4-Iodobenzonitrile as Effective Solid Additive for High-Efficiency Polymer Solar Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, Y.; Yao, H.; Zhang, J.; Xian, K.; Zhang, T.; Hong, L.; Wang, Y.; Xu, Y.; Ma, K.; An, C.; et al. Single-Junction Organic Photovoltaic Cells with Approaching 18% Efficiency. Adv. Mater. 2020, 32, 1908205. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Cheng, Y.; Fang, Y.; Zhang, L.; Hu, X.; Jeong, S.Y.; Zhang, H.; Woo, H.Y.; Wu, F.; Chen, L. A molecular weight-regulated sequential deposition strategy enabling semitransparent organic solar cells with the light utilization efficiency of over 5%. Energy Environ. Sci. 2022, 15, 4776–4788. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, J.; Meng, L.; Sun, C.; Qin, S.; Zhu, C.; Zhang, J.; Li, J.; Wei, Z.; Li, Y. 18.55% Efficiency Polymer Solar Cells Based on a Small Molecule Acceptor with Alkylthienyl Outer Side Chains and a Low-Cost Polymer Donor PTQ10. CCS Chem. 2023, 5, 841–850. [Google Scholar] [CrossRef]
- Li, C.; Yao, G.; Gu, X.; Lv, J.; Hou, Y.; Lin, Q.; Yu, N.; Abbasi, M.S.; Zhang, X.; Zhang, J.; et al. Highly efficient organic solar cells enabled by suppressing triplet exciton formation and non-radiative recombination. Nat. Commun. 2024, 15, 8872. [Google Scholar] [CrossRef]
- Shao, Y.; Gao, Y.; Sun, R.; Zhang, M.; Min, J. A Versatile and Low-Cost Polymer Donor Based on 4-Chlorothiazole for Highly Efficient Polymer Solar Cells. Adv. Mater. 2023, 35, 2208750. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Fu, Y.; Guo, C.; Li, D.; Cheng, J.; Sun, W.; Gan, Z.; Sun, Y.; Zhou, B.; et al. Donor–acceptor mutually diluted heterojunctions for layer-by-layer fabrication of high-performance organic solar cells. Nat. Energy 2024, 9, 208–218. [Google Scholar] [CrossRef]
- Xie, C.; Liu, Y.; Wei, W.; Zhou, Y. Large-Area Flexible Organic Solar Cells with a Robust Silver Nanowire-Polymer Composite as Transparent Top Electrode. Adv. Funct. Mater. 2023, 33, 2210675. [Google Scholar] [CrossRef]
