Advancing the Multifaceted Performance of Chemical-Grafted Silicone Rubbers via Molecular Simulation
Abstract
1. Introduction
2. Theoretical Models and Calculation Methodology
2.1. Molecular/Material Models and Calculation Schemes
2.2. Oxidation Reaction Pathway
- Molecular structures of the SiR constituent and the grafted agents are fully optimized to obtain their ground-state geometries.
- Oxidation reaction pathways are identified by constructing a series of intermediate structures representing the reaction coordinate through transition state searching with the quadratic synchronous transit method, and they are finally refined by the nudged elastic band (NEB) method to provide minimum energy pathways.
- The energy barrier or released energy is evaluated by determining the energy difference between the reactant and the transition state or product along the reaction pathway.
2.3. Reactive Molecular Dynamics Simulation
3. Results and Discussion
3.1. Charge Traps Introduced by Chemical Grafts
3.2. Oxidation Stability
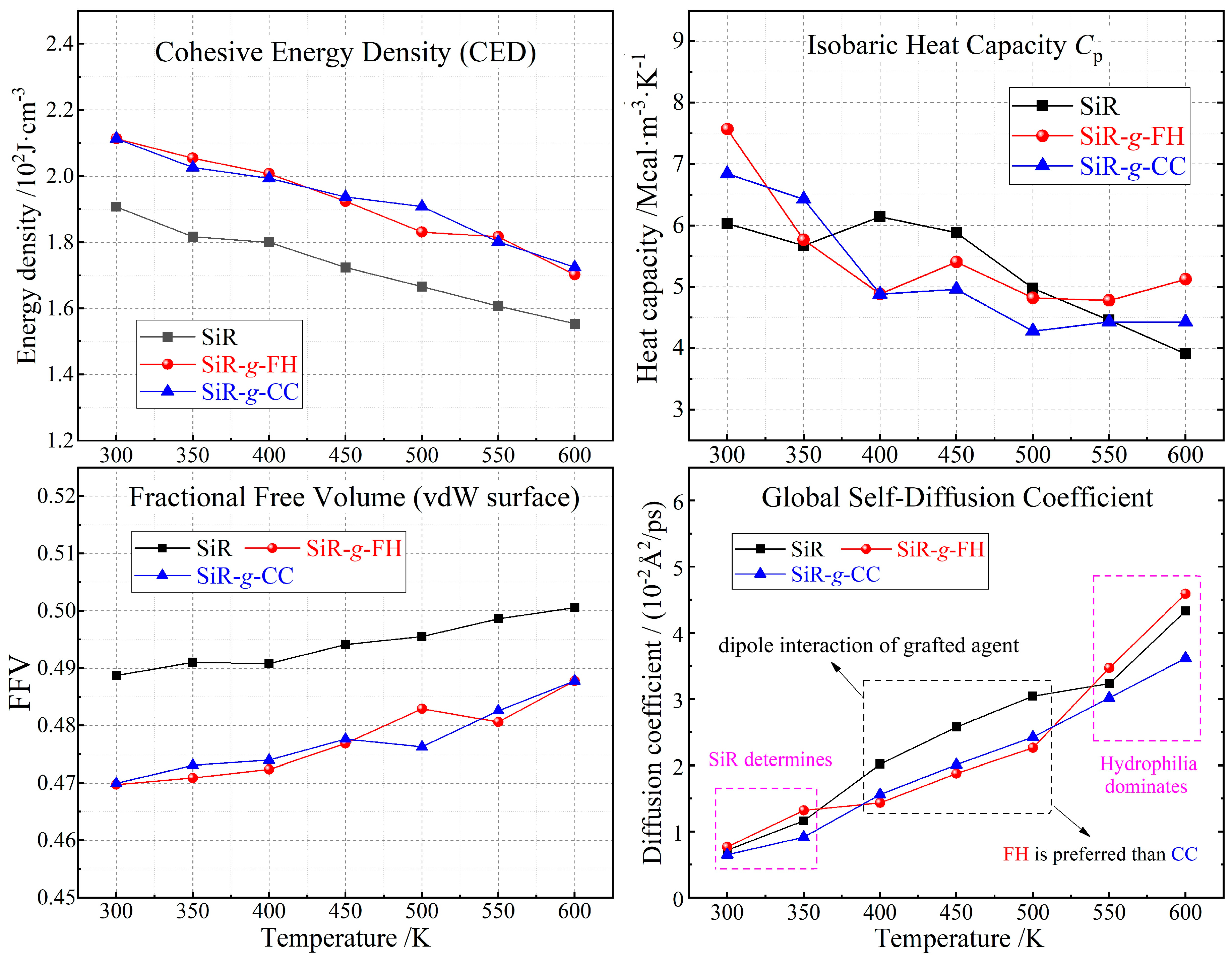
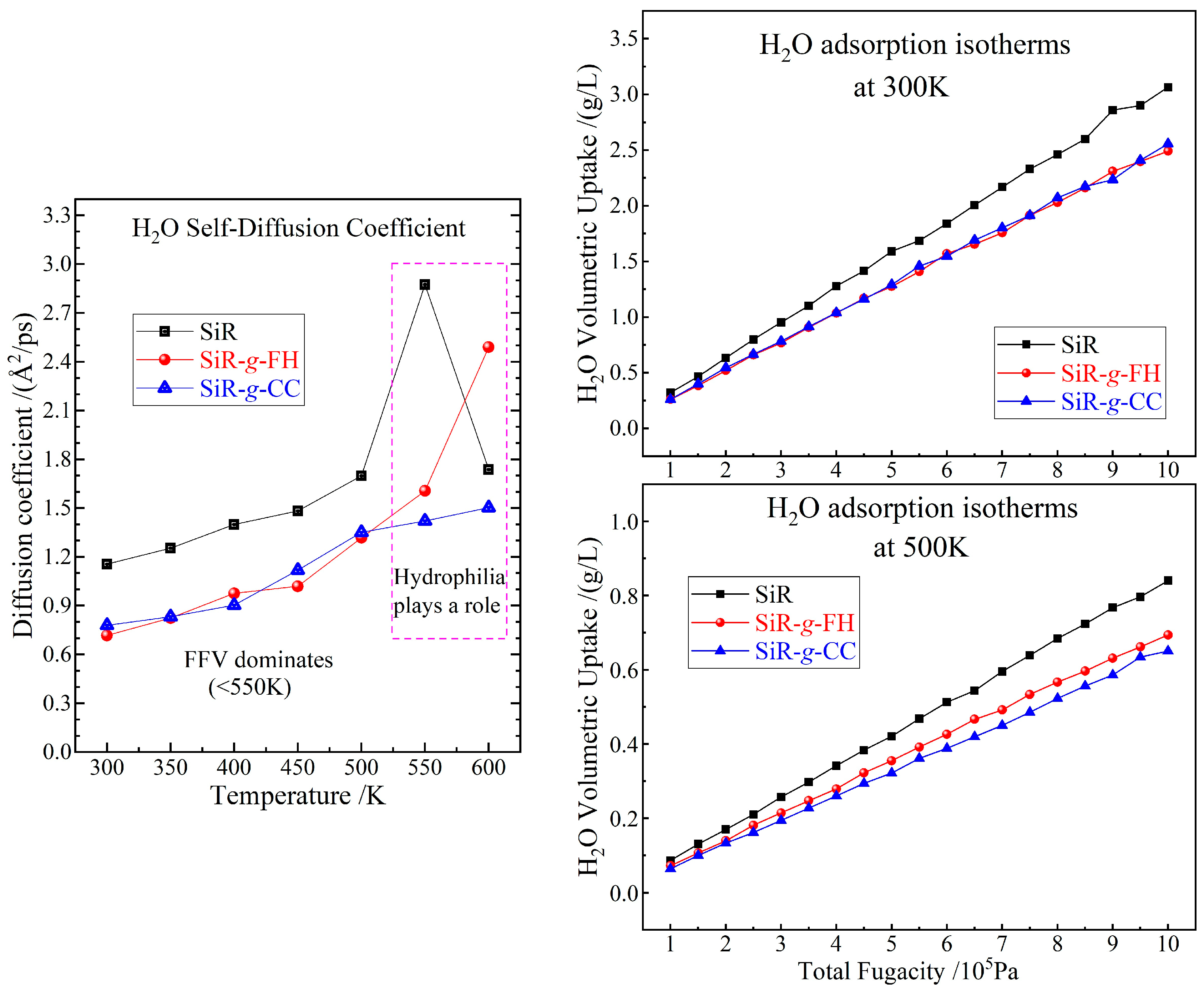
3.3. Thermal Stability and Moisture Resistance
3.4. Pyrolysis from Thermal Spikes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, S.; Yang, L.; Zhang, P.; Wang, H.; Cui, J. Tough omni-dynamic silicone rubbers with excellent self-healing, elasticity, remoldability, and degradability. Polymer 2022, 239, 124434. [Google Scholar] [CrossRef]
- Baranovskii, E.M.; Khistiaeva, V.V.; Deriabin, K.V.; Petrovskii, S.K.; Koshevoy, I.O.; Kolesnikov, I.E.; Grachova, E.V.; Islamova, R.M. Re (I) Complexes as Backbone Substituents and Cross-Linking Agents for Hybrid Luminescent Polysiloxanes and Silicone Rubbers. Molecules 2021, 26, 6866. [Google Scholar] [CrossRef] [PubMed]
- Karambar, S.; Tenbohlen, S. Compatibility Study of Silicone Rubber and Mineral Oil. Energies 2021, 14, 5899. [Google Scholar] [CrossRef]
- Cho, E.; Chiu, L.L.Y.; Lee, M.; Naila, D.; Sadanand, S.; Waldman, S.D.; Sussman, D. Characterization of Mechanical and Dielectric Properties of Silicone Rubber. Polymers 2021, 13, 1831. [Google Scholar] [CrossRef]
