Oxidation Resistance, Ablation Resistance, and Ablation Mechanism of HfC–B4C-Modified Carbon Fiber/Boron Phenolic Resin Ceramizable Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
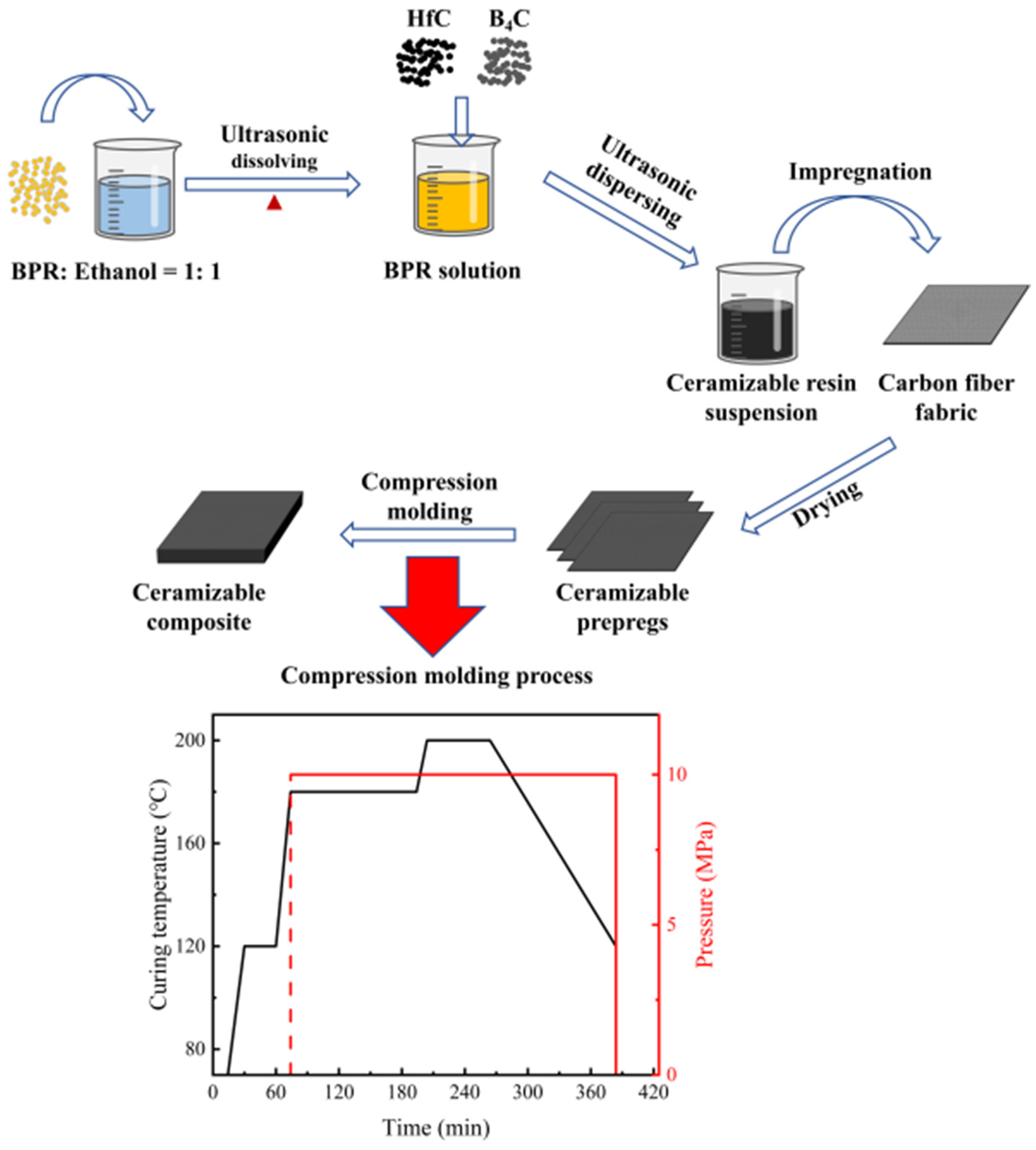
2.2. Fabrication of Ceramizable Composites
2.3. Flexural Test
2.4. Oxy-Acetylene Ablation Test
2.5. Characterizations
3. Results and Discussion
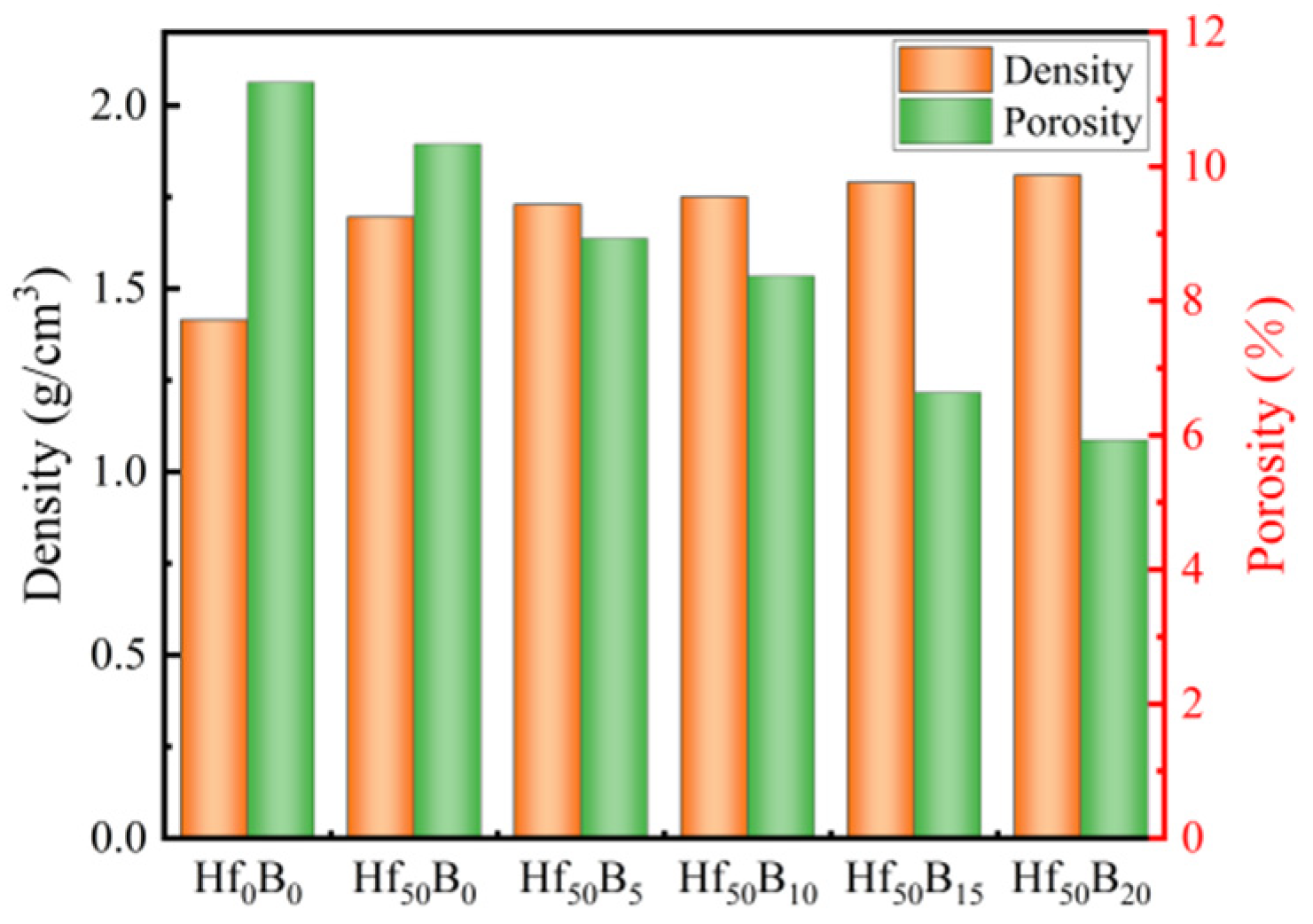
3.1. Density and Porosity of the Ceramizable Composites
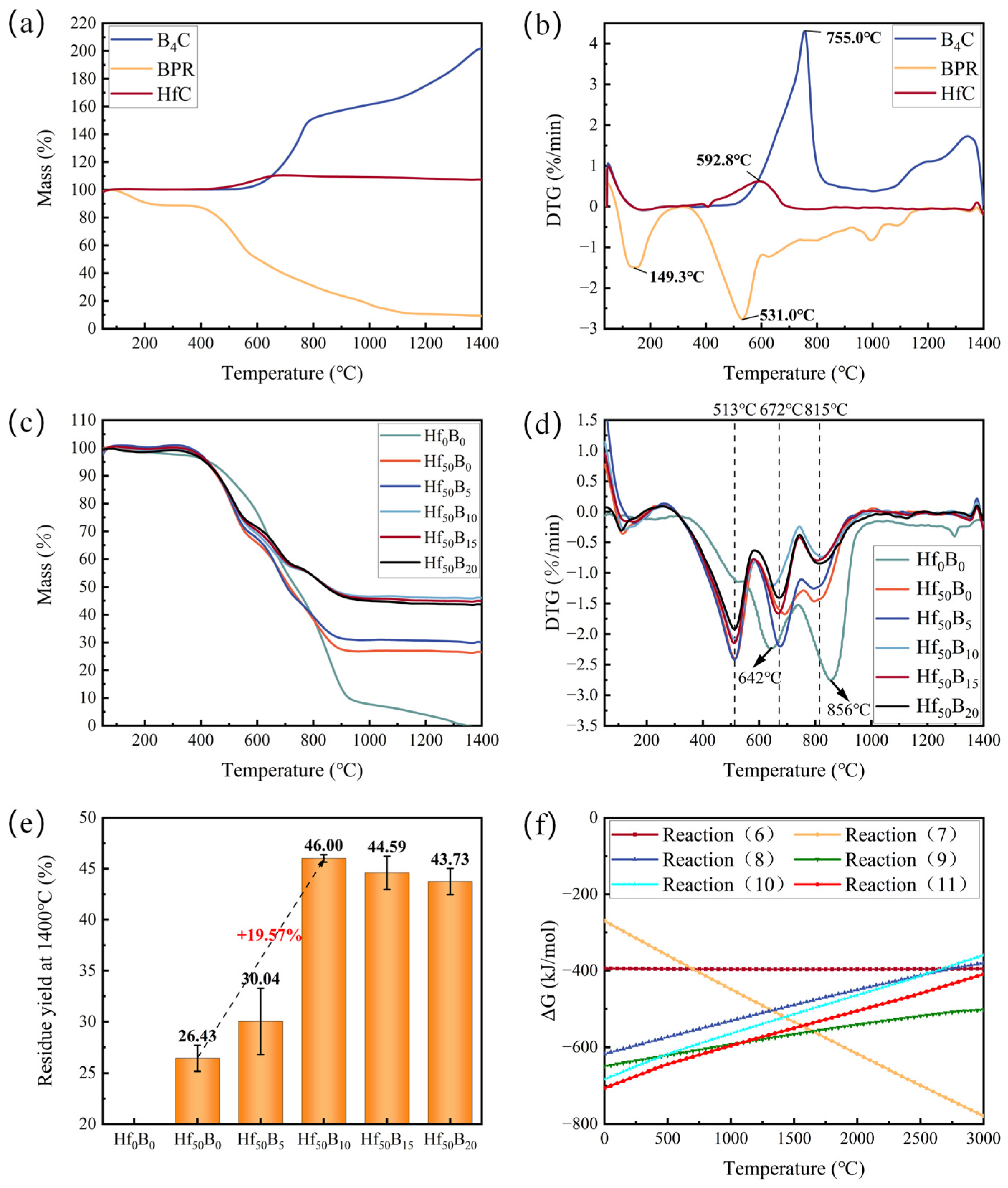
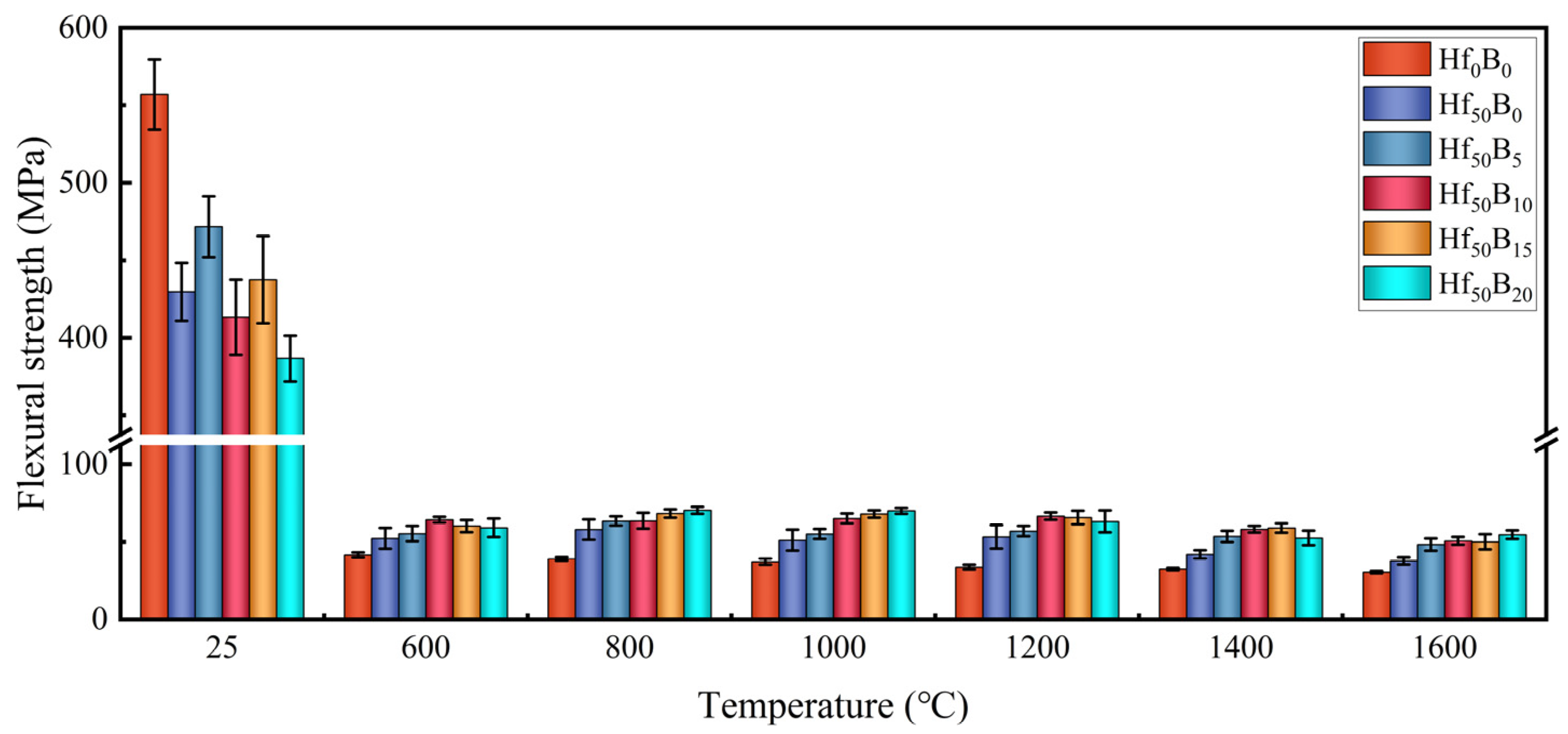
3.2. Thermal Stability, Oxidation Resistance, and Flexural Strength of Ceramizable Composites
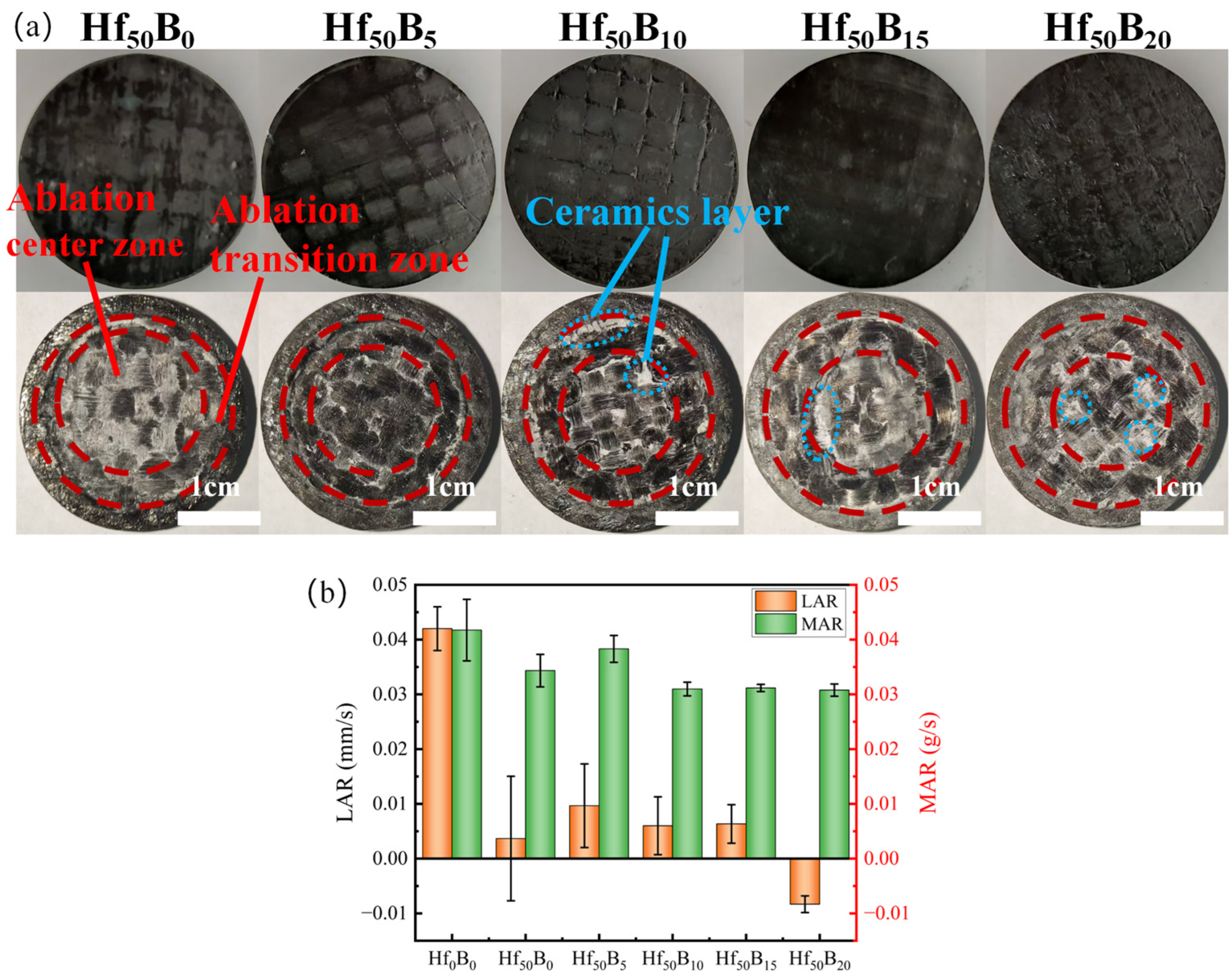
3.3. Ablation Resistance of Ceramizable Composites
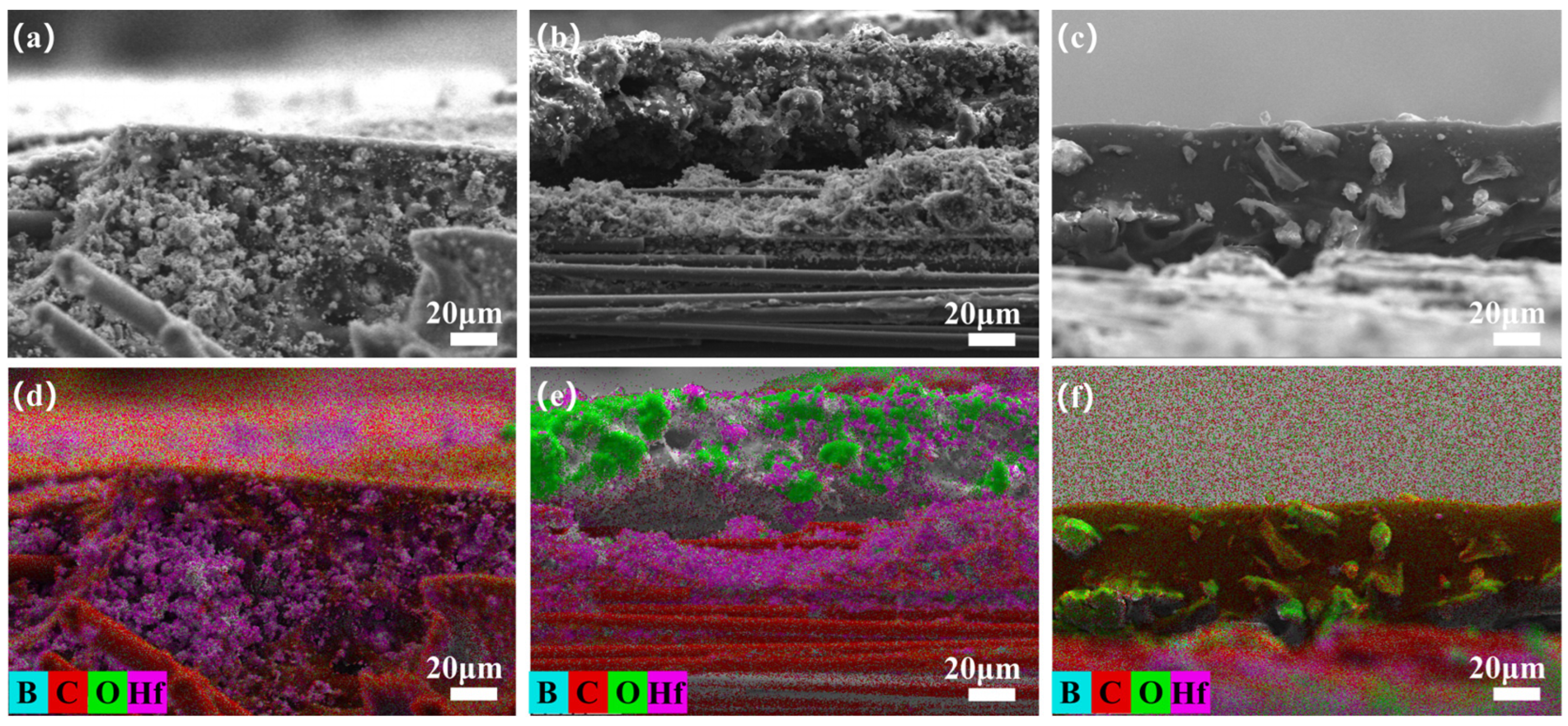
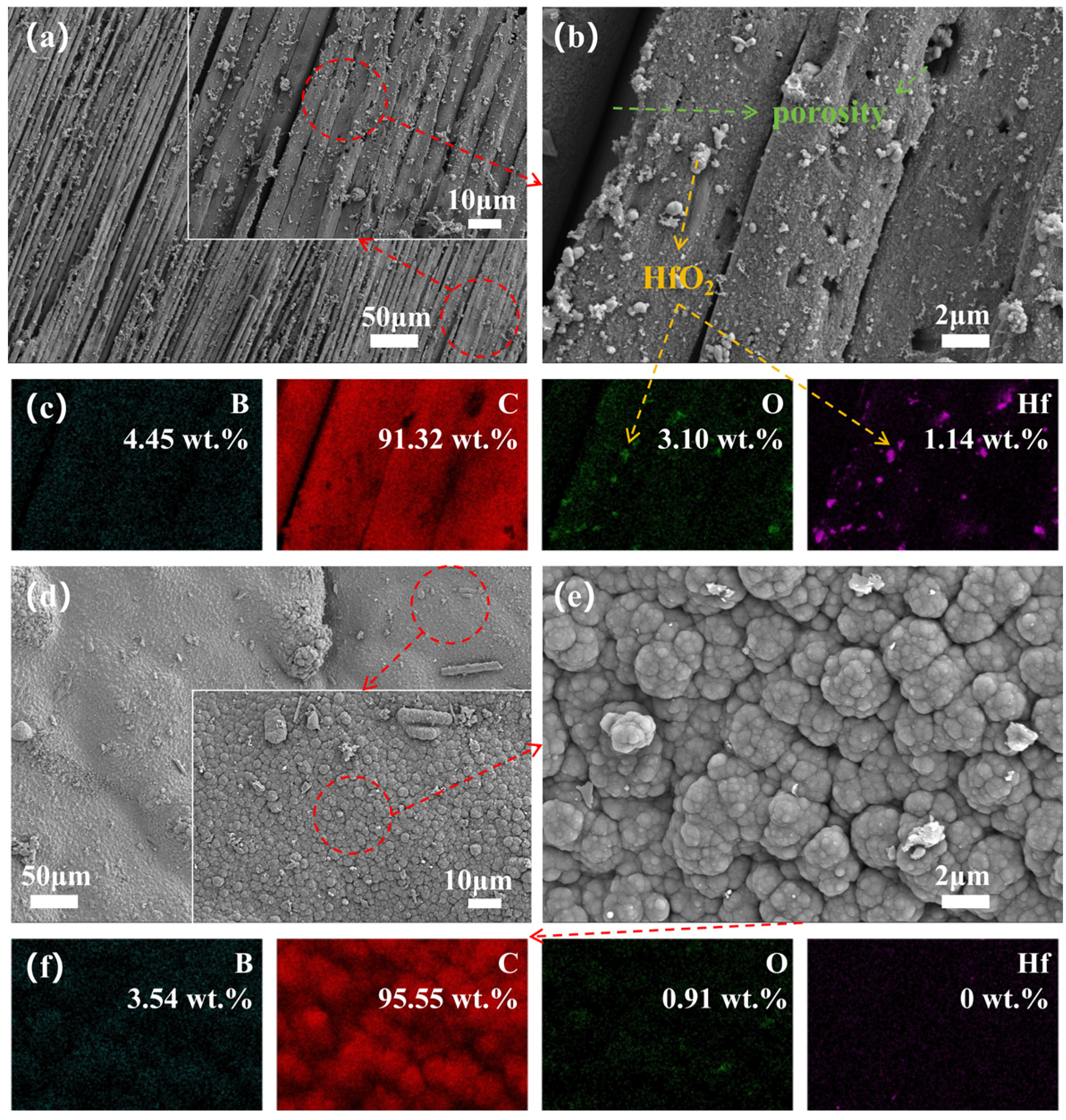
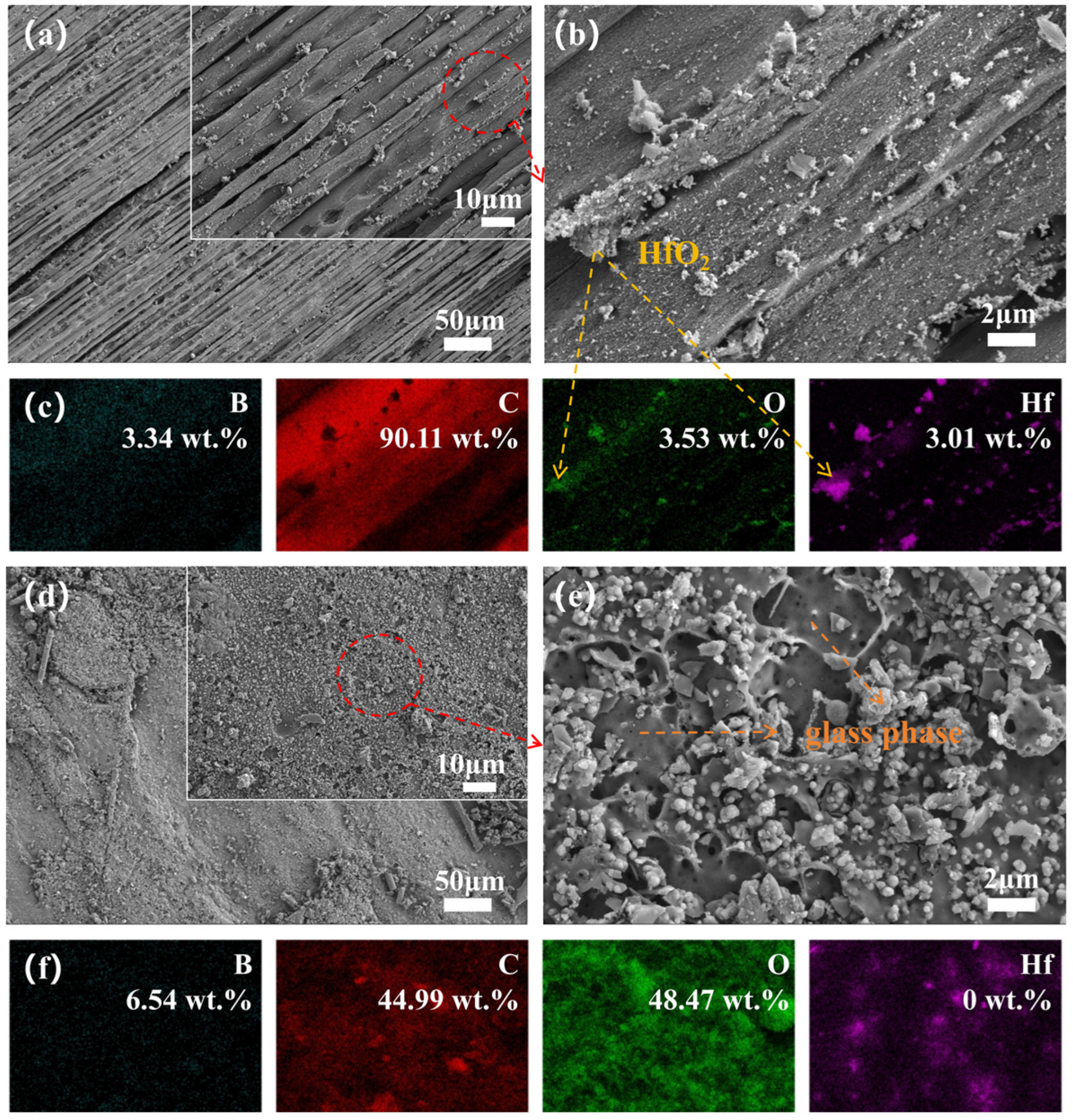
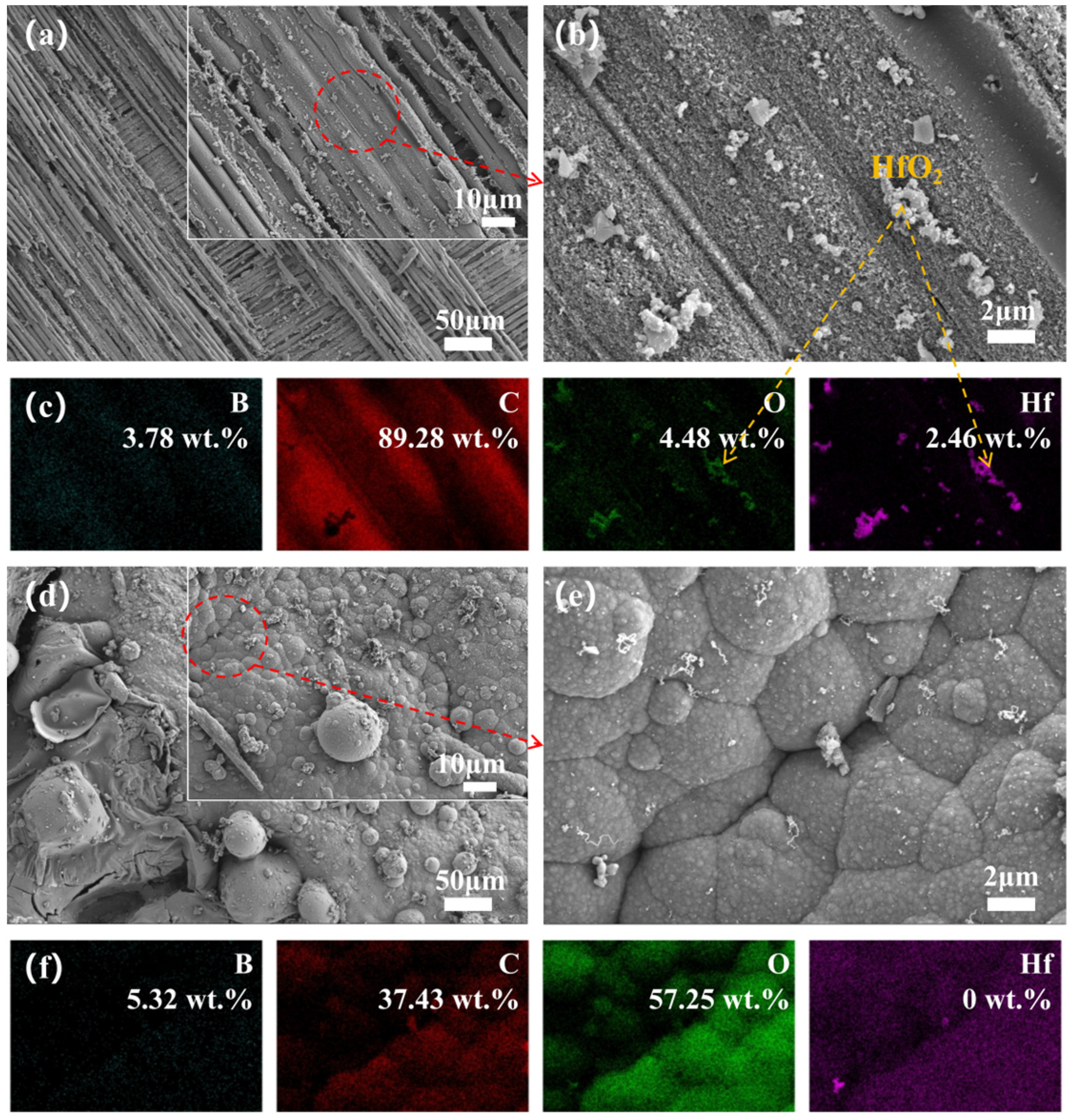
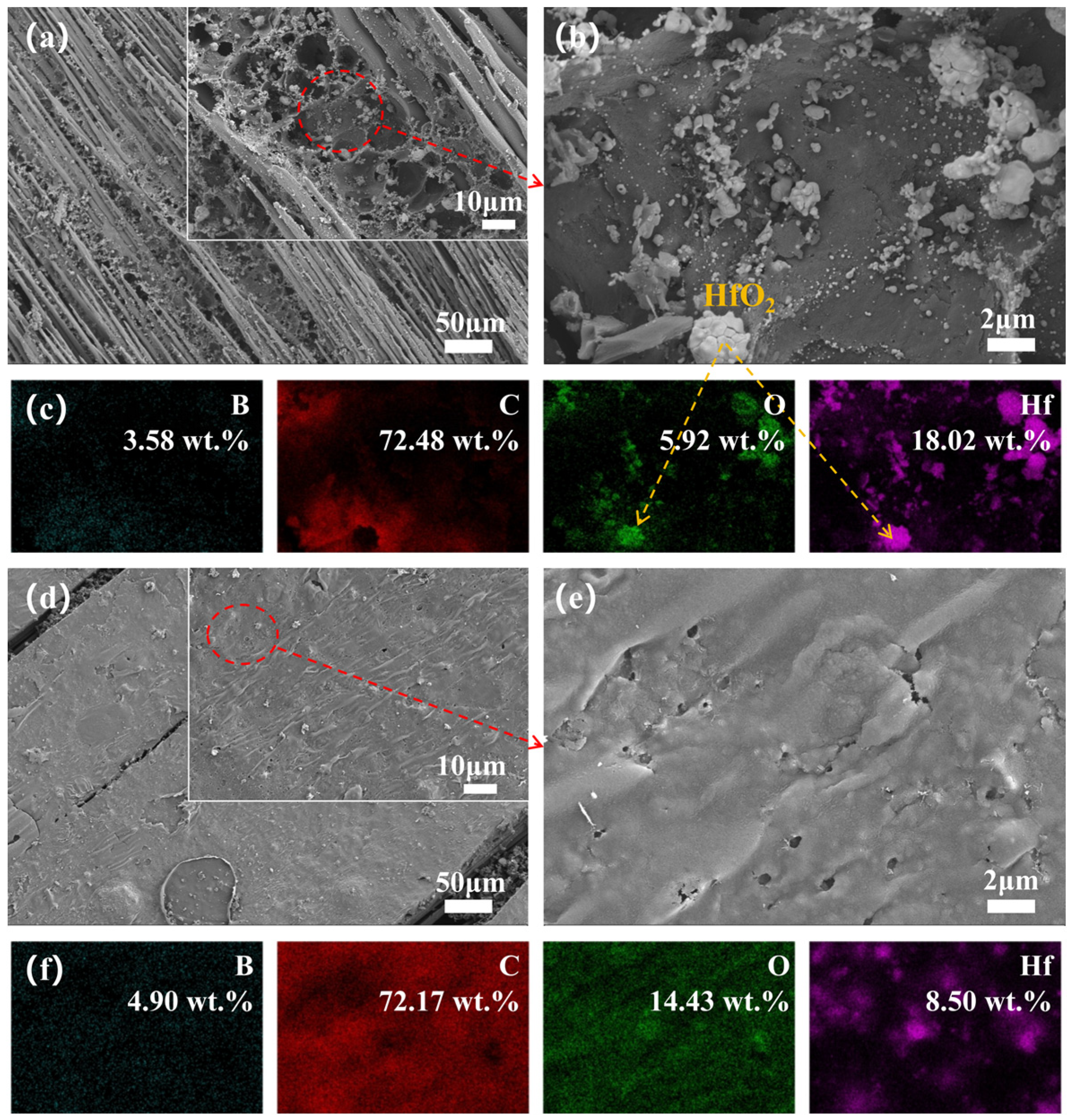
3.4. Microstructure Evolution
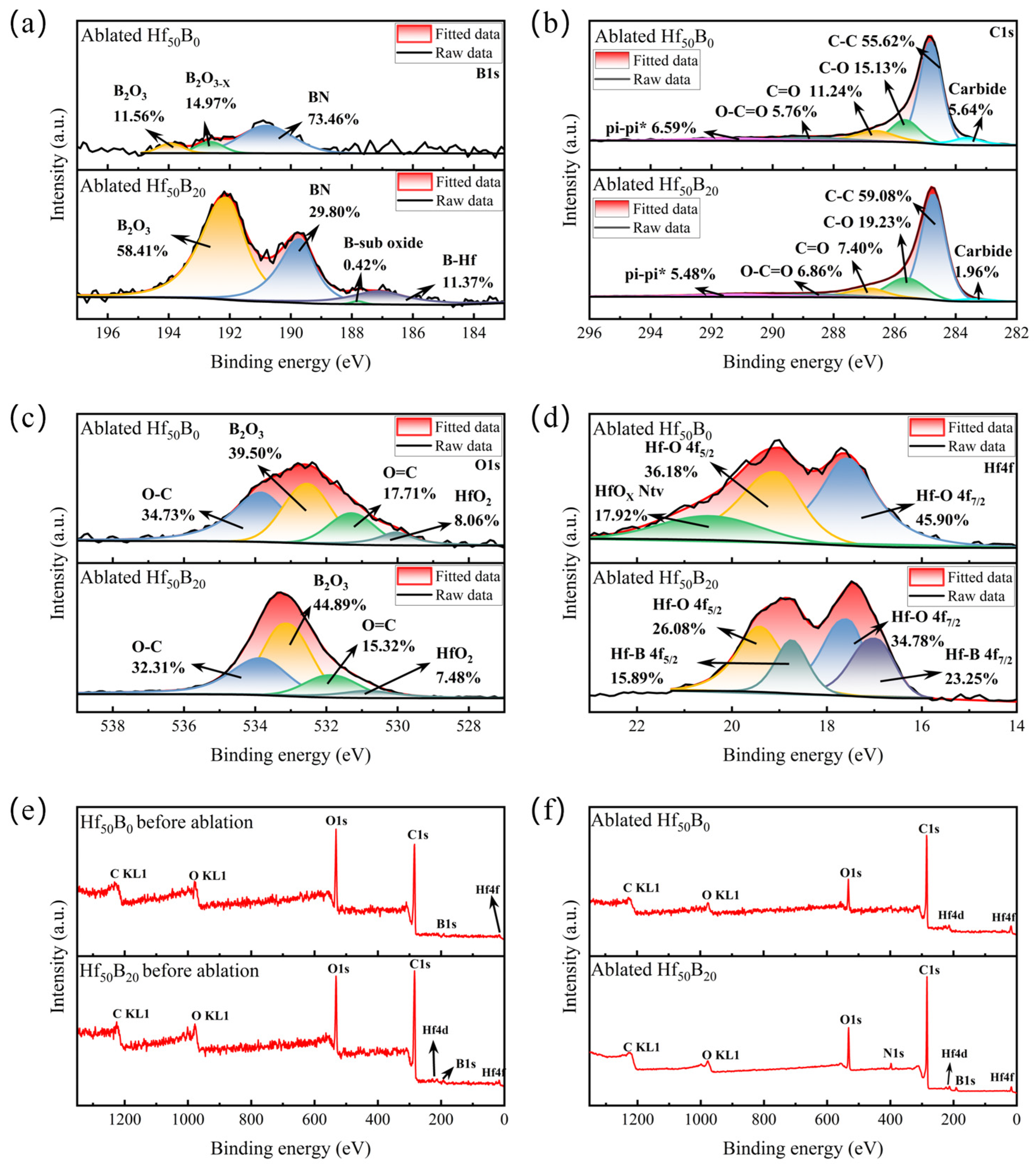
3.5. Phase Evolution
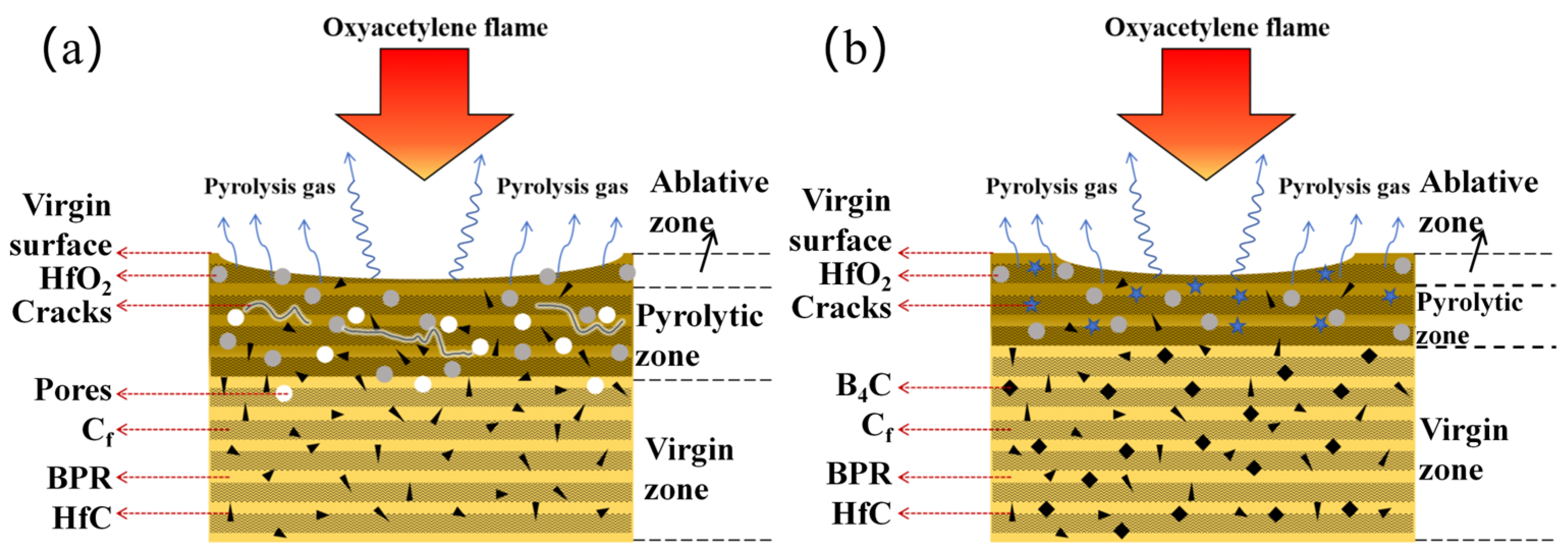
