Abstract
Vitamins are crucial micro-nutrients for overall well-being, making continuous monitoring essential. There are demands to provide an alternative detection, especially using a portable detection or a point-of-care-testing (POCT) device. One promising approach is employing an in situ electro-polymerised MIP (eMIP), which offers a straightforward polymerisation technique on screen-printed electrodes (SPEs). Here, we report a review based on three databases (PubMed, Scopus, and Web of Science) from 2014 to 2024 using medical subject heading (MeSH) terms “electrochemical polymerisation” OR “electropolymerisation” crossed with the terms “molecularly imprinted polymer” AND “vitamin A” OR “vitamin D” OR “vitamin E” OR “vitamin K” OR “fat soluble vitamin” OR “vitamin B” OR “vitamin C” OR “water soluble vitamin”. The resulting 12 articles covered the detection of vitamins in ascorbic acid, riboflavin, cholecalciferol, calcifediol, and menadione using monomers of catechol (CAT), 3,4-ethylenedioxythiophene (EDOT), o-aminophenol (oAP), o-phenylenediamine (oPD), pyrrole, p-aminophenol (pAP), p-phenylenediamine (pPD), or resorcinol (RES), using common bare electrodes including graphite rod electrode (GRE), glassy carbon electrode (GCE), gold electrode (GE), and screen-printed carbon electrode (SPCE). The most common electrochemical detections were differential pulse voltammetry (DPV) and linear sweep voltammetry (LSV). The imprinting factor (IF) of the eMIP-modified electrodes were from 1.6 to 21.0, whereas the cross-reactivity was from 0.0% to 29.9%. Several types of food and biological samples were tested, such as supplement tablets, poultry and pharmaceutical drugs, soft drinks, beverages, milk, infant formula, human and calf serum, and human plasma. However, more discoveries and development of detection methods needs to be performed, especially for the vitamins that have not been studied yet. This will allow the improvement in the application of eMIPs on portable-based detection and POCT devices.
1. Introduction
Vitamins are essential micro-nutrients that the human body requires to maintain normal growth and activity of the body, as well as overall health [1]. Vitamins are obtained naturally from plant and animal foods [2], although some of them can be produced naturally from our bodies [3,4]. Vitamins are generally divided into two categories: water-soluble vitamins (WSVs) including vitamin B1 (thiamine), vitamin B2 (riboflavin), vitamin B3 (niacin), vitamin B5 (pantothenic acid), vitamin B6 (pyridoxine), vitamin B7 (biotin), vitamin B9 (folic acid), vitamin B12 (cobalamin), and vitamin C (ascorbic acid); and fat-soluble vitamins (FSVs) including vitamin A (retinol, β-carotene), vitamin D2 (ergocalciferol), vitamin D3 (cholecalciferol), vitamin E (α-tocopherol), vitamin K1 (phylloquinone), vitamin K2 (menaquinone), and vitamin K3 (menadione). WSVs dissolve easily in water and are not stored in the body, which makes regular dietary intake crucial, whereas in contrast, FSVs dissolve in fats and oils, allowing them to be stored in the liver before they are absorbed in the bloodstream [4]. However, most vitamins cannot be produced naturally in the body, and the ones that are naturally synthesised produce insufficient amounts, necessitating the need for regular dietary intake to meet the recommended levels.
Each vitamin is important and has its role in the human body. Avitaminosis, or vitamin deficiency, can lead to many serious diseases. For example, vitamin B1 deficiency can cause beriberi (cardiomyopathy with oedema and lactic acidosis), Wernicke–Korsakoff syndromes, or even several neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease [5]. A deficiency in vitamin C can lead to scurvy, which causes symptoms including muscle weakness, swollen and bleeding gums, loss of teeth, petechial haemorrhaging, spontaneous ecchymoses, anaemia, impaired wound healing, hyperkeratosis, weakness, myalgia, arthralgia, and weight loss [6]. Vitamin D, which regulates calcium balance and supports bone mineralisation, can cause rickets in infants or children and osteomalacia in adults if deficient [3]. These consequences emphasise the importance of maintaining optimal vitamin levels to prevent deficiency-related diseases.
The summary of vitamins with their respective active metabolites form, dietary sources, and roles in the human body are simplified in Table 1. For example, WSVs are commonly found in fruits, vegetables, whole grains, and fortified cereals, whereas FSVs are typically present in high-fat foods such as dairy products, fish, eggs, liver, and plant oils.

Table 1.
List of WSVs and FSVs with their respective names, dietary sources, and roles in the human body. The information in the table were adapted from reported studies [1,7,8].
The detection of most analytes and other metabolites, including vitamins, biogenic amines, amino acids, and even proteins or peptides, commonly relied on laboratory-based instrumentation [9,10] such as High-Performance Liquid Chromatography (HPLC), which was typically coupled with several detectors, including ultraviolet (UV) or mass spectrometry (MS) [11,12]. This was because HPLC was considered a reference standard due to its high reliability, exceptional sensitivity, and selectivity, although the laboratory-based instrumentation incurred significant acquisition and maintenance costs. Furthermore, operating these instruments required specialised skills, encompassing method development, sample preparation, and data analysis, which often rendered the technique less practical for routine or on-site testing [13].
Consequently, there was a growing demand for alternative, portable devices that could complement or even replace traditional laboratory-based systems. These devices included portable electrochemical sensors, nonlinear optical (NLO) sensors, and point-of-care testing (POCT) platforms [14,15,16]. They offered numerous advantages such as simplicity of operation, high sensitivity, and lower costs. These features enabled real-time monitoring of various analytes, including vitamins, across different sample matrices, such as food products and biological fluids. These benefits alleviated the complexities and limitations associated with traditional analytical methods. In this context, molecularly imprinted polymer (MIP)-based electrochemical sensors emerged as highly promising candidates. MIP-based sensors provided excellent selectivity by mimicking the functions of biological recognition elements while remaining cost-effective, robust, and highly adaptable for integration into portable systems and POCT devices.
An MIP is a polymer specifically designed to match the shape of a template molecule. While the target compound itself is commonly used as the template, a structurally similar compound can also serve this purpose [17]. The general overview of MIP formation is illustrated in Figure 1 below. The polymerisation solution of MIP can be prepared by mixing several key ingredients, including the monomer(s), the template, a crosslinker (if required), and an electrolyte [18]. In the polymerisation solution, one or several monomers can be used to form MIP, depending on the desired MIP design. The easiest monomer(s) to be used was the self-polymerising monomer, which is a monomer that can interlink with the same monomer without the presence of any crosslinker [19]. This can reduce the use of too many parameters for optimisation of the MIP procedure, thereby shortening the overall optimisation steps. The template removal, one of the critical steps in the formation of effective MIPs, was enacted to leach out any template residual on the MIP structure, leaving a space or cavity, specific to the target compound. This contributed to the high specificity of the MIP [20]. Also, the introduction of the electrolyte in the polymerisation solution was required to facilitate ion movement to the working electrode surface [21]. Any electrolyte can be used depending on its solubility on the prepared polymerisation solution composition.
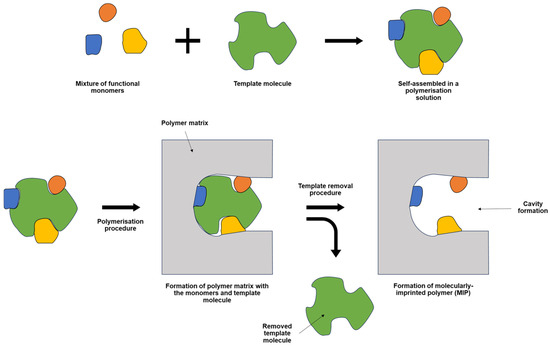
Figure 1.
The general overview for the synthesis of MIP.
On the other hand, an electrochemical detection platform is always a good alternative for detection, as the technique can be applied using a miniaturised and portable design. The detection of an electrochemical sensor deals with the study of electron transfer between species involving redox reactions, where losses of electrons (oxidation) and gains of electrons (reduction) result in the oxidation states of one species [22,23]. These changes can be detected through the observed current signal produced by the sensor device, which acts as an electrolytic cell. There are several techniques to observe the current signal for sample detection, including cyclic voltammetry (CV), linear sweep voltammetry (LSV), differential pulse voltammetry (DPV), and square-wave voltammetry (SWV) [22]. Typically, DPV and SWV gained much more attention for analyte detection at trace levels due to their significant improvement in signal through background noise reduction, which significantly enhance the signal-to-noise ratios, thereby indirectly lowering the detection limit much further and improving detection sensitivity [24,25]. Nevertheless, depending on the application of the study, these techniques are utilised to obtain an improved and optimum detection signal for quantification [26,27]. This capability makes them invaluable tools in modern analytical chemistry.
Thus, the combination of electrochemical detection using electrodes that were modified with MIP has recently gained popularity due to the high specificity results from MIP [18]. Moreover, the electrochemical polymerisation (electro-polymerisation) of MIP, denoted as eMIP, is also possible to be designed using an electrochemical technique, making the combination of eMIP and electrochemical detection a good option to explore for an alternative, affordable, reliable, and portable detection of vitamin with simple electro-polymerisation procedures. However, the technique also relies on the type of target compound to be detected. Not every target compound can be detected directly by electrochemical detection due to its electrochemically active nature.
