Carboxymethyl Chitosan Cinnamaldehyde Coated SilverNanocomposites for Antifungal Seed Priming in Wheat: A Dual-Action Approach Toward Sustainable Crop Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
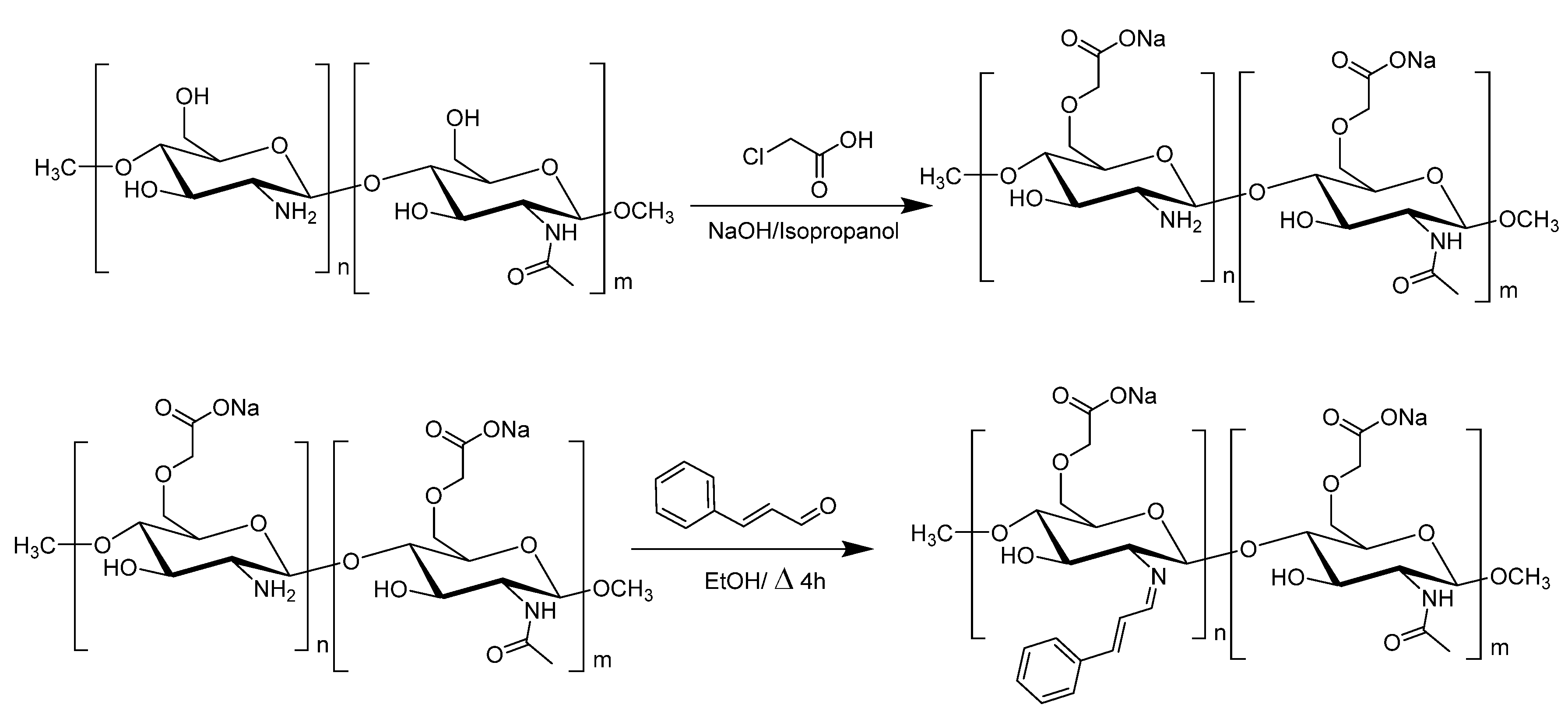
2.2. Synthesis of CMC
2.3. Synthesis of CMC=CIN and AgNP-CMC=CIN
2.3.1. Synthesis of CMC=CIN
2.3.2. Synthesis of Ag-CMC=CIN
2.4. Instrumental Characterization of Ag-CMC=CIN
2.4.1. UV-Spectrum of CMC=CIM and Ag-CMC=CIN
2.4.2. In Vitro Release Studies
2.4.3. DLS AgNP and AgNP-CMC=CIN
2.5. In Vitro Antifungal Activity of Ag-CMC=CIN
2.5.1. Antifungal Effect in Spore Germination
2.5.2. Antifungal Evaluation of Nanoparticles in Infected Seeds
Assessment of Plant Growth Dynamics
Flavonoid, Polyphenol, Chlorophyll, Carotenoid, and Malondialdehyde Content in Treated Plants
2.6. Statistics
3. Results and Discussion
3.1. Spectroscopy Characterization of Ag-CMC=CIN
Uv-Vis Spectroscopy
3.2. pH-Responsive Behavior of the Active Coating
3.3. Nanoparticle Characterization
3.4. Morphological and Size Evaluation of Nanoformulation by Electron Microscope
3.5. Evaluation of Antifungal Activity of CMC=CIN
3.5.1. Increase in Antifungal Activity Nanoparticles by Active Coating
3.5.2. Successful Antifungal Activity of AgNP-CMC=CIN in Infected Seeds
3.5.3. Morphological Studies of Treated Plants
3.5.4. Physiological Evaluation in Plants: Flavonoid, Polyphenol, and Chlorophyll Content in Treated Plants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tudor, V.C.; Stoicea, P.; Chiurciu, I.-A.; Soare, E.; Iorga, A.M.; Dinu, T.A.; David, L.; Micu, M.M.; Smedescu, D.I.; Dumitru, E.A. The Use of Fertilizers and Pesticides in Wheat Production in the Main European Countries. Sustainability 2023, 15, 3038. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Shakoor, N.; Wang, Q.; Zhu, G.; Jiang, Y.; Wang, Q.; Azeem, I.; Sun, Y.; Zhao, W.; et al. Metal-Organic Frameworks for Sustainable Crop Disease Management: Current Applications, Mechanistic Insights, and Future Challenges. J. Agric. Food Chem. 2024, 72, 22985–23007. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wang, Z.; Wang, C.; Yue, L.; Tao, M.; Elmer, W.H.; White, J.C.; Cao, X.; Xing, B. Nanomaterial Size and Surface Modification Mediate Disease Resistance Activation in Cucumber (Cucumis sativus). ACS Nano 2023, 17, 4871–4885. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiong, Z.; Fan, Y.; Wang, H.; An, C.; Wang, B.; Yang, M.; Li, X.; Wang, Y.; Wang, Y. Lignin/Surfactin Coacervate as an Eco-Friendly Pesticide Carrier and Antifungal Agent against Phytopathogen. ACS Nano 2024, 18, 22415–22430. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, Y.; Wang, C.; Yue, L.; Li, X.; Cao, X.; White, J.C.; Wang, Z.; Xing, B. Selenium Nanomaterials Enhance Sheath Blight Resistance and Nutritional Quality of Rice: Mechanisms of Action and Human Health Benefit. ACS Nano 2024, 18, 13084–13097. [Google Scholar] [CrossRef] [PubMed]
