Polymeric Membrane Contactors for CO2 Separation: A Systematic Literature Analysis of the Impact of Absorbent Temperature
Abstract
1. Introduction
1.1. Background
1.2. CO2 Capture Using a Physical Absorption Process
1.3. CO2 Capture Using a Chemical Absorption Process
1.3.1. Aqueous Alkaline Solvents
1.3.2. Alkali Salt Solvents
1.3.3. Aqueous Amine Solvents
1.4. Outline
2. Methodology
- CO2 capture utilizing polymeric HFM devices;
- the influence of liquid feed temperature on CO2 absorption performance.
- HFM contactors: A candidate study must focus on polymeric HFM contactors for CO2 removal;
- Data presentation: The selected study must also present CO2 separation efficiency (%) or CO2 absorption flux (mol/m2s) as a function of the liquid-phase temperature of a CO2-poor solution. These data need to be displayed graphically or reported in tabular form;
- Additional information: The study must clearly report important information such as the nature of the polymeric HFM, its physical characteristics, and geometrical factors (e.g., geometry, number of fibers, HFM dimensions, etc.).
3. Results
- Polytetrafluoroethylene (PTFE) HFM: from 20 to 60 °C (ΔT = 40 °C) [37];
- Polyvinylidene fluoride (PVDF) HFM: from 10 to 40 °C (ΔT = 30 °C) [38];
- Polyvinylidene fluoride (PVDF) HFM: from 30 to 60 °C (ΔT = 30 °C) [40];
- Polyvinylidene fluoride (PVDF) HFM: from 20 to 60 °C (ΔT = 40 °C) [43];
- Polytetrafluoroethylene (PTFE) HFM: from 25 to 40 °C (ΔT = 15 °C) [44];
- Polypropylene (PP) HFM: from 30 to 50 °C (ΔT = 20 °C) [45].
4. Discussion
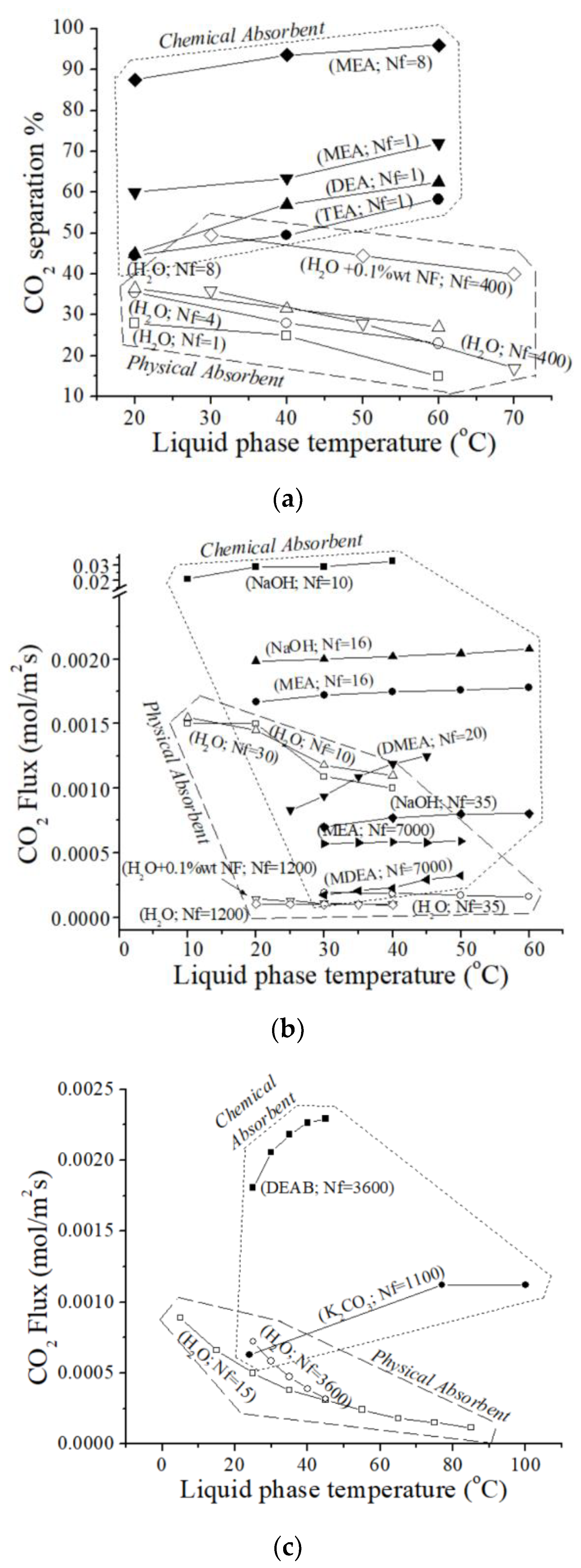
- In contrast to chemical absorption, physical absorption generally shows a decrease in CO2 absorption flux as liquid-phase temperatures increase. For example, Saidi et al. [46] observed that increasing the temperature in a DEAB system from 25 °C to 45 °C led to a maximum CO2 flux of 8.28 mol/m2h. In contrast, in the same HFM device using water as the absorbent, increasing the temperature from 25 °C to 45 °C resulted in a decrease in CO2 absorption capacity from 2.57 to 1.27 mol/m2h [46]. This trend aligns with findings from other studies, which suggest that temperature has a greater influence on reaction rate than other factors limiting CO2 absorption, such as gas and liquid flow rates.
