Formation of Neurointerfaces Based on Electrically Conductive Biopolymers by Two-Photon Polymerization Method
Abstract
1. Introduction
2. Materials and Methods
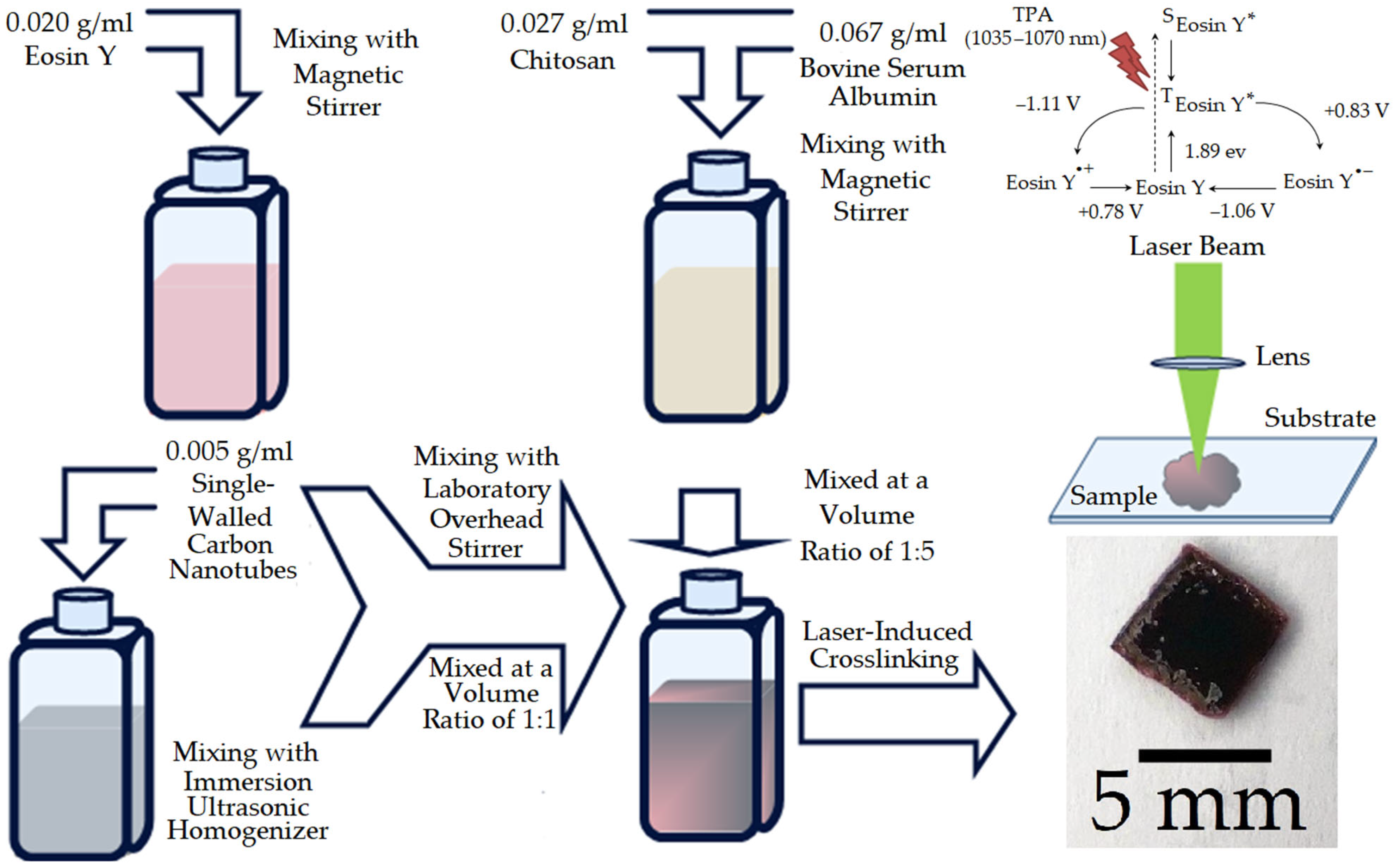
2.1. Materials and Preparation of CS/BSA/Eosin Y/SWCNTs Composite
2.2. Evaluation of Nonlinear Optical Properties
2.3. Spectral Studies
2.4. Determination of Electrical Conductivity of the Formed Composite
2.5. In Vitro Experiments
3. Results
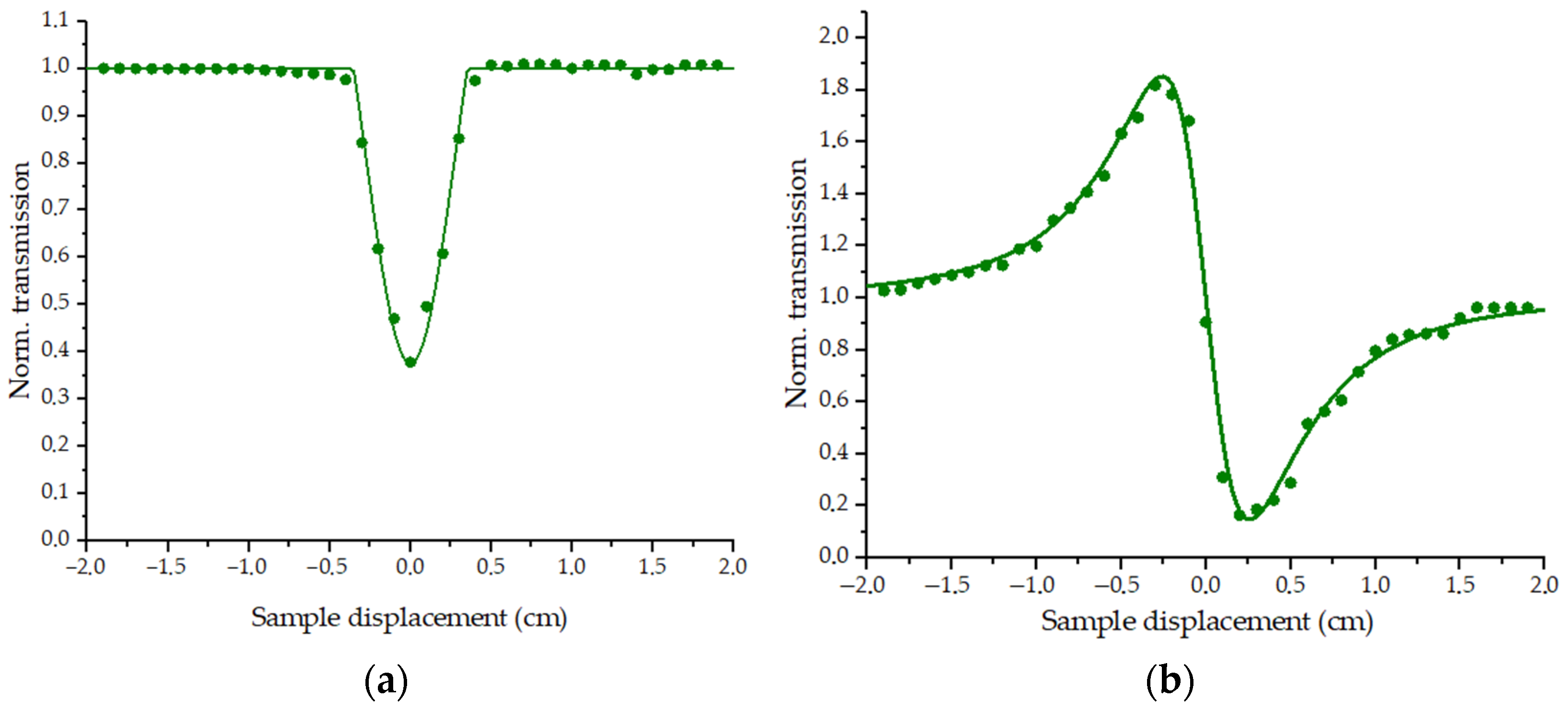
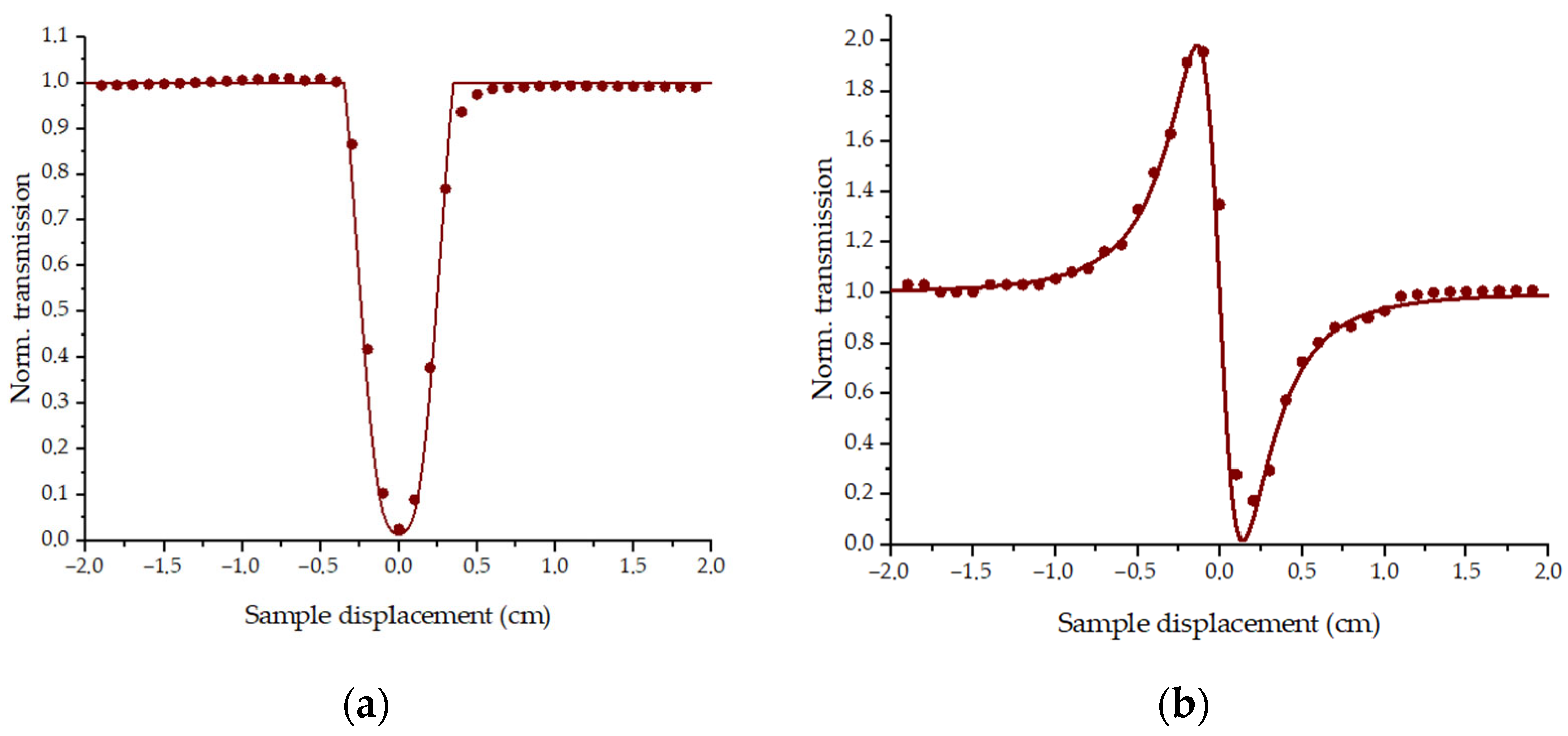
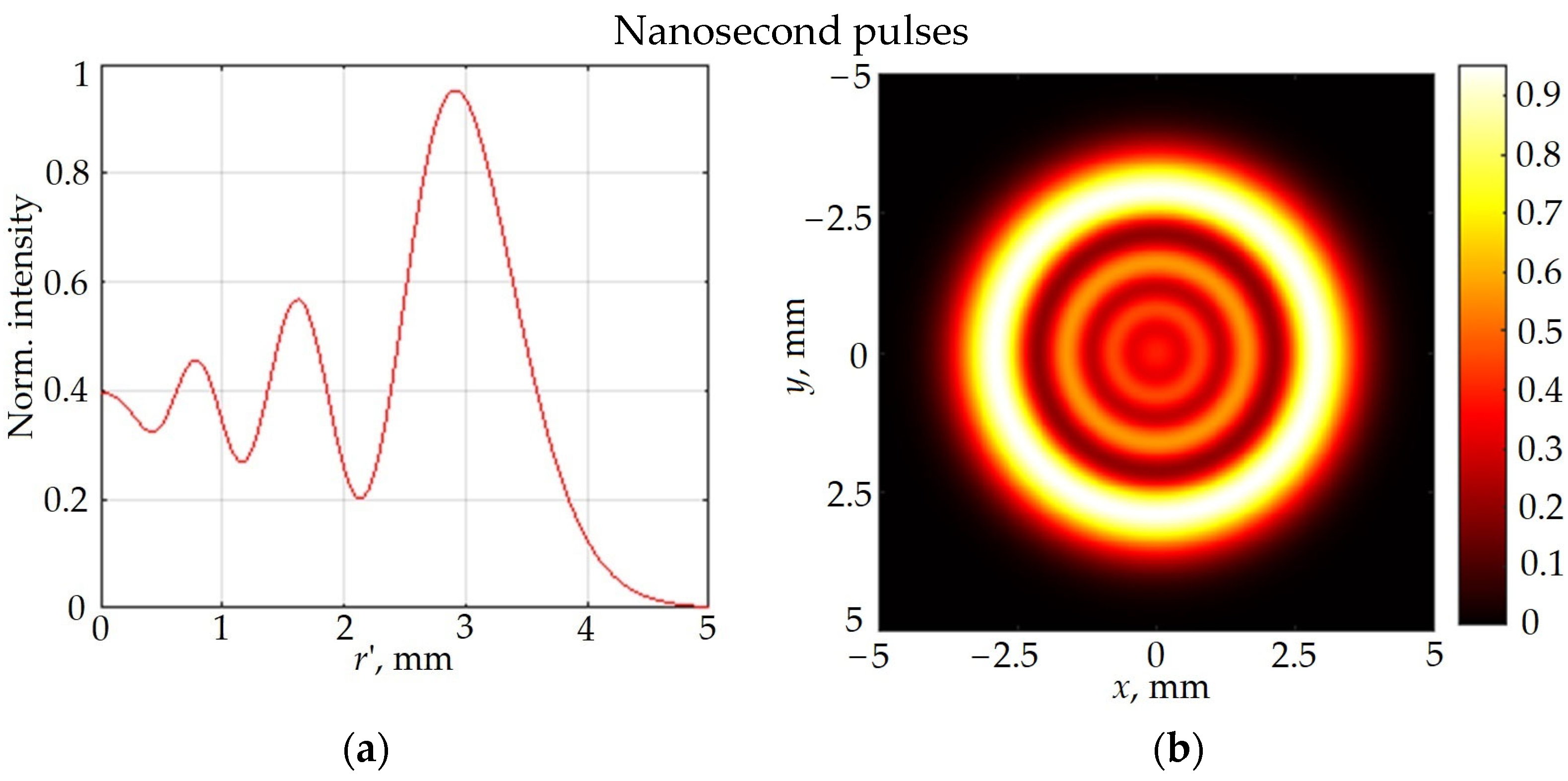
3.1. Nonlinear Optical Properties
3.1.1. Experiments
3.1.2. Theoretical Research
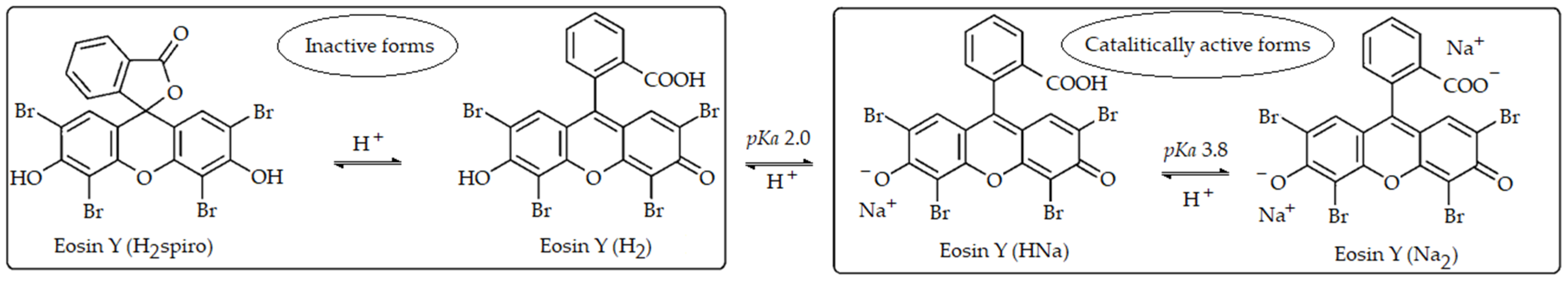
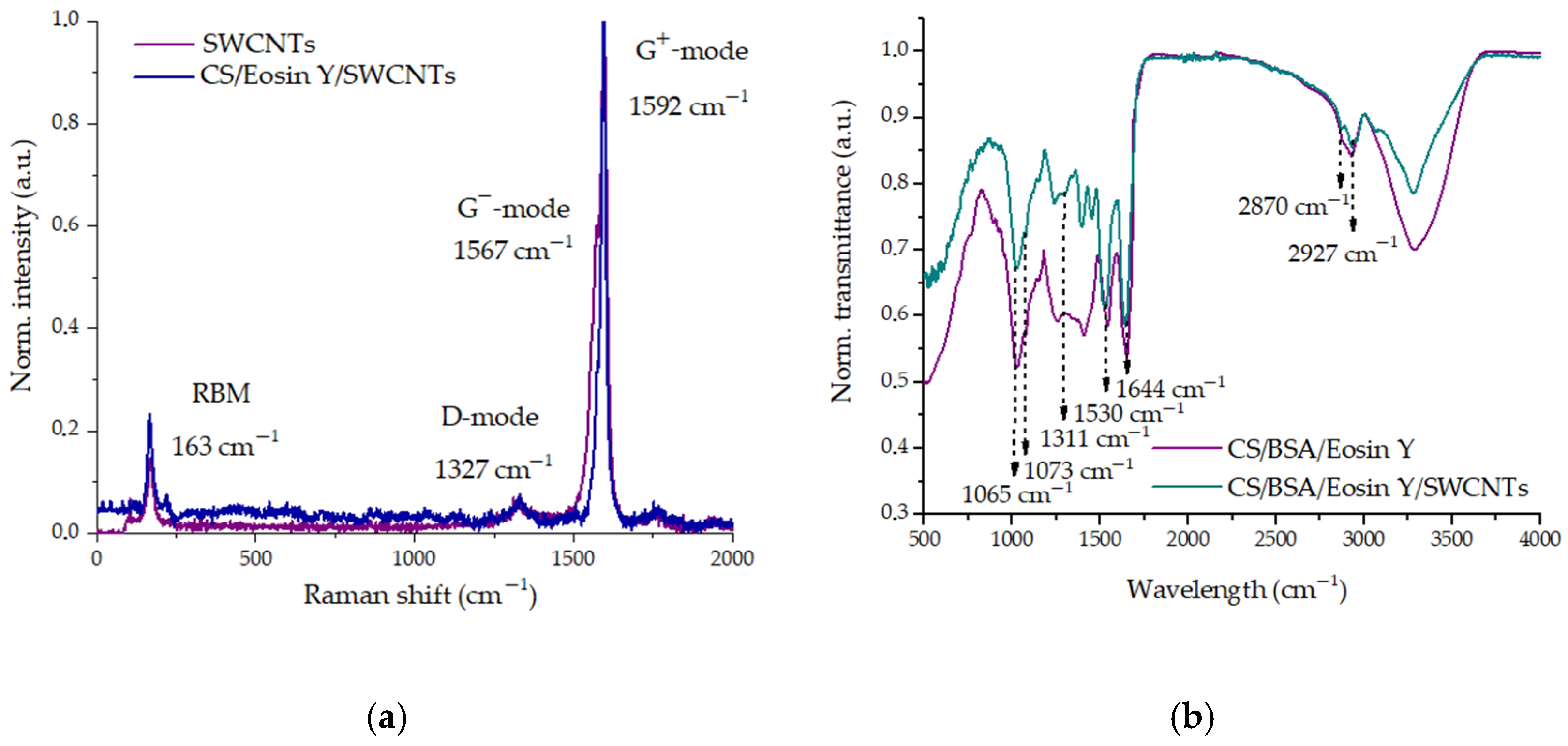
3.2. Spectral Analysis
3.3. Electrical Conductivity of the Formed Composite
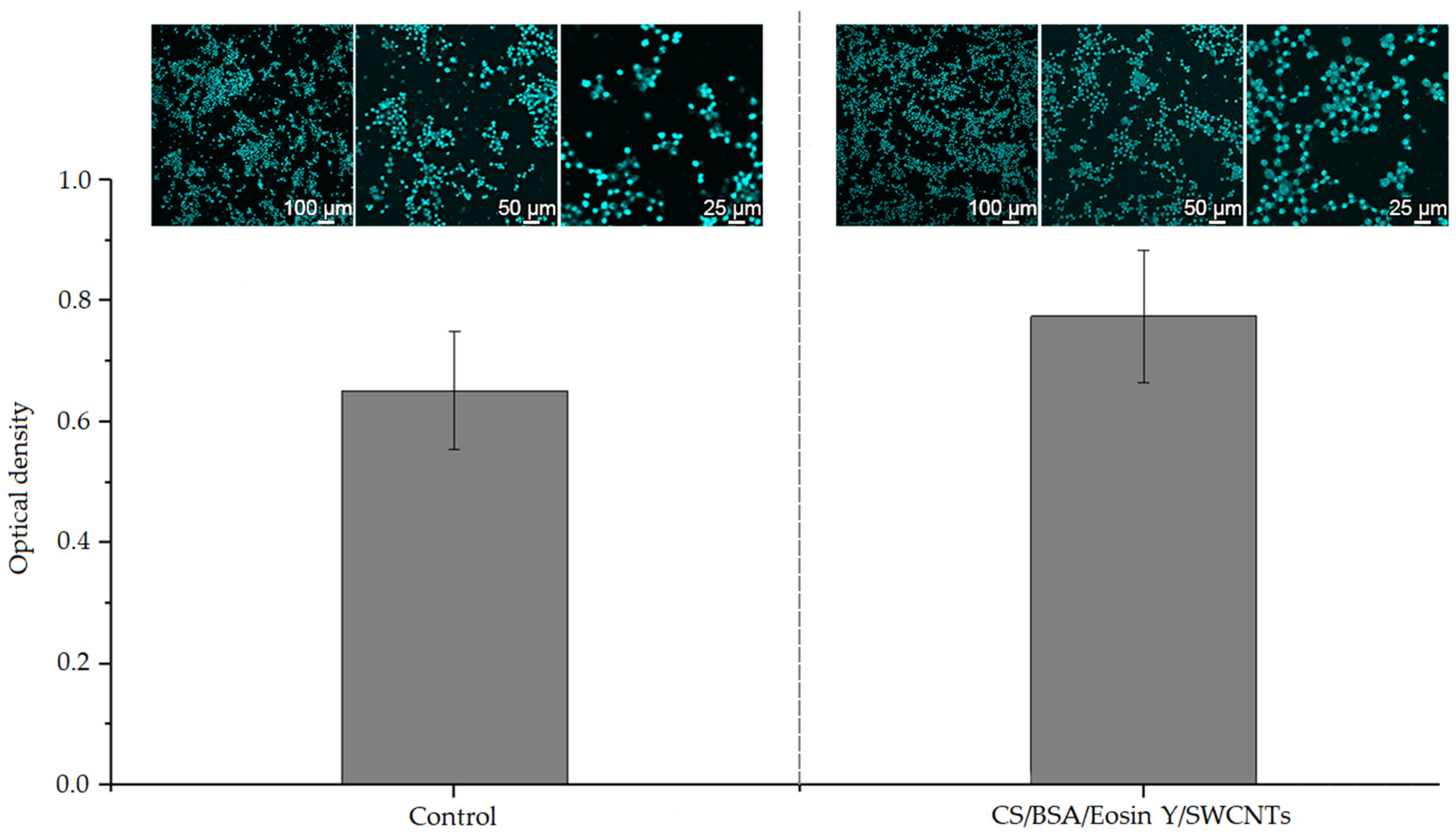
3.4. Biocompatibility of Formed Composite Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | Bovine serum albumin |
| SWCNTs | Single wall carbon nanotubes |
| FDA | Food and Drug Administration |
| CS | Chitosan |
| Eosin-Y | Eosin Yellow |
| TPA | Two-photon absorption |
| FTIR | Fourier transform infrared spectroscopy |
| ATR | Attenuated total reflection |
| SEM | Scanning electron microscope |
| MTT | Colorimetric assay for assessing cell metabolic activity |
| UV-Vis-Nir | Ultraviolet-Visible-Near Infrared |
| C-form | Cationic forms |
| D-form | Dianionic form |
| RBM | Radial breathing mode |
| G mode | Mode of graphite |
| D mode | Mode of diamond |
References
- Savelyev, M.S.; Kuksin, A.V.; Murashko, D.T.; Otsupko, E.P.; Kurilova, U.E.; Selishchev, S.V.; Gerasimenko, A.Y. Conductive Biocomposite Made by Two-Photon Polymerization of Hydrogels Based on BSA and Carbon Nanotubes with Eosin-Y. Gels 2024, 10, 711. [Google Scholar] [CrossRef] [PubMed]
- Kleeva, D.; Soghoyan, G.; Biktimirov, A.; Piliugin, N.; Matvienko, Y.; Sintsov, M.; Lebedev, M. Modulations in High-Density EEG during the Suppression of Phantom-Limb Pain with Neurostimulation in Upper Limb Amputees. Cereb. Cortex 2024, 34, bhad504. [Google Scholar] [CrossRef]
- Papadimitriou, L.; Manganas, P.; Ranella, A.; Stratakis, E. Biofabrication for Neural Tissue Engineering Applications. Mater. Today Bio 2020, 6, 100043. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Cheng, R.; Cao, Y.; Yan, Y.; Shen, Z.; Zhao, Y.; Han, Y. Biocompatible Chitosan/Polyethylene Glycol/Multi-Walled Carbon Nanotube Composite Scaffolds for Neural Tissue Engineering. J. Zhejiang Univ. B 2022, 23, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chang, L.; Shao, Y.; Yu, D.; Parajuli, J.; Xu, C.; Ying, G.; Yetisen, A.K.; Yin, Y.; Jiang, N. Conductive Ionic Liquid/Chitosan Hydrogels for Neuronal Cell Differentiation. Eng. Regen. 2022, 3, 1–12. [Google Scholar] [CrossRef]