For instance, certain vitamins, especially their metabolites such as calcifediol [28] and cobalamin [29], are unable to produce a measurable current signal due to their electrochemical inactivity. This inactivity is caused by their non-polar structures and the lack of strongly redox-active functional groups, such as phenolic hydroxyls [30], or quinone-like structures [31], which limit their ability to undergo oxidation or reduction within the typical potential range of most electrodes. Additionally, these metabolites exhibit high electron transfer overpotentials and do not have easily ionisable groups, which makes direct electrochemical detection challenging without modification or the use of mediators [32,33].
Nevertheless, this limitation can be addressed by monitoring the signal of a mediator such as a redox indicator on an eMIP-modified electrode before and after incubation with non-electrochemically active vitamins. The alteration in the redox indicator’s response demonstrates the binding interaction between the target metabolites and the MIP, thus facilitating indirect electrochemical detection. Therefore, the aim of this review was to evaluate the most applicable technique for performing electro-polymerisation on a variety of electrode materials, including carbon-based, metal, and composite electrodes, and to discover the need for developing innovative electrochemical-sensing strategies to detect vitamins in different samples.
2. Results
In the initial stage of screening, the bibliometric search results found 46 publications where 28 out of the 46 articles were excluded based on inclusion and exclusion criteria. Articles that have met the requirements, including articles with full text, reported in English, and peer-reviewed, were included to ensure the selection of high-quality and relevant research. The exclusion of articles were letters to the editor, books or book chapters, review papers, or duplicates, as these did not contribute original research data or were redundant. The search strategy also applied a filter for articles that were published from 10 years ago up until the present day (2014–present). This was to ensure our review reflects the current and latest knowledge.
Based on the article searches from the databases, and following the rules set within the inclusion and exclusion criteria, 18 articles were found. Only 12 full-text publications were deemed relevant after full screening for each article was performed (Figure 2).
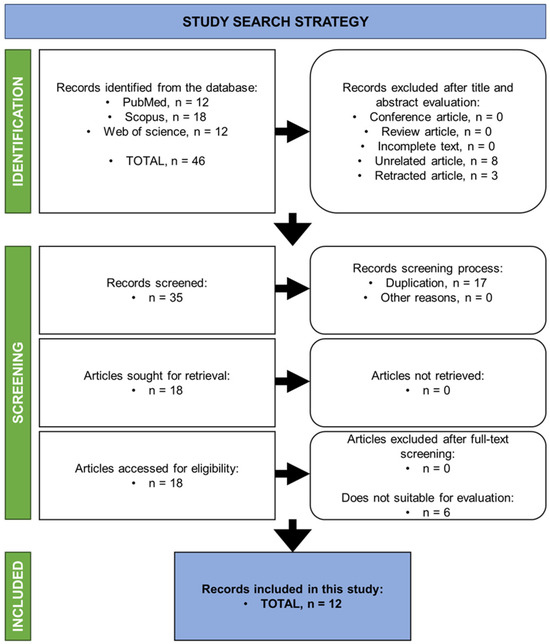
Figure 2.
The summarised search strategy of the study.
Furthermore, a summary of each article was tabulated in Table 2 below to better understand the whole context of the articles. The related information was categorised according to the aim, methodology, findings, and conclusion for a more thorough analysis of the articles. It is important to note that the Results and Discussion sections specify the chemical names of the vitamins to clearly identify the exact target compounds used in the study.

Table 2.
Finalised included articles for this study.
3. Discussion
The electro-polymerisation technique is one of the simplest approaches for polymerisation, which can also be utilised to form an MIP by introducing a template into the polymerisation solution [18]. The spontaneous formation of MIP on the working electrode surface after the electro-polymerisation procedures were performed led to an in situ formation of eMIP. Studies have also reported the polymerisation of MIP conducted elsewhere, typically through chemical reaction, where the formed MIP was collected and modified on the sensing electrode [45,46]. This process requires additional preparation steps.
The polymerisation of the monomer(s) can be initiated using an electrochemical technique through oxidation or reduction [21,47]. This occurs when a specific potential was applied to the polymerisation solution. A bare conventional electrode is then immersed in the polymerisation solution, after which the electrochemical technique is applied to initiate polymerisation. In the case of screen-printed electrodes (SPEs), they can be immersed in the polymerisation solution, or a portion of the polymerisation can be placed on the SPEs’ surface prior to electro-polymerisation.
To assess the specificity of the formed MIP, the same polymerisation solution is typically prepared and modified on the same bare electrode but without the template, which is known as a non-imprinted polymer (NIP). The specificity of the eMIP-modified electrode can be compared by calculating the MIP and NIP ratio, often referred to as the imprinting factor (IF). An IF of greater than one (IF > 1) indicates that the MIP was specific [48], although a higher IF value is preferable. However, an excessively high IF could indicate a higher binding affinity of the target compound towards the MIP, which might present challenges for template or target compound removal [49]. Residual target compound, due to ineffective template removal procedures, can lead to poor sensitivity of the eMIP-modified electrode [50].
In this review, the reported studies were compared by several criteria, including the MIP preparation technique, the detection of target compound, the concentration range of the detection, the detection technique, the methods of template removal, the type of real sample used, the imprinting factor (IF), and the cross-reactivity study.
The IF is usually determined by the formula shown in Equation (1):
where is the signal produced by the target compound using the MIP-modified electrode, and is the signal produced by the target compound using the NIP-modified electrode. The IF value represents a measure of the interaction affinity of a target compound towards its respective MIP in the imprinted cavity [51]. The supposedly non-interacted target compound with the NIP gives a lower signal, leading to a higher IF value. Thus, a higher IF value indicates good specificity of the synthesised MIP. However, a too-high IF value can also mean that the affinity of the target compound with the MIP is too strong, posing a challenge to removing the target compound from the MIP.
On the other hand, the cross-reactivity is calculated based on the formula given in Equation (2) [52]:
where the is the signal produced by the interfering compound using the MIP-modified electrode and is the signal produced by the target compound using the MIP-modified electrode. The cross-reactivity study was only discussed if the interference study was reported. The cross-reactivity study in MIP synthesis is a measure of non-selective interaction of non-target compounds with the imprinted cavity of the synthesised MIP. A higher percentage of the cross-reactivity value indicates that the MIP is becoming less selective towards the target compound. Typically, non-selectivity occurs when the chemical structure of the non-target compound contains a similar major backbone structure to that of the target compound, mimicking the interaction with the MIP cavity and leading to a false positive signal [52,53]. This contributes to a higher cross-reactivity percentage.
Based on the final results of scoping, there were limited studies reported regarding the detection of vitamins using the electro-polymerisation technique, in which the most common electrode used was the conventional type of electrodes such as graphite rod electrode (GRE), GCE, or even gold electrode (GE). Only very few studies utilised the use of SPEs.
For instance, the determination of ascorbic acid was successful by using the MIP of polypyrrole (PPY) on an electrospun-modified cellulose acetate nanofibre membrane (CA)/multi-walled carbon nanotubes (MWCNTs)/polyvinylpyrrolidone (PVP) on bare GCE (PPY/CA/MWCNTs/PVP/GCE), which was electro-polymerised using CV (−0.6 V to 0.8 V, 7 cycles, 100 mV/s) [34]. The polymerisation solution contains pyrrole and ascorbic acid with a molar ratio of 2.5:1 in lithium perchlorate (LiClO4, 0.1 M). The removal of the template was performed by immersing the eMIP-modified electrode in phosphate buffer (PB) (0.05 M, pH 8.5) solution for 15 min. The direct detection (visualised E vs. silver/silver chloride, Ag/AgCl at 0.0 V) of ascorbic acid was performed using DPV (−0.2 V to 0.4 V) after 5 min of immersion of the eMIP-modified electrode in the standard solution containing PB (0.05 M, pH 8.5). An extensive range of linear calibration was observed for ascorbic acid concentrations ranging from 20 µM to 1000 µM with an R2 of 0.9988. The real sample of six chewable vitamin C tablets was tested, and the relative standard deviation (RSD) was averaged at only 3.4%. It was also visualised that the IF was 3.5 using 100 µM ascorbic acid, whereas the mean cross-reactivity of several interferences, including L-histidine, uric acid, L-tryptophan, and glucose, was 17.9%.
Another PPY-based MIP has also been reported for ascorbic acid detection, which was modified on a new two-dimensional (2D)-layered, graphene-like black phosphorene quantum dots (BPQDs) on poly(3,4-ethylenedioxythiophene) nanorod (PEDOTNR) layers that were modified on GCE (PPY-BPQDs/PEDOTNRs/GCE) [35]. The imprinting was performed using CV (−0.5 V to 0.8 V, 10 cycles, 50 mV/s), using pyrrole as the monomer and ascorbic acid as the template in a 5:1 monomer–template molar ratio in 0.01 M LiClO4. Then, the template was removed by CV from −0.3 V to 0.5 V in phosphate buffer (pH 8.5). The detection of ascorbic acid was found to be linear from 0.01 mM to 4 mM (R2 = 0.9912) with a detection limit of 3.3 µM. The IF was calculated at 1.6 based on the reported sensitivity of both MIP and NIP. The mean cross-reactivity was 5.0% when ascorbic acid (0.1 mM) was tested with glucose, nicotinic acid, caffeic acid, and folic acid at 1:50 molar ratios of each interfering compound. The analysis of soft drink samples resulted in 94% to 99% recoveries.