- Vedhanarayanan, B.; Lakshmi, K.C.S.; Lin, T.-W. Interfacial Tuning of Polymeric Composite Materials for High-Performance Energy Devices. Batteries 2023, 9, 487. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Xia, W.; Qiu, K.; Guo, C.; Gan, Z.; Zhou, J.; Sun, Y.; Liu, D.; Li, W.; et al. Molecular interaction induced dual fibrils towards organic solar cells with certified efficiency over 20%. Nat. Commun. 2024, 15, 6865. [Google Scholar] [CrossRef]
- Chen, Z.; Ge, J.; Song, W.; Tong, X.; Liu, H.; Yu, X.; Li, J.; Shi, J.; Xie, L.; Han, C.; et al. 20.2% Efficiency Organic Photovoltaics Employing a π-Extension Quinoxaline-Based Acceptor with Ordered Arrangement. Adv. Mater. 2024, 36, 2406690. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, S.; Xu, R.; Liu, F.; Miao, X.; Ran, G.; Liu, K.; Yi, Y.; Zhang, W.; Zhu, X. Non-fullerene acceptor with asymmetric structure and phenyl-substituted alkyl side chain for 20.2% efficiency organic solar cells. Nat. Energy 2024, 9, 975–986. [Google Scholar] [CrossRef]
- Cheng, B.; Xia, X.; Cheng, S.; Han, C.; Sun, F.; Fu, Z.; Hou, W.; Hua, F.; Wang, H.; Sun, W.; et al. Precise Control Over Crystallization Kinetics by Combining Nucleating Agents and Plasticizers for 20.1% Efficiency Organic Solar Cells. Adv. Mater. 2025, 37, 2500357. [Google Scholar] [CrossRef]
- Dai, X.; Li, Y.; Li, H.; Zhou, W.; Xu, X.; Deng, M.; Liao, C.; Peng, Q. Double Hole Transport Layers Enable 20.42% Efficiency Organic Solar Cells by Aggregation Control of Self-Assembled Molecules on Cobalt Salt Surfaces. Small 2025, 2411457. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, K.; Liu, F.; Ran, G.; Wang, M.; Zhang, T.; Xu, R.; Liu, H.; Zhang, W.; Wei, Z.; et al. 20.6% Efficiency Organic Solar Cells Enabled by Incorporating a Lower Bandgap Guest Nonfullerene Acceptor Without Open-Circuit Voltage Loss. Adv. Mater. 