3.6. Ablation Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Cf | Carbon fiber |
| Ph | Phenolic resin |
| HfC | Hafnium carbide |
| B4C | Boron carbide |
| LAR | Linear ablation rate |
| MAR | Mass ablation rate |
| TPS | Thermal protection system |
| UHTCs | Ultra-high-temperature ceramics |
| BPR | Boron phenolic resin |
| SEM | Scanning electron microscope |
| EDS | Energy-dispersive X-ray |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectrometer |
| TGA | Thermogravimetric analysis |
| DTG | Derivative thermogravimetry |
| PyC | Pyrolytic carbon |
| ΔG | Gibbs free energy change |
| ΔH | Enthalpy change |
References
- Li, W.; Huang, J.; Zhang, Z.; Huang, H.; Liang, J.; Wang, L. Evaluation Method and Key Factor Analysis for Thermal Protection Performance of Multifunctional Integrated Ablative Materials. Polym. Compos. 2020, 41, 5043–5058. [Google Scholar] [CrossRef]
- Wu, D.; Lin, L.; Ren, H. Thermal/Vibration Joint Experimental Investigation on Lightweight Ceramic Insulating Material for Hypersonic Vehicles in Extremely High-Temperature Environment up to 1500 °C. Ceram. Int. 2020, 46, 14439–14447. [Google Scholar] [CrossRef]