- Kim, E.; Jeon, S.; An, H.-K.; Kianpour, M.; Yu, S.-W.; Kim, J.; Rah, J.-C.; Choi, H. A Magnetically Actuated Microrobot for Targeted Neural Cell Delivery and Selective Connection of Neural Networks. Sci. Adv. 2020, 6, eabb5696. [Google Scholar] [CrossRef]
- Jain, D.; Mattiassi, S.; Goh, E.; Yim, E.F. Extracellular Matrix and Biomimetic Engineering Microenvironment for Neuronal Differentiation. Neural Regen. Res. 2020, 15, 573. [Google Scholar] [CrossRef]
- Kittana, N.; Assali, M.; Zimmermann, W.-H.; Liaw, N.; Santos, G.L.; Rehman, A.; Lutz, S. Modulating the Biomechanical Properties of Engineered Connective Tissues by Chitosan-Coated Multiwall Carbon Nanotubes. Int. J. Nanomed. 2021, 16, 989–1000. [Google Scholar] [CrossRef]
- Shokrgozar, M.A.; Mottaghitalab, F.; Mottaghitalab, V.; Farokhi, M. Fabrication of Porous Chitosan/Poly(Vinyl Alcohol) Reinforced Single-Walled Carbon Nanotube Nanocomposites for Neural Tissue Engineering. J. Biomed. Nanotechnol. 2011, 7, 276–284. [Google Scholar] [CrossRef]
- Iftekhar, S.; Wasayh, M.A.; Asif, M.B.; Lehto, V.-P.; Sillanpää, M. Methods for Synthesis of Nanobiopolymers. In Biopolymeric Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 13–35. [Google Scholar]
- Azad, U.P.; Chandra, P. (Eds.) Handbook of Nanobioelectrochemistry; Springer Nature Singapore: Singapore, 2023; ISBN 978-981-19-9436-4. [Google Scholar]
- Wang, Z.; Duan, H.; Hao, F.; Hao, P.; Zhao, W.; Gao, Y.; Gu, Y.; Song, J.; Li, X.; Yang, Z. Circuit Reconstruction of Newborn Neurons after Spinal Cord Injury in Adult Rats via an NT3-Chitosan Scaffold. Prog. Neurobiol. 2023, 220, 102375. [Google Scholar] [CrossRef]
- Jahromi, H.K.; Farzin, A.; Hasanzadeh, E.; Barough, S.E.; Mahmoodi, N.; Najafabadi, M.R.H.; Farahani, M.S.; Mansoori, K.; Shirian, S.; Ai, J. Enhanced Sciatic Nerve Regeneration by Poly-L-Lactic Acid/Multi-Wall Carbon Nanotube Neural Guidance Conduit Containing Schwann Cells and Curcumin Encapsulated Chitosan Nanoparticles in Rat. Mater. Sci. Eng. C 2020, 109, 110564. [Google Scholar] [CrossRef]
- Hosney, A.; Ullah, S.; Barčauskaitė, K. A Review of the Chemical Extraction of Chitosan from Shrimp Wastes and Prediction of Factors Affecting Chitosan Yield by Using an Artificial Neural Network. Mar. Drugs 2022, 20, 675. [Google Scholar] [CrossRef]