- Vinod, P.; Desai, B.M.A.; Sarathi, R.; Kornhuber, S. Investigation on the thermal properties, space charge and charge trap characteristics of silicone rubber nano–micro composites. Electr. Eng. 2021, 103, 1779–1790. [Google Scholar] [CrossRef]
- Wang, W.; Yang, F.; Zhang, P.; Luo, Z.; Li, F.; Jiang, J.; Zhang, J.; Li, W.; Liu, A.; Qin, C. Preparation of Polyurethane-Modified Silicone Rubber Insulating Coating and Its Application in 10 kV Overhead Bare Wire Wrapping. Coatings 2023, 13, 837. [Google Scholar] [CrossRef]
- Gao, L.; Li, Y.; Fu, W.; Zhou, L.; Fang, S. Preparation and Performance of Silicone Rubber Composites Modified by Polyurethane. Polymers 2023, 15, 3920. [Google Scholar] [CrossRef]
- Vinod, P.; Babu, M.S.; Sarathi, R.; Kornhuber, S. Impact of Salt Fog Aging on Space Charge and Charge Trap-Controlled Mobility Characteristics of Silicone Rubber Micro-nanocomposites. J. Electron. Mater. 2021, 50, 5881–5890. [Google Scholar] [CrossRef]
- Wen, Q.; Yang, X.; He, D.; Fu, H. Thermal stability of cyanate ester modified silicone rubber as matrix of heat-resistant composite coatings. J. Coat. Technol. Res. 2023, 20, 2113–2123. [Google Scholar] [CrossRef]
- Sun, W.; Jin, S.; Zhang, A.; Huang, J.; Li, Y.; Liu, X.; Chen, H. Vascular cell responses to silicone surfaces grafted with heparin-like polymers: Surface chemical composition vs. topographic patterning. RSC Adv. 2020, 10, 45144–45152. [Google Scholar] [CrossRef]
- Gao, M.Z.; Li, Z.Y.; Sun, W.F. Nonlinear Conductivity and Space Charge Characteristics of SiC/Silicone Rubber Nanocomposites. Polymers 2022, 14, 2726. [Google Scholar] [CrossRef] [PubMed]
- Thangabalan, B.; Naveen, J.; Danikas, M.G.; Sarathi, R. Analysis of Surface Potential Decay and Charge Trap Characteristics of Water Diffused Silicone Rubber Nanocomposites. In Proceedings of the IEEE International Conference on the Properties and Applications of Dielectric Materials, Johor Bahru, Malaysia, 12–14 July 2021; pp. 282–285. [Google Scholar]
- Duan, W.; Guo, G.; Zhu, G.; Zhao, X.; Pu, L.; Ju, Z.; Sun, H.; Gao, J. Chemical Defects and Impurities Introduce Charge Traps to Silicone Rubber: A Quantum Chemistry Study. J. Phys. Conf. Ser. 2022, 2404, 012005. [Google Scholar] [CrossRef]
