Revealing the Calcium Assisted Partial Catalytic Graphitization of Lignin-Derived Hard Carbon Anode and Its Electrochemical Behaviors in Sodium Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Material Characterization
2.3. Electrochemical Analysis
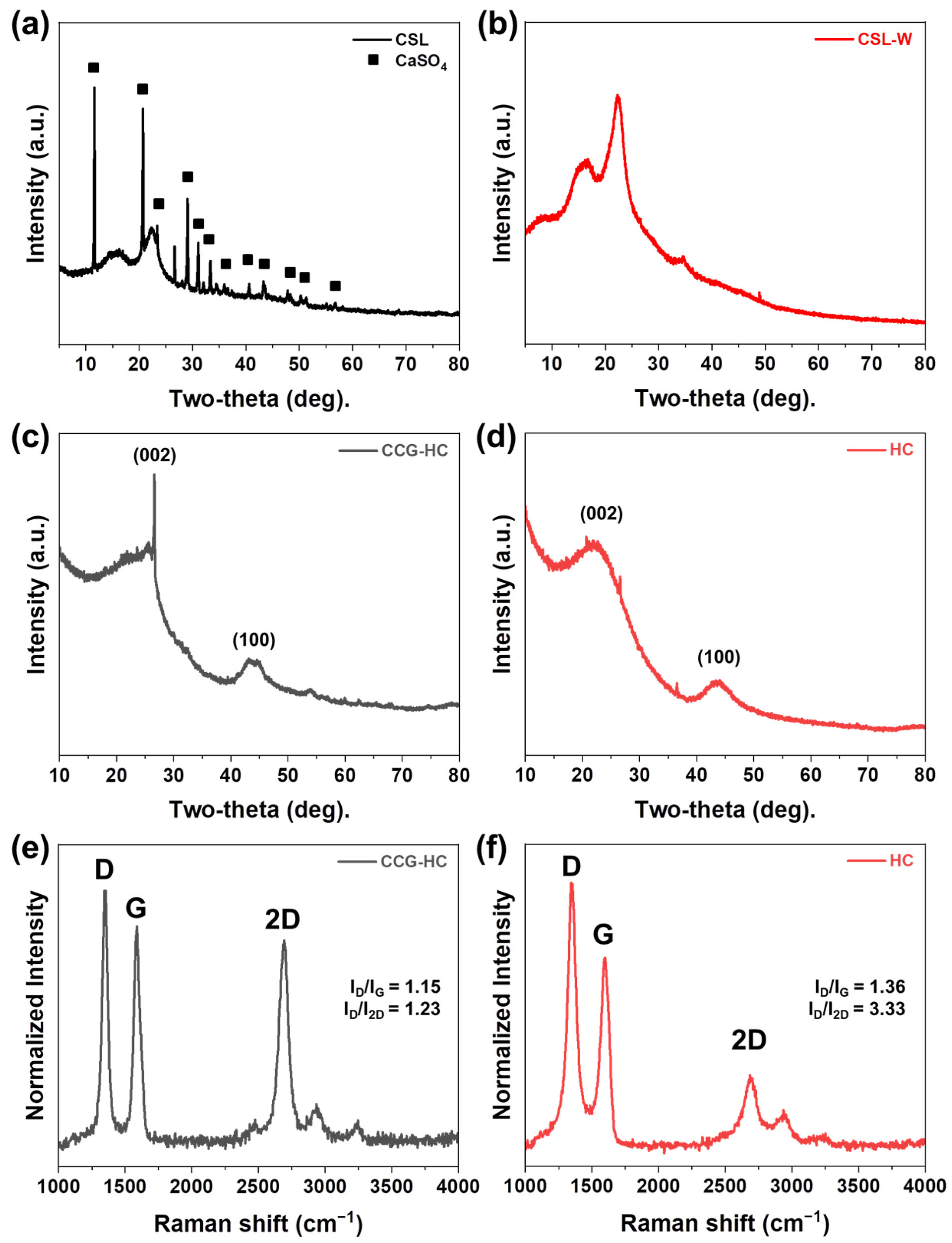
