Polydopamine-Coated Magnetic Nanoplatform for Magnetically Guided Penetration and Enhanced Antibacterial Efficacy in Root Canal Biofilm Elimination
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
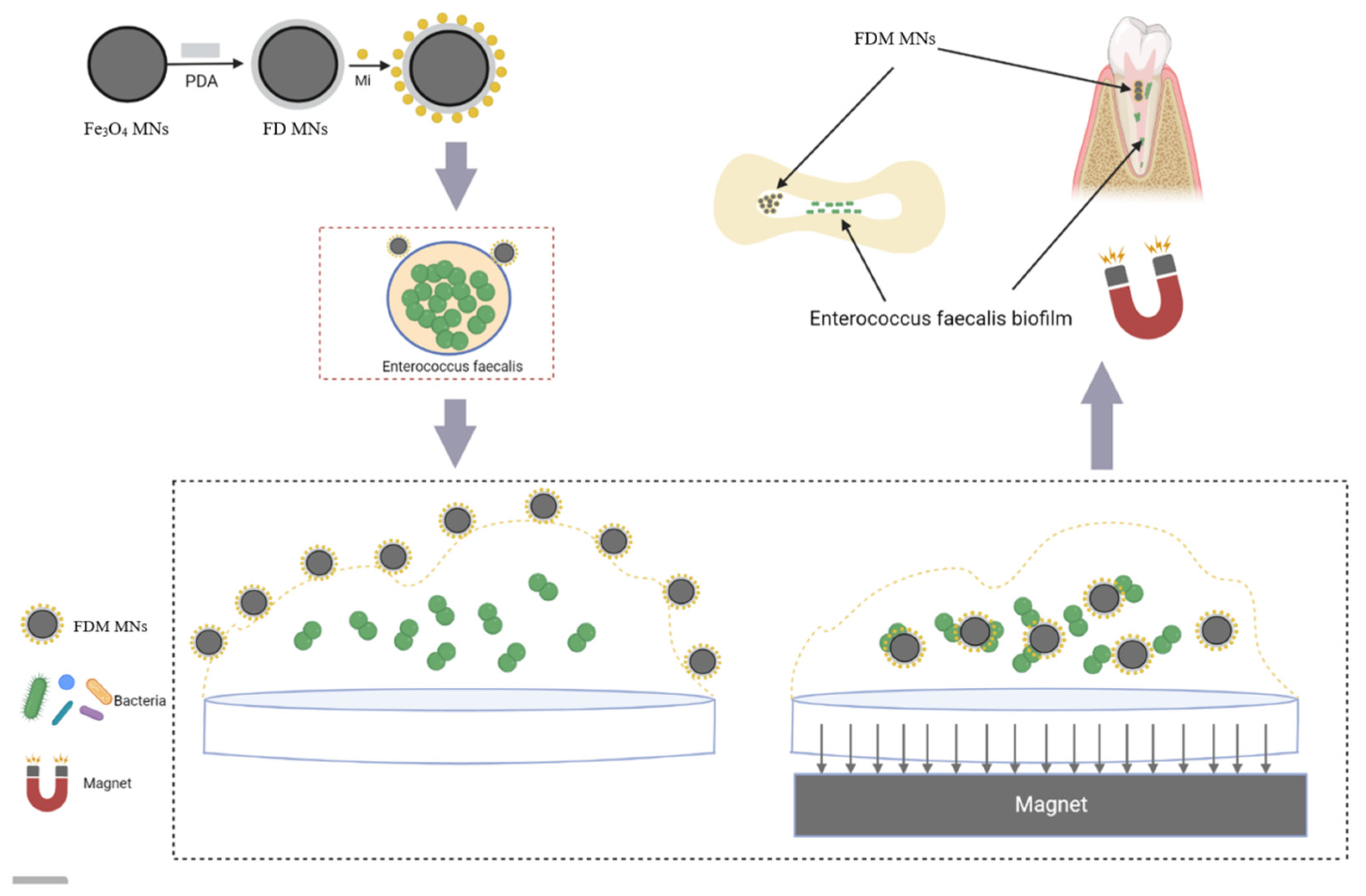
2.2. Preparation of FDM MNs
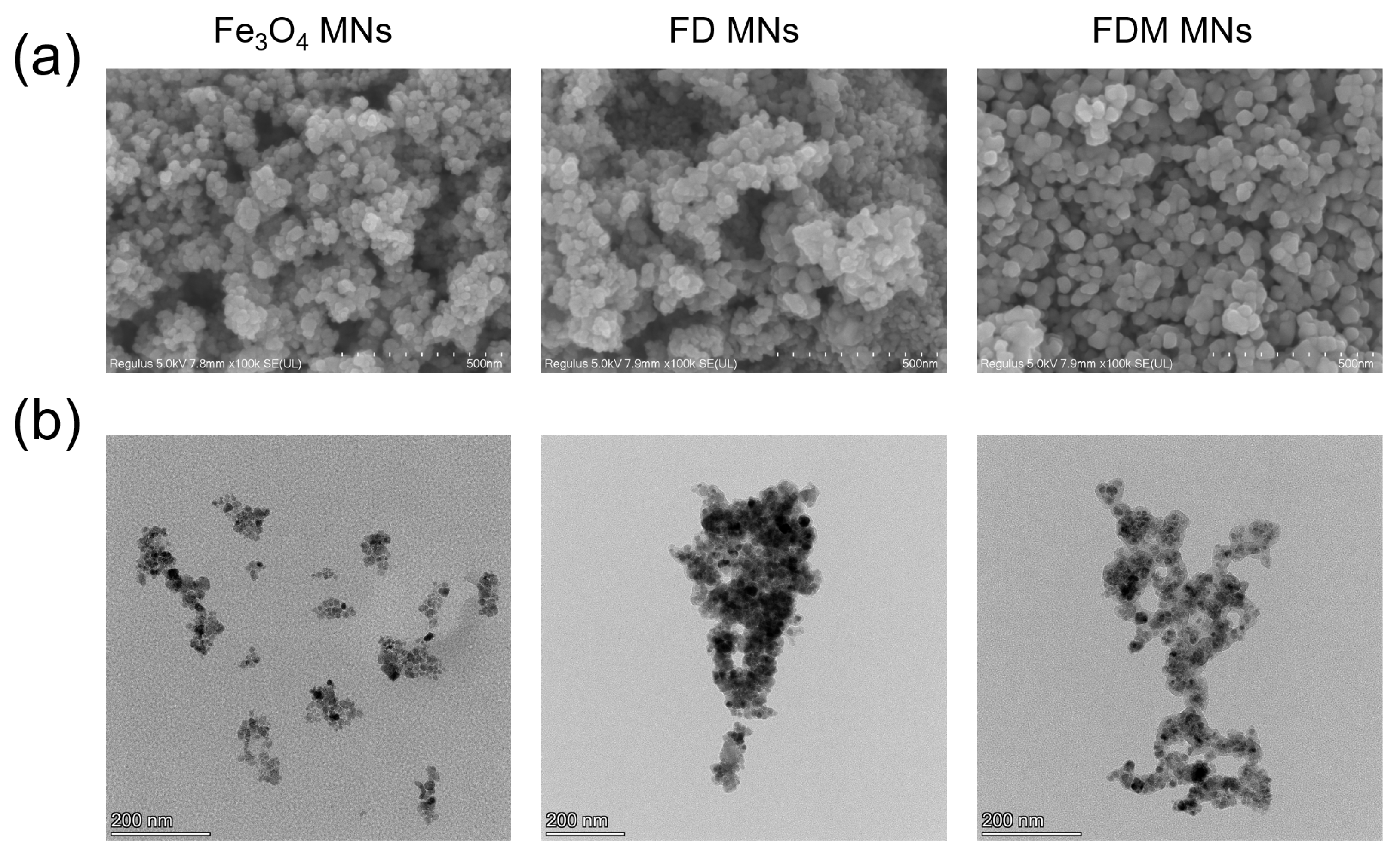
2.3. The Characteristics of FDM MNs
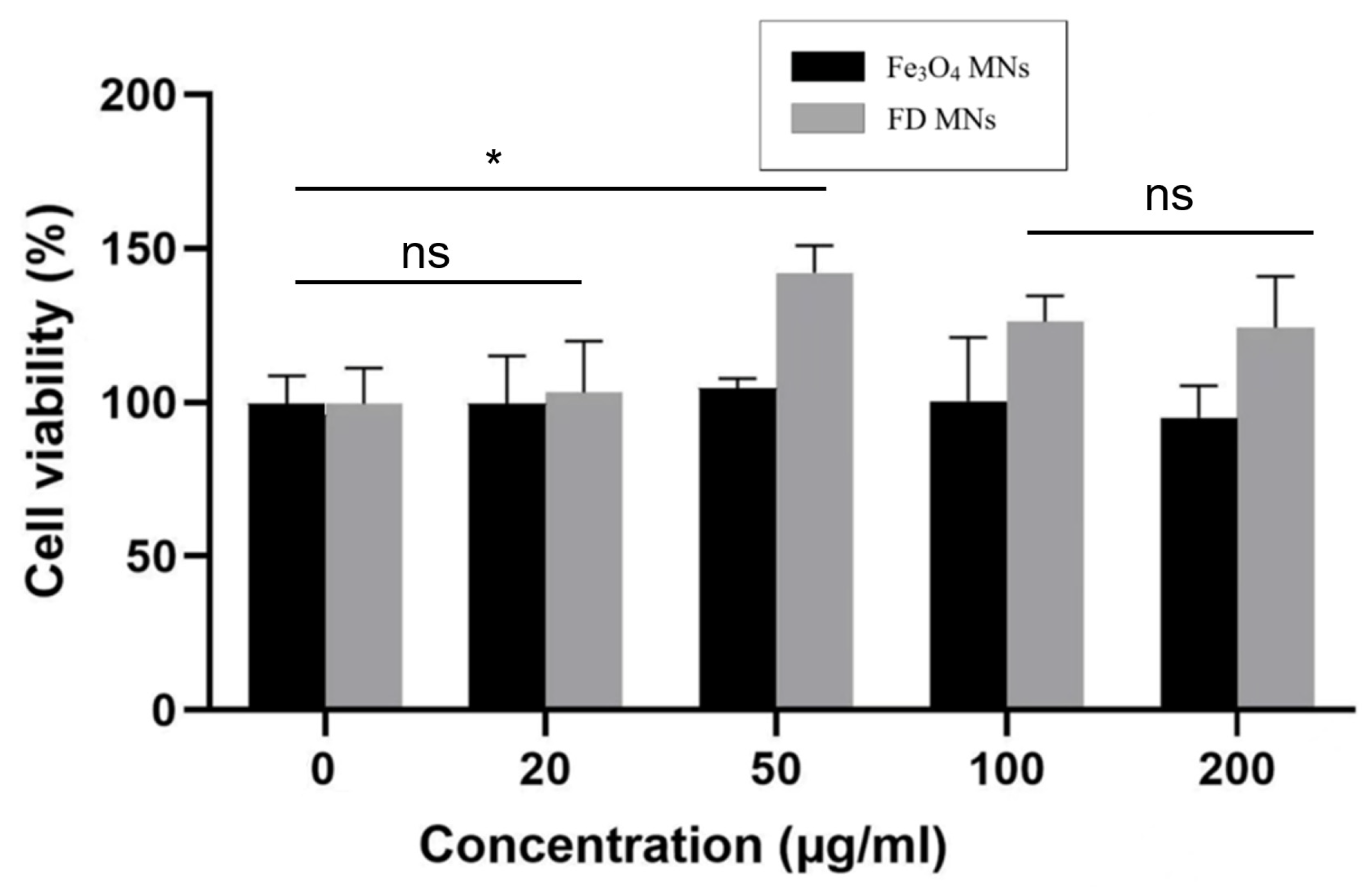
2.4. Cytotoxicity Assay
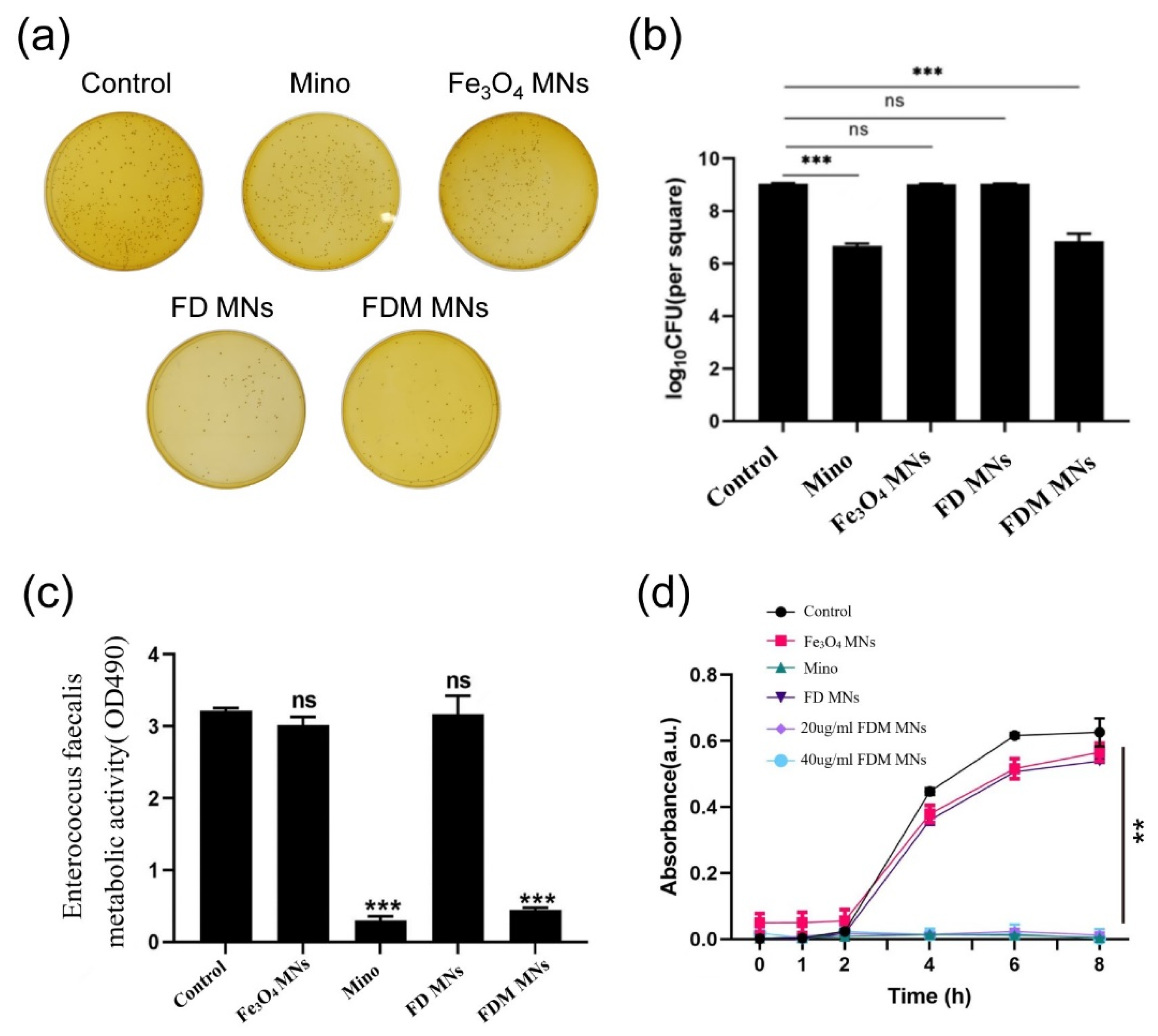
2.5. The Planktonic Antibacterial Assessment of FDM MNs
2.5.1. Colony-Forming Units (CFUs)
2.5.2. Bacterial Growth Curve
2.5.3. Metabolic Activity
2.6. The Anti-Biofilm Assay of FDM MNs
2.6.1. Forming Biofilms in Hydroxyapatite (HA) Disk
2.6.2. CFU, Metabolic Activity, and Live/Dead Staining
2.7. Anti-Biofilm Assay in Dentin Disk
2.8. Statistical Analysis
3. Results
3.1. Characteristics of FDM MNs
3.2. In Vitro Biocompatibility
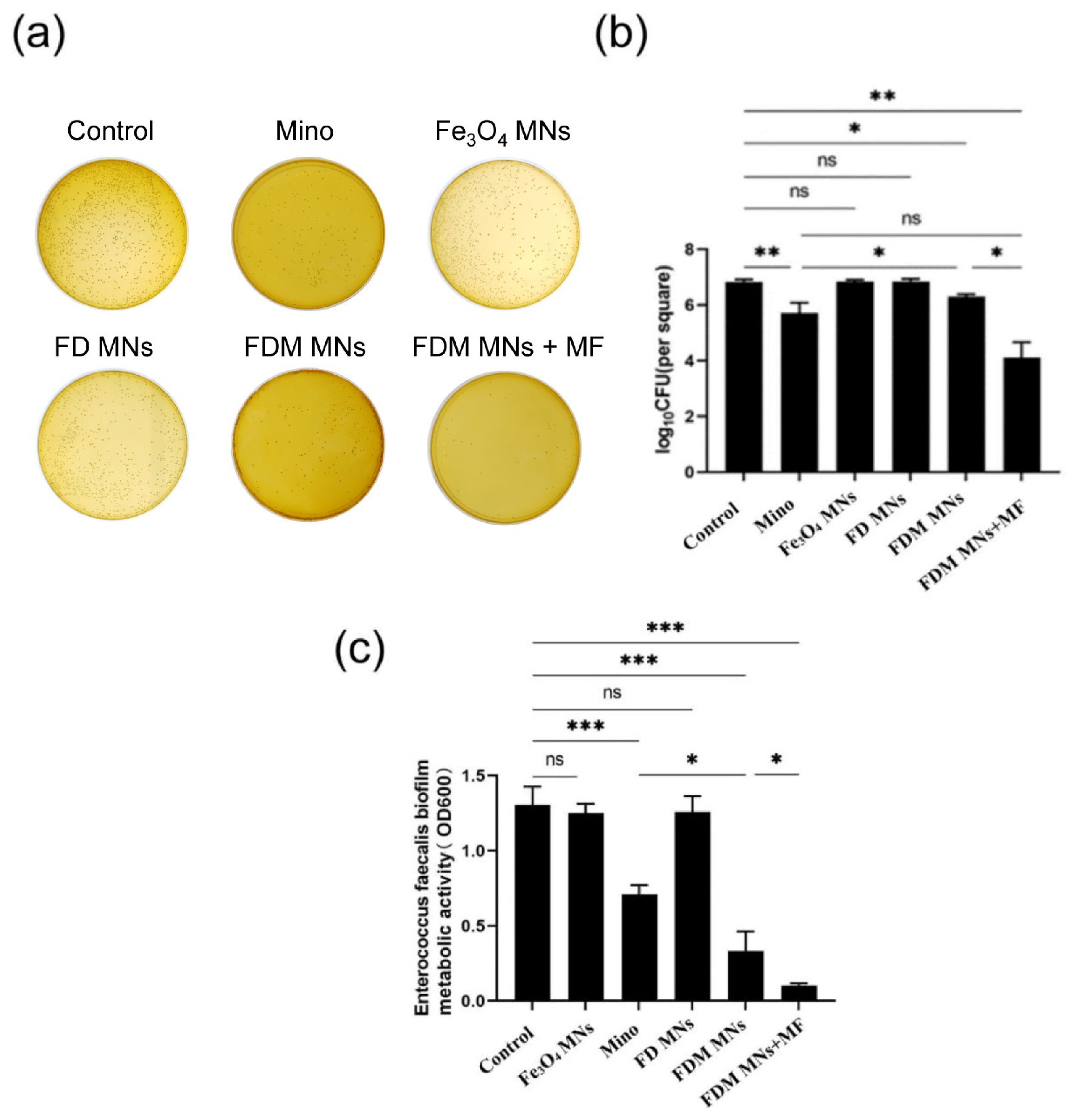