- Di Lisa, D.; Muzzi, L.; Pepe, S.; Dellacasa, E.; Frega, M.; Fassio, A.; Martinoia, S.; Pastorino, L. On the Way Back from 3D to 2D: Chitosan Promotes Adhesion and Development of Neuronal Networks onto Culture Supports. Carbohydr. Polym. 2022, 297, 120049. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Miller, A.L.; Park, S.; Waletzki, B.E.; Zhou, Z.; Terzic, A.; Lu, L. Functionalized Carbon Nanotube and Graphene Oxide Embedded Electrically Conductive Hydrogel Synergistically Stimulates Nerve Cell Differentiation. ACS Appl. Mater. Interfaces 2017, 9, 14677–14690. [Google Scholar] [CrossRef]
- Ghosh, S.; Shrivastava, A.; Jha, P.; Roy, P.; Lahiri, D. Analysis of Neural Cell Behaviour on Anisotropic Electrically Conductive Polymeric Biodegradable Scaffolds Reinforced with Carbon Nanotubes. Med. Devices Sens. 2021, 4, e10152. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Chitosan/Carbon Nanotube Hybrids: Recent Progress and Achievements for Industrial Applications. New J. Chem. 2021, 45, 3756–3777. [Google Scholar] [CrossRef]
- Wisdom, K.S.; Bhat, I.A.; Chanu, T.I.; Kumar, P.; Pathakota, G.-B.; Nayak, S.K.; Walke, P.; Sharma, R. Chitosan Grafting onto Single-Walled Carbon Nanotubes Increased Their Stability and Reduced the Toxicity in Vivo (Catfish) Model. Int. J. Biol. Macromol. 2020, 155, 697–707. [Google Scholar] [CrossRef]
- Vakhrusheva, T.V.; Gusev, A.A.; Gusev, S.A.; Vlasova, I.I. Albumin Reduces Thrombogenic Potential of Single-Walled Carbon Nanotubes. Toxicol. Lett. 2013, 221, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Zadeh Mehrizi, T.; Shafiee Ardestani, M. Application of Non-Metal Nanoparticles, as a Novel Approach, for Improving the Stability of Blood Products: 2011–2021. Prog. Biomater. 2022, 11, 137–161. [Google Scholar] [CrossRef]
- Parkatzidis, K.; Chatzinikolaidou, M.; Kaliva, M.; Bakopoulou, A.; Farsari, M.; Vamvakaki, M. Multiphoton 3D Printing of Biopolymer-Based Hydrogels. ACS Biomater. Sci. Eng. 2019, 5, 6161–6170. [Google Scholar] [CrossRef]
- Xing, X.; Su, J.; Liu, Y.; Lin, H.; Wang, Y.; Cheng, H. A Novel Visible Light-Curing Chitosan-Based Hydrogel Membrane for Guided Tissue Regeneration. Colloids Surf. B Biointerfaces 2022, 218, 112760. [Google Scholar] [CrossRef]
- Dobos, A.; Steiger, W.; Theiner, D.; Gruber, P.; Lunzer, M.; Van Hoorick, J.; Van Vlierberghe, S.; Ovsianikov, A. Screening of Two-Photon Activated Photodynamic Therapy Sensitizers Using a 3D Osteosarcoma Model. Analyst 2019, 144, 3056–3063. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Shih, H.; Muňoz, Z.; Kemp, A.; Lin, C.-C. Visible Light Cured Thiol-Vinyl Hydrogels with Tunable Degradation for 3D Cell Culture. Acta Biomater. 