane modified Fe3O4 nanoparticles: A versatile integration of high catalytic activity and facile recovery. J. Mol. Struct. 2014, 1072, 53–60. [Google Scholar] [CrossRef]
- Azizi, N.; Abbasi, F.; Abdoli-Senejani, M. Thiamine immobilized on silane-functionalized magnetic nanoparticles for catalytic synthesis of 2,3-dihydroquinazolin-4(1H)-ones in water. Mater. Chem. Phys. 2017, 196, 118–125. [Google Scholar] [CrossRef]
- Buxadera-Palomero, J.; Godoy-Gallardo, M.; Molmeneu, M.; Punset, M.; Gil, F.J. Antibacterial properties of triethoxysilylpropyl succinic anhydride silane (TESPSA) on titanium dental implants. Polymers 2020, 12, 773. [Google Scholar] [CrossRef]
- Réthoré, G.; Boyer, C.; Kouadio, K.; Toure, A.; Lesoeur, J.; Halgand, B.; Jordana, F.; Guicheux, J.; Weiss, P. Silanization of chitosan and hydrogel preparation for skeletal tissue engineering. Polymers 2020, 12, 2823. [Google Scholar] [CrossRef] [PubMed]
- Levenez, B.; Gil-Cortes, T.; Rodríguez-Fuentes, N.; Jiménez, J.E.; Herrera-Kao, W.; Loría-Bastarrachea, M.I.; May-Pat, A.; Guerrero-Bermea, C.; Uribe-Calderón, J.; Cervantes-Uc, J.M. Silanized graphene oxide as a reinforcing agent for acrylic bone cements: Physicochemical, mechanical and biological characterization. J. Biomater. Sci. Polym. Ed. 2021, 32, 1736–1753. [Google Scholar] [CrossRef]
- Khan, A.; Hussain, A.; Sidra, L.; Sarfraz, Z.; Khalid, H.; Khan, M.; Manzoor, F.; Shahzadi, L.; Yar, M.; Rehman, I. Fabrication and in vivo evaluation of hydroxyapatite/carbon nanotube electrospun fibers for biomedical/dental application. Mater. Sci. Eng. C 2017, 80, 387–396. [Google Scholar] [CrossRef]
- Langeroudi, M.P.; Binaeian, E. Tannin-APTES modified Fe3O4 nanoparticles as a carrier of Methotrexate drug: Kinetic, isotherm and thermodynamic studies. Mater. Chem. Phys. 2018, 218, 210–217. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, F.; Men, K.; Huang, R.; Zhou, B.; Zhang, R.; Zou, R.; Yang, L. Modified Fe3O4 Magnetic Nanoparticle Delivery of CpG Inhibits Tumor Growth and Spontaneous Pulmonary Metastases to Enhance Immunotherapy. Nanoscale Res. Lett. 2018, 13, 240. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, Y.; Shayesteh, H.; Haghshenas, N.; Safarzadeh Khosrowshahi, M. Investigation of grafting silane coupling agents on superhydrophobicity of carbonyl iron/SiO2 particles for efficient oil/water mixture and emulsion separation. Sci. Rep. 2023, 13, 788. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, H.; Hosseini, F. Fabrication of high-performance mixed matrix blend membranes comprising PES and TPU reinforced with APTS functionalized-graphene oxide via VIPS-NIPS technique for aqueous dye treatment and antifouling properties. J. Taiwan Inst. Chem. Eng. 2023, 142, 104609. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, Q.; Li, X.; Yun, M.; Xu, R.; Wang, S.; Li, Y.; Lin, L.; Ding, X.; Ye, H. Mixed matrix membranes comprising aminosilane-functionalized graphene oxide for enhanced CO2 separation. J. Membr. Sci. 2019, 570, 343–354. [Google Scholar] [CrossRef]