3.3. The Planktonic Antibacterial Capacity of FDM MNs
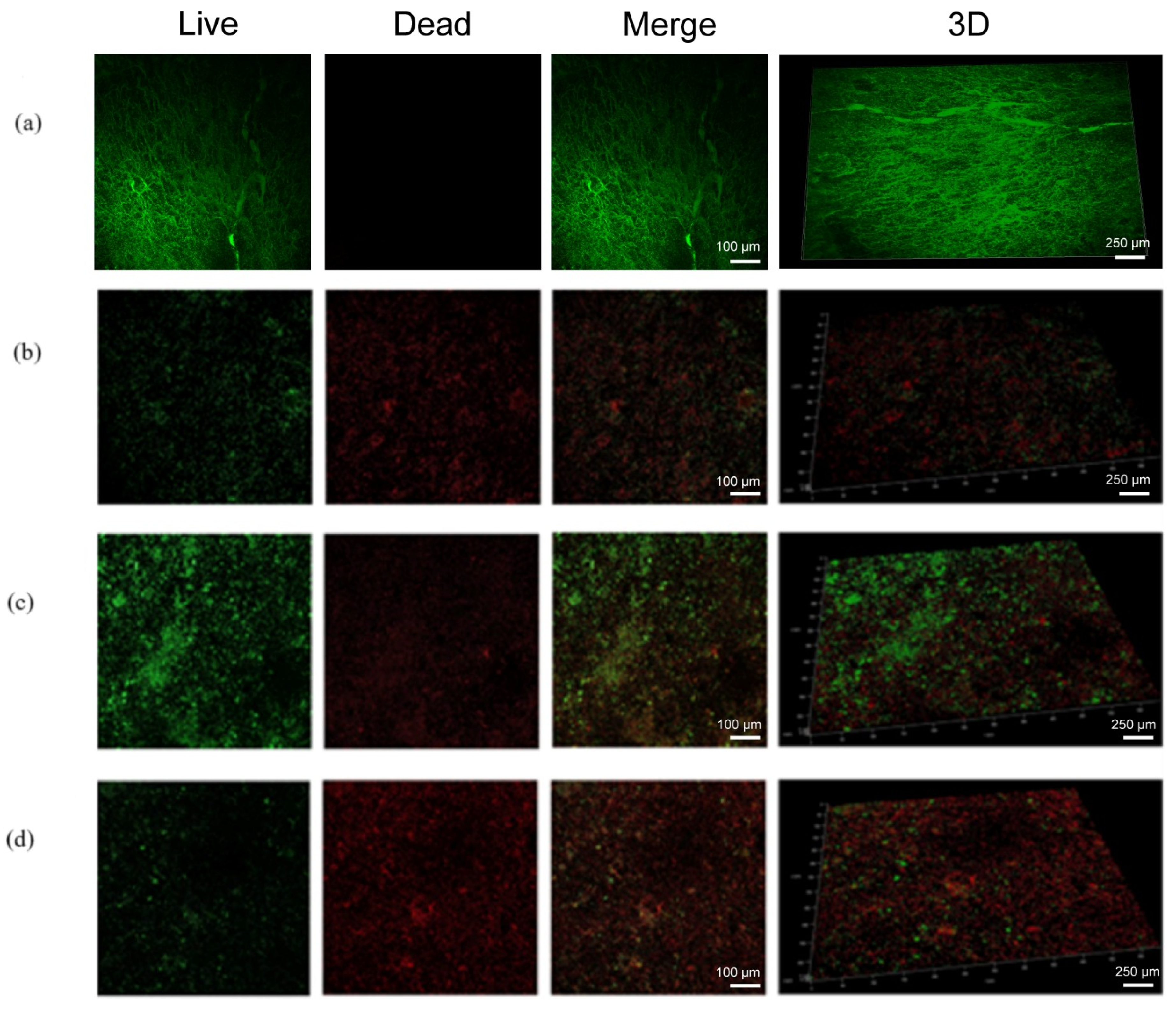
3.4. The Anti-Biofilm Capacity of FDM MNs
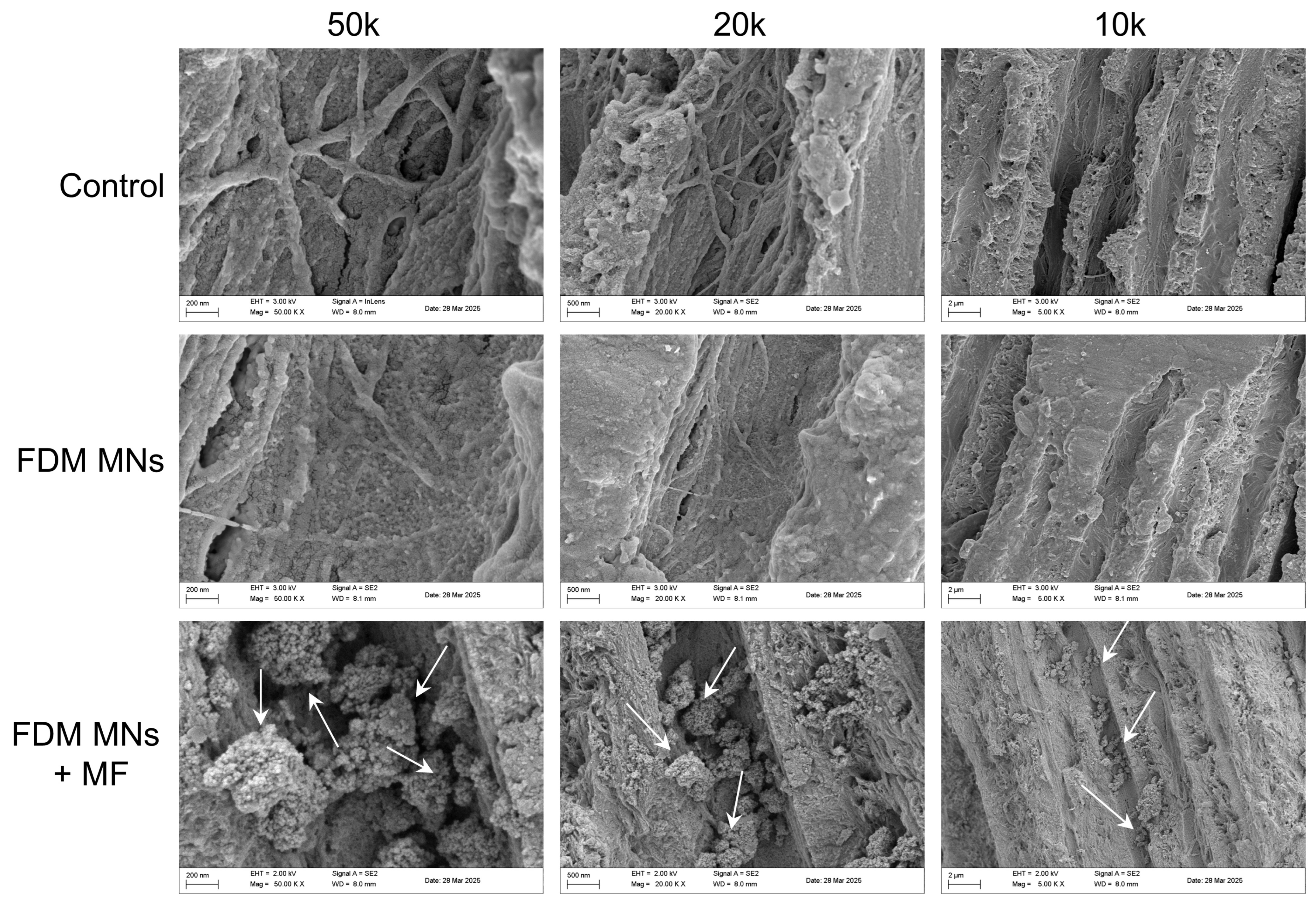
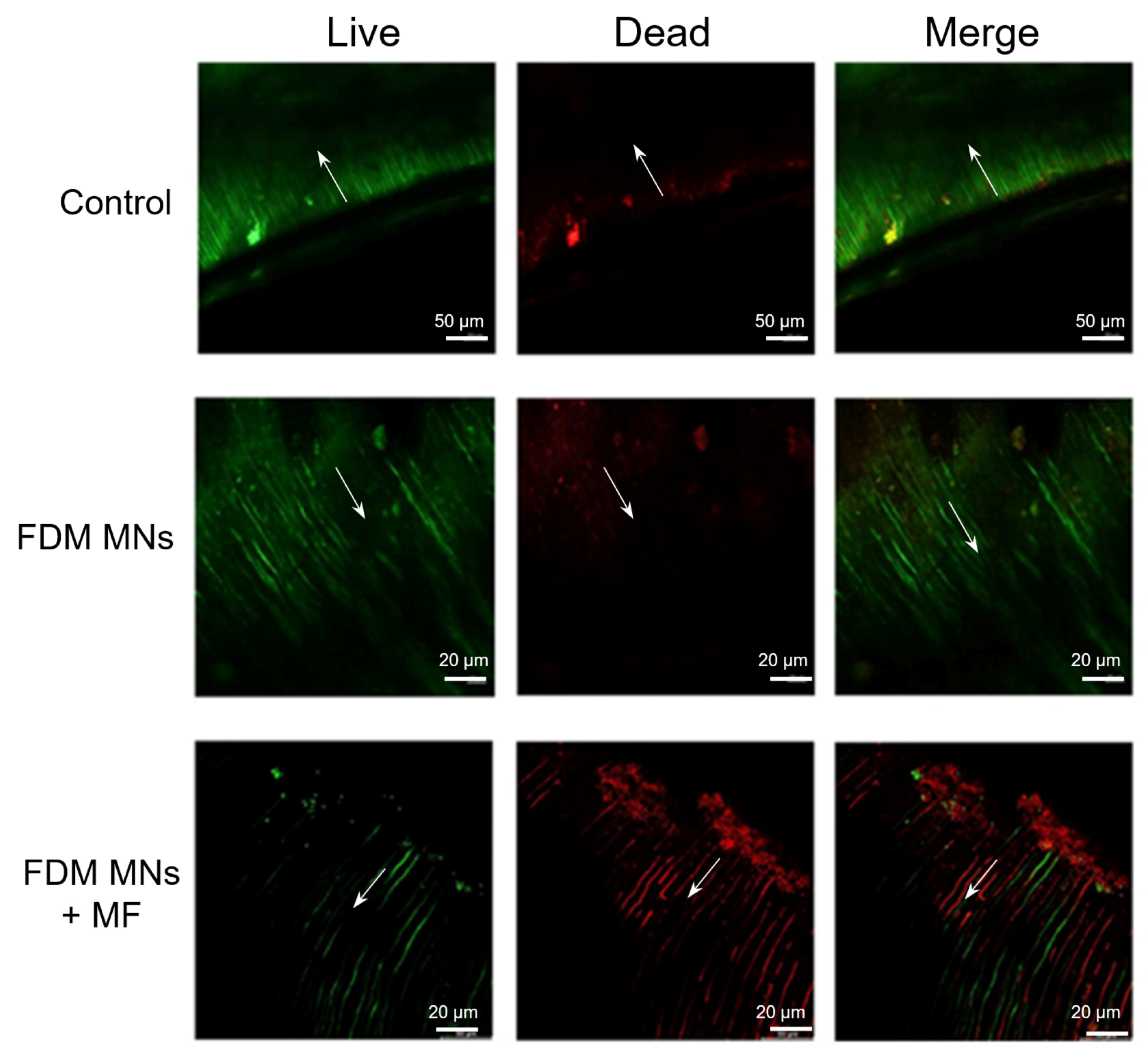
3.5. Anti-Biofilm Capacity of FDM MNs in Dentin Disk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFU | Colony-forming unit |
| ESD | Energy dispersive spectroscopy |
| EPS | Extracellular polymers |
| FD MNs | Dopamine on the surface of Fe3O4 magnetic nanoparticles |
| FDM MNs | Minocycline-loaded magnetic nanoparticles |
| MNs | Magnetic nanoparticles |
| Mino | Minocycline |
| OD | Optical density |
| PDA | Polydopamine |
References
- Li, X.-L.; Fan, W.; Fan, B. Dental pulp regeneration strategies: A review of status quo and recent advances. Bioact. Mater. 2024, 38, 258–275. [Google Scholar] [CrossRef]