- Sandri, S.; Hussein, H.; Alshyab, N.; Sagatowski, J. The European Green Deal: Challenges and opportunities for the Southern Mediterranean. Mediterr. Politics 2023, 30, 196–207. [Google Scholar] [CrossRef]
- Biswas, S.; Seal, P.; Majumder, B.; Biswas, A.K. Efficacy of seed priming strategies for enhancing salinity tolerance in plants: An overview of the progress and achievements. Plant Stress 2023, 9, 100186. [Google Scholar] [CrossRef]
- Dhaka, A.; Mali, S.C.; Sharma, S.; Trivedi, R. A review on biological synthesis of silver nanoparticles and their potential applications. Results Chem. 2023, 6, 101108. [Google Scholar] [CrossRef]
- Matei, P.M.; Buzón-Durán, L.; Pérez-Lebeña, E.; Martín-Gil, J.; Iacomi, B.M.; Ramos-Sánchez, M.C.; Martín-Ramos, P. In Vitro Antifungal Activity of Chitosan-Polyphenol Conjugates against Phytophthora cinnamomi. AgriEngineering 2020, 2, 72–77. [Google Scholar] [CrossRef]
- Molina-Hernández, J.B.; Scroccarello, A.; Della Pelle, F.; De Flaviis, R.; Compagnone, D.; Del Carlo, M.; Paparella, A.; Lόpez, C.C. Synergistic antifungal activity of catechin and silver nanoparticles on Aspergillus niger isolated from coffee seeds. LWT 2022, 169, 113990. [Google Scholar] [CrossRef]
- Francis, D.V.; Abdalla, A.K.; Mahakham, W.; Sarmah, A.K.; Ahmed, Z.F.R. Interaction of plants and metal nanoparticles: Exploring its molecular mechanisms for sustainable agriculture and crop improvement. Environ. Int. 2024, 190, 108859. [Google Scholar] [CrossRef] [PubMed]
- Mondéjar-López, M.; García-Simarro, M.P.; Navarro-Simarro, P.; Gómez-Gómez, L.; Ahrazem, O.; Niza, E. A review on the encapsulation of ‘eco-friendly’ compounds in natural polymer-based nanoparticles as next generation nano-agrochemicals for sustainable agriculture and crop management. Int. J. Biol. Macromol. 2024, 280, 136030. [Google Scholar] [CrossRef] [PubMed]
- Mondéjar-lópez, M.; López-jiménez, A.J.; Martínez, J.C.G.; Ahrazem, O.; Gómez-gómez, L.; Niza, E. Thymoquinone loaded chitosan nanoparticles as new ‘ eco-friendly ’ preservative agent in cosmetic products. Int. J. Mol. Sci. 2022, 23, 898. [Google Scholar] [CrossRef] [PubMed]
- Mondéjar-López, M.; García-Martínez, J.C.; Gómez-Gómez, L.; Ahrazem, O.; Niza, E. New gel from a water-soluble Carboxymethyl chitosan-Cinnamaldehyde Schiff base derivative as an effective preservative against soft rot in ginger. Food Chem. 2024, 461, 140970. [Google Scholar] [CrossRef] [PubMed]
- Palmero, D.; Rubio-Moraga, A.; Galvez-Patón, L.; Nogueras, J.; Abato, C.; Gómez-Gómez, L.; Ahrazem, O. Pathogenicity and genetic diversity of Fusarium oxysporum isolates from corms of Crocus sativus. Ind. Crop. Prod. 2014, 61, 186–192. [Google Scholar] [CrossRef]
- Chen, X.G.; Park, H.J. Chemical characteristics of O-carboxymethyl chitosans related to the preparation conditions. Carbohydr. Polym. 2003, 53, 355–359. [Google Scholar] [CrossRef]
