Visualization of Single Polymer Chains with Atomic Force Microscopy: A Review
Abstract
1. Introduction
2. Utilization of Single-Chain AFM to Verify Synthesis and Confirm the Molecular Structure
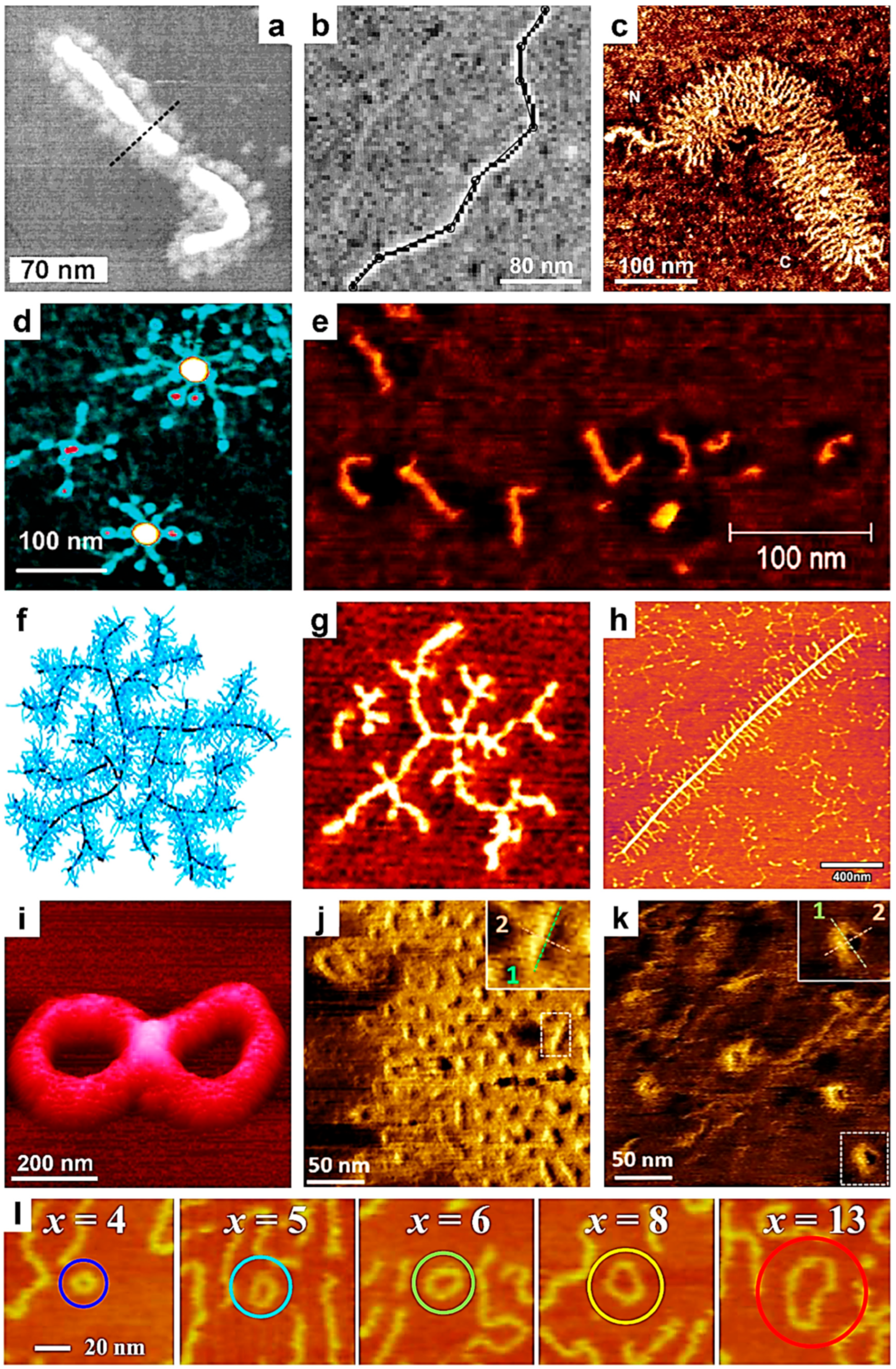
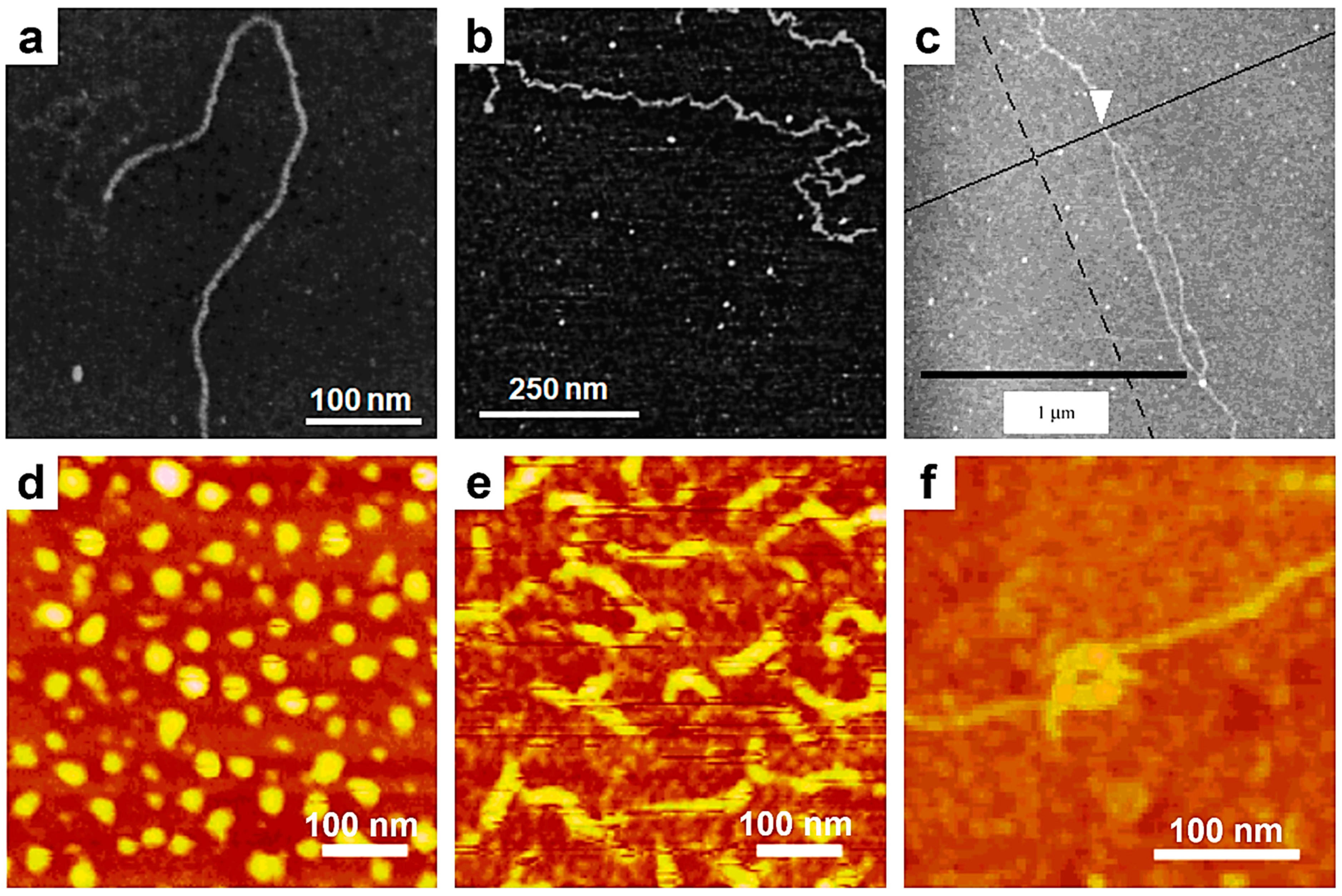
3. Using Single-Chain AFM to Monitor Chain Conformation and Conformational Transitions
4. Understanding Crystallization and Self-Assembly Processes by Using Single-Chain AFM
5. Revealing Polymer Adsorption and Desorption Properties with Single-Chain AFM
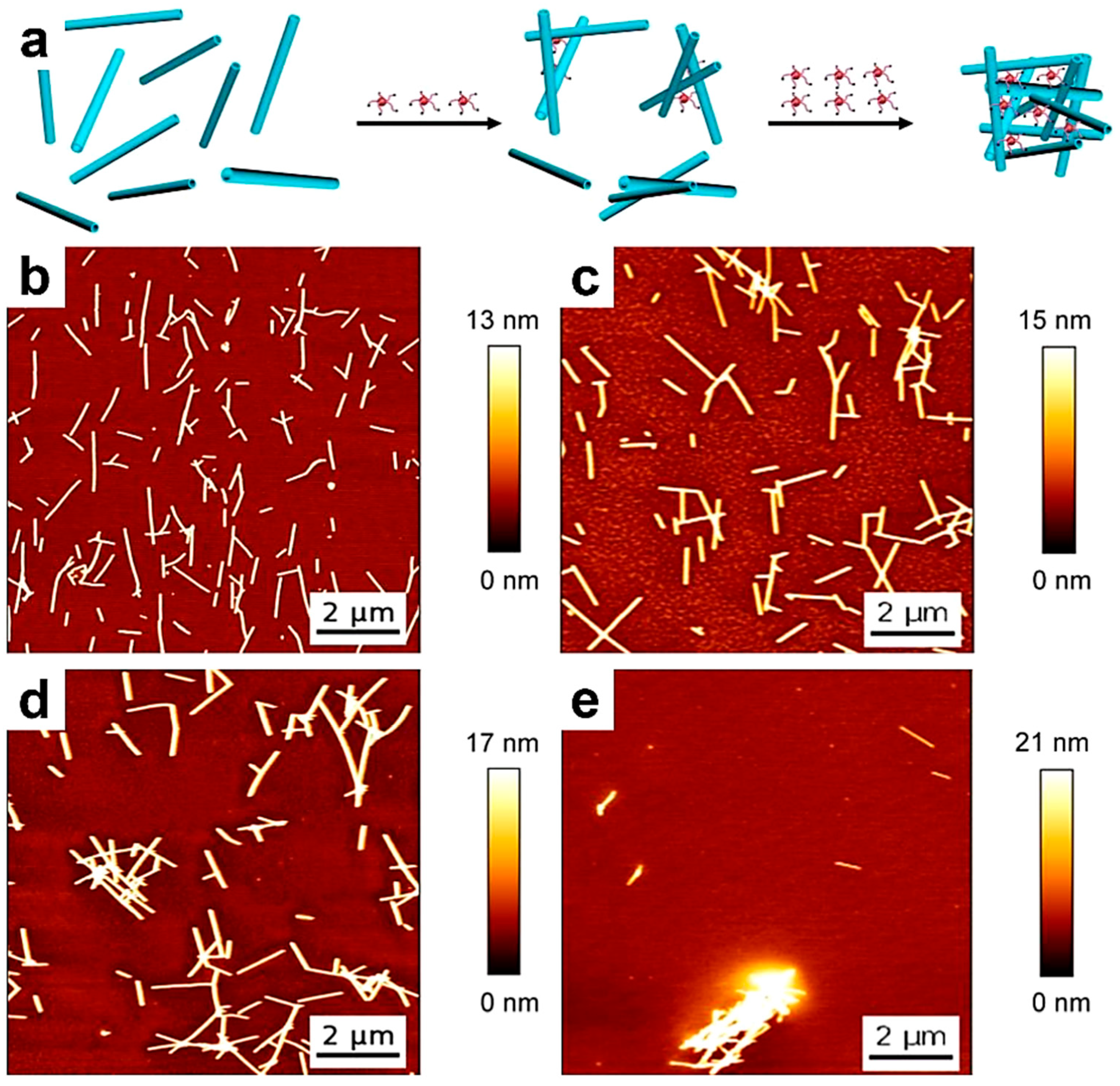
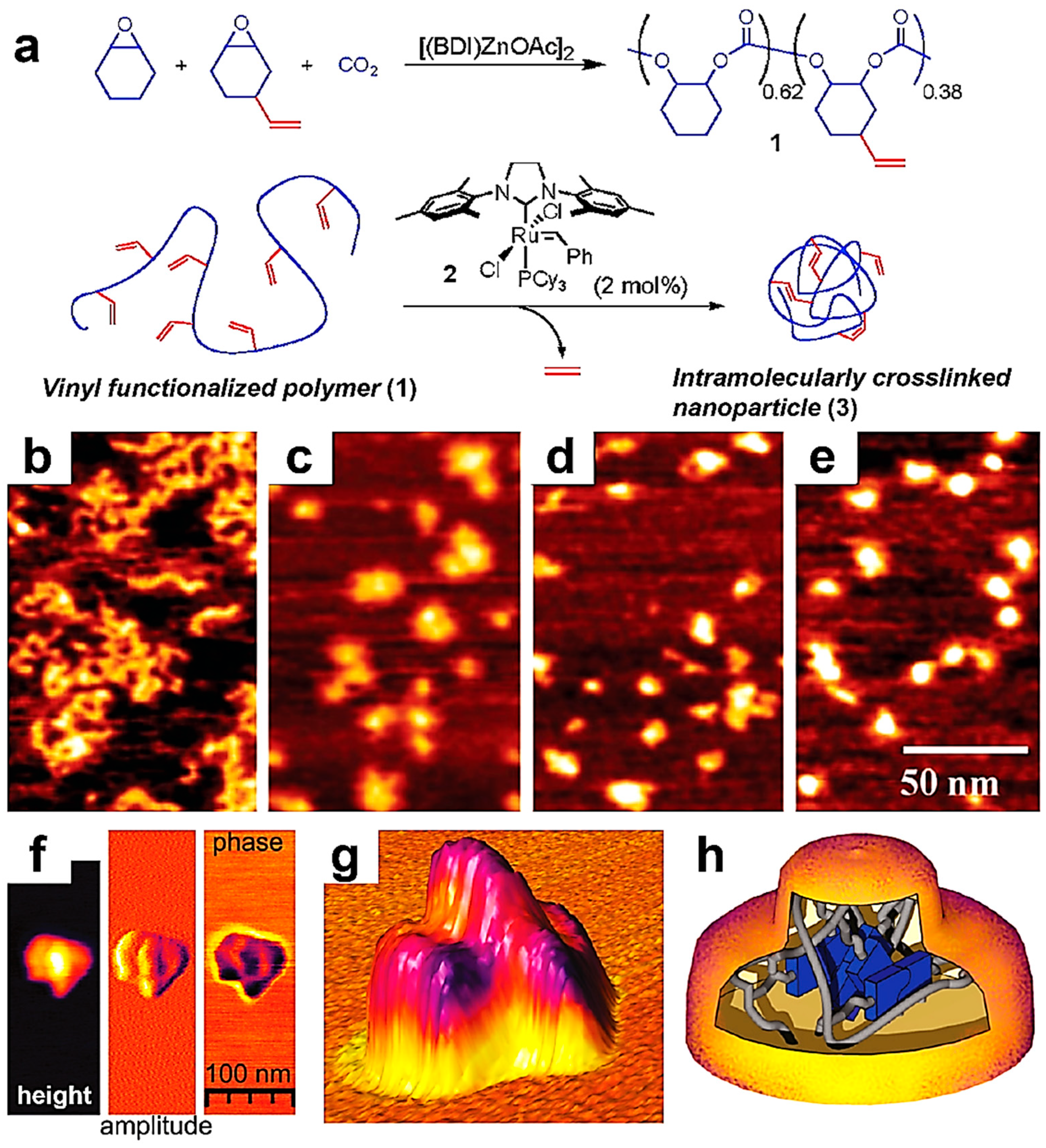
6. Employing AFM to Monitor the Generation of Single-Chain Nanoparticles
7. Using Single-Chain AFM to Determine Chain Stiffness and Probe Chemical Contrasting
8. Other Applications of Single-Chain AFM
9. Limitations and Challenges of Single-Chain AFM
10. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yao, G.; Duan, T.; An, M.; Xu, H.; Tian, F.; Wang, Z. The Influence of Epitaxial Crystallization on the Mechanical Properties of a High Density Polyethylene/Reduced Graphene Oxide Nanocomposite Injection Bar. RSC Adv. 2017, 7, 21918–21925. [Google Scholar] [CrossRef]
- Yamaoka, I.; Kimura, M. Effects of Morphology on Mechanical Properties of a SBS Triblock Copolymer. Polymer 1993, 34, 4399–4409. [Google Scholar] [CrossRef]
- Honeker, C.C.; Thomas, E.L. Impact of Morphological Orientation in Determining Mechanical Properties in Triblock Copolymer Systems. Chem. Mater. 1996, 8, 1702–1714. [Google Scholar] [CrossRef]
- Besford, Q.A.; Cavalieri, F.; Caruso, F. Glycogen as a Building Block for Advanced Biological Materials. Adv. Mater. 2020, 32, 1904625. [Google Scholar] [CrossRef]
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-Based Biomaterials for Tissue Regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef]
- Suresh, D.; Suresh, A.; Kannan, R. Engineering Biomolecular Systems: Controlling the Self-Assembly of Gelatin to Form Ultra-Small Bioactive Nanomaterials. Bioact. Mater. 2022, 18, 321–336. [Google Scholar] [CrossRef]
- Nita, L.E.; Croitoriu, A.; Serban, A.M.; Bercea, M.; Rusu, A.G.; Ghilan, A.; Butnaru, M.; Mititelu-Tartau, L.; Chiriac, A.P. New Hydrogels Based on Agarose/Phytagel and Peptides. Macromol. Biosci. 2023, 23, 2200451. [Google Scholar] [CrossRef]
- Ke, H.; Yang, H.; Zhao, Y.; Li, T.; Xin, D.; Gai, C.; Jiang, Z.; Wang, Z. 3D Gelatin Microsphere Scaffolds Promote Functional Recovery after Spinal Cord Hemisection in Rats. Adv. Sci. 2023, 10, 2204528. [Google Scholar] [CrossRef]
- Tudureanu, R.; Handrea-Dragan, I.M.; Boca, S.; Botiz, I. Insight and Recent Advances into the Role of Topography on the Cell Differentiation and Proliferation on Biopolymeric Surfaces. Int. J. Mol. Sci. 2022, 23, 7731. [Google Scholar] [CrossRef]
- Botiz, I.; Freyberg, P.; Leordean, C.; Gabudean, A.-M.; Astilean, S.; Yang, A.C.-M.; Stingelin, N. Emission Properties of MEH-PPV in Thin Films Simultaneously Illuminated and Annealed at Different Temperatures. Synth. Met. 2015, 199, 33–36. [Google Scholar] [CrossRef]
- Botiz, I.; Astilean, S.; Stingelin, N. Altering the Emission Properties of Conjugated Polymers. Polym. Int. 2016, 65, 157–163. [Google Scholar] [CrossRef]
- Roy, D.; Brooks, W.L.A.; Sumerlin, B.S. New Directions in Thermoresponsive Polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar] [CrossRef] [PubMed]
- Tarcan, R.; Handrea-Dragan, M.; Leordean, C.-I.; Cioban, R.C.; Kiss, G.-Z.; Zaharie-Butucel, D.; Farcau, C.; Vulpoi, A.; Simon, S.; Botiz, I. Development of Polymethylmethacrylate/Reduced Graphene Oxide Composite Films as Thermal Interface Materials. J. Appl. Polym. Sci. 2022, 139, e53238. [Google Scholar] [CrossRef]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Groth, T. Thermoresponsive Polymers and Their Biomedical Application in Tissue Engineering—A Review. J. Mater. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef]
- Mosbach, K.; Schröder, U. Preparation and Application of Magnetic Polymers for Targeting of Drugs. FEBS Lett. 1979, 102, 112–116. [Google Scholar] [CrossRef]
- Foster, D.P.; Majumdar, D. Critical Behavior of Magnetic Polymers in Two and Three Dimensions. Phys. Rev. E 2021, 104, 024122. [Google Scholar] [CrossRef]
- Garel, T.; Orland, H.; Orlandini, E. Phase Diagram of Magnetic Polymers. Eur. Phys. J. B—Condens. Matter Complex Syst. 1999, 12, 261–268. [Google Scholar] [CrossRef]
- Philippova, O.; Barabanova, A.; Molchanov, V.; Khokhlov, A. Magnetic Polymer Beads: Recent Trends and Developments in Synthetic Design and Applications. Eur. Polym. J. 2011, 47, 542–559. [Google Scholar] [CrossRef]
- Dimov, I.B.; Moser, M.; Malliaras, G.G.; McCulloch, I. Semiconducting Polymers for Neural Applications. Chem. Rev. 2022, 122, 4356–4396. [Google Scholar] [CrossRef]
- He, Y.; Kukhta, N.A.; Marks, A.; Luscombe, C.K. The Effect of Side Chain Engineering on Conjugated Polymers in Organic Electrochemical Transistors for Bioelectronic Applications. J. Mater. Chem. C 2022, 10, 2314–2332. [Google Scholar] [CrossRef]
- Pham, Q.-T.; Chern, C.-S. Applications of Polymers in Lithium-Ion Batteries with Enhanced Safety and Cycle Life. J. Polym. Res. 2022, 29, 124. [Google Scholar] [CrossRef]
- Yarali, E.; Baniasadi, M.; Zolfagharian, A.; Chavoshi, M.; Arefi, F.; Hossain, M.; Bastola, A.; Ansari, M.; Foyouzat, A.; Dabbagh, A.; et al. Magneto-/Electro-responsive Polymers toward Manufacturing, Characterization, and Biomedical/Soft Robotic Applications. Appl. Mater. Today 2022, 26, 101306. [Google Scholar] [CrossRef]
- Angel, N.; Li, S.; Yan, F.; Kong, L. Recent Advances in Electrospinning of Nanofibers from Bio-Based Carbohydrate Polymers and Their Applications. Trends Food Sci. Technol. 2022, 120, 308–324. [Google Scholar] [CrossRef]
- Handrea-Dragan, M.; Botiz, I. Multifunctional Structured Platforms: From Patterning of Polymer-Based Films to Their Subsequent Filling with Various Nanomaterials. Polymers 2021, 13, 445. [Google Scholar] [CrossRef]