- Sabet, N.E.; Lutfy, R.A. Ultrastructural morphologic evaluation of root canal walls prepared by two rotary nickel-titanium systems: A comparative study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 106, e59–e66. [Google Scholar] [CrossRef]
- Kang, W.; Zou, T.; Liang, Y.; Lei, H.; Zhang, R.; Kang, J.; Sun, Z.; Li, X.; Ge, S.; Zhang, C. An integrated preventive and therapeutic magnetic nanoparticle loaded with rhamnolipid and vancomycin for combating subgingival biofilms. Dent. Mater. 2024, 40, 1808–1822. [Google Scholar] [CrossRef]
- Semnani, S.J.; Moghadam, K.N.; Jafari, Z.; Chiniforush, N. Comparative effects of the conventional, ultrasonic, and laser-activated irrigation on penetration depth of three photosensitizers in the root canal system. Photodiagn. Photodyn. Ther. 2024, 49, 104286. [Google Scholar] [CrossRef] [PubMed]
- Belmabrouk, F.C.; Metref, Z.S.; Bouziani, N.; Gahlouz, S.I.; Tadja, S.M.H. Comparative study between manual and mechanized endodontic desobturation. Int. Dent. J. 2024, 74, S309–S310. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Afrashtehfar, K.I.; Corbella, S.; El-Kabbaney, A.; Perondi, I.; Taschieri, S. In Vivo and In Vitro Effectiveness of Rotary Nickel-Titanium vs. Manual Stainless Steel Instruments for Root Canal Therapy: Systematic Review and Meta-analysis. J. Évid. Based Dent. Pract. 2018, 18, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Stuart, C.; Schwartz, S.; Beeson, T.; Owatz, C. Enterococcus faecalis: Its Role in Root Canal Treatment Failure and Current Concepts in Retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Rôças, I.N.; Lopes, H.P. Patterns of microbial colonization in primary root canal infections. Oral Surgery, Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2002, 93, 174–178. [Google Scholar] [CrossRef]
- Mayattu, K.; Rajwade, J.; Ghormade, V. Development of erythromycin loaded PLGA nanoparticles for improved drug efficacy and sustained release against bacterial infections and biofilm formation. Microb. Pathog. 2024, 197, 107083. [Google Scholar] [CrossRef]