Another successful detection of ascorbic acid was achieved using an organic electrochemical transistor (OECT) with a bare GE that was modified with electro-polymerised poly(o-phenylenediamine) (poPD) (poPD/OECT/GE) using o-phenylenediamine (oPD) as the monomer and ascorbic acid as a template with a molar ratio of 1:2 in PB (0.2 M, pH 5.2) using CV (0.0 V to 0.8 V, no cycle number mentioned, 50 mV/s) [36]. The template was removed by immersing the eMIP-modified electrode in ultrapure water. The detection of ascorbic acid was based on the current change of the OECT gate electrodes with poly(3,4-ethylenedioxythiophene) doped with poly(styrenesulfonate) (PEDOT:PSS), modified on patterned gold–nickel (Au/Ni) source and drain electrodes, where it was able to detect ascorbic acid in vitamin C beverages diluted in PB (0.1 M, pH 7.0) from 1 µM to 100 µM (no R2 provided) with a 10 nM detection limit. The IF was calculated at 13.5 based on the visualised results. The selectivity of the eMIP-modified electrode for ascorbic acid (10 µM) was studied in contrast with other interfering compounds and ions, including aspartic acid (100 µM), glycine (100 µM), glucose (100 µM), uric acid (100 µM), glutathione (100 µM), hydrogen peroxide (100 µM), potassium ion (K+, 1 mM), sodium ion (Na+, 1 mM), calcium ion (Ca2+, 1 mM), magnesium ion (Mg2+, 1 mM), and iron (II) ion (Fe2+, 1 mM), where the mean cross-reactivity was 12.6%. Several ascorbic acid concentrations were spiked into the vitamin C beverage sample, resulting in a mean RSD of 10.0%.
A poPD-based MIP was also reported for ascorbic acid detection with different modifications using GCE [37]. The electro-polymerisation was performed using oPD as a monomer on the gold nanoparticles (AuNPs)-modified MWCNTs on the GCE surface (poPD/MWCNTs/AuNPs/GCE) by CV scan (−0.5 to 1.0 V, 20 cycles, 50 mV/s). The polymerisation solution was prepared in a 5:1 oPD-ascorbic acid as a monomer–template molar ratio containing 0.1 M KCl. The template was removed by immersing the poPD/MWCNTs/AuNPs/GCE in an ethanol–water mixture at a 4:1 (v/v) ratio for 12 min. The study reported a two-order calibration for ascorbic acid detection, i.e., 0.01 µM to 2 µM (R2 = 0.9912) and 2 µM to 100 µM (R2 = 0.9980), which shows a distinct ascorbic acid peak at around 0.0 V after a DPV scan. The two calibrations produced a detection limit of 2 nM. The study also compared the signal of ascorbic acid (20 µM) between MIP and NIP, which resulted in an IF of 21. The mean cross-reactivity of the eMIP-modified GCE was only 1.7% using D-glucose, caffeine, dopamine, and uric acid. Human serum was tested using the poPD/MWCNTs/AuNPs/GCE, which showed an excellent recovery of ascorbic acid from 95.6% to 108.3%.
A very different poPD-based MIP for ascorbic acid detection method was accomplished using ratiometric electrochemiluminescence (ECL) detection, combined with MIP, based on a closed bipolar electrode (BPE) [38]. The electrode surface was electro-polymerised by CV (0.0 V to 0.8 V, 15 cycles, 50 mV/s) using oPD as a monomer and ascorbic acid as a template in PB (0.1 M, pH 5.2) with a molar ratio of 1:2, respectively. The BPE was set up using zinc indium sulphide nanoflower (ZnIn2S4) as a cathode and the MIP of poPD as an anode; both were modified on a bare graphite rod electrode. The cathode was immersed in PB (pH 7) containing 0.1 M potassium persulfate (K2S2O8). In contrast, the anode was immersed in PB (0.1 M, pH 7) containing tris(bipyridine)ruthenium (II) ion (Ru(bpy)32+, 0.125 mM) and tri-n-propylamine (6.25 mM) during detection. The template removal was performed by immersing the eMIP-modified electrode with ultrapure water. The signal of ascorbic acid respective to its concentrations was studied by measuring the ECL current from the redox reaction of CV scans (0.0 V to 1.2 V, single scan, 50 mV/s) occurring on both the anode (observed wavelength, λ = 440 nm) and cathode (λ = 605 nm), simultaneously, after 2 min of incubation. The detection of ascorbic acid was calculated based on the current ratio of the cathode and the anode signal produced (Icat/Iano). In a real sample, the detection was performed on newborn calf serum with a linear detection range from 50 nM to 3 μM (R2 = 0.9820) and a detection limit of 20 nM, with a 0.7% mean RSD. The study also tested the effect of ascorbic acid signal versus other interfering compounds of 500 nM of urea, uric acid, dopamine, glucose, and their mixture solution with ascorbic acid, in which the mean cross-reactivity was 22.5%. However, there was no reported IF or any data to calculate the IF provided.
Another type of vitamin was also reported. Cholecalciferol was successfully detected in human plasma samples using the MIP originated from p-phenylenediamine (pPD)-resorcinol (RES) mixtures, which was modified on the screen-printed carbon electrode (SPCE) (pPD-RES/SPCE) [39]. The polymerisation solution containing 70% methanol was prepared by mixing pPD, RES, and cholecalciferol at a monomer–template molar ratio of 13.3:13.3:1, respectively, in the presence of acetate buffer (0.1 M, pH 5.2). The electro-polymerisation was performed using CV (0.0 V to 0.8 V, 7 cycles, 50 mV/s). The template was removed using acetonitrile–0.2 M hydrochloric acid (HCl) at a 10:1 (v/v) ratio. The linear detection of cholecalciferol was from 0.01 nM to 2 nM (R2 = 0.995), with a detection limit of 1 pM. The study compared the signal of MIP and NIP but only stated that NIP produced no signal. Similarly, the interference of 17-beta estradiol, dexamethasone, and betamethasone towards cholecalciferol was only described as non-interfering. However, the authors did not show any data to represent the value of IF and cross-reactivity study. The cholecalciferol detection on plasma samples exhibited an excellent recovery of 90.0% to 103.3% when spiked with 10 nM—200 nM of cholecalciferol.
The vitamin D metabolite was also studied for the detection of calcifediol in serum [28]. Initially, a GCE was modified with copper cobaltite combined with nitrogen-doped carbon nanotubes (CuCo2O4/N-CNTs) mixed with phosphorus-doped graphene oxide (P-GO). The GCE was modified by drop casting the nanocomposite on the GCE working electrode (CuCo2O4/N-CNTs/P-GO/GCE). This modified electrode was then electro-polymerised with calcifediol-imprinted PPY by 10 cycles of CV from −0.6 to 0.1 V at 10 mV/s, forming a PPY/CuCo2O4/N-CNTs/P-GO/GCE. The polymerisation solution was maintained in phosphate buffer (0.0075 M, pH 8.5) at a 1:2 template–monomer mole ratio. Subsequently, the template was removed by CV scan (0.1 V to 1.0 V, 20 cycles) in phosphate buffer (0.05 M, pH 7.0). This study highlighted the detection of calcifediol from 0.002 µM to 10 μM (R2 = 0.9972) with a detection limit of 0.38 nM. The serum sample analyses resulted in 80.0% to 106.4% recovery. Several compounds were tested in the selectivity study, including ergocalciferol, cholecalciferol, sodium deoxycholate, and cholesterol, and it was found that the mean cross-reactivity of the interferences was 17.0%, which may be attributed to the similarity in chemical structures of the interferences with calcifediol. However, no comparison of MIP and NIP signal was made in which the IF cannot be determined.
An eMIP-modified GCE was designed by using co-electro-polymerisation of poly(3,4-ethylenedioxythiophene) (PEDOT) with 2D-layered tungsten sulphide nanosheet (WS2) on a surface of single-walled carbon nanotubes (SWCNTs), with graphene oxide (GO)-modified GCE (PEDOT-WS2/SWCNTs-GO/GCE) for the detection of riboflavin in vitamin B2 tablets [40]. The electro-polymerisation was conducted using CV (0.0 V to 1.2 V, 5 cycles, 50 mV/s) with a polymerisation solution of 3,4-ethylenedioxythiophene (EDOT) mixed with riboflavin at a 2:1 molar ratio in a water solution containing 0.8 mg/mL WS2 and 0.04 M LiClO4. The template was then removed by soaking the PEDOT-WS2/SWCNT-GO/GCE in a 50 °C sodium hydroxide (NaOH) solution for 8 h. The calibration of riboflavin produced by LSV scan, recorded at –0.45 V of oxidation response, ranged from 0.002 µM to 0.9 µM (R2 = 0.9936) with a detection limit of 0.7 nM. Based on the provided calibration curve in both MIP and NIP, the IF was calculated at 3.0. In contrast, the mean cross-reactivity was found to be 4.0%, involving interfering compounds of thiamine, nicotinamide, pyridoxine, cyanocobalamin, ascorbic acid, UA, acetaminophen, and glucose, each at a 10:1 molar ratio with riboflavin. The real sample analysis recovered from 93.7% to 99.5% when spiked with riboflavin.