- De Leon, A.C.C.; da Silva, Í.G.M.; Pangilinan, K.D.; Chen, Q.; Caldona, E.B.; Advincula, R.C. High Performance Polymers for Oil and Gas Applications. React. Funct. Polym. 2021, 162, 104878. [Google Scholar] [CrossRef]
- Nagy-Simon, T.; Diaconu, O.; Focsan, M.; Vulpoi, A.; Botiz, I.; Craciun, A.-M. Pluronic Stabilized Conjugated Polymer Nanoparticles for NIR Fluorescence Imaging and Dual Phototherapy Applications. J. Mol. Struct. 2021, 1243, 130931. [Google Scholar] [CrossRef]
- Luppi, L.; Babut, T.; Petit, E.; Rolland, M.; Quemener, D.; Soussan, L.; Moradi, M.A.; Semsarilar, M. Antimicrobial Polylysine Decorated Nano-Structures Prepared through Polymerization Induced Self-Assembly (PISA). Polym. Chem. 2019, 10, 336–344. [Google Scholar] [CrossRef]
- Handrea-Dragan, I.M.; Botiz, I.; Tatar, A.-S.; Boca, S. Patterning at the Micro/Nano-Scale: Polymeric Scaffolds for Medical Diagnostic and Cell-Surface Interaction Applications. Colloids Surf. B Biointerfaces 2022, 218, 112730. [Google Scholar] [CrossRef]
- Nam, S.; Mooney, D. Polymeric Tissue Adhesives. Chem. Rev. 2021, 121, 11336–11384. [Google Scholar] [CrossRef]
- Gomez-Lopez, A.; Panchireddy, S.; Grignard, B.; Calvo, I.; Jerome, C.; Detrembleur, C.; Sardon, H. Poly(Hydroxyurethane) Adhesives and Coatings: State-of-the-Art and Future Directions. ACS Sustain. Chem. Eng. 2021, 9, 9541–9562. [Google Scholar] [CrossRef]
- Zhang, Z.; Liao, M.; Lou, H.; Hu, Y.; Sun, X.; Peng, H. Conjugated Polymers for Flexible Energy Harvesting and Storage. Adv. Mater. 2018, 30, 1704261. [Google Scholar] [CrossRef]
- Liang, Y.; Yu, L. Development of Semiconducting Polymers for Solar Energy Harvesting. Polym. Rev. 2010, 50, 454–473. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Zhang, Q.M.; Tan, D.Q. Advanced Dielectric Polymers for Energy Storage. Energy Storage Mater. 2022, 44, 29–47. [Google Scholar] [CrossRef]
- Rohland, P.; Schröter, E.; Nolte, O.; Newkome, G.R.; Hager, M.D.; Schubert, U.S. Redox-Active Polymers: The Magic Key towards Energy Storage—A Polymer Design Guideline Progress in Polymer Science. Prog. Polym. Sci. 2022, 125, 101474. [Google Scholar] [CrossRef]
- Rajak, D.K.; Wagh, P.H.; Kumar, A.; Behera, A.; Pruncu, C.I. Advanced Polymers in Aircraft Structures. In Materials, Structures and Manufacturing for Aircraft; Kuşhan, M.C., Gürgen, S., Sofuoğlu, M.A., Eds.; Sustainable Aviation; Springer International Publishing: Cham, Germany, 2022; pp. 65–88. ISBN 978-3-030-91873-6. [Google Scholar]
- Nikalin, D.M.; Merkulova, Y.I.; Zheleznyak, V.G. Fluorine-Containing Polymer Paints and Arnishes in Aviation. Russ. J. Gen. Chem. 2021, 91, 1171–1177. [Google Scholar] [CrossRef]
- Shah, V.; Bhaliya, J.; Patel, G.M.; Deshmukh, K. Advances in Polymeric Nanocomposites for Automotive Applications: A Review. Polym. Adv. Technol. 2022, 33, 3023–3048. [Google Scholar] [CrossRef]
- Salifu, S.; Desai, D.; Ogunbiyi, O.; Mwale, K. Recent Development in the Additive Manufacturing of Polymer-Based Composites for Automotive Structures—A Review. Int. J. Adv. Manuf. Technol. 2022, 119, 6877–6891. [Google Scholar] [CrossRef]
- Tian, B.; Xiao, D.; Hei, T.; Ping, R.; Hua, S.; Liu, J. The Application and Prospects of Cyclodextrin Inclusion Complexes and Polymers in the Food Industry: A Review. Polym. Int. 2020, 69, 597–603. [Google Scholar] [CrossRef]
- Nisticò, R. Polyethylene Terephthalate (PET) in the Packaging Industry. Polym. Test. 2020, 90, 106707. [Google Scholar] [CrossRef]
- Yaragatti, N.; Patnaik, A. A Review on Additive Manufacturing of Polymers Composites. Int. Conf. Adv. Mater. Process. Manuf. Appl. 2021, 44, 4150–4157. [Google Scholar] [CrossRef]
- Khokhar, D.; Jadoun, S.; Arif, R.; Jabin, S. Functionalization of Conducting Polymers and Their Applications in Optoelectronics. Polym.-Plast. Technol. Mater. 2021, 60, 465–487. [Google Scholar] [CrossRef]
- Wu, X.; Fu, W.; Chen, H. Conductive Polymers for Flexible and Stretchable Organic Optoelectronic Applications. ACS Appl. Polym. Mater. 2022, 4, 4609–4623. [Google Scholar] [CrossRef]
- Zhang, T.; Gregoriou, V.G.; Gasparini, N.; Chochos, C.L. Porous Organic Polymers in Solar Cells. Chem. Soc. Rev. 2022, 51, 4465–4483. [Google Scholar] [CrossRef] [PubMed]
- Hasseb, A.A.; Ghani, N.D.T.A.; Shehab, O.R.; El Nashar, R.M. Application of Molecularly Imprinted Polymers for Electrochemical Detection of Some Important Biomedical Markers and Pathogens. Curr. Opin. Electrochem. 2022, 31, 100848. [Google Scholar] [CrossRef]
- Ghoorchian, A.; Amouzegar, Z.; Moradi, M.; Khalili, S.; Afkhami, A.; Madrakian, T.; Ahmadi, M. Use of Conductive Polymers in Detection Stage of Analysis/Miniaturization Devices. In Conductive Polymers in Analytical Chemistry; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2022; Volume 1405, pp. 165–184. ISBN 978-0-8412-9786-9. [Google Scholar]
- He, W.; Ye, X.; Cui, T. Progress of Shrink Polymer Micro- and Nanomanufacturing. Microsyst. Nanoeng. 2021, 7, 88. [Google Scholar] [CrossRef]
- Handrea-Dragan, I.M.; Vulpoi, A.; Farcău, C.; Botiz, I. Spheres-in-Grating Assemblies with Altered Photoluminescence and Wetting Properties. Nanomaterials 2022, 12, 1084. [Google Scholar] [CrossRef]
- Chen, S.; Haehnle, B.; Van der Laan, X.; Kuehne, A.J.C.; Botiz, I.; Stavrinou, P.N.; Stingelin, N. Understanding Hierarchical Spheres-in-Grating Assembly for Bio-Inspired Colouration. Mater. Horiz. 2021, 8, 2230–2237. [Google Scholar] [CrossRef]
- Park, S.; Shou, W.; Makatura, L.; Matusik, W.; Fu, K.K. 3D Printing of Polymer Composites: Materials, Processes, and Applications. Matter 2022, 5, 43–76. [Google Scholar] [CrossRef]
- Mohd Yusoff, N.H.; Irene Teo, L.-R.; Phang, S.J.; Wong, V.-L.; Cheah, K.H.; Lim, S.-S. Recent Advances in Polymer-Based 3D Printing for Wastewater Treatment Application: An Overview. Chem. Eng. J. 2022, 429, 132311. [Google Scholar] [CrossRef]
- Fu, P.; Li, H.; Gong, J.; Fan, Z.; Smith, A.T.; Shen, K.; Khalfalla, T.O.; Huang, H.; Qian, X.; McCutcheon, J.R.; et al. 4D Printing of Polymers: Techniques, Materials, and Prospects. Prog. Polym. Sci. 2022, 126, 101506. [Google Scholar] [CrossRef]