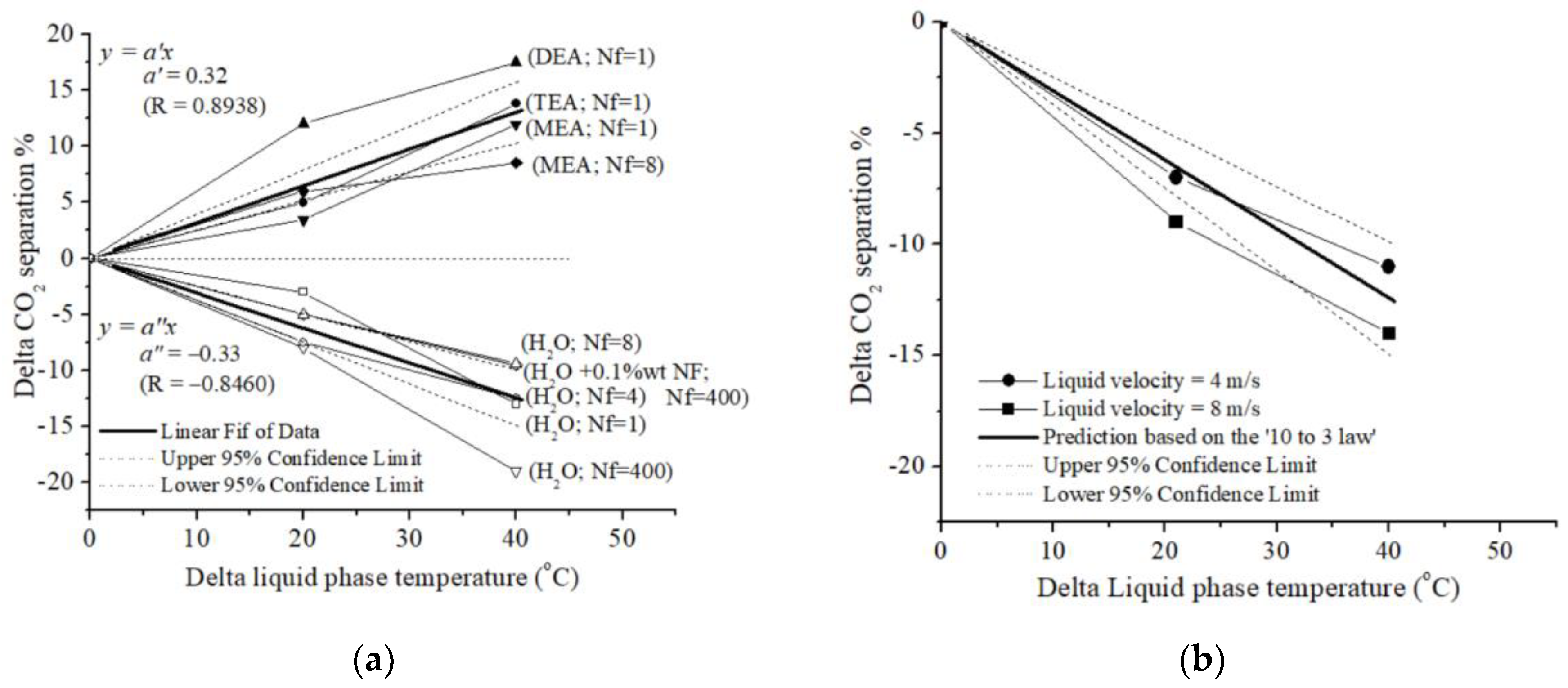
5. Conclusions
- Superiority of Chemical Absorption: CO2 chemical absorption consistently outperforms physical absorption using H2O as the absorbent;
- Temperature-Dependent Enhancement: CO2 chemical absorption flux exhibits a positive correlation with temperature, indicating enhanced performance at higher temperatures;
- Inverse Relationship with Temperature: In contrast, CO2 removal capacity in H2O decreases with increasing temperature.