- Ali, A.Y.; Alani, A.-A.K.; Ahmed, B.O.; Hamid, L.L. Effect of biosynthesized silver nanoparticle size on antibacterial and anti-biofilm activity against pathogenic multi-drug resistant bacteria. OpenNano 2024, 20, 100213. [Google Scholar] [CrossRef]
- Harish, V.; Ansari, M.; Tewari, D.; Yadav, A.B.; Sharma, N.; Bawarig, S.; García-Betancourt, M.-L.; Karatutlu, A.; Bechelany, M.; Barhoum, A. Cutting-edge advances in tailoring size, shape, and functionality of nanoparticles and nanostructures: A review. J. Taiwan Inst. Chem. Eng. 2023, 149, 105010. [Google Scholar] [CrossRef]
- Guo, J.; Wang, P.; Li, Y.; Liu, Y.; Ye, Y.; Chen, Y.; Kankala, R.K.; Tong, F. Advances in hybridized nanoarchitectures for improved oro-dental health. J. Nanobiotechnol. 2024, 22, 469. [Google Scholar] [CrossRef]
- Pavanello, L.; Cortês, I.T.; de Carvalho, R.D.P.; Picolo, M.Z.D.; Cavalli, V.; Silva, L.T.S.; Boaro, L.C.C.; Prokopovich, P.; Cogo-Müller, K. Physicochemical and biological properties of dental materials and formulations with silica nanoparticles: A narrative review. Dent. Mater. 2024, 40, 1729–1741. [Google Scholar] [CrossRef]
- Andreozzi, A.; Brunese, L.; Cafarchio, A.; Netti, P.; Vanoli, G. Effects of magnetic nanoparticle distribution in cancer therapy through hyperthermia. Int. J. Therm. Sci. 2025, 208, 109428. [Google Scholar] [CrossRef]
- Muthukumaran, T.; Philip, J. A review on synthesis, capping and applications of superparamagnetic magnetic nanopar-ticles. Adv. Colloid Interface Sci. 2024, 334, 103314. [Google Scholar] [PubMed]
- Yan, Y.; Li, Y.; You, J.; Shen, K.; Chen, W.; Li, L. Morphology-dependent magnetic hyperthermia characteristics of Fe3O4 nanoparticles. Mater. Chem. Phys. 2025, 329, 130045. [Google Scholar] [CrossRef]
- Sarah, B.; Dongyeop, K.; Yuan, L.; Bekir, K.; Hyun, K. Novel Endodontic Disinfection Approach Using Catalytic Nanoparticles. J. Endod. 2018, 44, 806–812. [Google Scholar] [CrossRef]
- Quan, K.; Zhang, Z.; Ren, Y.; Busscher, H.J.; van der Mei, H.C.; Peterson, B.W. On-demand pulling-off of magnetic nanoparticles from biomaterial surfaces through implant-associated infectious biofilms for enhanced antibiotic efficacy. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112526. [Google Scholar] [CrossRef]
- Quan, K.; Zhang, Z.; Ren, Y.; Busscher, H.J.; van der Mei, H.C.; Peterson, B.W. Possibilities and impossibilities of magnetic nanoparticle use in the control of infectious biofilms. J. Mater. Sci. Technol. 2021, 69, 69–78. [Google Scholar] [CrossRef]
- GB/T 16886.1-2022; Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing Within a Risk Management Process [S]. Standards Press of China: Beijing, China, 2022.