- Imrie, P.; Jin, J. Polymer 4D Printing: Advanced Shape-Change and Beyond. J. Polym. Sci. 2022, 60, 149–174. [Google Scholar] [CrossRef]
- Mallakpour, S.; Tabesh, F.; Hussain, C.M. A New Trend of Using Poly(Vinyl Alcohol) in 3D and 4D Printing Technologies: Process and Applications. Adv. Colloid Interface Sci. 2022, 301, 102605. [Google Scholar] [CrossRef] [PubMed]
- Noriega, R.; Rivnay, J.; Vandewal, K.; Koch, F.P.; Stingelin, N.; Smith, P.; Toney, M.F.; Salleo, A. A General Relationship between Disorder, Aggregation and Charge Transport in Conjugated Polymers. Nat. Mater. 2013, 12, 1038–1044. [Google Scholar] [CrossRef]
- Peng, Z.; Stingelin, N.; Ade, H.; Michels, J.J. A Materials Physics Perspective on Structure–Processing–Function Relations in Blends of Organic Semiconductors. Nat. Rev. Mater. 2023, 8, 439–455. [Google Scholar] [CrossRef]
- Botiz, I.; Freyberg, P.; Stingelin, N.; Yang, A.C.M.; Reiter, G. Reversibly Slowing Dewetting of Conjugated Polymers by Light. Macromolecules 2013, 46, 2352–2356. [Google Scholar] [CrossRef]
- Todor-Boer, O.; Petrovai, I.; Tarcan, R.; Vulpoi, A.; David, L.; Astilean, S.; Botiz, I. Enhancing Photoluminescence Quenching in Donor–Acceptor PCE11:PPCBMB Films through the Optimization of Film Microstructure. Nanomaterials 2019, 9, 1757. [Google Scholar] [CrossRef]
- Alam, M.M.; Tonzola, C.J.; Jenekhe, S.A. Nanophase-Separated Blends of Acceptor and Donor Conjugated Polymers. Efficient Electroluminescence from Binary Polyquinoline/Poly(2-Methoxy-5-(2′-Ethylhexyloxy)-1,4-Phenylenevinylene) and Polyquinoline/Poly(3-Octylthiophene) Blends. Macromolecules 2003, 36, 6577–6587. [Google Scholar] [CrossRef]
- Albalak, R.J.; Thomas, E.L. Microphase Separation of Block Copolymer Solutions in a Flow Field. J. Polym. Sci. Part B Polym. Phys. 1993, 31, 37–46. [Google Scholar] [CrossRef]
- Liang, Q.; Han, J.; Song, C.; Wang, Z.; Xin, J.; Yu, X.; Xie, Z.; Ma, W.; Liu, J.; Han, Y. Tuning Molecule Diffusion to Control the Phase Separation of the P-DTS(FBTTh2)2/EP-PDI Blend System via Thermal Annealing. J. Mater. Chem. C 2017, 5, 6842–6851. [Google Scholar] [CrossRef]
- MacFarlane, L.R.; Shaikh, H.; Garcia-Hernandez, J.D.; Vespa, M.; Fukui, T.; Manners, I. Functional Nanoparticles through π-Conjugated Polymer Self-Assembly. Nat. Rev. Mater. 2021, 6, 7–26. [Google Scholar] [CrossRef]
- Todor-Boer, O.; Petrovai, I.; Tarcan, R.; David, L.; Astilean, S.; Botiz, I. Control of Microstructure in Polymer: Fullerene Active Films by Convective Self-Assembly. Thin Solid Films 2020, 697, 137780. [Google Scholar] [CrossRef]
- Botiz, I.; Codescu, M.-A.; Farcau, C.; Leordean, C.; Astilean, S.; Silva, C.; Stingelin, N. Convective Self-Assembly of π-Conjugated Oligomers and Polymers. J. Mater. Chem. C 2017, 5, 2513–2518. [Google Scholar] [CrossRef]
- Verduzco, R.; Botiz, I.; Pickel, D.L.; Kilbey, S.M.; Hong, K.; Dimasi, E.; Darling, S.B. Polythiophene-Block-Polyfluorene and Polythiophene-Block-Poly(Fluorene-Co-Benzothiadiazole): Insights into the Self-Assembly of All-Conjugated Block Copolymers. Macromolecules 2011, 44, 530–539. [Google Scholar] [CrossRef]
- Le, T.P.; Smith, B.H.; Lee, Y.; Litofsky, J.H.; Aplan, M.P.; Kuei, B.; Zhu, C.; Wang, C.; Hexemer, A.; Gomez, E.D. Enhancing Optoelectronic Properties of Conjugated Block Copolymers through Crystallization of Both Blocks. Macromolecules 2020, 53, 1967–1976. [Google Scholar] [CrossRef]
- Yu, L.; Davidson, E.; Sharma, A.; Andersson, M.R.; Segalman, R.; Müller, C. Isothermal Crystallization Kinetics and Time–Temperature–Transformation of the Conjugated Polymer: Poly(3-(2′-Ethyl)Hexylthiophene). Chem. Mater. 2017, 29, 5654–5662. [Google Scholar] [CrossRef]
- Rahimi, K.; Botiz, I.; Stingelin, N.; Kayunkid, N.; Sommer, M.; Koch, F.P.V.; Nguyen, H.; Coulembier, O.; Dubois, P.; Brinkmann, M.; et al. Controllable Processes for Generating Large Single Crystals of Poly(3-Hexylthiophene). Angew. Chem. Int. Ed. 2012, 51, 11131–11135. [Google Scholar] [CrossRef]
- Marsh, H.S.; Reid, O.G.; Barnes, G.; Heeney, M.; Stingelin, N.; Rumbles, G. Control of Polythiophene Film Microstructure and Charge Carrier Dynamics through Crystallization Temperature. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 700–707. [Google Scholar] [CrossRef]
- Bustamante, C.J.; Chemla, Y.R.; Liu, S.; Wang, M.D. Optical Tweezers in Single-Molecule Biophysics. Nat. Rev. Methods Primer 2021, 1, 25. [Google Scholar] [CrossRef]
- Bustamante, C.; Alexander, L.; Maciuba, K.; Kaiser, C.M. Single-Molecule Studies of Protein Folding with Optical Tweezers. Annu. Rev. Biochem. 2020, 89, 443–470. [Google Scholar] [CrossRef]
- Wang, M.D.; Yin, H.; Landick, R.; Gelles, J.; Block, S.M. Stretching DNA with Optical Tweezers. Biophys. J. 1997, 72, 1335–1346. [Google Scholar] [CrossRef]
- Kuei, B.; Aplan, M.P.; Litofsky, J.H.; Gomez, E.D. New Opportunities in Transmission Electron Microscopy of Polymers. Mater. Sci. Eng. R Rep. 2020, 139, 100516. [Google Scholar] [CrossRef]
- Liu, Y.; Vancso, G.J. Polymer Single Chain Imaging, Molecular Forces, and Nanoscale Processes by Atomic Force Microscopy: The Ultimate Proof of the Macromolecular Hypothesis. Prog. Polym. Sci. 2020, 104, 101232. [Google Scholar] [CrossRef]
- Wang, D.; Russell, T.P. Advances in Atomic Force Microscopy for Probing Polymer Structure and Properties. Macromolecules 2018, 51, 3–24. [Google Scholar] [CrossRef]
- Kumaki, J.; Sakurai, S.; Yashima, E. Visualization of Synthetic Helical Polymers by High-Resolution Atomic Force Microscopy. Chem. Soc. Rev. 2009, 38, 737–746. [Google Scholar] [CrossRef]
- Bolinger, J.C.; Traub, M.C.; Brazard, J.; Adachi, T.; Barbara, P.F.; Vanden Bout, D.A. Conformation and Energy Transfer in Single Conjugated Polymers. Acc. Chem. Res. 2012, 45, 1992–2001. [Google Scholar] [CrossRef]
- Perkins, T.T.; Smith, D.E.; Chu, S. Direct Observation of Tube-Like Motion of a Single Polymer Chain. Science 1994, 264, 819–822. [Google Scholar] [CrossRef]