- Chemical absorption → CO2 separation (%) = +0.3 T (°C);
- Physical absorption → CO2 separation (%) = −0.3 T (°C).
6. Prospects
6.1. Economic Optimization of Liquid-Phase Temperature
- Decreased efficiency in physical absorption due to the reduced solubility of CO2 at lower temperatures;
- Enhanced efficiency in chemical absorption due to increased reaction kinetics at higher temperatures;
- Energy costs associated with liquid-phase temperature control;
- Energy costs associated with physical solvent regeneration (e.g., through temperature swing, pressure swing, or stripping with air or steam);
- Energy costs associated with chemical solvent regeneration via the introduction of heat to regenerate the original chemical solvent and obtain pure CO2.
6.2. High-Pressure Liquid-Phase Studies
- Chemical absorption: Elevated liquid-phase pressure can increase the density of the liquid, potentially enhancing mass transfer and CO2 chemical absorption [38].
6.3. Low- and High-Temperature Liquid-Phase Studies
6.4. Long-Term Stability Tests and Polymeric Nature
- Long-Term Stability Tests: Current research focuses on CO2 capture within a limited temperature range of the liquid phase. However, industrial applications may necessitate operation at higher or lower temperatures. Long-term stability tests at different liquid-phase temperatures are crucial to evaluate the long-term performance of traditional polymeric HFM contactors under these more demanding conditions.
- Membrane Wetting: Future studies should more explicitly investigate the potential for polymeric HFM wetting under varying operating conditions. This includes a systematic evaluation of the impact of elevated temperatures and the use of hydrophilic solvents on the long-term stability and performance of polymeric HFMs, as wetting can significantly hinder their efficiency.
- Advanced HFM Materials: Expanding the investigation beyond polymeric HFMs is essential. New materials, such as advanced ceramics, thin-film composites, facilitated transport membranes, and mixed-matrix membranes, offer potential advantages like enhanced durability and improved selectivity, potentially even at temperatures other than room temperature.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cigala, R.M.; De Luca, G.; Ielo, I.; Crea, F. Biopolymeric Nanocomposites for CO2 Capture. Polymers 2024, 16, 1063. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, G.; De Medeiros, J.; Araújo, O. Carbon Capture from CO2-Rich Natural Gas via Gas-Liquid Membrane Contactors with Aqueous-Amine Solvents: A Review. Gases 2022, 2, 98–133. [Google Scholar] [CrossRef]
- Kalmykov, D.; Shirokikh, S.; Grushevenko, E.A.; Legkov, S.A.; Bondarenko, G.N.; Anokhina, T.S.; Molchanov, S.; Bazhenov, S.D. Stability of Porous Polymeric Membranes in Amine Solvents for Membrane Contactor Applications. Membranes 2023, 13, 544. [Google Scholar] [CrossRef]
- Sreedhar, I.; Vaidhiswaran, R.; Kamani, B.M.; Venugopal, A. Process and Engineering Trends in Membrane Based Carbon Capture. Renew. Sustain. Energy Rev. 2017, 68, 659–684. [Google Scholar] [CrossRef]