2014, 10, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.; Lin, C. Visible-Light-Mediated Thiol-Ene Hydrogelation Using Eosin-Y as the Only Photoinitiator. Macromol. Rapid Commun. 2013, 34, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Nachlas, A.L.Y.; Li, S.; Jha, R.; Singh, M.; Xu, C.; Davis, M.E. Human IPSC-Derived Mesenchymal Stem Cells Encapsulated in PEGDA Hydrogels Mature into Valve Interstitial-like Cells. Acta Biomater. 2018, 71, 235–246. [Google Scholar] [CrossRef]
- Desmangles, A.-I.; Jordan, O.; Marquis-Weible, F. Interfacial Photopolymerization of β-Cell Clusters: Approaches to Reduce Coating Thickness Using Ionic and Lipophilic Dyes. Biotechnol. Bioeng. 2001, 72, 634–641. [Google Scholar] [CrossRef]
- Savelyev, M.S.; Gerasimenko, A.Y.; Vasilevsky, P.N.; Fedorova, Y.O.; Groth, T.; Ten, G.N.; Telyshev, D.V. Spectral Analysis Combined with Nonlinear Optical Measurement of Laser Printed Biopolymer Composites Comprising Chitosan/SWCNT. Anal. Biochem. 2020, 598, 113710. [Google Scholar] [CrossRef]
- Vasilevsky, P.N.; Savelyev, M.S.; Tereshchenko, S.A.; Selishchev, S.V.; Gerasimenko, A.Y. Nonlinear Optical Properties of Single-Walled Carbon Nanotubes/Water Dispersed Media Exposed to Laser Radiation with Nano- and Femtosecond Pulse Durations. Kondens. Sredy I Mezhfaznye Granitsy = Condens. Matter Interphases 2021, 23, 496–506. [Google Scholar] [CrossRef]
- Tremblay, R.G.; Sikorska, M.; Sandhu, J.K.; Lanthier, P.; Ribecco-Lutkiewicz, M.; Bani-Yaghoub, M. Differentiation of Mouse Neuro 2A Cells into Dopamine Neurons. J. Neurosci. Methods 2010, 186, 60–67. [Google Scholar] [CrossRef]
- Salto, R.; Vílchez, J.D.; Girón, M.D.; Cabrera, E.; Campos, N.; Manzano, M.; Rueda, R.; López-Pedrosa, J.M. β-Hydroxy-β-Methylbutyrate (HMB) Promotes Neurite Outgrowth in Neuro2a Cells. PLoS ONE 2015, 10, e0135614. [Google Scholar] [CrossRef]
- Dos Santos, M.G.; Gomes, J.R.; Costa, M.D.M. Methods Used to Achieve Different Levels of the Neuronal Differentiation Process in SH-SY5Y and Neuro2a Cell Lines: An Integrative Review. Cell Biol. Int. 2023, 47, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Evangelopoulos, M.E.; Weis, J.; Krüttgen, A. Signalling Pathways Leading to Neuroblastoma Differentiation after Serum Withdrawal: HDL Blocks Neuroblastoma Differentiation by Inhibition of EGFR. Oncogene 2005, 24, 3309–3318. [Google Scholar] [CrossRef]