2025, 37, 2500282. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zou, B.; Hai, Y.; Luo, Y.; Luo, Z.; Wu, J.; Yan, H.; Li, G. Triplet State Suppression for Energy Loss Reduction in 20% Nonhalogenated Solvent Processed Binary Organic Solar Cells. Adv. Mater. 2025, 37, 2500861. [Google Scholar] [CrossRef]
- Song, X.; Zhang, B.; Liu, X.; Mei, L.; Li, H.; Yin, S.; Zhou, X.; Chen, H.; Lin, Y.; Zhu, W.; et al. Low-Volatility Fused-Ring Solid Additive Engineering for Synergistically Elongating Exciton Lifetime and Mitigating Trap Density Toward Organic Solar Cells of 20.5% Efficiency. Adv. Mater. 2025, 37, 2418393. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, L.; Guo, C.; Xiao, J.; Liu, C.; Chen, C.; Xia, W.; Gan, Z.; Cheng, J.; Zhou, J.; et al. π-Extended Nonfullerene Acceptor for Compressed Molecular Packing in Organic Solar Cells To Achieve over 20% Efficiency. J. Am. Chem. Soc. 2024, 146, 12011–12019. [Google Scholar] [CrossRef]
- Wang, X.; Liang, Q.; Zhang, A.; Wei, N.; Jiang, H.; Cheng, Y.; Fang, H.; Li, S.; Lu, H.; Li, W.; et al. Amide-Based Cathode Interfacial Layer with Dual-Modification Mechanisms Enables Stable Organic Solar Cells with High Efficiency Achieving 20%. J. Am. Chem. Soc. 2025, 147, 9261–9272. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Ren, J.; Zhang, T.; Dai, J.; Wang, J.; Ma, L.; Qiao, J.; Hao, X.; Hou, J. Molecular Design for Vertical Phase Distribution Modulation in High-Performance Organic Solar Cells. Adv. Mater. 2024, 36, 2310390. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, Y.; Yao, H.; Bi, P.; Hong, L.; Zhang, J.; Zu, Y.; Zhang, T.; Qin, J.; Ren, J.; et al. Single-Junction Organic Photovoltaic Cell with 19% Efficiency. Adv. Mater. 2021, 33, 2102420. [Google Scholar] [CrossRef]