- Lee, Y.; Park, Y.-J.; Lee, J.; Bae, T.-H. Recent Advances and Emerging Applications of Membrane Contactors. Chem. Eng. J. 2023, 461, 141948. [Google Scholar] [CrossRef]
- Pabby, A.K.; Sastre, A.M. State-of-the-Art Review on Hollow Fibre Contactor Technology and Membrane-Based Extraction Processes. J. Membr. Sci. 2013, 430, 263–303. [Google Scholar] [CrossRef]
- Da Silva Biron, D.; Espíndola, J.C.; Subtil, E.L.; Mierzwa, J.C. A New Approach to the Development of Hollow Fiber Membrane Modules for Water Treatment: Mixed Polymer Matrices. Membranes 2023, 13, 613. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Kujawski, W.; Válek, R.; Koter, S. A Review—The Development of Hollow Fibre Membranes for Gas Separation Processes. Int. J. Greenh. Gas Control 2021, 104, 103195. [Google Scholar] [CrossRef]
- Li, J.-L.; Chen, B.-H. Review of CO2 Absorption Using Chemical Solvents in Hollow Fiber Membrane Contactors. Sep. Purif. Technol. 2005, 41, 109–122. [Google Scholar] [CrossRef]
- Li, L.; Ma, G.; Pan, Z.; Zhang, N.; Zhang, Z. Research Progress in Gas Separation Using Hollow Fiber Membrane Contactors. Membranes 2020, 10, 380. [Google Scholar] [CrossRef]
- Imtiaz, A.; Othman, M.H.D.; Jilani, A.; Khan, I.U.; Kamaludin, R.; Ayub, M.; Samuel, O.; Kurniawan, T.A.; Hashim, N.; Puteh, M.H. A Critical Review in Recent Progress of Hollow Fiber Membrane Contactors for Efficient CO2 Separations. Chemosphere 2023, 325, 138300. [Google Scholar] [CrossRef] [PubMed]
- Alammar, A.Y.; Choi, S.-H.; Buonomenna, M.G. Hollow Fiber Membrane Modification by Interfacial Polymerization for Organic Solvent Nanofiltration. Processes 2024, 12, 563. [Google Scholar] [CrossRef]
- Wojciechowski, C.; Wasyłeczko, M.; Lewińska, D.; Chwojnowski, A. A Comprehensive Review of Hollow-Fiber Membrane Fabrication Methods across Biomedical, Biotechnological, and Environmental Domains. Molecules 2024, 29, 2637. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Magnone, E.; Park, J.H. Preparation, Characterization and Laboratory-Scale Application of Modified Hydrophobic Aluminum Oxide Hollow Fiber Membrane for CO2 Capture Using H2O as Low-Cost Absorbent. J. Membr. Sci. 2015, 494, 143–153. [Google Scholar] [CrossRef]
- Gargari, S.F.; Mirfendereski, S.M. Enhanced CO2 Removal in Hollow Fiber Membrane Contactors Using Amine-Based Nanofluids. J. Ind. Eng. Chem. 2023, 128, 356–368. [Google Scholar] [CrossRef]
- Khan, U.; Ogbaga, C.C.; Abiodun, O.-A.O.; Adeleke, A.A.; Ikubanni, P.P.; Okoye, P.U.; Okolie, J.A. Assessing Absorption-Based CO2 Capture: Research Progress and Techno-Economic Assessment Overview. Carbon Capture Sci. Technol. 2023, 8, 100125. [Google Scholar] [CrossRef]
- De Carvalho Pinto, P.C.; Batista, T.V.; De Rezende Ferreira, G.; Voga, G.P.; Oliveira, L.C.A.; Oliveira, H.S.; De Souza, L.A.; Belchior, J.C. Chemical Absorption of CO2 Enhanced by Solutions of Alkali Hydroxides and Alkoxides at Room Temperature. ChemistrySelect 2022, 7, e202202731. [Google Scholar] [CrossRef]