- Mirzapour, M.A.; Haghighat, H.R.; Eslami, Z. Effect of Zirconia on Ablation Mechanism of Asbestos Fiber/Phenolic Composites in Oxyacetylene Torch Environment. Ceram. Int. 2013, 39, 9263–9272. [Google Scholar] [CrossRef]
- Biamino, S.; Antonini, A.; Eisenmenger-Sittner, C.; Fuso, L.; Pavese, M.; Fino, P.; Bauer, E.; Badini, C. Multilayer SiC for Thermal Protection System of Space Vehicles with Decreased Thermal Conductivity through the Thickness. J. Eur. Ceram. Soc. 2010, 30, 1833–1840. [Google Scholar] [CrossRef]
- Natali, M.; Kenny, J.M.; Torre, L. Science and Technology of Polymeric Ablative Materials for Thermal Protection Systems and Propulsion Devices: A Review. Prog. Mater. Sci. 2016, 84, 192–275. [Google Scholar] [CrossRef]
- Costa E Silva, S.F.; Machado, H.A.; Bittencourt, E. Effect of the Fiber Orientation Relatively to the Plasma Flow Direction in the Ablation Process of a Carbon-Phenolic Composite. J. Aerosp. Technol. Manag. 2015, 7, 43–52. [Google Scholar] [CrossRef]