Another GCE-based MIP was modified using mixed monomers of catechol (CAT) and p-aminophenol (pAP) in PB (0.01 M, pH 7) containing riboflavin, dopamine, and L-tryptophan as templates with a molar ratio of 1:5 between mixed monomers and each of the templates [41]. However, the authors did not mention the template removal technique. Nevertheless, the eMIP was performed using CV (−0.8 V to 1.2 V, 10 cycles, 50 mV/s) on the bare GCE, producing a modified electrode of CAT-pAP/GCE. The modified electrode successfully detects riboflavin (visualised E vs. Ag/AgCl at −0.5 V), dopamine (visual E vs. Ag/AgCl at 0.2 V), and L-tryptophan (visual E vs. Ag/AgCl at −0.7 V) in both milk and serum samples. The calibration range was reported from 0.005 µM to 500 µM (R2 = 0.9966) for riboflavin, 0.05 µM to 500 µM (R2 = 0.9949) for dopamine, and 0.1 µM to 250 µM (R2 = 0.9981) for L-tryptophan, using direct detection of DPV scan (−0.7 V to 0.8 V), with detection limits of 0.0016 µM, 0.016 µM, and 0.03 µM, respectively. The mean RSD of milk and serum samples was 2.3% for riboflavin, 3.0% for dopamine, and 2.5% for L-tryptophan. However, no comparison was made with the target compound signals using MIP versus NIP. Thus, no IF can be calculated. The interference study was conducted by introducing the three target compounds with varying concentrations for each compound. It was found that the mean cross-reactivity of riboflavin, dopamine, and L-tryptophan was at 0.0% due to the specificity of each target compound at a different potential, thus giving a very minimal effect on the signals. However, an inferior cross-reactivity result, near 100% based on visual observation, was noted for all three target compounds when each of the target compounds was studied against interference from compounds such as glucose, tyrosine, uric acid, and ascorbic acid. The study also mentioned that the concentration of each of the interfering compounds was 100-fold higher than the target compounds. A similar concentration ratio must be used to reflect the true representation of the interference study.
Folic acid had also been detected using a PPY-based MIP on a GCE modified with molybdenum carbide nanoparticles (PPY/Mo2C/GCE) [42]. This was performed by immersing the Mo2C/GCE into a polymerisation solution containing folic acid as a template and pyrrole (pH 7.2) as a monomer at a molar ratio of 4:1 between monomer and template in the presence of LiClO4 (0.1 M). Later on, electro-polymerisation was performed using CV (−0.9 V to 1.0 V, 10 cycles, 100 mV/s). The template was then removed by immersing the eMIP-modified electrode in an ethanol and acetic acid mixture solution (90:10, v/v) with mild stirring. A direct electrochemical detection for folic acid using DPV (scan range parameters were not revealed) was successful in a real sample of pharmaceutical drugs and serum in 0.1 M potassium chloride (KCl) and 0.1 M PB (pH 6.5) solution, with a concentration from 0.01 µM to 120 µM (R2 = 0.9960) and a detection limit of 4 nM. The RSD of the real sample analysis was 3.4% for pharmaceutical drugs and 4.3% for serum. The study also reported an excellent IF of 14.6 for the detection of folic acid using the eMIP-modified electrode. The selectivity study was employed with folic acid (1 µM) against progesterone, cortisol, lactate, uric acid, ascorbic acid, dopamine, and glucose, each at 50 µM of interfering compounds. The mean cross-reactivity study showed an outstanding result of only 6.3%.
One study also reported the development of eMIP for folic acid using o-aminophenol (oAP) as a monomer on reduced graphene oxide (rGO)-modified AuNPs on GCE, denoted as poAP/rGO/AuNPs/GCE [43]. This method, with its two orders of calibration ranging from 0.02 µM to 0.8 µM (R2 = 0.9860) and from 0.8 µM to 10 µM (R2 = 0.9888), as well as a detection limit of 2.8 nM of folic acid, has successfully detected folic acid (potential, E vs. Ag/AgCl at −0.7 V) in infant formula milk, multivitamin tablets, and serum samples using direct detection of differential pulse voltammetry (DPV) scans from −1.0 V to 0.0 V, which had a mean RSD of 3.1% for the three samples. The process involves the preparation of the MIP by first modifying GCE with chemically modified rGO/AuNPs. Then, it was electro-polymerised with oAP-containing folic acid at a 1:1 molar ratio in KCl (0.1 M) using CV (−0.5 V to 1.0 V, 20 cycles, 50 mV/s). The folic acid template was removed by immersing the poAP/rGO/AuNPs/GCE in a 4:1 (v/v) ethanol–water mixture for 12 min. The IF of folic acid was found to be around 15 (based on the visualised diagram). The selectivity of the modified sensor was tested using several compounds, including pteroic acid, caffeine, theobromine, and ascorbic acid, where the mean cross-reactivity of the interferences was about 6.8%, indicating a very high selectivity of the poAP/rGO/AuNPs/GCE towards folic acid against the tested interferences.
Lastly, the imprinting of poly(3,4-ethylenedioxythiophene) (PEDOT) using 3,4-ethylenedioxythiophene (EDOT) as monomer was successful on GCE for the trace analysis detection of vitamin K in the form of vitamin K3, also known as menadione, in poultry drug samples [44]. The eMIP was modified using the typical three-step procedure of (1) dissolving EDOT and menadione at a molar ratio of 1.3:1, respectively, in acetonitrile containing tetrabutylammonium tetrafluoroborate (Bu4NBF4, 0.2 M); followed by (2) electro-polymerisation of PEDOT on GCE using CV (0.0 V to 1.4 V, 20 cycles, 100 mV/s) to produce PEDOT/GCE; and finally (3) template removal by immersing the eMIP-modified electrode in ethanol for 15 min while stirring. The detection of menadione was performed directly using LSV (−0.50 V to 0.0 V) from 0.009 μM to 35 μM (R2 = 0.9923) with a detection limit of 0.31 nM in PB (0.1 M, pH 7.0), in which the oxidation peak was noted from −0.30 V to −0.4 V at the reported concentration range. The selectivity of the PEDOT/GCE electrode was studied using several compounds against menadione (0.5 μM), including vitamin K1 (phylloquinone), 2,3-dichloro-1,4-naphthoquine (DINA), and 2-amino-3-chloro-1,4-naphthoquine (ACNA) at 0.5 mM, after which the mean cross-reactivity was calculated at 29.9% when observed visually. Moreover, the IF of the eMIP-modified electrode was 3.4. The poultry drug resulted in a mean RSD of 1.7% when spiked with several menadione concentration levels.
Overall, only a few vitamins were explored for the past 10 years using an in situ electro-polymerisation technique, which only includes WSV of ascorbic acid, riboflavin, and folic acid, as well as FSV of cholecalciferol, calcifediol, and menadione. Except for calcifediol, all other vitamins were electrochemically active compounds (Table 3). This means the detection of these vitamins can be implemented directly based on the signal produced by the vitamin, making the detection procedure straightforward. On the other hand, the detection of non-electrochemically active vitamins would require observation of redox indicator signals such as ferrocyanide/ferricyanide mixtures for the leached eMIP-modified electrode, and after the eMIP-modified electrode was adsorbed with the target to obtain the net reading [54,55]. Moreover, the development of detection for electrochemically active vitamins can be adapted into a portable-based detector that can be brought to the site for real-time detection. Thus, a derivatisation step is recommended for non-electrochemically active vitamins to become electrochemically active.

Table 3.
The summarised information for the studied articles.
Typical monomers were employed for the electro-polymerisation, including CAT, EDOT, oAP, oPD, pyrrole, pAP, pPD, and RES. This was due to the nature of the listed monomers being able to undergo self-polymerisation when initiated electrochemically. The advantage of using self-polymerisation monomers is the ability of the monomer to form an interlink without the presence of a crosslinker [56,57]. During electro-polymerisation, the crosslinker would require additional experimental optimisation for the best molar ratio to be mixed with the monomer(s) and template, increasing the difficulty of the study. Thus, the utilisation of self-polymerised monomers was preferable.
The use of bare electrodes was important due to their contribution to sensitivity and the feasibility of further modification. Modifying electrodes was often performed to enhance their sensitivity by incorporating highly sensitive materials, including AuNPs, CA, CuCo2O4, GO, N-CNT, Mo2C, MWCNTs, P-GO, rGO, and WS2. Thus, the most popular electrode chosen in this review was GCE. This was due to the higher surface area of GCE compared to common graphite materials, and the cost is affordable [58,59].
However, not one study addressed the need to optimise the concentration of the polymerisation solution. This may be due to the use of a higher starting concentration of the polymerisation solution in the millimolar range (4–50 mM), which was sufficient enough to cover the surface of the working electrode during electro-polymerisation. Nevertheless, optimising the concentration of monomer in the polymerisation solution can be a good way to find the most effective adsorption of MIP on the working electrode surface, which in turn can maximise the eMIP-modified electrode detection ability. Utilising the optimisation software, such as response surface methodology (RSM), is also a viable option to save time and resources of the study [60,61].
Nonetheless, these reported studies were still able to produce a very good IF ranging from 1.6 to 21.0 and cross-reactivity of 0.0% to 29.9%. The IF value of more than 1 (IF > 1), indicating the specificity of detection towards the target compound, was good enough to differentiate between target and non-target compounds [48], whereas the low percentage of cross-reactivity indicated very selective detection for the target compound. These data further support that the use of monomers in a millimolar range was adequate.
The reported studies utilised several techniques for template removal, including immersing the eMIP-modified electrode in water, a water–solvent mixture, a pure solvent, acidic water, alkali, or a buffer solution with CV scans. The choice of method depended on the specific template and monomer used. Therefore, a thorough optimisation step is essential to enhance the accessibility of the MIP cavity, thereby improving the overall performance of the eMIP-modified electrode.