- Carroll, J.J.; Slupsky, J.D.; Mather, A.E. The Solubility of Carbon Dioxide in Water at Low Pressure. J. Phys. Chem. Ref. Data 1991, 20, 1201–1209. [Google Scholar] [CrossRef]
- Crovetto, R. Evaluation of Solubility Data of the System CO2–H2O from 273 K to the Critical Point of Water. J. Phys. Chem. Ref. Data 1991, 20, 575–589. [Google Scholar] [CrossRef]
- Wiebe, R.; Gaddy, V.L. The Solubility of Carbon Dioxide in Water at Various Temperatures from 12 to 40° and at Pressures to 500 Atmospheres. Critical Phenomena. J. Am. Chem. Soc. 1940, 62, 815–817. [Google Scholar] [CrossRef]
- Boributh, S.; Assabumrungrat, S.; Laosiripojana, N.; Jiraratananon, R. A Modeling Study on the Effects of Membrane Characteristics and Operating Parameters on Physical Absorption of CO2 by Hollow Fiber Membrane Contactor. J. Membr. Sci. 2011, 380, 21–33. [Google Scholar] [CrossRef]
- Versteeg, G.F.; Van Swaaij, W.P.M. Solubility and Diffusivity of Acid Gases (Carbon Dioxide, Nitrous Oxide) in Aqueous Alkanolamine Solutions. J. Chem. Eng. Data 1988, 33, 29–34. [Google Scholar] [CrossRef]
- Diamond, L.W.; Akinfiev, N.N. Solubility of CO2 in Water from −1.5 to 100 °C and from 0.1 to 100 MPa: Evaluation of Literature Data and Thermodynamic Modelling. Fluid Phase Equilibria 2003, 208, 265–290. [Google Scholar] [CrossRef]
- Zeman, F. Energy and Material Balance of CO2 Capture from Ambient Air. Environ. Sci. Technol. 2007, 41, 7558–7563. [Google Scholar] [CrossRef] [PubMed]
- Kucka, L.; Kenig, E.Y.; Górak, A. Kinetics of the Gas−liquid Reaction between Carbon Dioxide and Hydroxide Ions. Ind. Eng. Chem. Res. 2002, 41, 5952–5957. [Google Scholar] [CrossRef]
- Vázquez, G.; Alvarez, E.; Varela, R.; Cancela, A.; Navaza, J.M. Density and Viscosity of Aqueous Solutions of Sodium Dithionite, Sodium Hydroxide, Sodium Dithionite + Sucrose, and Sodium Dithionite + Sodium Hydroxide + Sucrose from 25 °C to 40 °C. J. Chem. Eng. Data 1996, 41, 244–248. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Kentish, S.E.; Stevens, G.W. Absorption of Carbon Dioxide into Aqueous Potassium Carbonate Promoted by Boric Acid. Energy Procedia 2009, 1, 1075–1081. [Google Scholar] [CrossRef]
- Saidi, M.; Heidarinejad, S.; Rahimpour, H.R.; Talaghat, M.R.; Rahimpour, M.R. Mathematical Modeling of Carbon Dioxide Removal Using Amine-Promoted Hot Potassium Carbonate in a Hollow Fiber Membrane Contactor. J. Nat. Gas Sci. Eng. 2014, 18, 274–285. [Google Scholar] [CrossRef]
- Mehdipour, M.; Karami, M.R.; Keshavarz, P.; Ayatollahi, S. Analysis of CO2 Separation with Aqueous Potassium Carbonate Solution in a Hollow Fiber Membrane Contactor. Energy Fuels 2013, 27, 2185–2193. [Google Scholar] [CrossRef]
- Lee, Y.; Noble, R.D.; Yeom, B.-Y.; Park, Y.-I.; Lee, K.-H. Analysis of CO2 Removal by Hollow Fiber Membrane Contactors. J. Membr. Sci. 2001, 194, 57–67. [Google Scholar] [CrossRef]
- Borhani, T.N.G.; Azarpour, A.; Akbari, V.; Wan Alwi, S.R.; Manan, Z.A. CO2 Capture with Potassium Carbonate Solutions: A State-of-the-Art Review. Int. J. Greenh. Gas Control 2015, 41, 142–162. [Google Scholar] [CrossRef]