- Thangabalan, B.; Sarathi, R.; Harid, N.; Griffiths, H. Analysis of space charge and charge trap characteristics of gamma-irradiated silicone nanocomposites. IET Nanodielectrics 2020, 3, 44–52. [Google Scholar] [CrossRef]
- Yu, Z.C.; Zhou, A.; Ning, W.Y.; Tam, L.-h. Molecular insights into the weakening effect of water on cement/epoxy interface. Appl. Surf. Sci. 2021, 553, 149493. [Google Scholar] [CrossRef]
- Guha, R.D.; Rahmani, F.; Berkowitz, K.; Pasquinelli, M.; Grace, L.R. Temporal evolution of the behavior of absorbed moisture in a damaged polymer-quartz composite: A molecular dynamics study. Comput. Mater. Sci. 2022, 214, 111690. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Li, J.; Li, L.; Hu, W. Surface-grafting polymers: From chemistry to organic electronics. Mater. Chem. Front. 2020, 4, 692–714. [Google Scholar] [CrossRef]
- Liu, A.; Ouyang, L.; Gong, D.; Zhang, C. A High-Temperature-Resistant and Conductive Flexible Silicone Rubber with High Phenyl Content Based on Silver-Coated Glass Fibers. Polymers 2025, 17, 1187. [Google Scholar] [CrossRef]
- Cao, K.; Li, B.; Jiao, Y.; Lu, Y.; Wang, L.; Guo, Y.; Dai, P. Enhancement of thermal and mechanical properties of silicone rubber with g-ray irradiationinduced polysilane-modified graphene oxide/carbon nanotube hybrid fillers. RSC Adv. 2021, 11, 33354. [Google Scholar] [CrossRef]
- Xing, Y.; Chi, J.; Xiao, M. Reactive molecular dynamics simulation on the pyrolysis characteristics of epoxy resin under the effect of partial discharge active products. High Perform. Polym. 2021, 33, 635–645. [Google Scholar] [CrossRef]
- Tawade, B.V.; Apata, I.E.; Pradhan, N.; Karim, A.; Raghavan, D. Recent Advances in the Synthesis of Polymer-Grafted Low-K and High-K Nanoparticles for Dielectric and Electronic Applications. Molecules 2021, 26, 2942. [Google Scholar] [CrossRef]
- Ko, H.; Kang, D.G.; Rim, M.; Koo, J.; Lim, S.I.; Jang, E.; Yu, D.; Yoo, M.J.; Kimc, N.; Jeong, K.U. Heat managing organic materials: Phase change materials with high thermal conductivity and shape stability. Polym. Chem. 2022, 13, 1152–1157. [Google Scholar] [CrossRef]