- Saragoni, L.; Hernández, P.; Maccioni, R.B. Differential Association of Tau with Subsets of Microtubules Containing Posttranslationally-Modified Tubulin Variants in Neuroblastoma Cells. Neurochem. Res. 2000, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, J.; Cuijpers, S.A.G.; Ferrari, E.; Niranjan, D.; Rust, A.; Leese, C.; O’Brien, J.A.; Binz, T.; Davletov, B. Botulinum Protease-cleaved SNARE Fragments Induce Cytotoxicity in Neuroblastoma Cells. J. Neurochem. 2014, 129, 781–791. [Google Scholar] [CrossRef] [PubMed]
- El Merhie, A.; Salis, B.; Ravasenga, T.; Tramontano, C.; Avugadda, S.K.; Pugliese, G.; Barberis, A.; Pellegrino, T.; Dante, S. Action Potentials as a Mean to Trigger the Specific Interaction of Negatively Charged Nanoparticles with Electrically Excitable Cells. ACS Appl. Nano Mater. 2024, 7, 18357–18366. [Google Scholar] [CrossRef]
- Martín, D.; Ruano, D.; Yúfera, A.; Daza, P. Electrical Pulse Stimulation Parameters Modulate N2a Neuronal Differentiation. Cell Death Discov. 2024, 10, 49. [Google Scholar] [CrossRef]
- Martín, D.; Bocio-Nuñez, J.; Scagliusi, S.F.; Pérez, P.; Huertas, G.; Yúfera, A.; Giner, M.; Daza, P. DC Electrical Stimulation Enhances Proliferation and Differentiation on N2a and MC3T3 Cell Lines. J. Biol. Eng. 2022, 16, 27. [Google Scholar] [CrossRef]
- Herbrik, F.; Camarero González, P.; Krstic, M.; Puglisi, A.; Benaglia, M.; Sanz, M.A.; Rossi, S. Eosin Y: Homogeneous Photocatalytic In-Flow Reactions and Solid-Supported Catalysts for In-Batch Synthetic Transformations. Appl. Sci. 2020, 10, 5596. [Google Scholar] [CrossRef]
- Mohamadpour, F. New role for photoexcited organic dye, Na2 eosin Y via the direct hydrogen atom transfer (HAT) process in photochemical visible-light-induced synthesis of spiroacenaphthylenes and 1H-pyrazolo[1,2-b] phthalazine-5,10-diones under air atmosphere. Dyes and Pigments 2021, 194, 109628. [Google Scholar] [CrossRef]
- Sheik-Bahae, M.; Said, A.A.; Wei, T.-H.; Hagan, D.J.; Van Stryland, E.W. Sensitive Measurement of Optical Nonlinearities Using a Single Beam. IEEE J. Quantum Electron. 1990, 26, 760–769. [Google Scholar] [CrossRef]
- Vasilevsky, P.N.; Savelyev, M.S.; Tolbin, A.Y.; Ryabkin, D.I.; Gerasimenko, A.Y. Spatial Self-Phase Modulation of Light in Liquid Dispersions Based on Conjugates of Phthalocyanines and Carbon Nanotubes. St. Petersburg Polytech. Univ. J. Phys. Math. 2023, 16, 31–35. [Google Scholar] [CrossRef]
- Jia, Y.; Liao, Y.; Wu, L.; Shan, Y.; Dai, X.; Cai, H.; Xiang, Y.; Fan, D. Nonlinear Optical Response, All Optical Switching, and All Optical Information Conversion in NbSe2 Nanosheets Based on Spatial Self-Phase Modulation. Nanoscale 2019, 11, 4515–4522. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.K.; Ahmed, M.S.; Murali, R.; Bhavani, B.; Prasanthkumar, S.; Giribabu, L.; Raavi, S.S.K. All-Optical Modulation and Photonic Diode Based on Spatial Self-Phase Modulation in Porphyrin–Napthalimide Molecules. J. Mater. Chem. C 2024, 12, 9841–9852. [Google Scholar] [CrossRef]