- Wasim, M.; Sabir, A.; Khan, R.U. Membranes with tunable graphene morphology prepared via Stöber method for high rejection of azo dyes. J. Environ. Chem. Eng. 2021, 9, 106069. [Google Scholar] [CrossRef]
- Zhang, Q.; Gu, T.; Shi, R.; Yan, J.; Wang, W.; Qian, W.; Zhai, B.; Zhou, M.; Chai, M.; Yang, R. Silane-functionalized carbon with super-hydrophobicity advancing microporous layer for proton exchange membrane fuel cells. J. Power Sources 2023, 555, 232342. [Google Scholar] [CrossRef]
- Ryu, H.; Yoon, H.-J.; Kim, S.-W. Hybrid Energy Harvesters: Toward Sustainable Energy Harvesting. Adv. Mater. 2019, 31, 1802898. [Google Scholar] [CrossRef]
- Yu, R.; Lin, Q.; Leung, S.-F.; Fan, Z. Nanomaterials and nanostructures for efficient light absorption and photovoltaics. Nano Energy 2012, 1, 57–72. [Google Scholar] [CrossRef]
- Khosroshahi, F.H.; Kordi, F.; Tohidian, M. Preparation of Cross-Linked Sponge With Piezoelectric Properties Based on Low Density Polyethylene/Poly(Ethylene- Co- Vinyl Acetate) and Barium Titanate: Relationship Between Mechanical Properties, and Cell Structure With Piezoelectric Coefficients. Polym. Adv. Technol. 2025, 36, e70084. [Google Scholar] [CrossRef]
- Sewvandi, G.A.; Tao, Z.; Kusunose, T.; Tanaka, Y.; Nakanishi, S.; Feng, Q. Modification of TiO2 Electrode with Organic Silane Interposed Layer for High-Performance of Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 5818–5826. [Google Scholar] [CrossRef] [PubMed]
- Sasi, S.; Chandran, A.; Sugunan, S.K.; Krishna, A.C.; Nair, P.R.; Peter, A.; Shaji, A.N.; Subramanian, K.R.V.; Pai, N.; Mathew, S. Flexible Nano-TiO2 Sheets Exhibiting Excellent Photocatalytic and Photovoltaic Properties by Controlled Silane Functionalization─Exploring the New Prospects of Wastewater Treatment and Flexible DSSCs. ACS Omega 2022, 7, 25094–25109. [Google Scholar] [CrossRef] [PubMed]
- Lai, B.-Y.; Lai, S.-N.; Lin, H.-Y.; Wu, J.M. Silane-modified MXene/PVA hydrogel for enhanced streaming vibration potential in high-performance flexible triboelectric nanogenerators. Nano Energy 2024, 125, 109554. [Google Scholar] [CrossRef]
- Bai, M.N.; Woo, I.; Subramanian, N.V.; Yoon, J.U.; Gajula, P.; Bae, J.W.; Arun, A.P. Influence of Pentaerythritol Core and Dimethylol Butanoic Acid Monomer-Based Aliphatic Hyperbranched Polyester on the Tribonegative Performance of Polyvinylidene Fluoride. ACS Appl. Electron. Mater. 2024, 6, 5586–5598. [Google Scholar] [CrossRef]
- Bindhu, A.; Arun, A.P.; Pathak, M. Review on polyvinylidene fluoride-based triboelectric nanogenerators for applications in health monitoring and energy harvesting. ACS Appl. Electron. Mater. 2024, 6, 47–72. [Google Scholar] [CrossRef]