- Pesci, P.G.S.; Araújo Machado, H.; De Paula E Silva, H.; Paterniani Rita, C.C.; Filho, G.P.; Botelho, E.C. Numerical-Experimental Analysis of a Carbon-Phenolic Composite via Plasma Jet Ablation Test. Mater. Res. Express 2018, 5, 065601. [Google Scholar] [CrossRef]
- Li, B.; Li, H.; Yao, X.; Zhu, X.; Liu, N. Preparation and Ablation Resistance of ZrC Nanowires-Reinforced CVD-ZrC Coating on Sharp Leading Edge C/C Composites. Appl. Surf. Sci. 2022, 584, 152617. [Google Scholar] [CrossRef]
- Liu, H.; Ma, H.; Chang, Y.; Zhou, X. Ablation and Mechanical Properties of ZrC-Reinforced PBI Resin Matrix Composites. Ceram. Int. 2023, 49, 15527–15535. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y.; Zhang, P.; Li, T.; Li, J.; Yang, Y. Ablation Resistance of HfC Coating Reinforced by HfC Nanowires in Cyclic Ablation Environment. J. Eur. Ceram. Soc. 2017, 37, 2759–2768. [Google Scholar] [CrossRef]
- Pan, X.; Niu, Y.; Liu, T.; Zhong, X.; Li, C.; Shi, M.; Zheng, X.; Ding, C. Ablation Behaviors of ZrC-TiC Coatings Prepared by Vacuum Plasma Spray: Above 2000 °C. J. Eur. Ceram. Soc. 2019, 39, 3292–3300. [Google Scholar] [CrossRef]