Also, there were several types of samples covered by the studies included in this review such as supplement tablets, poultry and pharmaceutical drugs, soft drinks, beverages, milk, infant formula, human and calf serum, and human plasma. The variety of samples indicates that the detection technique using in situ electro-polymerisation was applicable to any samples for vitamin detection, including food matrices and biological samples. These data provide insight into the wide possibility for the detection of vitamins in broader sample types.
In summary, Table 4 below presents a more detailed comparison of various eMIP-modified electrodes used for different types of samples to detect vitamins, along with their respective detection limits.

Table 4.
The tabulation of vitamin detection with their respective electrode modifications, type of samples, and detection limit.
The most-studied vitamin was ascorbic acid. This was presumably due to its electrochemically active nature, which could be detected directly using an electrochemical sensor, as well as the simplicity of the sample preparation procedure. The development of several different eMIP-modified electrodes, however, reported higher variability in detection limits ranging from 3000.00 nM down to 2.00 nM. The lowest detection limit was represented by poPD/MWCNTs/AuNPs/GCE, which was used to detect human serum. This highly sensitive detection resulted from the utilisation of nanomaterial layers of MWCNTs and AuNPs on the bare GCE. It was reported that the use of nanomaterials greatly enhanced the detection sensitivity of the electrodes due to their high surface area and fast electron transfer [62,63,64].
Another vitamin, cholecalciferol, was detected using pPD-RES/SPCE in human plasma with a detection limit of 0.01 nM. This study reported the simplest electrode modification thanks to the use of SPCE, which offered the closest example and most promising application for portable-based detection.
Calcifediol, a metabolite of cholecalciferol, was also studied in human serum using PPY/CuCo2O4/N-CNT/P-GO/GCE, producing a detection limit of 0.38 nM. Similarly, the use of nanomaterial layers of CuCo2O4, N-CNT, and P-GO on the bare GCE contributed to excellent detection sensitivity.
For riboflavin, the lowest detection limit of 0.70 nM was achieved by PEDOT-WS2/SWCNTs-GO/GCE for the detection of vitamin B2 tablets. The use of nanomaterials, such as SWCNTs-GO, also contributed to the sensitivity.
Additionally, the incorporation of rGO and AuNPs in poAP/rGO/AuNPs/GCE for folic acid detection yielded a detection limit of 2.80 nM in various samples of infant formula, multivitamin tablets, and human serum.
Lastly, another simple modification of PEDOT/GCE was reported for the detection of menadione in a poultry drug, with a detection limit of 0.31 nM.
These findings collectively highlighted the significant influence of electrode materials and sample types on detection sensitivity. Consistently, the application of nanomaterials and hybrid composites enhanced analytical performance across the different vitamins tested.
Be that as it may, minimal reported studies also limit this review’s findings. More studies need to be conducted to discover the potential of the in situ electro-polymerisation technique, as this will help improve future technology, especially in the application of portable-based detection and POCT devices.
As a whole, the procedures of the in situ electro-polymerisation technique were divided into six general steps (Figure 3). (1) The condition of the bare electrode surface: The surface of the working electrode was typically conditioned or pre-treated either through chemical or mechanical means. The use of conventional electrodes such as GCE or GRE often involved mechanical conditioning using gentle hand sanding with alumina slurry on a slightly rough padding [65]. On the other hand, chemical or electrochemical treatment was often used by the SPE for the surface conditioning of the working electrode [66,67]. The conditioning process roughened the working electrode surface, preparing it for the following steps. (2) Next was electrode modification. The modification of the working electrode using materials with higher surface area and higher electrochemical sensitivity was typically conducted, including the use of nanomaterials [36,43] or 2D-layered materials [28,35] to enhance the electrode performance. (3) The incubation of the polymerisation solution: Depending on the method optimisation, the electrodes could be incubated in the polymerisation solution for a certain period prior to electro-polymerisation. The incubation of monomer(s) onto the working electrode surface enhanced the possibility of the polymerisation being adsorbed by the working electrode. This also simultaneously increased the chance of interaction between the template and the monomer(s) [68]. (4) The removal of the template on the eMIP-modified electrode: The template needed to be removed from the working electrode surface before it could be used for detection. The higher efficiency of template removal increased the sensing capability of the eMIP-modified electrode. There were several methods to remove the template, including leaching using a highly polar solvent such as ethanol [44], leaching using a mixture of solvent and acid such as acetonitrile–HCl mixture [39], the use of alkali leaching such as NaOH [40], the use of buffers such as PB [34], and the use of electrochemical scanning such as a CV scan [35]. The method depended on the nature of the template used in the study. (5) The incubation of samples on the eMIP-modified electrode: The samples were usually incubated for a specific duration to increase the chance of interaction between the target and the working electrode. This increased the adsorption of the target on the working electrode, making it more sensitive for detection [69]. (6) The detection of the target using an electrochemical scan: The detection of the target could be achieved directly or indirectly. Indirect detection was often used to detect a non-electrochemically active target using a redox indicator such as ferrocyanide/ferricyanide solution [32] or methylene blue [33]. However, this approach had a more prolonged procedure as the signals observed had to be taken before and after the incubation of samples to obtain the net signal.
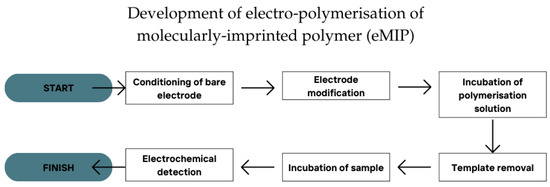
Figure 3.
The six steps of procedure for the development of eMIP.
The in situ electro-polymerisation was a simple and easy alternative for the preparation of MIP that can be directly used as a sensor for detection when combined with electrochemical techniques. However, there are many parameters that were quite hard to control during modification. One of the main challenges was to confirm the formation of the polymer on the working electrode surface. Unless the polymer of MIP was very dense, it was not easy to ascertain which part of the whole working electrode was covered by the MIP. Consequently, it was a very tedious procedure for physical characterisation, such as SEM or FTIR, to determine the presence of the MIP on the working electrode surface, as it had to be scanned at several different and random points. Additionally, the variability of occurrence of MIP formation on the working surface electrode can cause a poor reproducibility of the eMIP-modified electrodes, thus affecting the sensing capability for validation protocols. Several factors, including the conditioning of the bare electrode, the concentration of the polymerisation solution, and the electrochemical scans and parameters, controlled the occurrence of MIP on the working electrode [70,71].
Moreover, the imprinting effect might not be clearly represented by the aforementioned instruments due to poor NIP preparation (occurrence of monomer aggregation), which can cause an overestimation of IF [51].
Another challenge was the template removal procedure. The template was easy to remove when a highly polar solvent mixed with diluted acid was used [72]. This was true when using a conventional electrode. In the case of SPEs as bare electrodes, it will depend on how strongly the bare electrode printing attachment adheres to the base; otherwise, the whole printed material will peel off altogether. Moreover, the in situ electro-polymerisation can only utilise non-covalent bonding such as the hydrogen-bonding interaction between the template and the MIP [73]. Otherwise, it was not possible to remove the template using a harsher approach, such as saponification or hydrolysis [74], because it would destroy the electrode itself. This limits the applicability of the approach, although it also relies on the type of template used for detection.
The potential of converting the established eMIP-based electrochemical sensor offered a promising pathway for the POCT device. However, several challenges had to be addressed to facilitate its real-world application. One primary concern was the stability of the sensor when dealing with complex biological matrices, such as serum, plasma, or urine, or even in food matrices, where non-specific binding from the matrices could significantly affect the sensor’s analytical performance [75,76]. Furthermore, long-term use of the eMIP-modified electrode might have caused electrode fouling when it was exposed to complicated matrices over time [77,78]. Overcoming these challenges required several strategies, such as employing different electrochemical detection approaches [79,80] or adding more steps for sample preparation, which prolonged the overall detection procedures. The stability of the POCT device was also a crucial aspect of its feasibility. Therefore, future research should concentrate on overcoming the poor reproducibility by improving the developmental formulations of eMIPs that are suitable for large-scale production.
Nevertheless, in general, in situ electro-polymerisation is still a reliable approach with straightforward procedural steps that covered most of the analyte for detection, especially vitamins detection. The development involving vitamin detection in foods will provide a platform for an easy-to-use device that is portable, and a more affordable alternative to be used in the laboratory. Similarly, vitamin detections in their metabolite form in human serum or plasma can be further developed as a POCT device, widening the possibilities for reliable alternatives.
4. Conclusions and Future Direction
We have examined a total of 12 published articles related to the in situ electro-polymerisation technique for the detection of vitamins in ascorbic acid, riboflavin, cholecalciferol, calcifediol, and menadione, which involved the use of several common monomers including CAT, EDOT, oAP, oPD, pyrrole, pAP, pPD, and RES. Typical concentrations of these monomers range from 4 mM to 50 mM. These monomers were electro-polymerised using electrochemical CV using bare electrodes such as GRE, GCE, GE, and SPCE. These bare electrodes were often modified with AuNPs, CA, CuCo2O4, GO, N-CNT, Mo2C, MWCNTs, P-GO, rGO, and WS2 to enhance their performance, although the un-modified bare electrodes were still applicable. The most common electrochemical detection was DPV, and LSV. The reported IF was from 1.6 to 21.0 whereas the cross-reactivity was from 0.0% to 29.9%. Several types of food and biological samples were included, such as supplement tablets, poultry and pharmaceutical drugs, soft drinks, beverages, milk, infant formula, human and calf serum, and human plasma, indicating the versatility of vitamin detection using eMIP-modified electrodes across a variety of samples. However, this limited reported study urged the need of developing more detection methods using eMIP-modified electrodes for vitamins in both food and biological matrices, especially the vitamins that have not been studied yet. The development of eMIP-based electrochemical detection will enable interest in potential applications due to its enhanced specificity and selectivity, as well as its sensitivity, which eventually improves the application of portable-based detection and POCT devices, addressing existing gaps in nutritional monitoring, clinical diagnostics, and food quality control.