- Yu, C.; Zheng, D.; Xu, C.; Wang, T.; Xu, J. Global research trends of nanomaterials application in periodontitis and peri-implantitis: A bibliometric analysis. Heliyon 2024, 10, e36187. [Google Scholar] [CrossRef]
- Umapathy, V.R.; Natarajan, P.M.; SumathiJones, C.; Swamikannu, B.; Johnson, W.; Alagarsamy, V.; Milon, A.R. Current trends and future perspectives on dental nanomaterials—An overview of nanotechnology strategies in dentistry. J. King Saud Univ. Sci. 2022, 34, 102231. [Google Scholar] [CrossRef]
- Jandt, K.D.; Watts, D.C. Nanotechnology in dentistry: Present and future perspectives on dental nanomaterials. Dent. Mater. 2020, 36, 1365–1378. [Google Scholar] [CrossRef]
- Ionescu, A.; Harris, D.; Selvaganapathy, P.R.; Kishen, A. Electrokinetic transport and distribution of antibacterial nanoparticles for endodontic disinfection. Int. Endod. J. 2020, 53, 1120–1130. [Google Scholar] [CrossRef]
- Tanner, S.; Thibault, A.; Leprince, J.G.; Bouillaguet, S. Photothermal Effect of 970 nm Diode Laser Irradiation on Enterococcus faecalis Biofilms in Single-Rooted Teeth Ex Vivo. Dent. J. 2024, 12, 308. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, D.; Yue, X.; Shi, Y.; Li, C.; Wang, Y.; Chen, Y.; Liu, Q.; Ding, D.; Wang, D.; et al. Enhancing Root Canal Therapy with NIR-II Semiconducting Polymer AIEgen and Low-Concentration Sodium Hypochlorite Synergy. Adv. Healthc. Mater. 2024, 13, e2401434. [Google Scholar] [CrossRef]
- Fouad, E.M.; Fawzy, M.I.; Saafan, A.M.; Elhousiny, M.A. Regenerative endodontic therapy in immature teeth using photobiomodulation and photodynamic therapy; a histomorphological study in canine model. BMC Oral Health 2024, 24, 1430. [Google Scholar] [CrossRef]
- Kannan, K.P.; Gunasekaran, V.; Sreenivasan, P.; Sathishkumar, P. Recent updates and feasibility of nanodrugs in the prevention and eradication of dental biofilm and its associated pathogens—A review. J. Dent. 2024, 143, 104888. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Ghaedi, A.; Fooladfar, Z.; Bazrgar, A. Recent advance on nanoparticles or nanomaterials with anti-multidrug resistant bacteria and anti-bacterial biofilm properties: A systematic review. Heliyon 2023, 9, e22105. [Google Scholar] [CrossRef]
- Ertem, E.; Gutt, B.; Zuber, F.; Allegri, S.; Le Ouay, B.; Mefti, S.; Formentin, K.; Stellacci, F.; Ren, Q. Core–Shell Silver Nanoparticles in Endodontic Disinfection Solutions Enable Long-Term Antimicrobial Effect on Oral Biofilms. ACS Appl. Mater. Interfaces 2017, 9, 34762–34772. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, J.; Shen, J.; Xu, Y.; Li, L.; Wang, W.; Zhou, N.; Zhang, M. Chitosan modified magnetic nanocomposite for biofilm destruction and precise photothermal/photodynamic therapy. Int. J. Biol. Macromol. 2024, 259, 129402. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Wei, Y.; Guo, X.; Li, M.; Xia, Y.; Wu, Y.; Liao, M.; Wang, S.; Wang, H.; et al. Effects of a Novel Magnetic Nanomaterial on Oral Biofilms. Int. Dent. J. 2024, 75, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Wang, P.; Tong, F.; Liu, Y.; Hu, X.; Yang, J.; Guo, J. Polydopamine-Coated Magnetic Nanoplatform for Magnetically Guided Penetration and Enhanced Antibacterial Efficacy in Root Canal Biofilm Elimination. Polymers 2025, 17, 1305. https://doi.org/10.3390/polym17101305
Xu X, Wang P, Tong F, Liu Y, Hu X, Yang J, Guo J. Polydopamine-Coated Magnetic Nanoplatform for Magnetically Guided Penetration and Enhanced Antibacterial Efficacy in Root Canal Biofilm Elimination. Polymers. 2025; 17(10):1305. https://doi.org/10.3390/polym17101305
Chicago/Turabian StyleXu, Xingchen, Pei Wang, Fei Tong, Yifan Liu, Xinyang Hu, Jian Yang, and Jun Guo. 2025. "Polydopamine-Coated Magnetic Nanoplatform for Magnetically Guided Penetration and Enhanced Antibacterial Efficacy in Root Canal Biofilm Elimination" Polymers 17, no. 10: 1305. https://doi.org/10.3390/polym17101305
APA StyleXu, X., Wang, P., Tong, F., Liu, Y., Hu, X., Yang, J., & Guo, J. (2025). Polydopamine-Coated Magnetic Nanoplatform for Magnetically Guided Penetration and Enhanced Antibacterial Efficacy in Root Canal Biofilm Elimination. Polymers, 17(10), 1305. https://doi.org/10.3390/polym17101305