- Hugel, T.; Seitz, M. The Study of Molecular Interactions by AFM Force Spectroscopy. Macromol. Rapid Commun. 2001, 22, 989–1016. [Google Scholar] [CrossRef]
- Zhang, W.; Zou, S.; Wang, C.; Zhang, X. Single Polymer Chain Elongation of Poly(N-Isopropylacrylamide) and Poly(Acrylamide) by Atomic Force Microscopy. J. Phys. Chem. B 2000, 104, 10258–10264. [Google Scholar] [CrossRef]
- Marszalek, P.E.; Dufrêne, Y.F. Stretching Single Polysaccharides and Proteins Using Atomic Force Microscopy. Chem. Soc. Rev. 2012, 41, 3523–3534. [Google Scholar] [CrossRef]
- Börner, H.G.; Beers, K.; Matyjaszewski, K.; Sheiko, S.S.; Möller, M. Synthesis of Molecular Brushes with Block Copolymer Side Chains Using Atom Transfer Radical Polymerization. Macromolecules 2001, 34, 4375–4383. [Google Scholar] [CrossRef]
- Rivetti, C.; Codeluppi, S. Accurate Length Determination of DNA Molecules Visualized by Atomic Force Microscopy: Evidence for a Partial B- to A-Form Transition on Mica. Ultramicroscopy 2001, 87, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Sheiko, S.S.; da Silva, M.; Shirvaniants, D.; LaRue, I.; Prokhorova, S.; Moeller, M.; Beers, K.; Matyjaszewski, K. Measuring Molecular Weight by Atomic Force Microscopy. J. Am. Chem. Soc. 2003, 125, 6725–6728. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.; Grodzinsky, A.J.; Patwari, P.; Sandy, J.; Plaas, A.; Ortiz, C. Individual Cartilage Aggrecan Macromolecules and Their Constituent Glycosaminoglycans Visualized via Atomic Force Microscopy. J. Struct. Biol. 2003, 143, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Y.; Wang, L.; Xie, B.; Wang, H.; Deng, S. Visualization of Single and Aggregated Hulless Oat (Avena Nuda L.) (1→3),(1→4)-β-d-Glucan Molecules by Atomic Force Microscopy and Confocal Scanning Laser Microscopy. J. Agric. Food Chem. 2006, 54, 925–934. [Google Scholar] [CrossRef]
- Pyun, J.; Tang, C.; Kowalewski, T.; Fréchet, J.M.J.; Hawker, C.J. Synthesis and Direct Visualization of Block Copolymers Composed of Different Macromolecular Architectures. Macromolecules 2005, 38, 2674–2685. [Google Scholar] [CrossRef]
- Kiriy, A.; Gorodyska, G.; Minko, S.; Tsitsilianis, C.; Stamm, M. Atomic Force Microscopy Visualization of Single Star Copolymer Molecules. Polym. Mater. Sci. Eng. 2003, 88, 233. [Google Scholar]
- Kiriy, A.; Gorodyska, G.; Minko, S.; Stamm, M.; Tsitsilianis, C. Single Molecules and Associates of Heteroarm Star Copolymer Visualized by Atomic Force Microscopy. Macromolecules 2003, 36, 8704–8711. [Google Scholar] [CrossRef]
- Heidenreich, A.J.; Puskas, J.E.; Schappacher, M.; Ibarboure, E.; Deffieux, A. Visualization of Arborescent Architecture of Polystyrenes Prepared by Raft-Based Initiator-Monomer Polymerization Using Atomic Force Microscopy. J. Polym. Sci. Part Polym. Chem. 2012, 50, 1238–1247. [Google Scholar] [CrossRef]
- Kim, K.T.; Han, J.; Ryu, C.Y.; Sun, F.C.; Sheiko, S.S.; Winnik, M.A.; Manners, I. Synthesis, Characterization, and AFM Studies of Dendronized Polyferrocenylsilanes. Macromolecules 2006, 39, 7922–7930. [Google Scholar] [CrossRef]
- Rajaram, S.; Choi, T.-L.; Rolandi, M.; Fréchet, J.M.J. Synthesis of Dendronized Diblock Copolymers via Ring-Opening Metathesis Polymerization and Their Visualization Using Atomic Force Microscopy. J. Am. Chem. Soc. 2007, 129, 9619–9621. [Google Scholar] [CrossRef]
- Kang, E.-H.; Lee, I.S.; Choi, T.-L. Ultrafast Cyclopolymerization for Polyene Synthesis: Living Polymerization to Dendronized Polymers. J. Am. Chem. Soc. 2011, 133, 11904–11907. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.O.; Choi, T.-L. Synthesis of Rod-Like Dendronized Polymers Containing G4 and G5 Ester Dendrons via Macromonomer Approach by Living ROMP. ACS Macro Lett. 2012, 1, 445–448. [Google Scholar] [CrossRef]
- Kim, H.; Bang, K.-T.; Choi, I.; Lee, J.-K.; Choi, T.-L. Diversity-Oriented Polymerization: One-Shot Synthesis of Library of Graft and Dendronized Polymers by Cu-Catalyzed Multicomponent Polymerization. J. Am. Chem. Soc. 2016, 138, 8612–8622. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, J.; Li, Z.; Hou, W.; Li, Y.; Shi, Y.; Chen, Y. Synthesis and Visualization of Bottlebrush-Shaped Segmented Hyperbranched Polymers. Polym. Chem. 2022, 13, 4895–4900. [Google Scholar] [CrossRef]
- Yin, J.-F.; Hu, Y.; Wang, D.-G.; Yang, L.; Jin, Z.; Zhang, Y.; Kuang, G.-C. Cucurbit [8] Uril-Based Water-Soluble Supramolecular Dendronized Polymer: Evidence from Single Polymer Chain Morphology and Force Spectroscopy. ACS Macro Lett. 2017, 6, 139–143. [Google Scholar] [CrossRef]
- Ikeda, S.; Funami, T.; Zhang, G. Visualizing Surface Active Hydrocolloids by Atomic Force Microscopy. Carbohydr. Polym. 2005, 62, 192–196. [Google Scholar] [CrossRef]
- Round, A.N.; Rigby, N.M.; MacDougall, A.J.; Morris, V.J. A New View of Pectin Structure Revealed by Acid Hydrolysis and Atomic Force Microscopy. Carbohydr. Res. 2010, 345, 487–497. [Google Scholar] [CrossRef]
- Williams, M.A.K.; Cornuault, V.; Irani, A.H.; Symonds, V.V.; Malmström, J.; An, Y.; Sims, I.M.; Carnachan, S.M.; Sallé, C.; North, H.M. Polysaccharide Structures in the Outer Mucilage of Arabidopsis Seeds Visualized by AFM. Biomacromolecules 2020, 21, 1450–1459. [Google Scholar] [CrossRef]
- Paniagua, C.; Posé, S.; Morris, V.J.; Kirby, A.R.; Quesada, M.A.; Mercado, J.A. Fruit Softening and Pectin Disassembly: An Overview of Nanostructural Pectin Modifications Assessed by Atomic Force Microscopy. Ann. Bot. 2014, 114, 1375–1383. [Google Scholar] [CrossRef]
- Paniagua, C.; Kirby, A.R.; Gunning, A.P.; Morris, V.J.; Matas, A.J.; Quesada, M.A.; Mercado, J.A. Unravelling the Nanostructure of Strawberry Fruit Pectins by Endo-Polygalacturonase Digestion and Atomic Force Microscopy. Food Chem. 2017, 224, 270–279. [Google Scholar] [CrossRef]
- Schappacher, M.; Deffieux, A. Imaging of Catenated, Figure-of-Eight, and Trefoil Knot Polymer Rings. Angew. Chem. Int. Ed. 2009, 48, 5930–5933. [Google Scholar] [CrossRef]