- Acampora, L.; Grilletta, S.; Costa, G. The Integration of Carbon Capture, Utilization, and Storage (CCUS) in Waste-to-Energy Plants: A Review. Energies 2025, 18, 1883. [Google Scholar] [CrossRef]
- Hamdy, L.B.; Goel, C.; Rudd, J.A.; Barron, A.R.; Andreoli, E. The Application of Amine-Based Materials for Carbon Capture and Utilisation: An Overarching View. Mater. Adv. 2021, 2, 5843–5880. [Google Scholar] [CrossRef]
- El Hadri, N.; Quang, D.V.; Goetheer, E.L.V.; Abu Zahra, M.R.M. Aqueous Amine Solution Characterization for Post-Combustion CO2 Capture Process. Appl. Energy 2017, 185, 1433–1449. [Google Scholar] [CrossRef]
- Sartori, G.; Savage, D.W. Sterically Hindered Amines for Carbon Dioxide Removal from Gases. Ind. Eng. Chem. Fund. 1983, 22, 239–249. [Google Scholar] [CrossRef]
- Krzemień, A.; Więckol-Ryk, A.; Smoliński, A.; Koteras, A.; Więcław-Solny, L. Assessing the Risk of Corrosion in Amine-Based CO2 Capture Process. J. Loss Prev. Process Ind. 2016, 43, 189–197. [Google Scholar] [CrossRef]
- Ghobadi, J.; Ramirez, D.; Jerman, R.; Crane, M.; Khoramfar, S. CO2 Separation Performance of Different Diameter Polytetrafluoroethylene Hollow Fiber Membranes Using Gas-Liquid Membrane Contacting System. J. Membr. Sci. 2018, 549, 75–83. [Google Scholar] [CrossRef]
- Mansourizadeh, A.; Ismail, A.F.; Matsuura, T. Effect of Operating Conditions on the Physical and Chemical CO2 Absorption through the PVDF Hollow Fiber Membrane Contactor. J. Membr. Sci. 2010, 353, 192–200. [Google Scholar] [CrossRef]
- Mansourizadeh, A. Experimental Study of CO2 Absorption/Stripping via PVDF Hollow Fiber Membrane Contactor. Chem. Eng. Res. Des. 2012, 90, 555–562. [Google Scholar] [CrossRef]
- Atchariyawut, S.; Jiraratananon, R.; Wang, R. Separation of CO2 from CH4 by Using Gas–Liquid Membrane Contacting Process. J. Membr. Sci. 2007, 304, 163–172. [Google Scholar] [CrossRef]
- Golkhar, A.; Keshavarz, P.; Mowla, D. Investigation of CO2 Removal by Silica and CNT Nanofluids in Microporous Hollow Fiber Membrane Contactors. J. Membr. Sci. 2013, 433, 17–24. [Google Scholar] [CrossRef]
- Mohammaddoost, H.; Azari, A.; Ansarpour, M.; Osfouri, S. Experimental Investigation of CO2 Removal from N2 by Water/TiO2 NFs in PP Hollow Fiber Membrane Contactors. In Proceedings of the 9th International Chemical Engineering Congress & Exhibition (IChEC 2015), Shiraz, Iran, 26–28 December 2015. [Google Scholar]
- Rahim, N.A.; Ghasem, N.; Al-Marzouqi, M. Absorption of CO2 from Natural Gas Using Different Amino Acid Salt Solutions and Regeneration Using Hollow Fiber Membrane Contactors. J. Nat. Gas Sci. Eng. 2015, 26, 108–117. [Google Scholar] [CrossRef]
- Cao, F.; Gao, H.; Xiong, Q.; Liang, Z. Experimental Studies on Mass Transfer Performance for CO2 Absorption into Aqueous N,N-Dimethylethanolamine (DMEA) Based Solutions in a PTFE Hollow Fiber Membrane Contactor. Int. J. Greenh. Gas Control 2019, 82, 210–217. [Google Scholar] [CrossRef]
- Yan, S.; Fang, M.-X.; Zhang, W.-F.; Wang, S.-Y.; Xu, Z.-K.; Luo, Z.-Y.; Cen, K.-F. Experimental Study on the Separation of CO2 from Flue Gas Using Hollow Fiber Membrane Contactors without Wetting. Fuel Process. Technol. 2007, 88, 501–511. [Google Scholar] [CrossRef]