5. Limitation
This review was limited by the total output of the published articles since there were not many reported studies conducted. Also, this review was started in December 2024. Thus, any latest published articles beyond this period were not included to retain the feasibility of the report.
6. Materials and Methods
This review was performed based on the guidelines provided by the Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocols (PRISMA-ScR). Medical subject heading (MeSH) terms “electrochemical polymerisation” or “electropolymerisation” crossed with the terms “molecularly imprinted polymer” and “vitamin A” or “vitamin D” or “vitamin E” or “vitamin K” or “fat soluble vitamin” or “vitamin B” or “vitamin C” or “water soluble vitamin” were used to search for original papers in four databases (PubMed, Scopus, and Web of Science) from 2014 to 2024. Only available full-paper publication that were reported in English were examined. Letters to the editor, books or book chapters, reviews, and conference papers, however, were not included. Duplicate articles were also eliminated.
Author Contributions
Conceptualisation, M.A.J., Z.M.Z. and M.F.M.N.; methodology, M.A.J. and Z.M.Z.; software, M.A.J.; validation, M.A.J. and Z.M.Z.; formal analysis, M.A.J.; investigation, M.A.J. and Z.M.Z.; resources, M.A.J., Z.M.Z. and M.F.M.N.; data curation, M.A.J. and Z.M.Z.; writing—original draft preparation, M.A.J. and B.K.; writing—review and editing, B.K., Z.M.Z., K.P.S., F.S.M. and M.F.M.N.; visualisation, Z.M.Z. and M.F.M.N.; supervision, Z.M.Z., K.P.S., F.S.M. and M.F.M.N.; project administration, M.A.J. and M.F.M.N.; funding acquisition, M.A.J. and M.F.M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Health Malaysia under the Medical Research Grant (MRG) (NMRR ID-22-02161-STX).
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank the Director General of Health Malaysia for his permission to publish this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| 5-MTHF | 5-methyltetrahydrofolate |
| ACNA | 2-amino-3-chloro-1,4-naphthoquine |
| AFM | atomic force microscopy |
| Ag | silver |
| AgCl | silver chloride |
| Au | gold |
| AuNP | gold nanoparticle |
| BPE | bipolar electrode |
| BPQD | black phosphorene quantum dot |
| CA | cellulose acetate |
| Ca2⁺ | calcium ion |
| CAT | catechol |
| CuCo2O4 | copper cobaltite |
| CV | cyclic voltammetry |
| DINA | 2,3-dichloro-1,4-naphthoquine |
| DPV | differential pulse voltammetry |
| ECL | electro-chemiluminescence |
| EDOT | 3,4-ethylenedioxythiophene |
| EIS | electrochemical impedance spectroscopy |
| eMIP | electro-polymerised MIP |
| FAD | flavin adenine dinucleotide |
| Fe2⁺ | iron (II) ion |
| FESEM | field emission scanning electron microscopy |
| FMN | flavin mononucleotide |
| FSV | fat-soluble vitamin |
| FTIR | Fourier transform infrared |
| GCE | glassy carbon electrode |
| GE | gold electrode |
| GRE | graphite rod electrode |
| HCl | hydrochloric acid |
| HPLC | High-Performance Liquid Chromatography |
| IF | imprinting factor |
| K⁺ | potassium ion |
| K2S2O8 | potassium persulfate |
| KCl | potassium chloride |
| LiClO4 | lithium perchlorate |
| LSV | linear sweep voltammetry |
| MeSH | medical subject heading |
| Mg2⁺ | magnesium ion |
| Mo2C | molybdenum carbide |
| MS | mass spectrometry |
| MWCNT | multi-walled carbon nanotube |
| Na⁺ | sodium ion |
| NAD+ | nicotinamide adenine dinucleotide |
| NADP+ | nicotinamide adenine dinucleotide phosphate |
| NaOH | sodium hydroxide |
| N-CNT | nitrogen-doped carbon nanotube |
| Ni | nickel |
| NIP | non-imprinted polymer |
| oAP | o-aminophenol |
| OECT | organic electrochemical transistor sensor |
| oPD | o-phenylenediamine |
| pAP | para-aminophenol |
| PB | phosphate buffer |
| PEDOT | poly(3,4-ethylenedioxythiophene) |
| PEDOTNR | poly(3,4-ethylenedioxythiophene) nanorod |
| P-GO | phosphorus-doped graphene oxide |
| PLP | pyridoxal phosphate |
| POCT | point-of-care-testing |
| poPD | poly(o-phenylenediamine) |
| pPD | p-phenylenediamine |
| PPY | polypyrrole |
| PRISMA-ScR | Systematic Reviews and Meta-analysis Protocols |
| PSS | poly(styrenesulfonate) |
| PVP | polyvinylpyrrolidone |
| RES | resorcinol |
| rGO | reduced graphene oxide |
| RSD | relative standard deviation |
| RSM | response surface methodology |
| Ru(bpy)32+ | tris(bipyridine)ruthenium (II) ion |
| SEM | scanning electron microscope |
| SPCE | screen-printed carbon electrode |
| SPE | screen-printed electrode |
| SWCNT | Single-walled carbon nanotube |
| TCA | tricarboxylic acid |
| TPP | thiamine pyrophosphate |
| UV | ultraviolet |
| WS2 | tungsten sulfide |
| WSV | water-soluble vitamin |
| ZnIn2S4 | zinc indium sulphide nanoflower |
| α-CEHC | α-carboxyethylhydroxychroman |
References
- Gupta, U.; Gupta, S. Role of Vitamins in Human Health and Nutrition: Sources and Morbidity. Curr. Nutr. Food Sci. 2015, 11, 105–115. [Google Scholar] [CrossRef]
- Hoque, M.; Emon, K.; Malo, P.C.; Hossain, M.H.; Tannu, S.I.; Roshed, M.M. Comprehensive Guide to Vitamin and Mineral Sources with Their Requirements. Indiana J. Agric. Life Sci. 2023, 3, 23–31. [Google Scholar] [CrossRef]
- Chang, S.-W.; Lee, H.-C. Vitamin D and Health—The Missing Vitamin in Humans. Pediatr. Neonatol. 2019, 60, 237–244. [Google Scholar] [CrossRef]
- Bhalla, T.C. Savitri Vitamin B3, Niacin. In Industrial Biotechnology of Vitamins, Biopigments, and Antioxidants; Wiley: Hoboken, NJ, USA, 2016; pp. 41–66. ISBN 9783527681754. [Google Scholar]
- Mrowicka, M.; Mrowicki, J.; Dragan, G.; Majsterek, I. The Importance of Thiamine (Vitamin B1) in Humans. Biosci. Rep. 2023, 43, 374. [Google Scholar] [CrossRef] [PubMed]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P. Vitamin C—Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Ball, G.F.M. Vitamins: Their Role in the Human Body; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; ISBN 9781405148108. [Google Scholar]
- Combs, G.F.; McClung, J.P. The Vitamins; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780323904735. [Google Scholar]
- Chawla, G.; Chaudhary, K. A Review of HPLC Technique Covering Its Pharmaceutical, Environmental, Forensic, Clinical and Other Applications. Int. J. Pharm. Chem. Anal. 2019, 6, 27–39. [Google Scholar] [CrossRef]
- Swartz, M. HPLC DETECTORS: A BRIEF REVIEW. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 1130–1150. [Google Scholar] [CrossRef]
- Li, X.; Lv, H.; Luo, W.; Yang, W.; Kong, L.; Zhu, Q.; Zeng, L. Recent Advances in Detection Techniques for Vitamin Analysis: A Comprehensive Review. Food Chem. X 2025, 26, 102226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, W.; Yan, J.; Liu, M.; Zhou, Y.; Shen, X.; Ma, Y.; Feng, X.; Yang, J.; Li, G. A Review of the Extraction and Determination Methods of Thirteen Essential Vitamins to the Human Body: An Update from 2010. Molecules 2018, 23, 1484. [Google Scholar] [CrossRef]
- Rahimi, F.; Chatzimichail, S.; Saifuddin, A.; Surman, A.J.; Taylor-Robinson, S.D.; Salehi-Reyhani, A. A Review of Portable High-Performance Liquid Chromatography: The Future of the Field? Chromatographia 2020, 83, 1165–1195. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, J.; Ko, S.H. Electrochemical Biosensors for Point-of-Care Testing. Bio-Des. Manuf. 2024, 7, 548–565. [Google Scholar] [CrossRef]
- Asthana, D.; Pandey, R.; Mukhopadhyay, P. Urea-Based Constructs Readily Amplify and Attenuate Nonlinear Optical Activity in Response to H-Bonding and Anion Recognition. Chem. Commun. 2013, 49, 451–453. [Google Scholar] [CrossRef]
- Rodriguez, S.R.K. Enhancing the Speed and Sensitivity of a Nonlinear Optical Sensor with Noise. Phys. Rev. Appl. 2020, 13, 24032. [Google Scholar] [CrossRef]