- Schué, E.; Kopyshev, A.; Lutz, J.-F.; Börner, H.G. Molecular Bottle Brushes with Positioned Selenols: Extending the Toolbox of Oxidative Single Polymer Chain Folding with Conformation Analysis by Atomic Force Microscopy. J. Polym. Sci. 2020, 58, 154–162. [Google Scholar] [CrossRef]
- Narumi, A.; Yamada, M.; Unno, Y.; Kumaki, J.; Binder, W.H.; Enomoto, K.; Kikuchi, M.; Kawaguchi, S. Evaluation of Ring Expansion-Controlled Radical Polymerization System by AFM Observation. ACS Macro Lett. 2019, 8, 634–638. [Google Scholar] [CrossRef]
- Kawauchi, T.; Kumaki, J.; Yashima, E. Synthesis, Isolation via Self-Assembly, and Single-Molecule Observation of a [60]Fullerene-End-Capped Isotactic Poly(Methyl Methacrylate). J. Am. Chem. Soc. 2005, 127, 9950–9951. [Google Scholar] [CrossRef]
- Endo, M.; Sugiyama, H. Single-Molecule Imaging of Dynamic Motions of Biomolecules in DNA Origami Nanostructures Using High-Speed Atomic Force Microscopy. Acc. Chem. Res. 2014, 47, 1645–1653. [Google Scholar] [CrossRef]
- Hansma, H.G.; Vesenka, J.; Siegerist, C.; Kelderman, G.; Morrett, H.; Sinsheimer, R.L.; Elings, V.; Bustamante, C.; Hansma, P.K. Reproducible Imaging and Dissection of Plasmid DNA Under Liquid with the Atomic Force Microscope. Science 1992, 256, 1180–1184. [Google Scholar] [CrossRef]
- Kumaki, J.; Nishikawa, Y.; Hashimoto, T. Visualization of Single-Chain Conformations of a Synthetic Polymer with Atomic Force Microscopy. J. Am. Chem. Soc. 1996, 118, 3321–3322. [Google Scholar] [CrossRef]
- Moffat, J.; Morris, V.J.; Al-Assaf, S.; Gunning, A.P. Visualisation of Xanthan Conformation by Atomic Force Microscopy. Carbohydr. Polym. 2016, 148, 380–389. [Google Scholar] [CrossRef]
- Furukawa, K. End-Grafted PolysilaneAn Approach to Single Polymer Science. Acc. Chem. Res. 2003, 36, 102–110. [Google Scholar] [CrossRef]
- Kumaki, J.; Hashimoto, T. Conformational Change in an Isolated Single Synthetic Polymer Chain on a Mica Surface Observed by Atomic Force Microscopy. J. Am. Chem. Soc. 2003, 125, 4907–4917. [Google Scholar] [CrossRef]
- Schefer, L.; Adamcik, J.; Mezzenga, R. Unravelling Secondary Structure Changes on Individual Anionic Polysaccharide Chains by Atomic Force Microscopy. Angew. Chem. Int. Ed. 2014, 53, 5376–5379. [Google Scholar] [CrossRef] [PubMed]
- Kiriy, A.; Gorodyska, G.; Minko, S.; Jaeger, W.; Štěpánek, P.; Stamm, M. Cascade of Coil-Globule Conformational Transitions of Single Flexible Polyelectrolyte Molecules in Poor Solvent. J. Am. Chem. Soc. 2002, 124, 13454–13462. [Google Scholar] [CrossRef] [PubMed]
- Kumaki, J.; Kawauchi, T.; Yashima, E. “Reptational” Movements of Single Synthetic Polymer Chains on Substrate Observed by in-Situ Atomic Force Microscopy. Macromolecules 2006, 39, 1209–1215. [Google Scholar] [CrossRef]
- Anzai, T.; Kawauchi, M.; Kawauchi, T.; Kumaki, J. Crystallization Behavior of Single Isotactic Poly(Methyl Methacrylate) Chains Visualized by Atomic Force Microscopy. J. Phys. Chem. B 2015, 119, 338–347. [Google Scholar] [CrossRef]
- Ono, Y.; Kumaki, J. In Situ Real-Time Observation of Polymer Folded-Chain Crystallization by Atomic Force Microscopy at the Molecular Level. Macromolecules 2018, 51, 7629–7636. [Google Scholar] [CrossRef]
- Ono, Y.; Kumaki, J. In Situ AFM Observation of Folded-Chain Crystallization of a Low-Molecular-Weight Isotactic Poly(Methyl Methacrylate) in a Langmuir Monolayer at the Molecular Level. Macromol. Chem. Phys. 2021, 222, 2000372. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kumaki, J. In Situ Atomic Force Microscopy Observation of Folded-Chain Crystallization of Single Isolated Isotactic Poly(Methyl Methacrylate) Chains in Langmuir–Blodgett Monolayers. Macromolecules 2024, 57, 1147–1158. [Google Scholar] [CrossRef]
- McIntire, T.M.; Brant, D.A. Imaging of Individual Biopolymers and Supramolecular Assemblies Using Noncontact Atomic Force Microscopy. Biopolymers 1997, 42, 133–146. [Google Scholar] [CrossRef]
- Ueno, T.; Yokota, S.; Kitaoka, T.; Wariishi, H. Conformational Changes in Single Carboxymethylcellulose Chains on a Highly Oriented Pyrolytic Graphite Surface under Different Salt Conditions. Carbohydr. Res. 2007, 342, 954–960. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Zhang, W. A Single-Molecule Atomic Force Microscopy Study Reveals the Antiviral Mechanism of Tannin and Its Derivatives. Nanoscale 2019, 11, 16368–16376. [Google Scholar] [CrossRef]
- Hlushko, R.; Pozharski, E.; Prabhu, V.M.; Andrianov, A.K. Directly Visualizing Individual Polyorganophosphazenes and Their Single-Chain Complexes with Proteins. Commun. Mater. 2024, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Gunning, A.P.; Mackie, A.R.; Kirby, A.R.; Kroon, P.; Williamson, G.; Morris, V.J. Motion of a Cell Wall Polysaccharide Observed by Atomic Force Microscopy. Macromolecules 2000, 33, 5680–5685. [Google Scholar] [CrossRef]
- Roiter, Y.; Minko, S. AFM Single Molecule Experiments at the Solid−Liquid Interface: In Situ Conformation of Adsorbed Flexible Polyelectrolyte Chains. J. Am. Chem. Soc. 2005, 127, 15688–15689. [Google Scholar] [CrossRef]
- Roiter, Y.; Jaeger, W.; Minko, S. Conformation of Single Polyelectrolyte Chains vs. Salt Concentration: Effects of Sample History and Solid Substrate. Polymer 2006, 47, 2493–2498. [Google Scholar] [CrossRef]
- Zhao, F.; Du, Y.-K.; Yang, P.; Tang, J.; Li, X.-C. Single Polymer Molecules Adsorbed to Mica and the Oppositely Charged Polymer/Surfactant Complexes Formed at the Air–Water Interface Visualized by Atomic Force Microscopy. Colloid Polym. Sci. 2005, 283, 1361–1365. [Google Scholar] [CrossRef]
- Koestner, R.; Roiter, Y.; Kozhinova, I.; Minko, S. AFM Imaging of Adsorbed Nafion Polymer on Mica and Graphite at Molecular Level. Langmuir 2011, 27, 10157–10166. [Google Scholar] [CrossRef]
- Oda, Y.; Kawaguchi, D.; Morimitsu, Y.; Yamamoto, S.; Tanaka, K. Direct Observation of Morphological Transition for an Adsorbed Single Polymer Chain. Sci. Rep. 2020, 10, 20914. [Google Scholar] [CrossRef]
- Morimitsu, Y.; Matsuno, H.; Tanaka, K. Morphologies of Polymer Chains Spun onto Solid Substrates. Polym. J. 2024, 56, 1041–1050. [Google Scholar] [CrossRef]