- Chugh, B.; Singh, A.K.; Poddar, D.; Thakur, S.; Pani, B.; Jain, P. Relation of degree of substitution and metal protecting ability of cinnamaldehyde modified chitosan. Carbohydr. Polym. 2020, 234, 115945. [Google Scholar] [CrossRef] [PubMed]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef] [PubMed]
- Mondéjar-lópez, M.; Rubio-Moraga, A.; López-Jimenez, A.J.; Martínez, J.C.G.; Ahrazem, O.; Gómez-Gómez, L.; Niza, E. Chitosan nanoparticles loaded with garlic essential oil: A new alternative to tebuconazole as seed dressing agent. Carbohydr. Polym. 2022, 277, 118815. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Tan, A.S. Characterization of the Cellular Reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular Localization, Substrate Dependence, and Involvement of Mitochondrial Electron Transport in MTT Reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Mondéjar-López, M.; Castillo, R.; Jiménez, A.J.L.; Gómez-Gómez, L.; Ahrazem, O.; Niza, E. Polysaccharide film containing cinnamaldehyde-chitosan nanoparticles, a new eco-packaging material effective in meat preservation. Food Chem. 2024, 437, 137710. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhao, L.; Khan, I.M.; Yue, L.; Zhang, Y.; Wang, Z. Emerging chitosan grafted essential oil components: A review on synthesis, characterization, and potential application. Carbohydr. Polym. 2022, 297, 120011. [Google Scholar] [CrossRef] [PubMed]
- Cox, H.J.; Li, J.; Saini, P.; Paterson, J.R.; Sharples, G.J.; Badyal, J.P.S. Bioinspired and eco-friendly high efficacy cinnamaldehyde antibacterial surfaces. J. Mater. Chem. B 2021, 9, 2918–2930. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Wang, M.; Khan, I.M.; Xu, J.; Peng, C.; Wang, Z. Preparation, characterization, and antibiofilm activity of cinnamic acid conjugated hydroxypropyl chitosan derivatives. Int. J. Biol. Macromol. 2021, 189, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Su, Y.A.; Liu, T.Y.; Sheng, Y.J.; Lin, J.J. Thermo-responsive nanoarrays of silver nanoparticle, silicate nanoplatelet and PNiPAAm for the antimicrobial applications. Colloids Surf. B Biointerfaces 2017, 152, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Chen, X.; Chen, X.; Li, S.; Yuan, G.; Zhou, T.; Li, J.; Jia, Y.; Xiong, D.; Tan, H. Covalent Chitosan-Cellulose Hydrogels via Schiff-Base Reaction Containing Macromolecular Microgels for pH-Sensitive Drug Delivery and Wound Dressing. Macromol. Chem. Phys. 2019, 220, 1900399. [Google Scholar] [CrossRef]
- Dalei, G.; Das, S.; Jena, S.R.; Jena, D.; Nayak, J.; Samanta, L. In situ crosslinked dialdehyde guar gum-chitosan Schiff-base hydrogels for dual drug release in colorectal cancer therapy. Chem. Eng. Sci. 2023, 269, 118482. [Google Scholar] [CrossRef]