- Shichalin, O.O.; Ivanov, N.P.; Seroshtan, A.I.; Nadaraia, K.V.; Simonenko, T.L.; Gurin, M.S.; Kornakova, Z.E.; Shchitovskaya, E.V.; Barkhudarov, K.V.; Tsygankov, D.K.; et al. Spark Plasma Sintering of Ti2AlC/TiC MAX-Phase Based Composite Ceramic Materials and Study of Their Electrochemical Characteristics. Ceram. Int. 2024, 50, 53120–53128. [Google Scholar] [CrossRef]
- Xu, X.; Pan, X.; Niu, Y.; Li, H.; Ni, D.; Huang, S.; Zhong, X.; Zheng, X.; Sun, J. Difference evaluation on ablation behaviors of ZrC-based and ZrB2-based UHTCs coatings. Corros. Sci. 2021, 180, 109181. [Google Scholar] [CrossRef]
- Li, H.; Yao, D.; Fu, Q.; Liu, L.; Zhang, Y.; Yao, X.; Wang, Y.; Li, H. Anti-Oxidation and Ablation Properties of Carbon/Carbon Composites Infiltrated by Hafnium Boride. Carbon 2013, 52, 418–426. [Google Scholar] [CrossRef]
- Wang, D.; Ding, Z.; Zhang, C.; Zhao, J.; Lei, Y.; Zhang, G.; Zheng, G. Mechanical Properties and Thermal Shock Resistance of TiB2-B4C Composite Ceramic Tool Materials at Microscopic Scale. Int. J. Appl. Ceram. Technol. 2024, 21, 3485–3500. [Google Scholar] [CrossRef]