- Kiriy, A.; Gorodyska, G.; Minko, S.; Tsitsilianis, C.; Jaeger, W.; Stamm, M. Chemical Contrasting in a Single Polymer Molecule AFM Experiment. J. Am. Chem. Soc. 2003, 125, 11202–11203. [Google Scholar] [CrossRef]
- Maurstad, G.; Danielsen, S.; Stokke, B.T. Analysis of Compacted Semiflexible Polyanions Visualized by Atomic Force Microscopy: Influence of Chain Stiffness on the Morphologies of Polyelectrolyte Complexes. J. Phys. Chem. B 2003, 107, 8172–8180. [Google Scholar] [CrossRef]
- Fujita, R.; Furudate, K.; Kumaki, J. Atomic Force Microscopy of Single Polymer Chains on a Substrate at Temperatures above the Bulk Glass Transition Temperatures. Polymer 2019, 168, 21–28. [Google Scholar] [CrossRef]
- Sheiko, S.S.; Sun, F.C.; Randall, A.; Shirvanyants, D.; Rubinstein, M.; Lee, H.; Matyjaszewski, K. Adsorption-Induced Scission of Carbon–Carbon Bonds. Nature 2006, 440, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Cherian, A.E.; Sun, F.C.; Sheiko, S.S.; Coates, G.W. Formation of Nanoparticles by Intramolecular Cross-Linking: Following the Reaction Progress of Single Polymer Chains by Atomic Force Microscopy. J. Am. Chem. Soc. 2007, 129, 11350–11351. [Google Scholar] [CrossRef] [PubMed]
- Berda, E.B.; Foster, E.J.; Meijer, E.W. Toward Controlling Folding in Synthetic Polymers: Fabricating and Characterizing Supramolecular Single-Chain Nanoparticles. Macromolecules 2010, 43, 1430–1437. [Google Scholar] [CrossRef]
- Cassina, V.; Seruggia, D.; Beretta, G.L.; Salerno, D.; Brogioli, D.; Manzini, S.; Zunino, F.; Mantegazza, F. Atomic Force Microscopy Study of DNA Conformation in the Presence of Drugs. Eur. Biophys. J. 2011, 40, 59–68. [Google Scholar] [CrossRef]
- Balnois, E.; Stoll, S.; Wilkinson, K.J.; Buffle, J.; Rinaudo, M.; Milas, M. Conformations of Succinoglycan As Observed by Atomic Force Microscopy. Macromolecules 2000, 33, 7440–7447. [Google Scholar] [CrossRef]
- Camesano, T.A.; Wilkinson, K.J. Single Molecule Study of Xanthan Conformation Using Atomic Force Microscopy. Biomacromolecules 2001, 2, 1184–1191. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, H.; Yin, Y.; Nishinari, K. Conformation of Curdlan as Observed by Tapping Mode Atomic Force Microscopy. Colloid Polym. Sci. 2006, 284, 1371–1377. [Google Scholar] [CrossRef]
- Kumaki, J.; Kajitani, T.; Nagai, K.; Okoshi, K.; Yashima, E. Visualization of Polymer Chain Conformations in Amorphous Polyisocyanide Langmuir−Blodgett Films by Atomic Force Microscopy. J. Am. Chem. Soc. 2010, 132, 5604–5606. [Google Scholar] [CrossRef]
- Sugihara, K.; Kumaki, J. Visualization of Two-Dimensional Single Chain Conformations Solubilized in a Miscible Polymer Blend Monolayer by Atomic Force Microscopy. J. Phys. Chem. B 2012, 116, 6561–6568. [Google Scholar] [CrossRef]
- Minko, S.; Kiriy, A.; Gorodyska, G.; Lupitskyy, R.; Tsitsilianis, C.; Stamm, M. Conformational Transitions Visualized in Single Polyelectrolyte Molecule AFM Experiments. Polym. Prepr. 2004, 45, 258. [Google Scholar]
- Minko, S.; Roiter, Y. AFM Single Molecule Studies of Adsorbed Polyelectrolytes. Curr. Opin. Colloid Interface Sci. 2005, 10, 9–15. [Google Scholar] [CrossRef]
- Kumaki, J. Observation of Polymer Chain Structures in Two-Dimensional Films by Atomic Force Microscopy. Polym. J. 2016, 48, 3–14. [Google Scholar] [CrossRef]
- Magonov, S.N.; Yerina, N.A.; Ungar, G.; Reneker, D.H.; Ivanov, D.A. Chain Unfolding in Single Crystals of Ultralong Alkane C390H782 and Polyethylene: An Atomic Force Microscopy Study. Macromolecules 2003, 36, 5637–5649. [Google Scholar] [CrossRef]
- Prokhorov, V.V.; Nitta, K. The AFM Observation of Linear Chain and Crystalline Conformations of Ultrahigh Molecular Weight Polyethylene Molecules on Mica and Graphite. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 766–777. [Google Scholar] [CrossRef]
- Wang, D.; Liang, X.; Nakajima, K. Investigating Single-Chain Structure during the Crystallization Process by Atomic Force Microscopy. Polymer 2025, 316, 127883. [Google Scholar] [CrossRef]
- Ikeda, S.; Morris, V.J.; Nishinari, K. Microstructure of Aggregated and Nonaggregated κ-Carrageenan Helices Visualized by Atomic Force Microscopy. Biomacromolecules 2001, 2, 1331–1337. [Google Scholar] [CrossRef]
- Yokota, S.; Ueno, T.; Kitaoka, T.; Wariishi, H. Molecular Imaging of Single Cellulose Chains Aligned on a Highly Oriented Pyrolytic Graphite Surface. Carbohydr. Res. 2007, 342, 2593–2598. [Google Scholar] [CrossRef]
- Yermolenko, I.S.; Lishko, V.K.; Ugarova, T.P.; Magonov, S.N. High-Resolution Visualization of Fibrinogen Molecules and Fibrin Fibers with Atomic Force Microscopy. Biomacromolecules 2011, 12, 370–379. [Google Scholar] [CrossRef]
- Percec, V.; Rudick, J.G.; Wagner, M.; Obata, M.; Mitchell, C.M.; Cho, W.-D.; Magonov, S.N. AFM Visualization of Individual and Periodic Assemblies of a Helical Dendronized Polyphenylacetylene on Graphite. Macromolecules 2006, 39, 7342–7351. [Google Scholar] [CrossRef]
- Stals, P.J.M.; Li, Y.; Burdyńska, J.; Nicolaÿ, R.; Nese, A.; Palmans, A.R.A.; Meijer, E.W.; Matyjaszewski, K.; Sheiko, S.S. How Far Can We Push Polymer Architectures? J. Am. Chem. Soc. 2013, 135, 11421–11424. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Yamada, K.; Horimoto, N.N.; Ishida, Y. Supramolecular Self-Assembly of an ABA-Triblock Bottlebrush Polymer: Atomic-Force Microscopy Visualization of Discrete Oligomers. Polymer 2017, 120, 68–72. [Google Scholar] [CrossRef]
- Korolkov, V.V.; Summerfield, A.; Murphy, A.; Amabilino, D.B.; Watanabe, K.; Taniguchi, T.; Beton, P.H. Ultra-High Resolution Imaging of Thin Films and Single Strands of Polythiophene Using Atomic Force Microscopy. Nat. Commun. 2019, 10, 1537. [Google Scholar] [CrossRef]
- Gunning, A.P.; Kirby, A.R.; Mackie, A.R.; Kroon, P.; Williamson, G.; Morris, V.J. Watching Molecular Processes with the Atomic Force Microscope: Dynamics of Polymer Adsorption and Desorption at the Single Molecule Level. J. Microsc. 2004, 216, 52–56. [Google Scholar] [CrossRef]