- Dalençon, F.; Amjaud, Y.; Lafforgue, C.; Derouin, F.; Fessi, H. Atovaquone and rifabutine-loaded nanocapsules: Formulation studies. Int. J. Pharm. 1997, 153, 127–130. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, S.G.; Oh, E.J.; Chung, H.Y.; Han, S.I.; Kim, E.J.; Seo, S.Y.; Ghim, H.D.; Yeum, J.H.; Choi, J.H. Antimicrobial polyethyleneimine-silver nanoparticles in a stable colloidal dispersion. Colloids Surfaces B Biointerfaces 2011, 88, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.M.; Bizeto, M.A.; Ferrari, V.B.; Okamoto, D.N.; de Vasconcellos, S.P.; Camilo, F.F. Direct evaluation of microbial growth dynamics and colloidal stability of silver nanoparticles stabilized by poly(vinyl pyrrolidone) and poly(vinyl alcohol). J. Nanoparticle Res. 2020, 22, 137. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; López-Jimenez, A.J.; Ahrazem, O.; Gómez-Gómez, L.; Niza, E. Chitosan coated-biogenic silver nanoparticles from wheat residues as green antifungal and nanoprimig in wheat seeds. Int. J. Biol. Macromol. 2022, 225, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Dananjaya, S.H.S.; Erandani, W.K.C.U.; Kim, C.H.; Nikapitiya, C.; Lee, J.; De Zoysa, M. Comparative study on antifungal activities of chitosan nanoparticles and chitosan silver nano composites against Fusarium oxysporum species complex. Int. J. Biol. Macromol. 2017, 105, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.H.; Saied, E.; Amin, B.H.; Alotibi, F.O.; Al-Askar, A.A.; Arishi, A.A.; Elkady, F.M.; Elbahnasawy, M.A. Antifungal Activity of Biosynthesized Silver Nanoparticles (AgNPs) against Aspergilli Causing Aspergillosis: Ultrastructure Study. J. Funct. Biomater. 2022, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, D.; Rahman, H.B.H.; Dhandapani, R.; Palanivel, V.; Thangavelu, S.; Paramasivam, R.; Muthupandian, S. Coating of wallpaper with green synthesized silver nanoparticles from Passiflora foetida fruit and its illustrated antifungal mechanism. Process Biochem. 2022, 112, 177–182. [Google Scholar] [CrossRef]
- Radhakrishnan, V.S.; Mudiam, M.K.R.; Kumar, M.; Dwivedi, S.P.; Singh, S.P.; Prasad, T. Silver nanoparticles induced alterations in multiple cellular targets, which are critical for drug susceptibilities and pathogenicity in fungal pathogen (Candida albicans). Int. J. Nanomed. 2018, 13, 2647–2663. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Tu, Y.Y.; Lin, J.C. Studies of Mercaptosuccinic Acid-Crosslinked Chitosan Hydrogel with Grafted Cinnamaldehyde and Silver Nanoparticles for Antibacterial Biomedical Application. Int. J. Mol. Sci. 2022, 23, 14806. [Google Scholar] [CrossRef] [PubMed]
- Alvear, A.G.; Pineda-Aguilar, N.; Lozano, P.; Lárez-Velázquez, C.; Suppan, G.; Galeas, S.; Debut, A.; Vizuete, K.; De Lima, L.; Saucedo-Vázquez, J.P.; et al. Synergistic Antibacterial Properties of Silver Nanoparticles and Its Reducing Agent from Cinnamon Bark Extract. Bioengineering 2024, 11, 517. [Google Scholar] [CrossRef] [PubMed]