- Jiang, X.; Gillett, A.J.; Zheng, T.; Song, X.; Heger, J.E.; Sun, K.; Spanier, L.V.; Guo, R.; Liang, S.; Bernstorff, S.; et al. Operando study of the influence of small molecule acceptors on the morphology induced device degradation of organic solar cells with different degrees of π–π stacking. Energy Environ. Sci. 2023, 16, 5970–5981. [Google Scholar] [CrossRef]
- Si, X.; Huang, Y.; Shi, W.; Wang, R.; Ma, K.; Zhang, Y.; Wu, S.; Yao, Z.; Li, C.; Wan, X.; et al. Achieving Organic Solar Cells with an Efficiency of 18.80% by Reducing Nonradiative Energy Loss and Tuning Active Layer Morphology. Adv. Funct. Mater. 2023, 33, 2306471. [Google Scholar] [CrossRef]
- Zhu, S.; Lyu, L.; Li, Y.; Li, W.; Cui, Y.; Hu, H. Cyclization Engineering of Electron-Deficient Maleimide Unit for Nonfused Ring Electron Acceptors Enables Efficient Organic Solar Cells. ACS Appl. Mater. Interfaces 2024, 16, 33928–33934. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Lv, J.; Chen, Z.; Luo, Z.; Zhang, G.; Liu, H.; Huang, H.; Hu, D.; Lu, X.; Lu, S.; et al. Deciphering the Role of Side-Chain Engineering and Solvent Vapor Annealing for Binary All-Small-Molecule Organic Solar Cells. Adv. Funct. Mater. 2023, 33, 2210549. [Google Scholar] [CrossRef]
- Liang, W.; Chen, L.; Wang, Z.; Peng, Z.; Zhu, L.; Kwok, C.H.; Yu, H.; Xiong, W.; Li, T.; Zhang, Z.; et al. Oligothiophene Additive-Assisted Morphology Control and Recombination Suppression Enable High-Performance Organic Solar Cells. Adv. Energy Mater. 2024, 14, 2303661. [Google Scholar] [CrossRef]
- Lee, J.K.; Ma, W.L.; Brabec, C.J.; Yuen, J.; Moon, J.S.; Kim, J.Y.; Lee, K.; Bazan, G.C.; Heeger, A.J. Processing Additives for Improved Efficiency from Bulk Heterojunction Solar Cells. J. Am. Chem. Soc. 2008, 130, 3619–3623. [Google Scholar] [CrossRef]
- Hoven, C.V.; Dang, X.-D.; Coffin, R.C.; Peet, J.; Nguyen, T.-Q.; Bazan, G.C. Improved Performance of Polymer Bulk Heterojunction Solar Cells Through the Reduction of Phase Separation via Solvent Additives. Adv. Mater. 2010, 22, E63–E66. [Google Scholar] [CrossRef]