- Feng, T.; Rai, A.; Hun, D.E.; Shrestha, S. Molecular dynamics simulations of energy accommodation between gases and polymers for ultra-low thermal conductivity insulation. Int. J. Heat Mass Tran. 2021, 164, 120459. [Google Scholar] [CrossRef]
- Li, S.; Zhao, H.; Yue, T.; Zhang, L.; Chen, Y.; Liu, J. All-atom molecular dynamics simulation of structure, dynamics and mechanics of elastomeric polymer materials in a wide range of pressure and temperature. Mol. Syst. Des. Eng. 2024, 9, 264–277. [Google Scholar] [CrossRef]
- Qiao, B.; Yan, Z.; Li, W.; Yang, W.; Wu, S. Molecular dynamics simulation study of insulating material for high voltage cable system application. In Proceedings of the SPIE International Conference on Electronic Materials and Information Engineering (EMIE), Guangzhou, China, 14–16 July 2023; p. 129190I. [Google Scholar]
- Zhang, H.; Sundaresan, S.; Webb, M.A. Thermodynamic driving forces in contact electrification between polymeric materials. Nat. Commun. 2024, 15, 2616. [Google Scholar] [CrossRef]
- Hou, G.; Ren, R.; Shang, W.; Weng, Y.; Liu, J. Molecular Dynamics Simulation of Polymer Nanocomposites with Supramolecular Network Constructed via Functionalized Polymer End-Grafted Nanoparticles. Polymers 2023, 15, 3259. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xian, W.; Wang, Q.; Li, Y. Molecular simulation-guided and physics-informed mechanistic modeling of multifunctional polymers. Acta Mech. Sin. 2021, 37, 725–745. [Google Scholar] [CrossRef]
- Sato, M. First-Principles Modeling in the Context of Dielectric Materials Science and Design. In Proceedings of the IEEE Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Vancouver, BC, Canada, 12–15 December 2021; pp. 85–88. [Google Scholar]
- Ebadi, M.; Marchiori, C.; Mindemark, J.; Brandell, D.; Araujo, C.M. Assessing structure and stability of polymer/lithium-metal interfaces from first-principles calculations. J. Mater. Chem. A 2019, 7, 8394–8404. [Google Scholar] [CrossRef]
- Ma, T.; Chakraborty, P.; Guo, X.; Cao, L.; Wang, Y. First-principles Modeling of Thermal Transport in Materials: Achievements, Opportunities, and Challenges. Int. J. Thermophys. 2020, 41, 9. [Google Scholar] [CrossRef]