- Ottoni, C.A.; Neto, M.C.L.; Léo, P.; Ortolan, B.D.; Barbieri, E.; De Souza, A.O. Environmental impact of biogenic silver nanoparticles in soil and aquatic organisms. Chemosphere 2020, 239, 124698. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; McLean, J.E.; Martineau, N.; Britt, D.W.; Haverkamp, R.; Anderson, A.J. Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ. Sci. Technol. 2013, 47, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Courtois, P.; Rorat, A.; Lemiere, S.; Guyoneaud, R.; Attard, E.; Levard, C.; Vandenbulcke, F. Ecotoxicology of silver nanoparticles and their derivatives introduced in soil with or without sewage sludge: A review of effects on microorganisms, plants and animals. Environ. Pollut. 2019, 253, 578–598. [Google Scholar] [CrossRef] [PubMed]








| NP | Size (nm) | PDI | Zeta Potential |
|---|---|---|---|
| AgNP | 59.46 ± 12.63 | 0.29 ± 0.01 | −29.03 ± 0.25 |
| AgNP-CMC=CIN | 110.17 ± 4.74 | 0.27 ± 0.02 | −29.97 ± 2.99 |
| Fungi (3000 Spores) | ||||
|---|---|---|---|---|
| Germinated Spore Treatment | Fusarium oxysporum | Penicillium citrinum | Aspergillus niger | Aspergillus brasilensis |
| AgNP (μg/mL) | 708 | <18 | 71 | 71 |
| CIN (μg/mL) | 416.25 | 182.25 | 416.25 | 416.25 |
| CMC=CIN (μg/mL) | 159.25 | 273.25 | 387.03 | 364.34 |
| AgNP-CMC=CIN (μg/mL) | 83 | <21 | <21 | <21 |
| Tebuconazole (μg/mL) | 52 | <13 | <13 | <13 |
| Viability | ||||||
|---|---|---|---|---|---|---|
| Seed Priming Before Inoculation | 48 h | 72 h | 120 h | |||
| Germination Rate % | Fungal Infection % | Germination Rate % | Fungal Infection % | Germination Rate % | Fungal Infection % | |
| Control− | 96.7 | 10 | 100 | 16.7 | 100 | 23.3 |
| Control+ | 70 | 100 | 70 | 100 | 70 | 100 |
| CMC=CIN | 96.7 | 3.3 | 96.7 | 3.3 | 96.7 | 6.7 |
| AgNP | 85.5 | 20 | 85.5 | 20 | 85.5 | 20 |
| AgNP-CMC=CIN | 85 | 2.5 | 85 | 2.5 | 85 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondéjar-López, M.; García-Simarro, M.P.; Gómez-Gómez, L.; Ahrazem, O.; Niza, E. Carboxymethyl Chitosan Cinnamaldehyde Coated SilverNanocomposites for Antifungal Seed Priming in Wheat: A Dual-Action Approach Toward Sustainable Crop Protection. Polymers 2025, 17, 2031. https://doi.org/10.3390/polym17152031
Mondéjar-López M, García-Simarro MP, Gómez-Gómez L, Ahrazem O, Niza E. Carboxymethyl Chitosan Cinnamaldehyde Coated SilverNanocomposites for Antifungal Seed Priming in Wheat: A Dual-Action Approach Toward Sustainable Crop Protection. Polymers. 2025; 17(15):2031. https://doi.org/10.3390/polym17152031
Chicago/Turabian StyleMondéjar-López, María, María Paz García-Simarro, Lourdes Gómez-Gómez, Oussama Ahrazem, and Enrique Niza. 2025. "Carboxymethyl Chitosan Cinnamaldehyde Coated SilverNanocomposites for Antifungal Seed Priming in Wheat: A Dual-Action Approach Toward Sustainable Crop Protection" Polymers 17, no. 15: 2031. https://doi.org/10.3390/polym17152031
APA StyleMondéjar-López, M., García-Simarro, M. P., Gómez-Gómez, L., Ahrazem, O., & Niza, E. (2025). Carboxymethyl Chitosan Cinnamaldehyde Coated SilverNanocomposites for Antifungal Seed Priming in Wheat: A Dual-Action Approach Toward Sustainable Crop Protection. Polymers, 17(15), 2031. https://doi.org/10.3390/polym17152031