- Niranjana, V.S.; Yoon, J.U.; Woo, I.; Gajula, P.; Bae, J.W.; Prabu, A.A. Exploring a New Class of PVDF/3-Aminopropyltriethoxysilane (core) and 2,2-Bis (hydroxymethyl) butyric Acid (monomer)-Based Hyperbranched Polyester Hybrid Fibers by Electrospinning Technique for Enhancing Triboelectric Performance. Adv. Sustain. Syst. 2024, 8, 2400311. [Google Scholar] [CrossRef]
- Khan, M.U.; Dumbre, D.; Abbas, Y.; Rezeq, M.d.; Alazzam, A.; Alamoodi, N.; Khaleel, M.; Mohammad, B. Triboelectric nanogenerator based on silane-coupled LTA/PDMS for physiological monitoring and biomechanical energy harvesting. Microsyst. Nanoeng. 2024, 10, 152. [Google Scholar] [CrossRef]
- Jakmuangpak, S.; Prada, T.; Mongkolthanaruk, W.; Harnchana, V.; Pinitsoontorn, S. Engineering Bacterial Cellulose Films by Nanocomposite Approach and Surface Modification for Biocompatible Triboelectric Nanogenerator. ACS Appl. Electron. Mater. 2020, 2, 2498–2506. [Google Scholar] [CrossRef]
- Guo, Y.; Li, K.; Hou, C.; Li, Y.; Zhang, Q.; Wang, H. Fluoroalkylsilane-Modified Textile-Based Personal Energy Management Device for Multifunctional Wearable Applications. ACS Appl. Mater. Interfaces 2016, 8, 4676–4683. [Google Scholar] [CrossRef]
- Nie, S.; Fu, Q.; Lin, X.; Zhang, C.; Lu, Y.; Wang, S. Enhanced performance of a cellulose nanofibrils-based triboelectric nanogenerator by tuning the surface polarizability and hydrophobicity. Chem. Eng. J. 2021, 404, 126512. [Google Scholar] [CrossRef]
- Qu, M.; Deng, Y.; Liu, H.; Li, J.; Zhang, Y.; Dong, Y.; Wang, Y.; Zhang, R.; Feng, P.; He, J. Flexible, Eco-Friendly, and Superhydrophobic Textile-Based Triboelectric Nanogenerator for Biomechanical Energy Harvesting and Self-Powered Human Motion Sensing. ACS Appl. Electron. Mater. 2025, 7, 612–621. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, S.; Zu, J.; Inman, D. High-Performance Piezoelectric Energy Harvesters and Their Applications. Joule 2018, 2, 642–697. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J. Piezoelectric Nanogenerators Based on Zinc Oxide Nanowire Arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef]
- Park, K.-I.; Xu, S.; Liu, Y.; Hwang, G.-T.; Kang, S.-J.L.; Wang, Z.L.; Lee, K.J. Piezoelectric BaTiO3 Thin Film Nanogenerator on Plastic Substrates. Nano Lett. 2010, 10, 4939–4943. [Google Scholar] [CrossRef]
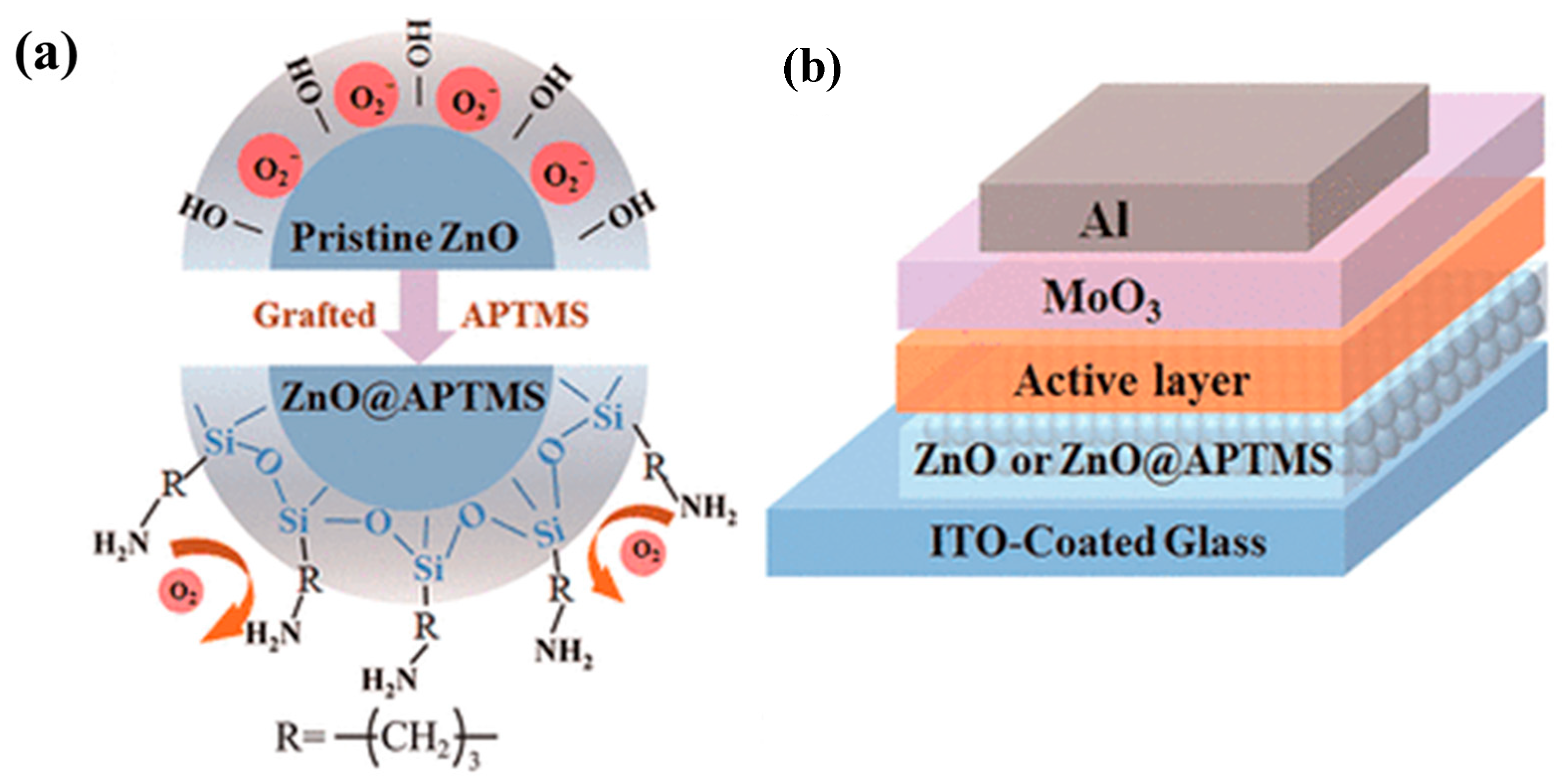
- Chandran, A.M.; Varun, S.; Karumuthil, S.C.; Varghese, S.; Mural, P.K.S. Zinc Oxide Nanoparticles Coated with (3-Aminopropyl)triethoxysilane as Additives for Boosting the Dielectric, Ferroelectric, and Piezoelectric Properties of Poly(vinylidene fluoride) Films for Energy Harvesting. ACS Appl. Nano Mater. 2021, 4, 1798–1809. [Google Scholar] [CrossRef]
- Bairagi, S.; Ali, S.W. Flexible lead-free PVDF/SM-KNN electrospun nanocomposite based piezoelectric materials: Significant enhancement of energy harvesting efficiency of the nanogenerator. Energy 2020, 198, 117385. [Google Scholar] [CrossRef]
- Bairagi, S.; Ali, S.W. Effects of surface modification on electrical properties of KNN nanorod-incorporated PVDF composites. J. Mater. Sci. 2019, 54, 11462–11484. [Google Scholar] [CrossRef]