- Gao, M.; Li, Z.; Sun, W. Molecular Simulation Insights into Chemical-Grafted EPDM for Improving Charge Traps, Moisture Resistance, and Pyrolysis Tolerance. ECS J. Solid State Sci. Technol. 2024, 13, 083009. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Chen, X.; Wen, H.; Xiao, S. Theoretical Study on Decomposition Mechanism of Insulating Epoxy Resin Cured by Anhydride. Polymers 2017, 9, 341. [Google Scholar] [CrossRef]
- Zhao, X.D.; Sun, W.F.; Zhao, H. Enhanced Insulation Performances of Crosslinked Polyethylene Modified by Chemically Grafting Chloroacetic Acid Allyl Ester. Polymers 2019, 11, 592. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, Y.; Zhou, Y.; Huang, X. Moisture Absorption Characteristics of Nanoparticle-Doped Silicone Rubber and Its Influence Mechanism on Electrical Properties. Polymers 2021, 13, 1474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Bie, Y.; Zhang, X. Preparation and Thermo Stable Functionalization of Phenyl Silicon Rubber. Aerosp. Manuf. Technol. 2017, 1, 44–50. [Google Scholar]
- Huang, Y.; Mu, Q.; Su, Z. High and Low Temperature Resistance of Phenyl silicone rubber. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1048, 012001. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, Y.; Xue, L.; Zhao, X.; Su, Z.; Wang, J. Study on the High and Low Temperature Properties of Methyl Phenyl Silicone Rubber. Silicone Mater. 2016, 30, 93–96. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Y.; Sun, W. Advancing the Multifaceted Performance of Chemical-Grafted Silicone Rubbers via Molecular Simulation. Polymers 2025, 17, 1308. https://doi.org/10.3390/polym17101308
Zou Y, Sun W. Advancing the Multifaceted Performance of Chemical-Grafted Silicone Rubbers via Molecular Simulation. Polymers. 2025; 17(10):1308. https://doi.org/10.3390/polym17101308
Chicago/Turabian StyleZou, Yu, and Weifeng Sun. 2025. "Advancing the Multifaceted Performance of Chemical-Grafted Silicone Rubbers via Molecular Simulation" Polymers 17, no. 10: 1308. https://doi.org/10.3390/polym17101308
APA StyleZou, Y., & Sun, W. (2025). Advancing the Multifaceted Performance of Chemical-Grafted Silicone Rubbers via Molecular Simulation. Polymers, 17(10), 1308. https://doi.org/10.3390/polym17101308