- Hergenhahn, L.; Padutsch, N.; Azawi, S.; Weiskirchen, R.; Liehr, T.; Rinčic, M. Cytogenomic Characterization of Murine Neuroblastoma Cell Line Neuro-2a and Its Two Derivatives Neuro-2a TR-Alpha and Neuro-2a TR-Beta. Cells 2024, 13, 1889. [Google Scholar] [CrossRef]
- Gu, Q. Neural Cell Lines (Lineage). In Neural Cell Biology; CRC Press: Boca Raton, FL, USA, 2017; pp. 169–186. [Google Scholar]
- Perreault, F.; Melegari, S.P.; da Costa, C.H.; de Oliveira Franco Rossetto, A.L.; Popovic, R.; Matias, W.G. Genotoxic Effects of Copper Oxide Nanoparticles in Neuro 2A Cell Cultures. Sci. Total Environ. 2012, 441, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Aluker, N.L.; Herrmann, M.; Suzdaltseva, J.M. Water Spectrophotometry in the UV and Visible Range as an Element of Water-Resource Ecoanalytics. Instrum. Exp. Tech. 2020, 63, 853–859. [Google Scholar] [CrossRef]
- Venugopal Rao, S.; Shuvan Prashant, T.; Swain, D.; Sarma, T.; Panda, P.K.; Tewari, S.P. Two-Photon and Three-Photon Absorption in Dinaphthoporphycenes. Chem. Phys. Lett. 2011, 514, 98–103. [Google Scholar] [CrossRef]
- Waheed, A.A.; Rao, K.S.; Gupta, P.D. Mechanism of Dye Binding in the Protein Assay Using Eosin Dyes. Anal. Biochem. 2000, 287, 73–79. [Google Scholar] [CrossRef]
- Chen, P.-H.; Chen, H.-H.; Anbarasan, R.; Kuo, L.-S. Synthesis and Characterization of Eosin Y Functionalized MWCNT. In Proceedings of the 2010 IEEE Nanotechnology Materials and Devices Conference, Monterey, CA, USA, 12–15 October 2010; IEEE: Piscataway, NJ, USA, 2010; pp. 325–327. [Google Scholar]
- Dresselhaus, M.S.; Dresselhaus, G.; Jorio, A.; Souza Filho, A.G.; Saito, R. Raman Spectroscopy on Isolated Single Wall Carbon Nanotubes. Carbon 2002, 40, 2043–2061. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman Spectroscopy of Carbon Nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Zhang, S.; Wang, Z.; Jia, H.; Oda, H.; Li, R. Fabrication and Characterization of the H/J-Type Aggregates Astaxanthin/Bovine Serum Albumin/Chitosan Nanoparticles. Int. J. Biol. Macromol. 2022, 223, 1186–1195. [Google Scholar] [CrossRef]