- Liu, H.; Ma, H.; Xu, Y.; Zhou, X. Ablation Properties of SiC-Reinforced PBI Resin Matrix Composites under High-Energy Continuous Laser Ablation. Prog. Org. Coat. 2024, 186, 107964. [Google Scholar] [CrossRef]
- Deng, Z.; Yang, X.; Yu, X.; Huang, Z.; Zhu, D.; Shi, M. High Temperature Flexural Strength, Microstructure, Phase Evolution and Anti-Oxidation Mechanism of Al-Coated Carbon Fiber/Boron Phenolic Resin Ceramizable Composite Modified with TiB2 and B4C. Ceram. Int. 2023, 49, 25003–25015. [Google Scholar] [CrossRef]
- Zou, Z.; Qin, Y.; Chang, K.; Huang, Z. Thermal Properties and Oxidative Corrosion Behaviour of HfB2-SiB6 Ceramicizable Phenolic Resin Matrix Composites. Corros. Sci. 2023, 221, 111340. [Google Scholar] [CrossRef]
- Deng, Z.; Wu, Y.; Shi, M.; Yang, X.; Huang, Z. Ablation Behavior, Microstructure, Phase Evolution and in Situ Ceramization Mechanism of ZrC-LaB6 Modified Carbon Fiber/Phenolic Resin Ceramizable Composite. Polym. Degrad. Stab. 2023, 218, 110584. [Google Scholar] [CrossRef]