- Roiter, Y.; Trotsenko, O.; Tokarev, V.; Minko, S. Single Molecule Experiments Visualizing Adsorbed Polyelectrolyte Molecules in the Full Range of Mono- and Divalent Counterion Concentrations. J. Am. Chem. Soc. 2010, 132, 13660–13662. [Google Scholar] [CrossRef]
- Aiertza, M.K.; Odriozola, I.; Cabañero, G.; Grande, H.-J.; Loinaz, I. Single-Chain Polymer Nanoparticles. Cell. Mol. Life Sci. 2012, 69, 337–346. [Google Scholar] [CrossRef]
- Artar, M.; Huerta, E.; Meijer, E.W.; Palmans, A.R.A. Dynamic Single Chain Polymeric Nanoparticles: From Structure to Function. In Sequence-Controlled Polymers: Synthesis, Self-Assembly, and Properties; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2014; Volume 1170, pp. 313–325. ISBN 978-0-8412-3001-9. [Google Scholar]
- Zhang, Y.; Xue, Y.; Gao, L.; Liao, R.; Wang, F.; Wang, F. Merging Non-Covalent and Covalent Crosslinking: En Route to Single Chain Nanoparticles. Chin. Chem. Lett. 2024, 35, 109217. [Google Scholar] [CrossRef]
- Altintas, O.; Willenbacher, J.; Wuest, K.N.R.; Oehlenschlaeger, K.K.; Krolla-Sidenstein, P.; Gliemann, H.; Barner-Kowollik, C. A Mild and Efficient Approach to Functional Single-Chain Polymeric Nanoparticles via Photoinduced Diels–Alder Ligation. Macromolecules 2013, 46, 8092–8101. [Google Scholar] [CrossRef]
- Karabacak, S.; Çoban, B.; Yıldız, A.A.; Yıldız, Ü.H. Near-Infrared Emissive Super Penetrating Conjugated Polymer Dots for Intratumoral Imaging in 3D Tumor Spheroid Models. Adv. Sci. 2024, 11, 2403398. [Google Scholar] [CrossRef]
- Rivetti, C.; Walker, C.; Bustamante, C. Polymer Chain Statistics and Conformational Analysis of DNA Molecules with Bends or Sections of Different flexibility11Edited by D. Draper. J. Mol. Biol. 1998, 280, 41–59. [Google Scholar] [CrossRef]
- Zhuang, W.; Ecker, C.; Metselaar, G.A.; Rowan, A.E.; Nolte, R.J.M.; Samorí, P.; Rabe, J.P. SFM Characterization of Poly(Isocyanodipeptide) Single Polymer Chains in Controlled Environments: Effect of Tip Adhesion and Chain Swelling. Macromolecules 2005, 38, 473–480. [Google Scholar] [CrossRef]
- Kiriy, A.; Gorodyska, G.; Kiriy, N.; Sheparovych, R.; Lupytsky, R.; Minko, S.; Stamm, M. AFM Imaging of Single Polycation Molecules Contrasted with Cyanide-Bridged Compounds. Macromolecules 2005, 38, 501–506. [Google Scholar] [CrossRef]
- Sukhanova, M.V.; Hamon, L.; Kutuzov, M.M.; Joshi, V.; Abrakhi, S.; Dobra, I.; Curmi, P.A.; Pastre, D.; Lavrik, O.I. A Single-Molecule Atomic Force Microscopy Study of PARP1 and PARP2 Recognition of Base Excision Repair DNA Intermediates. J. Mol. Biol. 2019, 431, 2655–2673. [Google Scholar] [CrossRef]
- Müller, D.J.; Helenius, J.; Alsteens, D.; Dufrêne, Y.F. Force Probing Surfaces of Living Cells to Molecular Resolution. Nat. Chem. Biol. 2009, 5, 383–390. [Google Scholar] [CrossRef]
- Drake, B.; Prater, C.B.; Weisenhorn, A.L.; Gould, S.A.C.; Albrecht, T.R.; Quate, C.F.; Cannell, D.S.; Hansma, H.G.; Hansma, P.K. Imaging Crystals, Polymers, and Processes in Water with the Atomic Force Microscope. Science 1989, 243, 1586–1589. [Google Scholar] [CrossRef]
- Radmacher, M.; Tillmann, R.W.; Fritz, M.; Gaub, H.E. From Molecules to Cells: Imaging Soft Samples with the Atomic Force Microscope. Science 1992, 257, 1900–1905. [Google Scholar] [CrossRef]
- Hu, J.; Gao, M.; Wang, Z.; Chen, Y. Review on the Applications of Atomic Force Microscopy Imaging in Proteins. Micron 2022, 159, 103293. [Google Scholar] [CrossRef]
- Xu, K.; Liu, Y. Studies of Probe Tip Materials by Atomic Force Microscopy: A Review. Beilstein J. Nanotechnol. 2022, 13, 1256–1267. [Google Scholar] [CrossRef]
- Anne, A.; Chovin, A.; Demaille, C.; Lafouresse, M. High-Resolution Mapping of Redox-Immunomarked Proteins Using Electrochemical–Atomic Force Microscopy in Molecule Touching Mode. Anal. Chem. 2011, 83, 7924–7932. [Google Scholar] [CrossRef]
- Anne, A.; Cambril, E.; Chovin, A.; Demaille, C. Touching Surface-Attached Molecules with a Microelectrode: Mapping the Distribution of Redox-Labeled Macromolecules by Electrochemical-Atomic Force Microscopy. Anal. Chem. 2010, 82, 6353–6362. [Google Scholar] [CrossRef]
- Yan, S.; Yuan, S.; Zhang, Q.; Luo, M.; Qiao, D.; Jiang, F.; Qian, H. Improved Sample Preparation Method on the Morphology Observation of Hydrophilic Polysaccharides for Atomic Force Microscopy (AFM). Food Hydrocoll. 2023, 144, 109048. [Google Scholar] [CrossRef]
- Aoki, H. Conformation and Dynamics of Single Polymer Chain Studied by Optical Microscopy Techniques beyond the Diffraction Limit. Microscopy 2017, 66, 223–233. [Google Scholar] [CrossRef] [PubMed]



| Polymer System | Benefits of Single-Chain AFM Conducted on Isolated Polymer Chains | Ref. |
|---|---|---|
| Plasmid DNA | First visualization of a conformation adopted by a single DNA chain | [108] |
| PS-b-PMMA | First visualization of a conformation of a single synthetic polymer chain | [109] |
| Xanthan polysaccharide | Demonstration of a double-stranded structure adopted by a xanthan helix | [110] |
| PBPEM-g-(PnBuA-b-PS) BCP | Visualization of BCPs adopting extended molecular conformations, along with their side chains | [82] |
| DNA | Precise determination of the length of a DNA molecule | [83] |
| PBA brushes | Accurate measurement of the molecular weight of a molecule | [84] |
| Cartilage aggrecan macromolecules | Visualization of a conformation adopted by individual monomers and their constituent parts; determination of the end-to-end length | [85] |
| Oat β-glucans | Exact determination of the contour length, end-to-end distance, persistence length, and Mw | [86] |
| PS7-P2VP7 heteroarm star copolymer | Confirming the number of P2VP arms and visualization of the unimolecular micellar structure | [89] |
| Polystyrene- and poly(butyl acrylate)-based hybrid BCPs | Validating the generation of hybrid BCPs and visualization of a single nanoparticle–coil copolymer architecture | [87] |
| Arborescent PS | Observing the molecular structure of tree-like high-molecular-weight PSs and validating the mechanism of inimer polymerization | [90] |