- Madikizela, L.; Tavengwa, N.; Pakade, V. Molecularly Imprinted Polymers for Pharmaceutical Compounds: Synthetic Procedures and Analytical Applications. In Recent Research in Polymerization; IntechOpen: London, UK, 2018; pp. 47–67. [Google Scholar]
- Herrera-Chacón, A.; Cetó, X.; del Valle, M. Molecularly Imprinted Polymers—Towards Electrochemical Sensors and Electronic Tongues. Anal. Bioanal. Chem. 2021, 413, 6117–6140. [Google Scholar] [CrossRef]
- Panagiotopoulou, M.; Beyazit, S.; Nestora, S.; Haupt, K.; Tse Sum Bui, B. Initiator-Free Synthesis of Molecularly Imprinted Polymers by Polymerization of Self-Initiated Monomers. Polymer 2015, 66, 43–51. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Kazemifard, N.; Saberi Dehkordi, Z. Parameters That Affect Molecular Imprinting Polymers. In Molecularly Imprinted Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 21–48. ISBN 9780128199527. [Google Scholar]
- Fomo, G.; Waryo, T.; Feleni, U.; Baker, P.; Iwuoha, E. Electrochemical Polymerization; Springer Nature: Berlin/Heidelberg, Germany, 2019; pp. 1–28. [Google Scholar]
- Westbroek, P. Electrochemical Methods. In Analytical Electrochemistry in Textiles; Elsevier: Amsterdam, The Netherlands, 2005; pp. 37–69. ISBN 9781855739192. [Google Scholar]
- Phogat, P.; Sharma, S.; Jha, R.; Singh, S. Electrochemical Devices; In Engineering Materials; Springer Nature: Singapore, 2024; Volume 14, pp. 6–22. ISBN 978-981-96-0526-2. [Google Scholar]
- Chen, A.; Shah, B. Electrochemical Sensing and Biosensing Based on Square Wave Voltammetry. Anal. Methods 2013, 5, 2158. [Google Scholar] [CrossRef]
- Kashyap, B.; Kumar, R. A Novel Multi-Set Differential Pulse Voltammetry Technique for Improving Precision in Electrochemical Sensing. Biosens. Bioelectron. 2022, 216, 114628. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, X.; Zhang, P.; Wang, H.; Ma, Q.; Tao, X. Comparison between Voltammetric Detection Methods for Abalone-Flavoring Liquid. Open Life Sci. 2021, 16, 354–361. [Google Scholar] [CrossRef]
- Hussain, G.; Silvester, D.S. Comparison of Voltammetric Techniques for Ammonia Sensing in Ionic Liquids. Electroanalysis 2018, 30, 75–83. [Google Scholar] [CrossRef]
- Sheikh Beig Goharrizi, M.A.; Kazemi Oskuee, R.; Aleyaghoob, G.; Mohajeri, T.; Mohammadinejad, A.; Rezayi, M. A New Molecularly Imprinted Polymer Electrochemical Sensor Based on CuCo2O4/N-Doped CNTs/P-Doped GO Nanocomposite for Detection of 25-Hydroxyvitamin D3 in Serum Samples. Biotechnol. Appl Biochem. 2023, 70, 357–373. [Google Scholar] [CrossRef]
- Parvin, M.H.; Azizi, E.; Arjomandi, J.; Lee, J.Y. Highly Sensitive and Selective Electrochemical Sensor for Detection of Vitamin B12 Using an Au/PPy/FMNPs@TD-Modified Electrode. Sens. Actuators B Chem. 2018, 261, 335–344. [Google Scholar] [CrossRef]
- da Silva, L.V.; de Almeida, A.K.; Xavier, J.A.; Lopes, C.B.; dos Santos Silva, F.D.A.; Lima, P.R.; dos Santos, N.D.; Kubota, L.T.; Goulart, M.O. Phenol Based Redox Mediators in Electroanalysis. J. Electroanal. Chem. 2018, 827, 230–252. [Google Scholar] [CrossRef]
- Silva, T.L.; Maria de Lourdes, S.G.; Ferreira, F.R.; Santos, D.C.; Amatore, C.; Goulart, M.O. Quinone-Based Molecular Electrochemistry and Their Contributions to Medicinal Chemistry: A Look at the Present and Future. Curr. Opin. Electrochem. 2020, 24, 79–87. [Google Scholar] [CrossRef]
- Amiri, M.; Arshi, S. An Overview on Electrochemical Determination of Cholesterol. Electroanalysis 2020, 32, 1391–1407. [Google Scholar] [CrossRef]
- Lin, X.; Ni, Y.; Kokot, S. An Electrochemical DNA-Sensor Developed with the Use of Methylene Blue as a Redox Indicator for the Detection of DNA Damage Induced by Endocrine-Disrupting Compounds. Anal. Chim. Acta 2015, 867, 29–37. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, D.; Liu, H.; Zeng, Y.; Yin, Z.; Li, L. Electrochemical Molecular Imprinted Sensors Based on Electrospun Nanofiber and Determination of Ascorbic Acid. Anal. Sci. 2015, 31, 793–798. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Xu, J.; Wen, Y. Electropolymerized Molecularly Imprinted Polypyrrole Decorated with Black Phosphorene Quantum Dots onto Poly(3,4-Ethylenedioxythiophene) Nanorods and Its Voltammetric Sensing of Vitamin C. J. Electroanal. Chem. 2018, 814, 153–160. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, G.; Wu, D.; Xiong, C.; Zheng, L.; Ding, Y.; Lu, H.; Zhang, G.; Qiu, L. Highly Selective and Sensitive Sensor Based on an Organic Electrochemical Transistor for the Detection of Ascorbic Acid. Biosens. Bioelectron. 2018, 100, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Miao, Q. Molecularly Imprinted Sensor for Ascorbic Acid Based on Gold Nanoparticles and Multiwalled Carbon Nanotubes. Curr. Anal. Chem. 2020, 16, 905–913. [Google Scholar] [CrossRef]
- Hu, Y.; He, Y.; Peng, Z.; Li, Y. A Ratiometric Electrochemiluminescence Sensing Platform for Robust Ascorbic Acid Analysis Based on a Molecularly Imprinted Polymer Modified Bipolar Electrode. Biosens. Bioelectron. 2020, 167, 112490. [Google Scholar] [CrossRef]
- Kia, S.; Bohlooli, S.; Bahar, S. A Novel Electrochemical Sensor Based on Plastic Antibodies for Vitamin D3 Detection in Real Samples. IEEE Sens. J. 2019, 19, 4752–4757. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, J.; Wen, Y.; Wang, T. A Highly-Sensitive VB2 Electrochemical Sensor Based on One-Step Co-Electrodeposited Molecularly Imprinted WS2-PEDOT Film Supported on Graphene Oxide-SWCNTs Nanocomposite. Mater. Sci. Eng. C 2018, 92, 77–87. [Google Scholar] [CrossRef]
- Mahdi, N.; Roushani, M.; Karazan, Z.M. Electrochemical Sensor Based on Molecularly Imprinted Copolymer for Selective and Simultaneous Determination of Riboflavin, Dopamine, and L-tryptophan. J. Mol. Recognit. 2023, 36, 3053. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Zaidi, S.A.; Vikraman, D.; Kim, H.S.; Jung, J. Facile Preparation of Molybdenum Carbide (Mo2C) Nanoparticles and Its Effective Utilization in Electrochemical Sensing of Folic Acid via Imprinting. Biosens. Bioelectron. 2019, 140, 111330. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, X. Selective and Sensitive Determination of Folic Acid Based on Molecularly Imprinted Poly (O-Aminophenol) and Reduced Graphene Oxide Decorated with Au Nanoparticles. Curr. Anal. Chem. 2021, 17, 1201–1210. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, J.; Wen, Y.; Zhang, J.; Ding, W. The Electro-Synthesized Imprinted PEDOT Film as a Simple Voltammetric Sensor for Highly Sensitive and Selective Detection of Vitamin K3 in Poultry Drug Samples. Synth. Met. 2017, 230, 79–88. [Google Scholar] [CrossRef]
- Khan, S.; Wong, A.; Rychlik, M.; Sotomayor, M.D.P.T. A Novel Synthesis of a Magnetic Porous Imprinted Polymer by Polyol Method Coupled with Electrochemical Biomimetic Sensor for the Detection of Folate in Food Samples. Chemosensors 2022, 10, 473. [Google Scholar] [CrossRef]
- Alizadeh, T.; Akhoundian, M. An Ultra-Sensitive and Highly Selective Impedimetric Sensor for Vitamin D Measurement Based on a Novel Imprinted Polymer Synthesized Utilizing Template-Derived Functional Monomer. Anal. Chim. Acta 2022, 1223, 340206. [Google Scholar] [CrossRef]
- Zembrzuska, D.; Kalecki, J.; Cieplak, M.; Lisowski, W.; Borowicz, P.; Noworyta, K.; Sharma, P.S. Electrochemically Initiated Co-Polymerization of Monomers of Different Oxidation Potentials for Molecular Imprinting of Electroactive Analyte. Sens. Actuators B Chem. 2019, 298, 126884. [Google Scholar] [CrossRef]
- Ansell, R.J. Characterization of the Binding Properties of Molecularly Imprinted Polymers. In Advances in Biochemical Engineering/Biotechnology; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2015; Volume 150, pp. 51–93. [Google Scholar]