- Deng, J.; Hu, K.; Lu, B.; Zheng, B.; Fan, S.; Zhang, L.; Cheng, L. Influence of B4C on Oxidation Resistance of PSN/Borosilicate Glass-B4C Field-Based Repair Coating of C/C Aircraft Brake Materials at 700–900 °C. Ceram. Int. 2019, 45, 20860–20872. [Google Scholar] [CrossRef]
- Houssain, H.; Islak, S.; Okay, F.; Cabbar, T. Microstructure, Mechanical, and Oxidation Properties of Mo-SiC/B4C Hybrid Composites. Ceram. Int. 2025, 51, 10375–10382. [Google Scholar] [CrossRef]
- Guo, Q.; Song, J.; Liu, L.; Zhang, B. Factors Influencing Oxidation Resistance of B4C/C Composites with Self-Healing Properties. Carbon 1998, 36, 1597–1601. [Google Scholar] [CrossRef]
- Zhu, D.; Huang, Z.; Shi, M.; Qin, Y.; Zou, Z.; Deng, Z. High Char Yield BPR Modified with ZrSi2 and B4C: Pyrolysis Kinetic Behavior and Structure Evolution. J. Therm. Anal. Calorim. 2023, 148, 789–805. [Google Scholar] [CrossRef]
- GB/T 1449-2005; Fibre-Reinforced Plastic Composites-Determination of Flexural Properties. China Building Materials Federation: Beijing, China, 2005.