- Li, H.; Song, Y.-S.; Kim, T.-W.; Lee, M.-H.; Lim, S. Fully Printed Flexible Piezoelectric Nanogenerators with Triethoxyvinylsilane (TEVS) Coated Barium Titanate (BTO) Nanoparticles for Energy Harvesting and Self-Powered Sensing. Macromol. Mater. Eng. 2022, 307, 2200235. [Google Scholar] [CrossRef]
- Li, H.; Lim, S. Screen Printing of Surface-Modified Barium Titanate/Polyvinylidene Fluoride Nanocomposites for High-Performance Flexible Piezoelectric Nanogenerators. Nanomaterials 2022, 12, 2910. [Google Scholar] [CrossRef]
- Lewis, N.S. Toward Cost-Effective Solar Energy Use. Science 2007, 315, 798–801. [Google Scholar] [CrossRef]
- Oktik, Ş. The Holy Triangle of Science, Technology and Industry for Photovoltaic Solar Energy Conversion. In Renewable Energy Based Solutions; Uyar, T.S., Javani, N., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 51–80. [Google Scholar]
- Prabakaran, K.; Mohanty, S.; Nayak, S.K. PEO/PVdF–HFP electrolytes for natural dye sensitized solar cell applications: Effect of modified nano-TiO2 on electrochemical and photovoltaic performance. J. Mater. Sci. Mater. Electron. 2015, 26, 3887–3897. [Google Scholar] [CrossRef]
- Prabakaran, K.; Mohanty, S.; Nayak, S.K. Solid state metal-free eosin-Y dye sensitized solar cell based on PVdF-HFP electrolytes: Combined effect of surface modified TiO2 and plasticizer on electrochemical and photovoltaic properties. J. Solid State Electrochem. 2015, 19, 2465–2479. [Google Scholar] [CrossRef]
- Wei, J.; Ji, G.; Zhang, C.; Yan, L.; Luo, Q.; Wang, C.; Chen, Q.; Yang, J.; Chen, L.; Ma, C.-Q. Silane-Capped ZnO Nanoparticles for Use as the Electron Transport Layer in Inverted Organic Solar Cells. ACS Nano 2018, 12, 5518–5529. [Google Scholar] [CrossRef] [PubMed]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niranjana, V.S.; Ponnan, S.; Mukundan, A.; Prabu, A.A.; Wang, H.-C. Emerging Trends in Silane-Modified Nanomaterial–Polymer Nanocomposites for Energy Harvesting Applications. Polymers 2025, 17, 1416. https://doi.org/10.3390/polym17101416
Niranjana VS, Ponnan S, Mukundan A, Prabu AA, Wang H-C. Emerging Trends in Silane-Modified Nanomaterial–Polymer Nanocomposites for Energy Harvesting Applications. Polymers. 2025; 17(10):1416. https://doi.org/10.3390/polym17101416
Chicago/Turabian StyleNiranjana, Vadakkaveedu Subramanian, Sathiyanathan Ponnan, Arvind Mukundan, Arun Anand Prabu, and Hsiang-Chen Wang. 2025. "Emerging Trends in Silane-Modified Nanomaterial–Polymer Nanocomposites for Energy Harvesting Applications" Polymers 17, no. 10: 1416. https://doi.org/10.3390/polym17101416
APA StyleNiranjana, V. S., Ponnan, S., Mukundan, A., Prabu, A. A., & Wang, H.-C. (2025). Emerging Trends in Silane-Modified Nanomaterial–Polymer Nanocomposites for Energy Harvesting Applications. Polymers, 17(10), 1416. https://doi.org/10.3390/polym17101416