- Zhang, F.; Shi, F.; Ma, W.; Gao, F.; Jiao, Y.; Li, H.; Wang, J.; Shan, X.; Lu, X.; Meng, S. Controlling Adsorption Structure of Eosin Y Dye on Nanocrystalline TiO2 Films for Improved Photovoltaic Performances. J. Phys. Chem. C 2013, 117, 14659–14666. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Shi, Y.; Wan, D.; Chen, J.; Xiao, S. Adsorption of Toxic Dye Eosin Y from Aqueous Solution by Clay/Carbon Composite Derived from Spent Bleaching Earth. Water Environ. Res. 2021, 93, 159–169. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Wang, R.; He, Y.; Dang, X. Fabrication, Structure and Surface Charges of Albumin-Chitosan Hybrids. Sci. China Chem. 2012, 55, 1788–1795. [Google Scholar] [CrossRef]
- Karimi, M.; Avci, P.; Mobasseri, R.; Hamblin, M.R.; Naderi-Manesh, H. The Novel Albumin–Chitosan Core–Shell Nanoparticles for Gene Delivery: Preparation, Optimization and Cell Uptake Investigation. J. Nanopart. Res. 2013, 15, 1651. [Google Scholar] [CrossRef]
- Volkov, D.S.; Krivoshein, P.K.; Proskurnin, M.A. Detonation Nanodiamonds: A Comparison Study by Photoacoustic, Diffuse Reflectance, and Attenuated Total Reflection FTIR Spectroscopies. Nanomaterials 2020, 10, 2501. [Google Scholar] [CrossRef]
- Purves, D.; Augustine, G.; Fitzpatrick, D.; Hall, W.; LaMantia, A.-S.; White, L. Neuroscience, 5th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2012; ISBN 978-0878936953. [Google Scholar]
- Blanco, V.; Camelo, J.L.; Carri, N.G. Growth inhibition, morphological differentiation and stimulation of survival in neuronal cell type (Neuro-2a) treated with trophic molecules. Cell Biol. Int. 2014, 25, 909. [Google Scholar] [CrossRef]
- Neupane, T.; Tabibi, B.; Kim, W.-J.; Seo, F.J. Spatial Self-Phase Modulation in Graphene-Oxide Monolayer. Crystals 2023, 13, 271. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, S.; Umran, F.A.; Cheng, X.; Dong, N.; Coghlan, D.; Cheng, Y.; Zhang, L.; Blau, W.J.; Wang, J. Tunable Effective Nonlinear Refractive Index of Graphene Dispersions during the Distortion of Spatial Self-Phase Modulation. Appl. Phys. Lett. 2014, 104, 141909. [Google Scholar] [CrossRef]
- Shi, B.; Miao, L.; Wang, Q.; Du, J.; Tang, P.; Liu, J.; Zhao, C.; Wen, S. Broadband Ultrafast Spatial Self-Phase Modulation for Topological Insulator Bi2Te3 Dispersions. Appl. Phys. Lett. 2015, 107, 151101. [Google Scholar] [CrossRef]
- Burkins, P.; Kuis, R.; Basaldua, I.; Johnson, A.M.; Swaminathan, S.R.; Zhang, D.; Trivedi, S. Thermally Managed Z-Scan Methods Investigation of the Size-Dependent Nonlinearity of Graphene Oxide in Various Solvents. J. Opt. Soc. Am. B 2016, 33, 2395. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, B.; Chen, Y. Recent Progress in Two-Dimensional Nanomaterials for Laser Protection. Chemistry 2019, 1, 17–43. [Google Scholar] [CrossRef]