- Saidi, M. Mathematical Modeling of CO2 Absorption into Novel Reactive DEAB Solution in Hollow Fiber Membrane Contactors; Kinetic and Mass Transfer Investigation. J. Membr. Sci. 2017, 524, 186–196. [Google Scholar] [CrossRef]
- Privalova, E.I.; Mäki-Arvela, P.; Murzin, D.Y.; Mikkhola, J.P. Capturing CO2: Conventional versus Ionic-Liquid Based Technologies. Russ. Chem. Rev. 2012, 81, 435–457. [Google Scholar] [CrossRef]
- Faisal Elmobarak, W.; Almomani, F.; Tawalbeh, M.; Al-Othman, A.; Martis, R.; Rasool, K. Current Status of CO2 Capture with Ionic Liquids: Development and Progress. Fuel 2023, 344, 128102. [Google Scholar] [CrossRef]
- Boucif, N.; Roizard, D.; Corriou, J.-P.; Favre, E. To What Extent Does Temperature Affect Absorption in Gas-Liquid Hollow Fiber Membrane Contactors? Sep. Sci. Technol. 2015, 50, 1331–1343. [Google Scholar] [CrossRef]
- Pandya, J.D. Adiabatic Gas Absorption and Stripping with Chemical Reaction in Packed Tower. Chem. Eng. Commun. 1983, 19, 343–361. [Google Scholar] [CrossRef]
- Jalali, A.; Lotfi, M.; Mohebbi, S.; Baghban, A. Modeling of CO2 Capture from Gas Stream Emissions of Petrochemical Industries by Membrane Contactor. Pet. Sci. Technol. 2018, 36, 1446–1454. [Google Scholar] [CrossRef]
- Chen, G.; Chen, G.; Cao, F.; Zhang, R.; Gao, H.; Liang, Z. Mass Transfer Performance and Correlation for CO2 Absorption into Aqueous 3-Diethylaminopropylamine Solution in a Hollow Fiber Membrane Contactor. Chem. Eng. Process.-Process Intensif. 2020, 152, 107932. [Google Scholar] [CrossRef]
- Luqmani, B.; Brookes, A.; Moore, A.; Vale, P.; Pidou, M.; McAdam, E.J. The Role of Solvent Temperature and Gas Pressure on CO2 Mass Transfer during Biogas Upgrading within Porous and Dense-Skin Hollow Fibre Membrane Contactors. J. Membr. Sci. 2023, 685, 121967. [Google Scholar] [CrossRef]
- Wang, B.; Shao, Y.; Yang, L.; Guo, K.; Li, X.; Sun, M.; Hao, Y. Sustainable Hydrogen Production with Negative Carbon Emission through Thermochemical Conversion of Biogas/Biomethane. Energies 2025, 18, 1804. [Google Scholar] [CrossRef]
- Singh, D.; Sirini, P.; Lombardi, L. Review of Reforming Processes for the Production of Green Hydrogen from Landfill Gas. Energies 2024, 18, 15. [Google Scholar] [CrossRef]
- Feng, C.Y.; Khulbe, K.C.; Matsuura, T.; Ismail, A.F. Recent Progresses in Polymeric Hollow Fiber Membrane Preparation, Characterization and Applications. Sep. Purif. Technol. 2013, 111, 43–71. [Google Scholar] [CrossRef]
- Dindore, V.Y.; Brilman, D.W.F.; Feron, P.H.M.; Versteeg, G.F. CO2 Absorption at Elevated Pressures Using a Hollow Fiber Membrane Contactor. J. Membr. Sci. 2004, 235, 99–109. [Google Scholar] [CrossRef]

| T (°C) | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 |
|---|---|---|---|---|---|---|---|---|---|---|
| Solubility | 1.362 | 0.962 | 0.704 | 0.531 | 0.409 | 0.319 | 0.247 | 0.185 | 0.127 | 0.067 |
| Ref. | [37] | [37] | [37] | [38] | [39] | [40] | [41] | [42] | [42] |
|---|---|---|---|---|---|---|---|---|---|
| Fiber | PTFE | PTFE | PTFE | PVDF | PVDF | PVDF | PP | PP | PP |