| B-SHBPs | Visualization of the molecular structure of various SHBPs | [96] |
| Dendronized PFSs | Observing unimolecular spherical cocoons and other elongated structures comprising single PFS chains | [91] |
| Dendronized polynorbornenes | Detecting unimolecular tadpoles | [92] |
| Visualization of single random coil and rigid rod structures | [94] | |
| Dendronized conjugated di-BCPs | Deciphering the structure of single molecular wires comprising a regioregular backbone surrounded by bulky dendrons | [93] |
| Gum arabic and soybean polysaccharides | Validating a previous structural model assuming the existence of long sugar side chains on the main backbone; differentiating the linear and branched appearances of gum arabic and soybean polysaccharides | [98] |
| Pectin heteropolysaccharide | Confirming the branched appearance of polysaccharides and the minimal impact of the loss of neutral sugars on the size or the branching density of the individual chains | [99] |
| Polysaccharide rhamnogalacturonan I | Demonstrating the existence of regular side chains on isolated polysaccharide molecules | [100] |
| PCEVE-based polymers | Observing the linear, cyclic, and tadpole-shaped dimer molecules consisting of a ring and a linear chain, trefoil knot rings, and figure-of-eight dimer rings, as well as catenane molecular structures | [103] |
| Grafted polymers based on selenol moieties | Monitoring the collapse of single polymer chains containing selenol moieties | [104] |
| Polymer brushes | Visualization of molecular cycles made of cyclic polymers and their fusions into multimers | [105] |
| Polysilane | Observing dots of globular conformation and ropes with rigid rod conformations | [111] |
| PS-b-PMMA | Visualizing the transition from single non-aggregated PMMA chains to aggregated PMMA chains, generating a condensed monolayer, to PMMA chains adopting an expanded coil conformation | [112] |
| Carrageenan polysaccharides | Visualization of a coil to helix transition in an iota-Na-carrageenan single chain on mica and demonstrating the intramolecular generation of a unimeric helix from such a chain | [113] |
| DNA | Monitoring the dynamic motions of DNA single molecules and their structural changes within DNA origami surface relief structures | [107] |
| PMB | Revealing multiple conformational changes from a coil to a pearl necklace–globule, to a globule of single PMB chains on mica | [114] |
| it-PMMA | Demonstrating the “reptational” movements of PMMA flexible chains along their chain axis on mica | [115] |
| Observing, for the first time, the crystallization behavior of a single polymeric chain at the molecular level | [116] | |
| Demonstrating the folding of isolated it-PMMA chains upon their crystallization | [117] | |
| Monitoring the growth of crystals from unimolecular to multichain structures | [118] | |
| Visualizing the stem-level crystallization of a single it-PMMA chain into a folded-chain crystal | [119] | |
| Collagen, k-carrageenan, xanthan, gellan, scleroglucan | Monitoring the assembly of individual chains of various biopolymers into supramolecular (fibrillar) entities | [120] |
| Cellulose | Demonstrating the transition from aligned single chains to globular aggregates | [121] |
| Tannin/TMV | Studying the effect of tannin on the aggregation of TMV particles and its antiviral mechanism | [122] |
| PCPP/antigenic proteins | Revealing the ability of antigenic proteins to bind at single PCPP chains to generate single-chain compact spherical complexes or stiffened coils | [123] |
| Polysaccharide | Observing the motion of a polysaccharide under an aqueous buffer solution | [124] |
| P2VP | Watching the transition from extended coils to compressed globules of adsorbed P2VP single chains | [125] |
| PMB | Seeing the transition from extended coils to pearl necklace-like globules of adsorbed PMB single chains | [126] |
| PSS | Revealing how PSS single chains adsorbed on mica are adopting a wormlike coil conformation | [127] |
| Nafion | Showing how autoclaved Nafion single chains adsorbed on mica and graphite adopt a conformation resembling compact globules | [128] |
| PMMA | Monitoring the rigidification of single PMMA chains with temperature increases | [129] |
| DNA | Observing the transition from 2D random coils to stretched DNA conformations | [130] |
| PMB, P2VP, PS7-P2VP7 | Demonstrating the first enhancement of resolution of single-chain AFM on positively charged polymeric chains by using contrasting agents | [131] |
| Alginate, circular plasmid DNA, acetan, xanthan | Showing that the complexation of polyanions with chitosan generates torus-like morphologies | [132] |
| Isotactic and atactic PMMA, P2VP | Determining the glass transition temperature of isolated polymer chains by monitoring their movements at various temperatures | [133] |
| PBA-based brushes | Monitoring the rupture of covalent bonds in the main chain backbone | [134] |
| Polycarbonate | Monitoring the cross-linking reactions that generate SCPNPs | [135] |
| UPy-functionalized PMMA | Observing the chain folding upon the formation of individual SCPNPs | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, M.; Todor-Boer, O.; Botiz, I. Visualization of Single Polymer Chains with Atomic Force Microscopy: A Review. Polymers 2025, 17, 1397. https://doi.org/10.3390/polym17101397
Pop M, Todor-Boer O, Botiz I. Visualization of Single Polymer Chains with Atomic Force Microscopy: A Review. Polymers. 2025; 17(10):1397. https://doi.org/10.3390/polym17101397
Chicago/Turabian StylePop, Maria, Otto Todor-Boer, and Ioan Botiz. 2025. "Visualization of Single Polymer Chains with Atomic Force Microscopy: A Review" Polymers 17, no. 10: 1397. https://doi.org/10.3390/polym17101397
APA StylePop, M., Todor-Boer, O., & Botiz, I. (2025). Visualization of Single Polymer Chains with Atomic Force Microscopy: A Review. Polymers, 17(10), 1397. https://doi.org/10.3390/polym17101397