- Dissanayake, D.M.A.S.; Cifuentes, M.P.; Humphrey, M.G. Optical Limiting Properties of (Reduced) Graphene Oxide Covalently Functionalized by Coordination Complexes. Coord. Chem. Rev. 2018, 375, 489–513. [Google Scholar] [CrossRef]
- Krivenkov, R.Y.; Mogileva, T.N.; Mikheev, K.G.; Okotrub, A.V.; Mikheev, G.M. Heat-Induced Dip of Optical Limiting Threshold in Carbon Nanotube Aqueous Suspension. J. Phys. Chem. C 2018, 122, 16339–16345. [Google Scholar] [CrossRef]
- Anand, B.; Podila, R.; Ayala, P.; Oliveira, L.; Philip, R.; Sankara Sai, S.S.; Zakhidov, A.A.; Rao, A.M. Nonlinear Optical Properties of Boron Doped Single-Walled Carbon Nanotubes. Nanoscale 2013, 5, 7271. [Google Scholar] [CrossRef]
- Zhao, B.; Cao, B.; Zhou, W.; Li, D.; Zhao, W. Nonlinear Optical Transmission of Nanographene and Its Composites. J. Phys. Chem. C 2010, 114, 12517–12523. [Google Scholar] [CrossRef]
- Dong, Y.; Semin, S.; Feng, Y.; Xu, J.; Rasing, T. Solvent Induced Enhancement of Nonlinear Optical Response of Graphdiyne. Chin. Chem. Lett. 2021, 32, 525–528. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savelyev, M.S.; Kuksin, A.V.; Murashko, D.T.; Otsupko, E.P.; Suchkova, V.V.; Popovich, K.D.; Vasilevsky, P.N.; Vasilevskaya, Y.O.; Kurilova, U.E.; Eganova, E.M.; et al. Formation of Neurointerfaces Based on Electrically Conductive Biopolymers by Two-Photon Polymerization Method. Polymers 2025, 17, 1300. https://doi.org/10.3390/polym17101300
Savelyev MS, Kuksin AV, Murashko DT, Otsupko EP, Suchkova VV, Popovich KD, Vasilevsky PN, Vasilevskaya YO, Kurilova UE, Eganova EM, et al. Formation of Neurointerfaces Based on Electrically Conductive Biopolymers by Two-Photon Polymerization Method. Polymers. 2025; 17(10):1300. https://doi.org/10.3390/polym17101300
Chicago/Turabian StyleSavelyev, Mikhail S., Artem V. Kuksin, Denis T. Murashko, Ekaterina P. Otsupko, Victoria V. Suchkova, Kristina D. Popovich, Pavel N. Vasilevsky, Yulia O. Vasilevskaya, Ulyana E. Kurilova, Elena M. Eganova, and et al. 2025. "Formation of Neurointerfaces Based on Electrically Conductive Biopolymers by Two-Photon Polymerization Method" Polymers 17, no. 10: 1300. https://doi.org/10.3390/polym17101300
APA StyleSavelyev, M. S., Kuksin, A. V., Murashko, D. T., Otsupko, E. P., Suchkova, V. V., Popovich, K. D., Vasilevsky, P. N., Vasilevskaya, Y. O., Kurilova, U. E., Eganova, E. M., Edelbekova, P. A., Selishchev, S. V., Pavlov, A. A., & Gerasimenko, A. Y. (2025). Formation of Neurointerfaces Based on Electrically Conductive Biopolymers by Two-Photon Polymerization Method. Polymers, 17(10), 1300. https://doi.org/10.3390/polym17101300