- GJB 323A-1996; Test Methods for Ablation of Ablators. Commission for Science, Technology and Industry for National Defense: Beijing, China, 1996.
- Weng, Y.; Yang, X.; Chen, F.; Zhang, X.; Shi, A.; Yan, J.; Huang, Q. Effect of CVI SiC Content on Ablation and Mechanism of C/C-SiC-ZrC-Cu Composites. Ceram. Int. 2022, 48, 7937–7950. [Google Scholar] [CrossRef]
- Niu, Z.; Xin, Y.; Wang, L.; Shen, S.; Ma, X.; Chen, B.; Wang, C.; Chen, F.; Zhang, C.; Hou, X. Two Birds with One Stone: Construction of Bifunctional-POSS Hybridized Boron-Silicon Ceramicized Phenolic Composites and Its Ablation Behavior. J. Mater. Sci. Technol. 2023, 141, 199–208. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, Y.; Wang, R.; Fu, Q. Ablation Behaviour of C/C-HfC-SiC Composites Prepared by Joint Route of Precursor Infiltration and Pyrolysis and Gaseous Silicon Infiltration. Chin. J. Aeronaut. 2023, 36, 426–436. [Google Scholar] [CrossRef]
- Su, Y.; Chen, S.; Shi, Y. Effects of LaB6 on the Ablation Properties and Mechanism of C/HfC-SiC Composites. Ceram. Int. 2025, 51, 8507–8515. [Google Scholar] [CrossRef]
- Lu, J.; Zhu, S.; Liu, Y.; Xie, M. Effects of Gd2O3 Content on the Infrared Emissivity and Ablation Resistance of HfB2/SiC/TaSi2 Coating at 4400 kW/m2. Coatings 2023, 13, 1397. [Google Scholar] [CrossRef]
- Gai, W.; Zhang, Y.; Zhang, J.; Chen, H.; Chen, G.; Kong, J.; Fu, Y. A Strategy to Improve the Anti-Ablation Performance of C/C Composites under Cyclic Oxyacetylene Flame: SiC/HfB2 Multi-Layer Alternating Coatings Prepared by CVD. J. Alloys Compd. 2025, 1015, 178803. [Google Scholar] [CrossRef]
- Gai, W.; Zhang, Y.; Chen, G.; Kong, J.; Zhang, P.; Zhu, X.; Li, T. HfB2 Coating on C/C Composites Prepared by Chemical Vapor Deposition: Thermodynamics and Experimental Investigation. Ceram. Int. 2022, 48, 31354–31362. [Google Scholar] [CrossRef]
- Ren, J.; Dou, J.; Zhang, L.; He, S.; Qin, Y.; Fu, H. Enhancement of the Ablation Properties of Silica/Phenolic Resin Composites by SiC/Si3N4 Multiphase Structures. J. Macromol. Sci. B 2024, 63, 998–1012. [Google Scholar] [CrossRef]
- Fu, H.; Qin, Y.; Peng, Z.; Dou, J.; Huang, Z. A Novel Co-Continuous Si–Zr Hybrid Phenolic Aerogel Composite with Excellent Antioxidant Ablation Enabled by Sea-Island-like Ceramic Structure at High Temperature. Ceram. Int. 2024, 50, 21008–21019. [Google Scholar] [CrossRef]
- Duan, L.; Zhao, X.; Wang, Y. Oxidation and Ablation Behaviors of Carbon Fiber/Phenolic Resin Composites Modified with Borosilicate Glass and Polycarbosilane Interface. J. Alloys Compd. 2020, 827, 154277. [Google Scholar] [CrossRef]
- Tong, M.; Fu, Q.; Feng, T.; Dai, Y.; Hou, W.; He, P. Effect on BN Interphase Thickness upon SiCnws @BN/HfC Coating Performance under Impact and Ablation Environment. Int. J. Appl. Ceram. Technol. 2024, 21, 240–253. [Google Scholar] [CrossRef]
- Ghosh, B.; Xu, F.; Grant, D.M.; Giangrande, P.; Gerada, C.; George, M.W.; Hou, X. Highly Ordered BN⊥ –BN⊥ Stacking Structure for Improved Thermally Conductive Polymer Composites. Adv. Electron. Mater. 2020, 6, 2000627. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Zhou, Y.; Li, D.; Ying, G. The Ablation Performance of Silicon Nitride/Boron Nitride Fibrous Monolithic Ceramics under an Oxyacetylene Combustion Torch. Materials 2023, 16, 6703. [Google Scholar] [CrossRef]
- Xue, X.; Peng, Y.; Huang, J.; Li, L.; Ni, Y.; Ma, Z.; Gao, L.; Chen, W.; Chen, G.; Ma, C. Design and Preparation of Resin Matrix Composite Coating with Good Ablation Resistance Performance under High-Energy Laser Irradiation. Ceram. Int. 2023, 49, 18962–18968. [Google Scholar] [CrossRef]


| Samples | Mass/g | |||
|---|---|---|---|---|
| BPR | Cf | HfC | B4C | |
| Hf0B0 | 100 | 100 | 0 | 0 |
| Hf50B0 | 100 | 100 | 50 | 0 |
| Hf50B5 | 100 | 100 | 50 | 5 |
| Hf50B10 | 100 | 100 | 50 | 10 |
| Hf50B15 | 100 | 100 | 50 | 15 |
| Hf50B20 | 100 | 100 | 50 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, H.; Zhang, W.; Deng, Z.; Yang, X.; Huang, W. Oxidation Resistance, Ablation Resistance, and Ablation Mechanism of HfC–B4C-Modified Carbon Fiber/Boron Phenolic Resin Ceramizable Composites. Polymers 2025, 17, 1412. https://doi.org/10.3390/polym17101412
Wen H, Zhang W, Deng Z, Yang X, Huang W. Oxidation Resistance, Ablation Resistance, and Ablation Mechanism of HfC–B4C-Modified Carbon Fiber/Boron Phenolic Resin Ceramizable Composites. Polymers. 2025; 17(10):1412. https://doi.org/10.3390/polym17101412
Chicago/Turabian StyleWen, Hairun, Wei Zhang, Zongyi Deng, Xueyuan Yang, and Wenchao Huang. 2025. "Oxidation Resistance, Ablation Resistance, and Ablation Mechanism of HfC–B4C-Modified Carbon Fiber/Boron Phenolic Resin Ceramizable Composites" Polymers 17, no. 10: 1412. https://doi.org/10.3390/polym17101412
APA StyleWen, H., Zhang, W., Deng, Z., Yang, X., & Huang, W. (2025). Oxidation Resistance, Ablation Resistance, and Ablation Mechanism of HfC–B4C-Modified Carbon Fiber/Boron Phenolic Resin Ceramizable Composites. Polymers, 17(10), 1412. https://doi.org/10.3390/polym17101412