- Pratama, K.F.; Manik, M.E.R.; Rahayu, D.; Hasanah, A.N. Effect of the Molecularly Imprinted Polymer Component Ratio on Analytical Performance. Chem. Pharm. Bull. 2020, 68, 1013–1024. [Google Scholar] [CrossRef]
- Valentino, M.; Imbriano, A.; Tricase, A.; Della Pelle, F.; Compagnone, D.; Macchia, E.; Torsi, L.; Bollella, P.; Ditaranto, N. Electropolymerized Molecularly Imprinted Polypyrrole Film for Dimethoate Sensing: Investigation on Template Removal after the Imprinting Process. Anal. Methods 2023, 15, 1250–1253. [Google Scholar] [CrossRef] [PubMed]
- Ndunda, E.N. Molecularly Imprinted Polymers—A Closer Look at the Control Polymer Used in Determining the Imprinting Effect: A Mini Review. J. Mol. Recognit. 2020, 33, 2855. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Billing, J.; Nilsson, C.; Boyd, B.; Kecili, R.; Nivhede, D.; Axelsson, S.; Rees, A. Utilizing the Cross-Reactivity of MIPs. In Advances in Biochemical Engineering/Biotechnology; Springer Science and Business Media Deutschland GmbH: Berlin, Germany, 2015; Volume 150, pp. 167–182. [Google Scholar]
- Yulianti, E.S.; Rahman, S.F.; Whulanza, Y. Molecularly Imprinted Polymer-Based Sensor for Electrochemical Detection of Cortisol. Biosensors 2022, 12, 1090. [Google Scholar] [CrossRef]
- Imai, K.; Okazaki, T.; Hata, N.; Taguchi, S.; Sugawara, K.; Kuramitz, H. Simultaneous Multiselective Spectroelectrochemical Fiber-Optic Sensor: Demonstration of the Concept Using Methylene Blue and Ferrocyanide. Anal. Chem. 2015, 87, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Arman, A.; Üzer, A.; Sağlam, Ş.; Erçağ, E.; Apak, R. Indirect Electrochemical Determination of Antioxidant Capacity with Hexacyanoferrate(III) Reduction Using a Gold Nanoparticle-Coated o -Phenylenediamine-Aniline Copolymer Electrode. Anal. Lett. 2019, 52, 1282–1297. [Google Scholar] [CrossRef]
- Zhou, W.; Qu, Q.; Yu, W.; An, Z. Single Monomer for Multiple Tasks: Polymerization Induced Self-Assembly, Functionalization and Cross-Linking, and Nanoparticle Loading. ACS Macro. Lett. 2014, 3, 1220–1224. [Google Scholar] [CrossRef]
- Li, L.; Yang, L.; Teng, Y.; Zhong, M.; Lu, X.; Kan, X. Preparation and Application of Imprinted Electrochemical Sensor Based on Dopamine Self-Polymerization. J. Electrochem. Soc. 2014, 161, B312–B316. [Google Scholar] [CrossRef]
- Sharma, S. Glassy Carbon: A Promising Material for Micro- and Nanomanufacturing. Materials 2018, 11, 1857. [Google Scholar] [CrossRef]
- Lakhera, P.; Chaudhary, V.; Jha, A.; Singh, R.; Kush, P.; Kumar, P. Recent Developments and Fabrication of the Different Electrochemical Biosensors Based on Modified Screen Printed and Glassy Carbon Electrodes for the Early Diagnosis of Diverse Breast Cancer Biomarkers. Mater. Today Chem. 2022, 26, 101129. [Google Scholar] [CrossRef]
- Qronfla, M.M.; Jamoussi, B.; Chakroun, R.; Al-Mur, B.A.; Halawani, R.F.; Aloufi, F.A. Synthesis of a New Molecularly Imprinted Polymer and Optimisation of Phenylglyoxylic Acid Extraction from Human Urine Samples Using a Central Composite Design within the Response Surface Methodology. Polymers 2023, 15, 3279. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Dang, Y.; Chen, Z.; Zhang, R.; Li, Y.; Ye, B.C. A Robust Electrochemical Sensing of Molecularly Imprinted Polymer Prepared by Using Bifunctional Monomer and Its Application in Detection of Cypermethrin. Biosens. Bioelectron. 2019, 127, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Harun-Ur-Rashid, M.; Imran, A.B.; Foyez, T. Voltammetric Sensors Modified with Nanomaterials: Applications in Rapid Detection of Bioactive Compounds for Health and Safety Monitoring. Discov. Electrochem. 2025, 2, 14. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Cinti, S.; Arduini, F.; Carbone, M.; Sansone, L.; Cacciotti, I.; Moscone, D.; Palleschi, G. Screen-Printed Electrodes Modified with Carbon Nanomaterials: A Comparison among Carbon Black, Carbon Nanotubes and Graphene. Electroanalysis 2015, 27, 2230–2238. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Palanisamy, S.; Chen, S.; Sayee Kannan, R. Alumina Polished Glassy Carbon Electrode as a Simple Electrode for Lower Potential Electrochemical Detection of Dopamine in Its Sub-micromolar Level. Electroanalysis 2016, 28, 425–430. [Google Scholar] [CrossRef]
- González-Sánchez, M.I.; Gómez-Monedero, B.; Agrisuelas, J.; Iniesta, J.; Valero, E. Highly Activated Screen-Printed Carbon Electrodes by Electrochemical Treatment with Hydrogen Peroxide. Electrochem. Commun. 2018, 91, 36–40. [Google Scholar] [CrossRef]
- González-Sánchez, M.I.; Gómez-Monedero, B.; Agrisuelas, J.; Iniesta, J.; Valero, E. Electrochemical Performance of Activated Screen Printed Carbon Electrodes for Hydrogen Peroxide and Phenol Derivatives Sensing. J. Electroanal. Chem. 2019, 839, 75–82. [Google Scholar] [CrossRef]
- Safaryan, A.H.M.; Smith, A.M.; Bedwell, T.S.; Piletska, E.V.; Canfarotta, F.; Piletsky, S.A. Optimisation of the Preservation Conditions for Molecularly Imprinted Polymer Nanoparticles Specific for Trypsin. Nanoscale. Adv. 2019, 1, 3709–3714. [Google Scholar] [CrossRef]
- Lim, K.F.; Holdsworth, C.I. Effect of Formulation on the Binding Efficiency and Selectivity of Precipitation Molecularly Imprinted Polymers. Molecules 2018, 23, 2996. [Google Scholar] [CrossRef]
- Romanholo, P.V.V.; Razzino, C.A.; Raymundo-Pereira, P.A.; Prado, T.M.; Machado, S.A.S.; Sgobbi, L.F. Biomimetic Electrochemical Sensors: New Horizons and Challenges in Biosensing Applications. Biosens. Bioelectron. 2021, 185, 113242. [Google Scholar] [CrossRef]
- Marć, M.; Wieczorek, P.P. Introduction to MIP Synthesis, Characteristics and Analytical Application. In Comprehensive Analytical Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2019; Volume 86, pp. 1–15. ISBN 9780444642660. [Google Scholar]
- Garg, M.; Pamme, N. Strategies to Remove Templates from Molecularly Imprinted Polymer (MIP) for Biosensors. TrAC Trends Anal. Chem. 2024, 170, 117437. [Google Scholar] [CrossRef]
- Moreno Bondi, M.C.; Urraca, J.L.; Carrasco, S.; Navarro-Villoslada, F. Handbook of Molecularly Imprinted Polymers “Ch 2: Preparation of Molecularly Imprinted Polymers”; Alvarez-Lorenzo, C., Concheiro, A., Eds.; Smithers Rapra Technology: Shrewsbury, UK, 2013; ISBN 9781847359599. [Google Scholar]
- Belbruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, A.; Gáspár, S.; Gheorghiu, M.; Polonschii, C.; Banciu, R.M.; David, S.; Gheorghiu, E.; Marty, J.L. Promising Solutions to Address the Non-Specific Adsorption in Biosensors Based on Coupled Electrochemical-Surface Plasmon Resonance Detection. Chemosensors 2025, 13, 92. [Google Scholar] [CrossRef]
- Hosseinikebria, S.; Khazaei, M.; Dervisevic, M.; Judicpa, M.A.; Tian, J.; Razal, J.M.; Voelcker, N.H.; Nilghaz, A. Electrochemical Biosensors: The Beacon for Food Safety and Quality. Food Chem. 2025, 475, 143284. [Google Scholar] [CrossRef]
- Hanssen, B.L.; Siraj, S.; Wong, D.K.Y. Recent Strategies to Minimise Fouling in Electrochemical Detection Systems. Rev. Anal. Chem. 2016, 35, 1–28. [Google Scholar] [CrossRef]
- Szunerits, S.; Pagneux, Q.; M’Barek, Y.B.; Vassal, S.; Boukherroub, R. Do Not Let Electrode Fouling Be the Enemy of Bioanalysis. Bioelectrochemistry 2023, 153, 108479. [Google Scholar] [CrossRef]
- Spring, S.A.; Goggins, S.; Frost, C.G. Ratiometric Electrochemistry: Improving the Robustness, Reproducibility and Reliability of Biosensors. Molecules 2021, 26, 82130. [Google Scholar] [CrossRef]
- Chen, G.; Chen, W.; Xu, L.; Jin, H.; Sun, W.; Lan, J.; Wu, F.; Zhang, X.; Zhang, J.; Chen, J. Sensitive, Highly Stable, and Anti-Fouling Electrode with Hexanethiol and Poly-A Modification for Exosomal MicroRNA Detection. Anal. Chem. 2022, 94, 5382–5391. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).