- Schmidt, K.; Tassone, C.J.; Niskala, J.R.; Yiu, A.T.; Lee, O.P.; Weiss, T.M.; Wang, C.; Fréchet, J.M.; Beaujuge, P.M.; Toney, M.F. A mechanistic understanding of processing additive-induced efficiency enhancement in bulk heterojunction organic solar cells. Adv. Mater. 2014, 26, 300–305. [Google Scholar] [CrossRef]
- Bao, S.; Yang, H.; Fan, H.; Zhang, J.; Wei, Z.; Cui, C.; Li, Y. Volatilizable Solid Additive-Assisted Treatment Enables Organic Solar Cells with Efficiency over 18.8% and Fill Factor Exceeding 80%. Adv. Mater. 2021, 33, 2105301. [Google Scholar] [CrossRef]
- Xu, H.; Han, J.; Sharma, A.; Paleti, S.H.K.; Hultmark, S.; Yazmaciyan, A.; Müller, C.; Baran, D. Progress in the Stability of Small Molecule Acceptor-Based Organic Solar Cells. Adv. Mater. 2025, 37, 2407119. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, Y.; Yao, H.; Ren, J.; Zhang, T.; Qiao, J.; Zhu, S.; Lin, R.; Hao, X.; Hou, J. Local Dipole Modulation Toward High Fill Factor in Organic Solar Cells. Adv. Mater. 2024, 36, 2408858. [Google Scholar] [CrossRef]
- Guan, H.; Liao, Q.; Huang, T.; Geng, S.; Cao, Z.; Zhang, Z.; Wang, D.; Zhang, J. Solid Additive Enables Organic Solar Cells with Efficiency up to 18.6%. ACS Appl. Mater. Interfaces 2023, 15, 25774–25782. [Google Scholar] [CrossRef]
- Xie, J.; Deng, J.; Pei, Y.; Jeong, S.Y.; Huang, B.; Zhou, D.; Woo, H.Y.; Xu, J.; Wu, F.; Chen, L. Synergistic Regulation of Crystallization Kinetics of Donor/Acceptor by New Volatile Additives for High Performance Organic Solar Cells. Adv. Funct. Mater. 2024, 34, 2402281. [Google Scholar] [CrossRef]
- Liu, B.; Xu, W.; Ma, R.; Lee, J.-W.; Dela Peña, T.A.; Yang, W.; Li, B.; Li, M.; Wu, J.; Wang, Y.; et al. Isomerized Green Solid Additive Engineering for Thermally Stable and Eco-Friendly All-Polymer Solar Cells with Approaching 19% Efficiency. Adv. Mater. 2023, 35, 2308334. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Xu, J.; Wang, Z.; Lee, S.; Wang, L.; Hu, Y.; Zhao, X.; Yang, C.; Zhou, E.; Yuan, Z. Volatile Imide Additives with Large Dipole and Special Film Formation Kinetics Enable High-Performance Organic Solar Cells. Angew. Chem. Int. Ed. 2025, 64, e202501816. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Shen, H.; Jiang, Z.; Liu, X.; Chen, H.; Peng, Q.; Yang, J.; Chen, Y.; Ding, J.; et al. Balancing carrier mobility with solvent-solid hybrid additives for high-performance non-fullerene organic solar cells. Chem. Eng. J. 2025, 508, 161037. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, H.; Wang, J.; Zhang, J.; Zhang, T.; Li, Z.; Qiao, J.; Xiu, S.; Hao, X.; Hou, J. Restrained energetic disorder for high-efficiency organic solar cells via a solid additive. Energy Environ. Sci. 2023, 16, 2637–2645. [Google Scholar] [CrossRef]
- Zhao, J.; Chung, S.; Li, H.; Zhao, Z.; Zhu, C.; Yin, J.; Cho, K.; Kan, Z. Impact of Intermolecular Interactions between Halogenated Volatile Solid Additives and the Nonfullerene Acceptor in Organic Solar Cells. Adv. Funct. Mater. 2023, 33, 2307355. [Google Scholar] [CrossRef]
- Cui, X.; Ji, Y.; Liu, Y.; Ma, X.; Li, H.; Cheng, P.; Huang, W.; Bo, Z. Optimizing Molecular Packing and Film Morphology in Organic Solar Cells via Additive-Modulated Growth Processes. Adv. Energy Mater. 2025, 15, 2403077. [Google Scholar] [CrossRef]
- Zhang, H.; Ran, G.; Cui, X.; Liu, Y.; Yin, Z.; Li, D.; Ma, X.; Liu, W.; Lu, H.; Liu, R.; et al. Mitigating Exciton Recombination Losses in Organic Solar Cells by Engineering Nonfullerene Molecular Crystallization Behavior. Adv. Energy Mater. 2023, 13, 2302063. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, H.; Du, J.; He, Z.; Du, X.; Lin, H.; Zheng, C.; Yang, G.; Li, X.; Tao, S. Solid additive modulates acceptor crystallization to achieve 19.11% efficiency and high storage stability in organic solar cells. J. Energy Chem. 2025, 103, 19–26. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988, 37, 785. [Google Scholar] [CrossRef] [PubMed]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Wu, L.; Yun, M.; Qin, J.; Huang, S.; Ou, Z.; Li, Q.; Wang, X.; Yuan, W.; Dong, L.; Cheng, X.; et al. Morphology Optimization by Non-Halogenated and Twisted Volatile Solid Additive for High-Efficiency Organic Solar Cells. Small 2024, 20, e2408610. [Google Scholar] [CrossRef]
- Lv, J.; Tang, H.; Huang, J.; Yan, C.; Liu, K.; Yang, Q.; Hu, D.; Singh, R.; Lee, J.; Lu, S.; et al. Additive-induced miscibility regulation and hierarchical morphology enable 17.5% binary organic solar cells. Energy Environ. Sci. 2021, 14, 3044–3052. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, Y.; Li, M.; Ye, L.; Geng, Y. Molecular Engineering and Morphology Control of Polythiophene:Nonfullerene Acceptor Blends for High-Performance Solar Cells. Adv. Energy Mater. 2020, 10, 2002572. [Google Scholar] [CrossRef]
- Liao, X.; Li, Q.; Ye, J.; Li, Z.; Ren, J.; Zhang, K.; Xu, Y.; Cai, Y.-P.; Liu, S.; Huang, F. Solid-liquid convertible fluorinated terthiophene as additives in mediating morphology and performance of organic solar cells. Chem. Eng. J. 2023, 453, 139489. [Google Scholar] [CrossRef]
- Zhang, F.; Dai, T.; Li, X.; Li, M.; Liu, Y.; Wang, D.; Xu, D.; Hu, R. Dual Additive-Assisted Layer-by-Layer Processing for 19.59% Efficiency Quasi-Bulk Heterojunction Organic Solar Cells. Adv. Funct. Mater. 2025, 35, 2414260. [Google Scholar] [CrossRef]
- Xia, Z.; Gao, C.; Xie, Z.; Wu, M.; Chen, H.; Li, T.; Zhou, J.; Cai, T.; Hu, H.; Shuai, J.; et al. Isomerization-Controlled Aggregation in Photoactive Layer: An Additive Strategy for Organic Solar Cells with Over 19.5 % Efficiency. Angew. Chem. Int. Ed. Engl. 2025, 64, e202421953. [Google Scholar] [CrossRef]
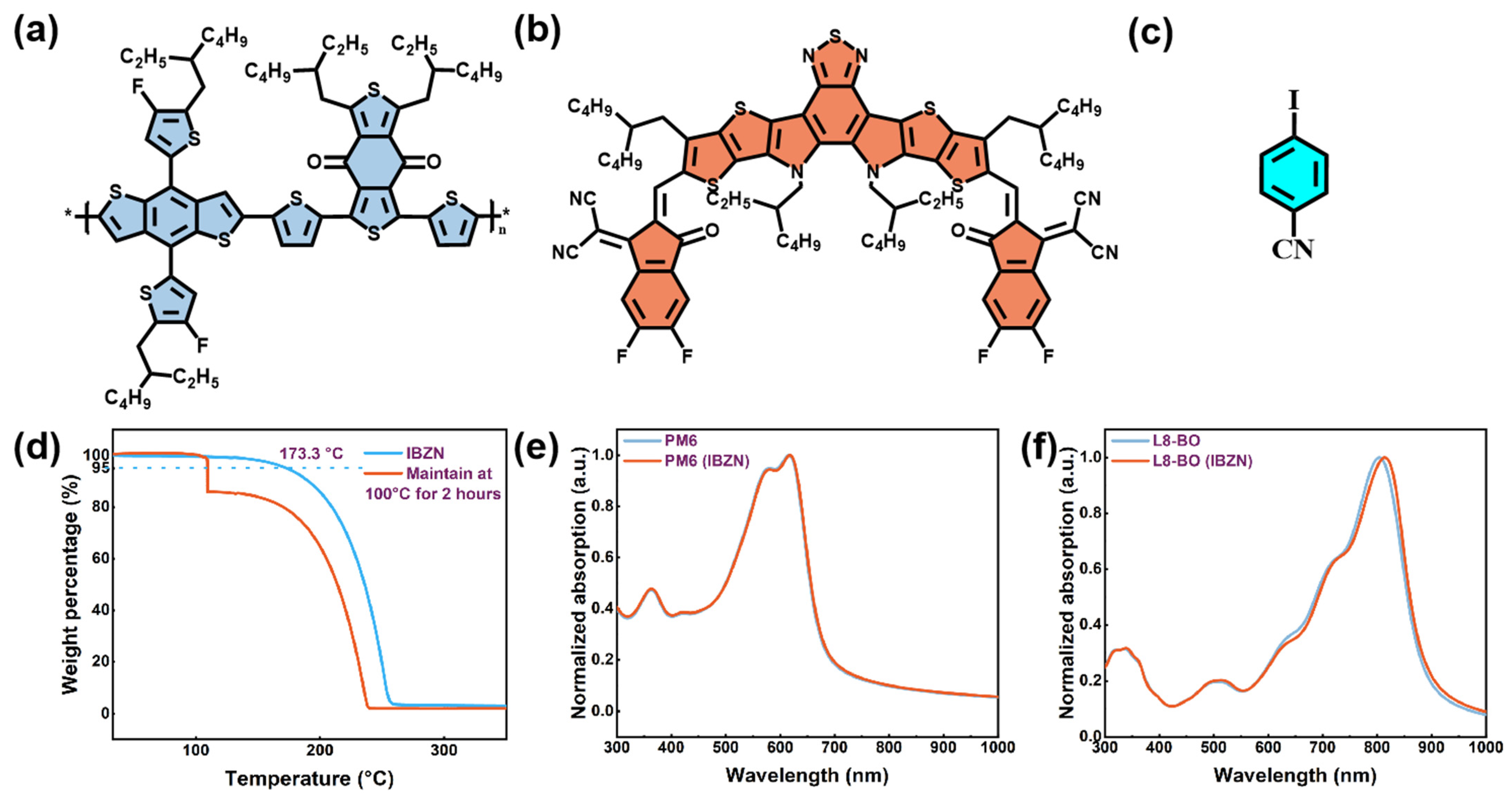
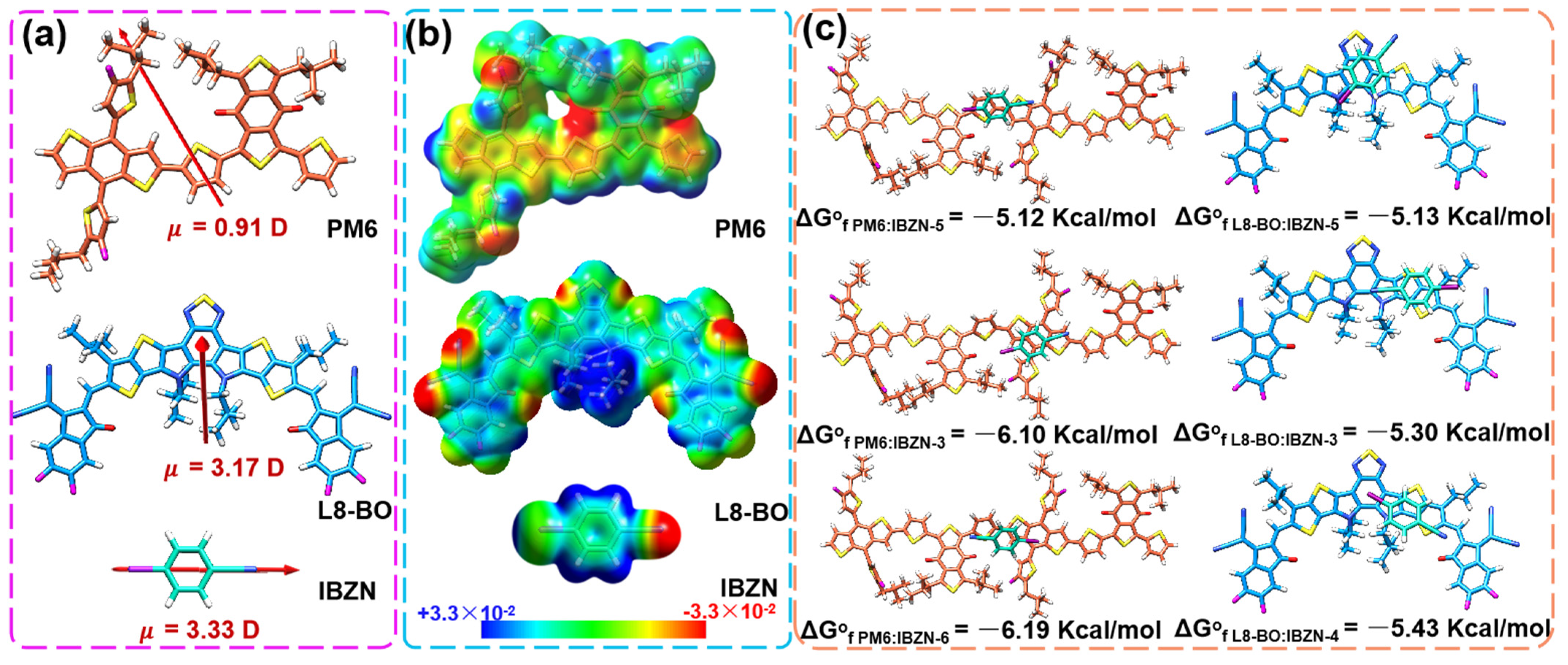
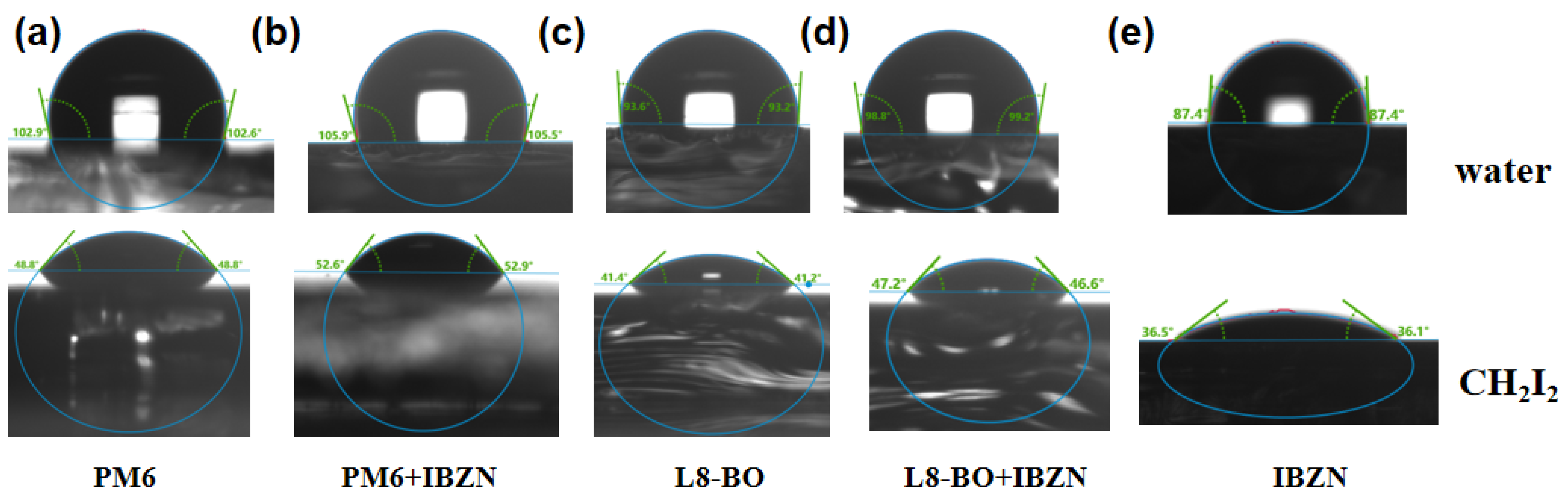
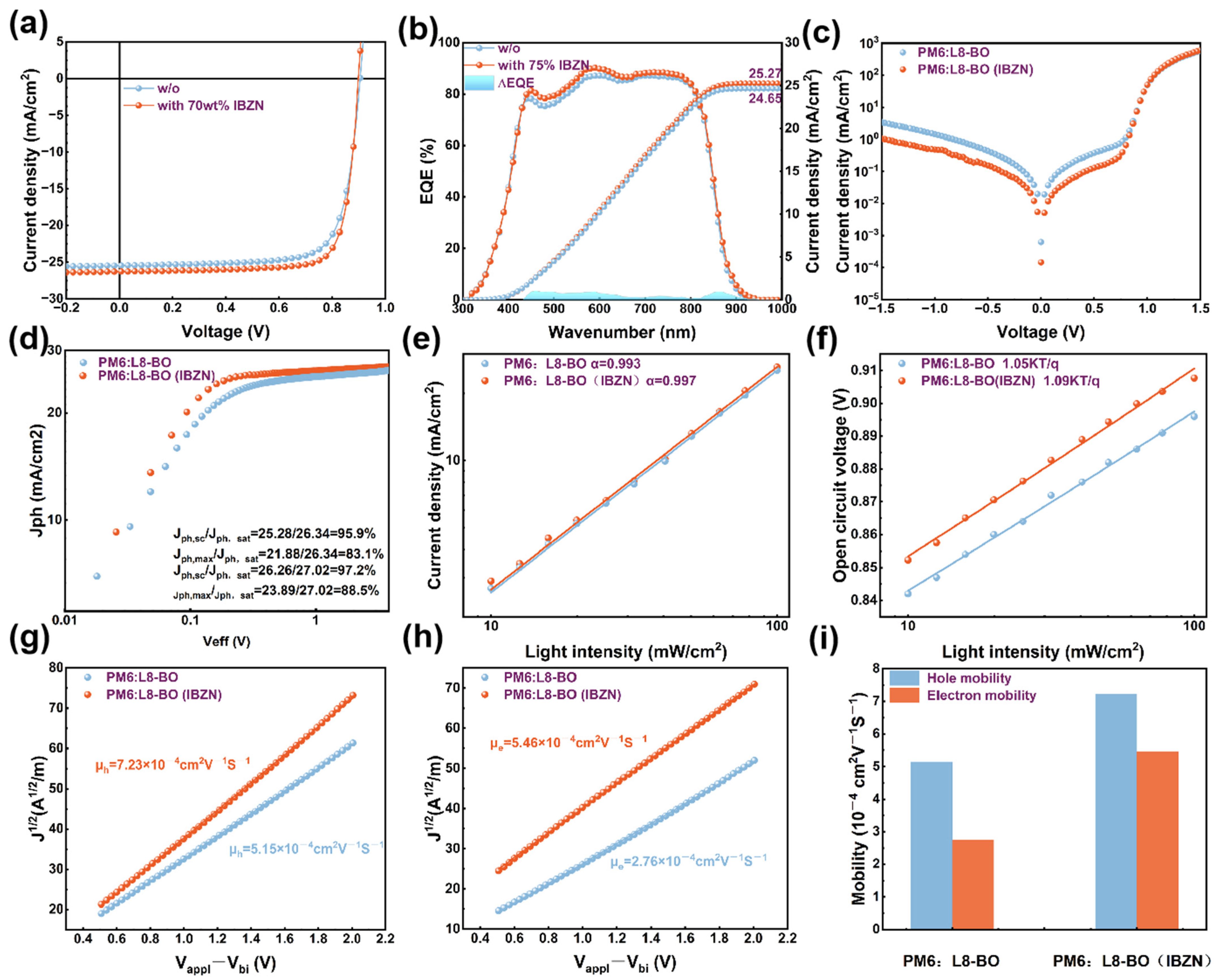