| Liquid | H2O | H2O | H2O | H2O | H2O | H2O | H2O NF | H2O NF | H2O NF |
| Ti (°C) | 20 | 20 | 20 | 10 | 10 | 30 | 30 | 20 | 20 |
| Tf (°C) | 60 | 60 | 60 | 40 | 40 | 60 | 70 | 40 | 40 |
| Nf | 1 | 4 | 8 | 10 | 30 | 35 | 400 | 1200 | 1200 |
| F.Od (mm) | 3.52 | 1.84 | 1.2 | 1 | 0.95 | 1 | 0.45 | 0.4 | 0.4 |
| F.Id (mm) | 2.96 | 1.46 | 0.68 | 0.55 | 0.6 | 0.65 | 0.32 | 0.3 | 0.3 |
| F.l (mm) | 150 | 150 | 400 | 250 | 250 | ||||
| M.Pd (µm) | 0.51 | 0.58 | 0.48 | 0.2 | 0.15 | 0.2 | 0.2 | ||
| M.P (%) | 52 | 52 | 52 | 77.1 | 0.75 * | 50 | 50 | ||
| B.p (psi) | 12.3 | 14 | 14.8 | ||||||
| M.Od (mm) | 6.35 | 6.35 | 6.35 | ||||||
| M.Id (mm) | 4.78 | 4.78 | 4.78 | 14 | 14 | 10 | 20 | 34 | 34 |
| M.l (mm) | 230 | 230 | 230 | 270 | 270 | 270 | |||
| C.A. (cm2) | 42 | 88 | 115 | 190 | 1600 | ||||
| P.D. | 54 | 59 | 51 | 204 | 582 |
| Ref. | [37] | [37] | [37] | [37] | [38] | [43] | [43] | [44] | [40] | [45] | [45] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fiber | PTFE | PTFE | PTFE | PTFE | PVDF | PVDF | PVDF | PTFE | PVDF | PP | PP |
| Liquid | TEA | DEA | MEA | MEA | NaOH | MEA | NaOH | DMEA | NaOH | MDEA | MEA |
| Ti (°C) | 20 | 20 | 20 | 20 | 10 | 20 | 20 | 25 | 30 | 30 | 30 |
| Tf (°C) | 60 | 60 | 60 | 60 | 40 | 60 | 60 | 44 | 60 | 50 | 50 |
| Nf | 1 | 1 | 1 | 8 | 10 | 16 | 16 | 20 | 35 | 7000 | 7000 |
| F.Od (mm) | 3.52 | 3.52 | 3.52 | 3.52 | 1 | 1.1 | 1.1 | 1.7 | 1 | 0.442 | 0.442 |
| F.Id (mm) | 2.96 | 2.96 | 2.96 | 2.96 | 0.55 | 0.42 | 0.42 | 1 | 0.65 | 0.344 | 0.344 |
| F.l (mm) | 150 | 800 | 800 | ||||||||
| M.Pd (µm) | 0.51 | 0.51 | 0.51 | 0.5 | |||||||
| M.P (%) | 52 | 52 | 52 | 52 | 50 | 0.75 * | >45 | >45 | |||
| B.p (psi) | 12.3 | 12.3 | 12.3 | 12.3 | |||||||
| M.Od (mm) | 6.35 | 6.35 | 6.35 | 6.35 | 15 | 15 | |||||
| M.Id (mm) | 4.78 | 4.78 | 4.78 | 4.78 | 14 | 11 | 11 | 1.8 | 10 | 80 | 80 |
| M.l (mm) | 230 | 230 | 230 | 230 | 270 | 160 | 160 | 200 | 270 | 1000 | 1000 |
| C.A. (cm2) | 42 | 42 | 42 | 42 | 34 | 34 | 190 | 60,500 | 60,500 | ||
| P.D. | 54 | 54 | 54 | 54 | 204 | 17 * | 17 * | 21.4 | 21.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnone, E.; Shin, M.C.; Park, J.H. Polymeric Membrane Contactors for CO2 Separation: A Systematic Literature Analysis of the Impact of Absorbent Temperature. Polymers 2025, 17, 1387. https://doi.org/10.3390/polym17101387
Magnone E, Shin MC, Park JH. Polymeric Membrane Contactors for CO2 Separation: A Systematic Literature Analysis of the Impact of Absorbent Temperature. Polymers. 2025; 17(10):1387. https://doi.org/10.3390/polym17101387
Chicago/Turabian StyleMagnone, Edoardo, Min Chang Shin, and Jung Hoon Park. 2025. "Polymeric Membrane Contactors for CO2 Separation: A Systematic Literature Analysis of the Impact of Absorbent Temperature" Polymers 17, no. 10: 1387. https://doi.org/10.3390/polym17101387
APA StyleMagnone, E., Shin, M. C., & Park, J. H. (2025). Polymeric Membrane Contactors for CO2 Separation: A Systematic Literature Analysis of the Impact of Absorbent Temperature. Polymers, 17(10), 1387. https://doi.org/10.3390/polym17101387