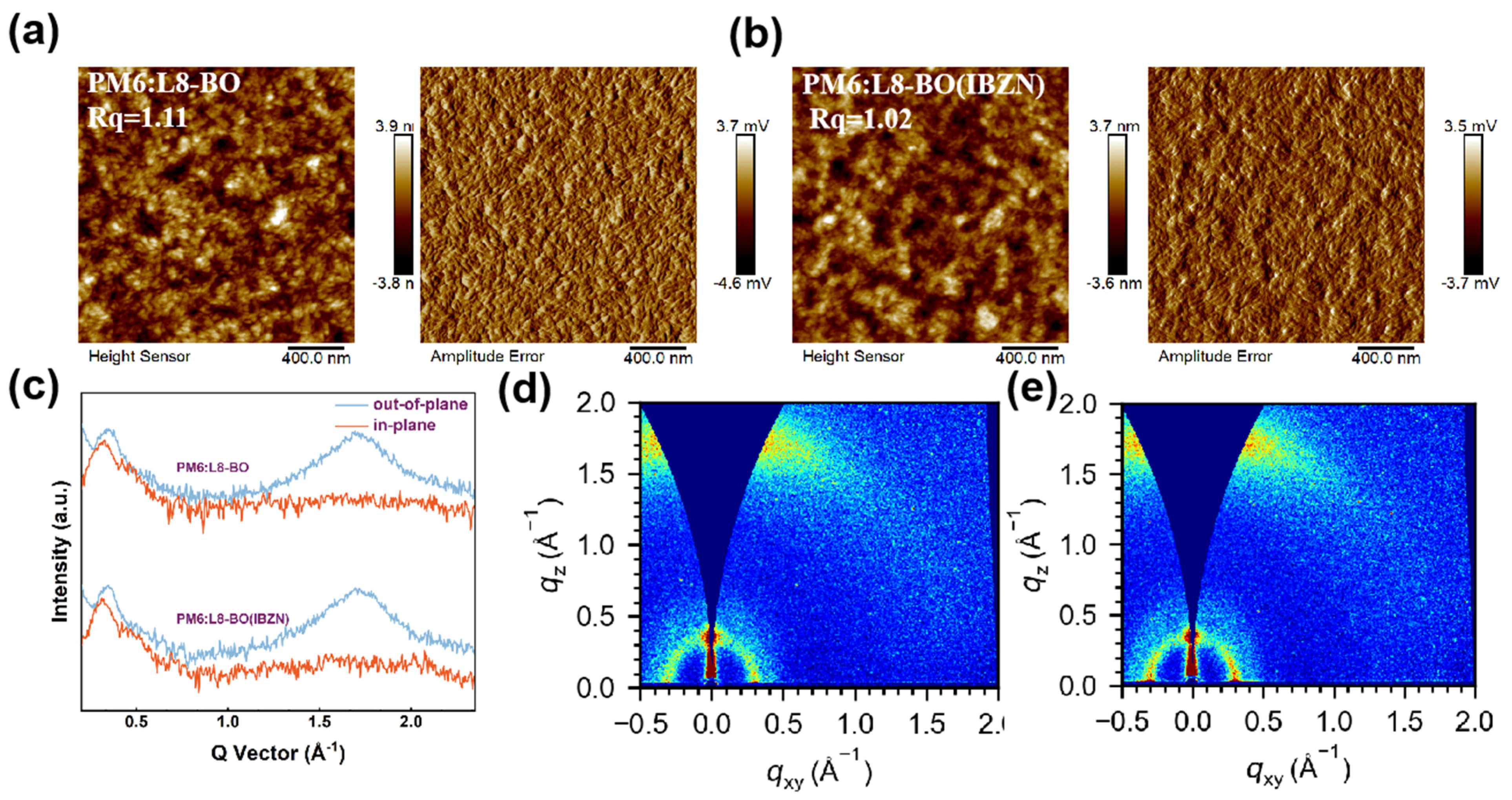
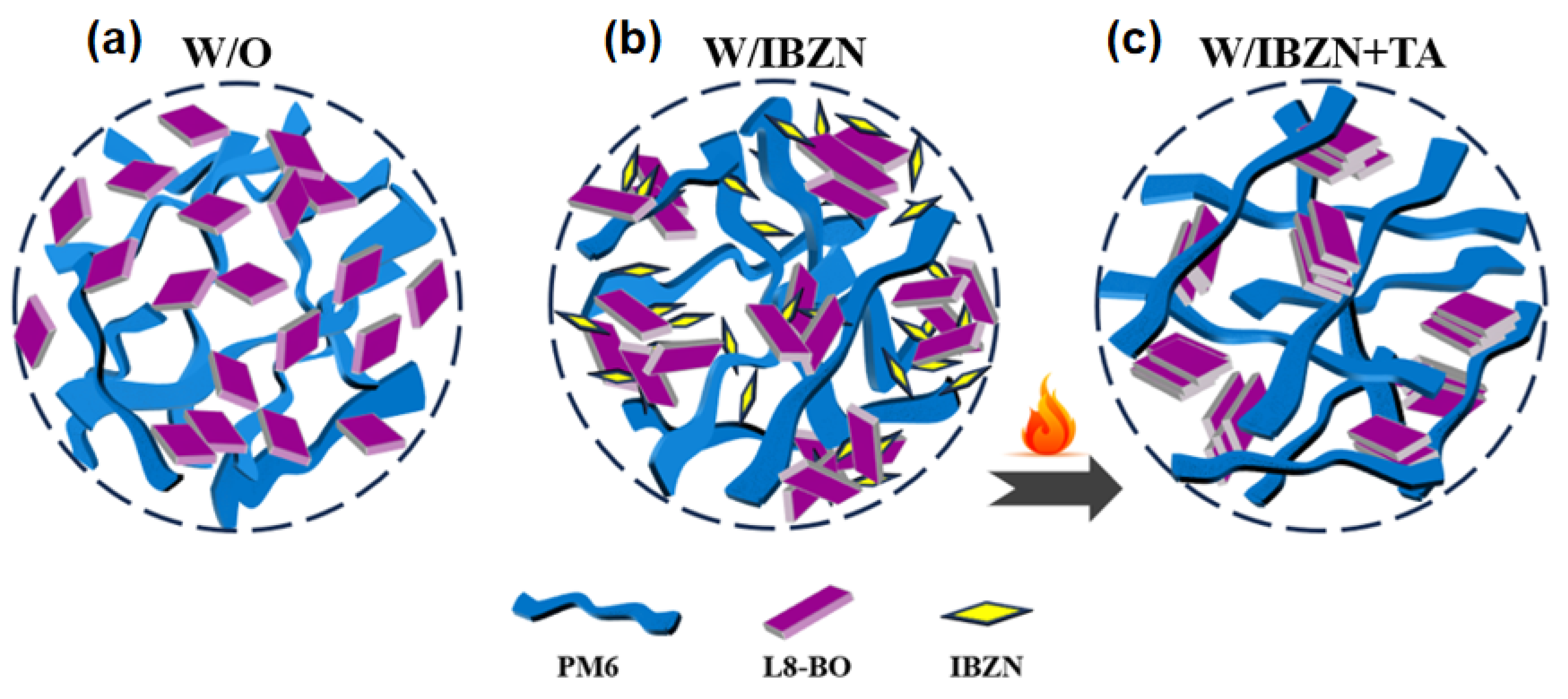
| Conditions | VOC (V) | JSC (mA/cm2) | JSC a (mA/cm2) | FF (%) | PCE b (%) Max. | PCE c (%) Avg. |
|---|---|---|---|---|---|---|
| PM6: L8-BO (w/o) | 0.905 | 25.46 | 24.65 | 75.87 | 17.49 | 17.31 ± 0.18 |
| PM6: L8-BO (w/IBZN) | 0.898 | 26.28 | 25.27 | 79.54 | 18.77 | 18.65 ± 0.22 |
| Concentration of IBZN | VOC (V) | JSC (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| 40 wt% | 0.901 | 25.69 | 77.3 | 17.91 |
| 70 wt% | 0.898 | 26.28 | 79.54 | 18.77 |
| 100 wt% | 0.894 | 26.04 | 79.09 | 18.42 |
| 150 wt% | 0.889 | 25.96 | 78.55 | 18.14 |
| Annealing Temperature | VOC (V) | JSC (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| 90 °C | 0.901 | 26.10 | 77.77 | 18.30 |
| 100 °C | 0.898 | 26.28 | 79.54 | 18.77 |
| 110 °C | 0.894 | 25.91 | 78.17 | 18.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Cai, C.; Li, Y.; Ma, C.; Gámez-Valenzuela, S.; Liu, Y.; Li, J.; Wang, X.; Li, Y. 4-Iodobenzonitrile as Effective Solid Additive for High-Efficiency Polymer Solar Cells. Polymers 2025, 17, 1386. https://doi.org/10.3390/polym17101386
Li J, Cai C, Li Y, Ma C, Gámez-Valenzuela S, Liu Y, Li J, Wang X, Li Y. 4-Iodobenzonitrile as Effective Solid Additive for High-Efficiency Polymer Solar Cells. Polymers. 2025; 17(10):1386. https://doi.org/10.3390/polym17101386
Chicago/Turabian StyleLi, Jiayu, Chuanchen Cai, Yuechen Li, Changbiao Ma, Sergio Gámez-Valenzuela, Yixiao Liu, Jianfeng Li, Xiaochen Wang, and Yongfang Li. 2025. "4-Iodobenzonitrile as Effective Solid Additive for High-Efficiency Polymer Solar Cells" Polymers 17, no. 10: 1386. https://doi.org/10.3390/polym17101386
APA StyleLi, J., Cai, C., Li, Y., Ma, C., Gámez-Valenzuela, S., Liu, Y., Li, J., Wang, X., & Li, Y. (2025). 4-Iodobenzonitrile as Effective Solid Additive for High-Efficiency Polymer Solar Cells. Polymers, 17(10), 1386. https://doi.org/10.3390/polym17101386