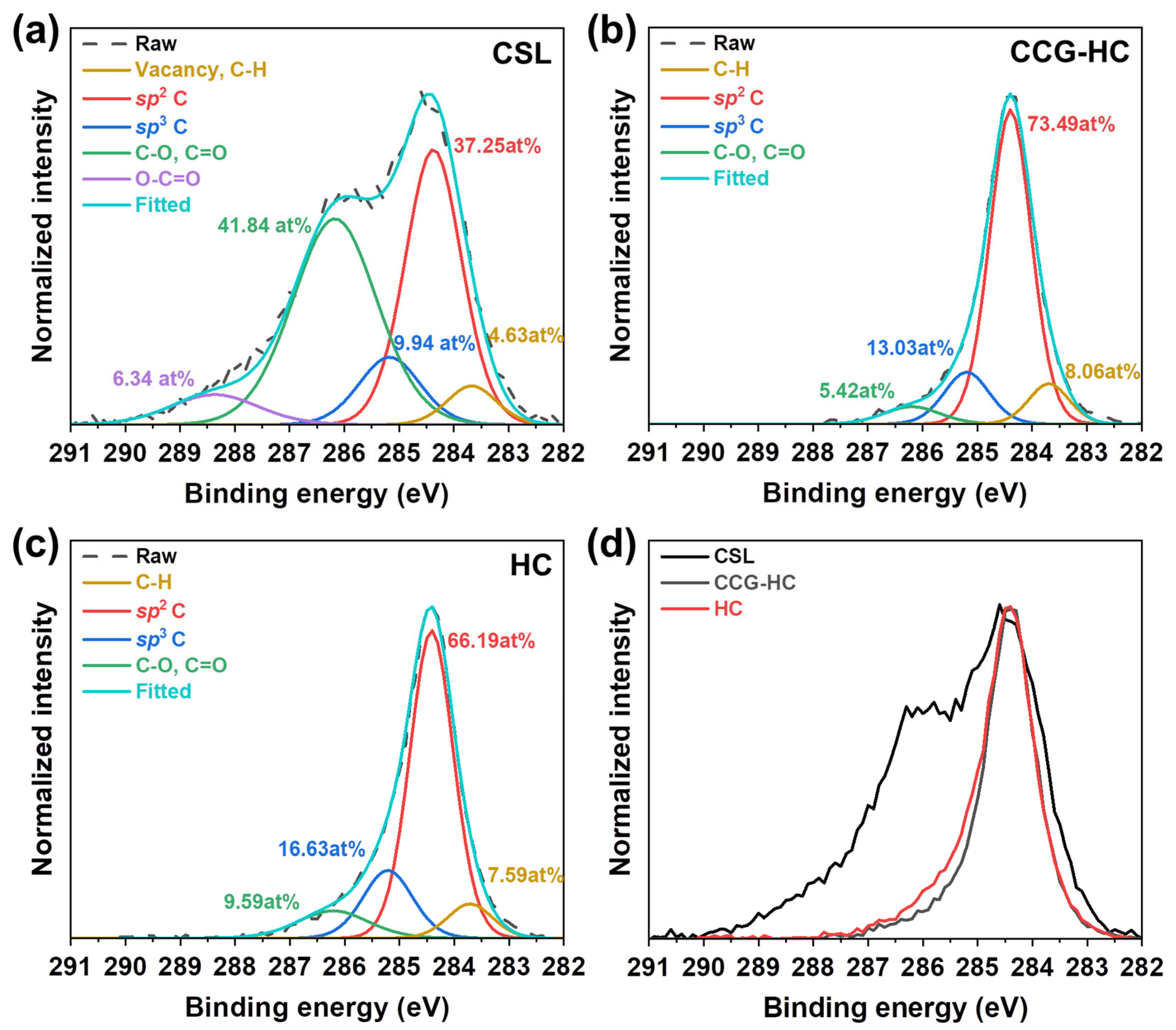
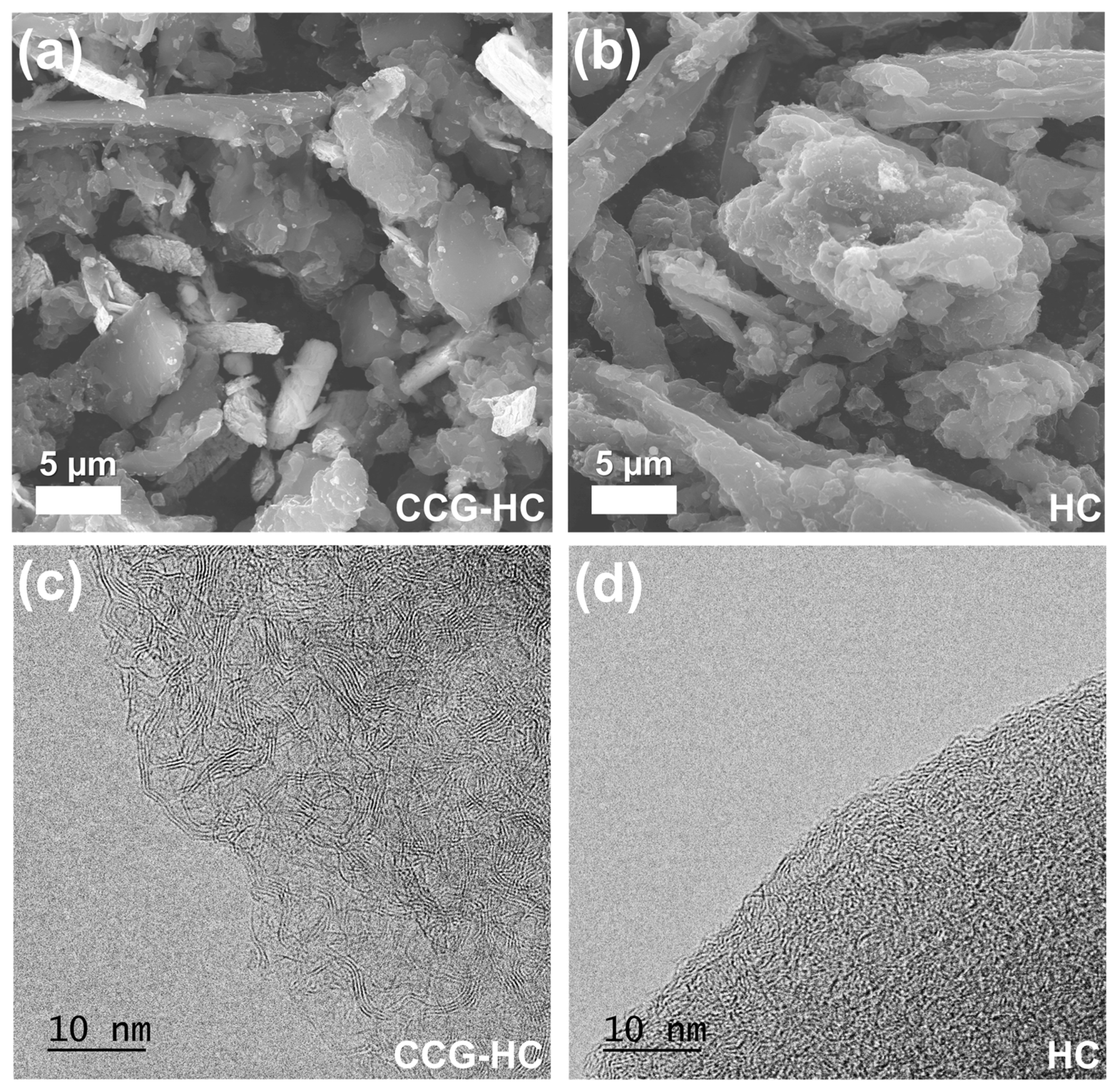
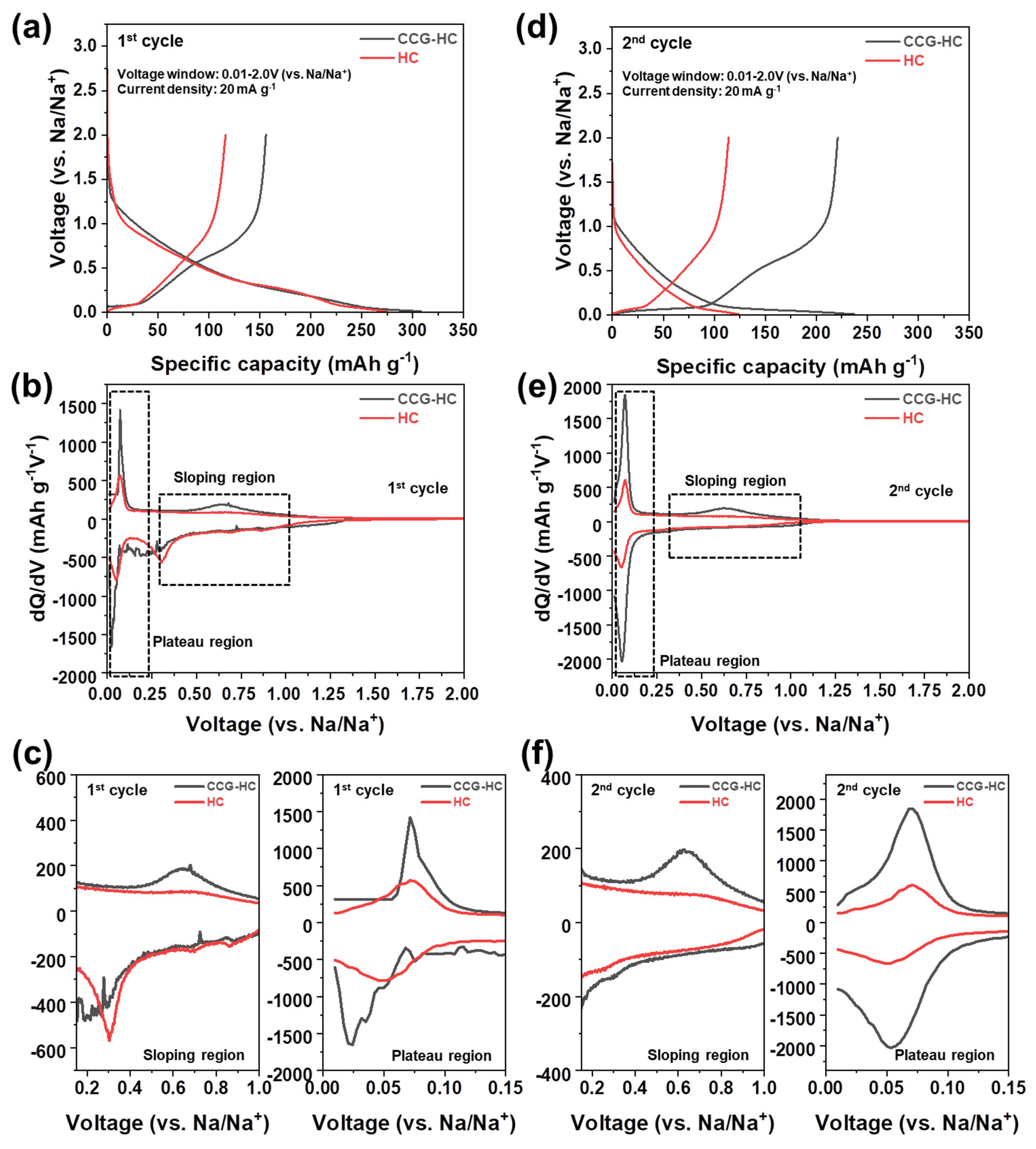
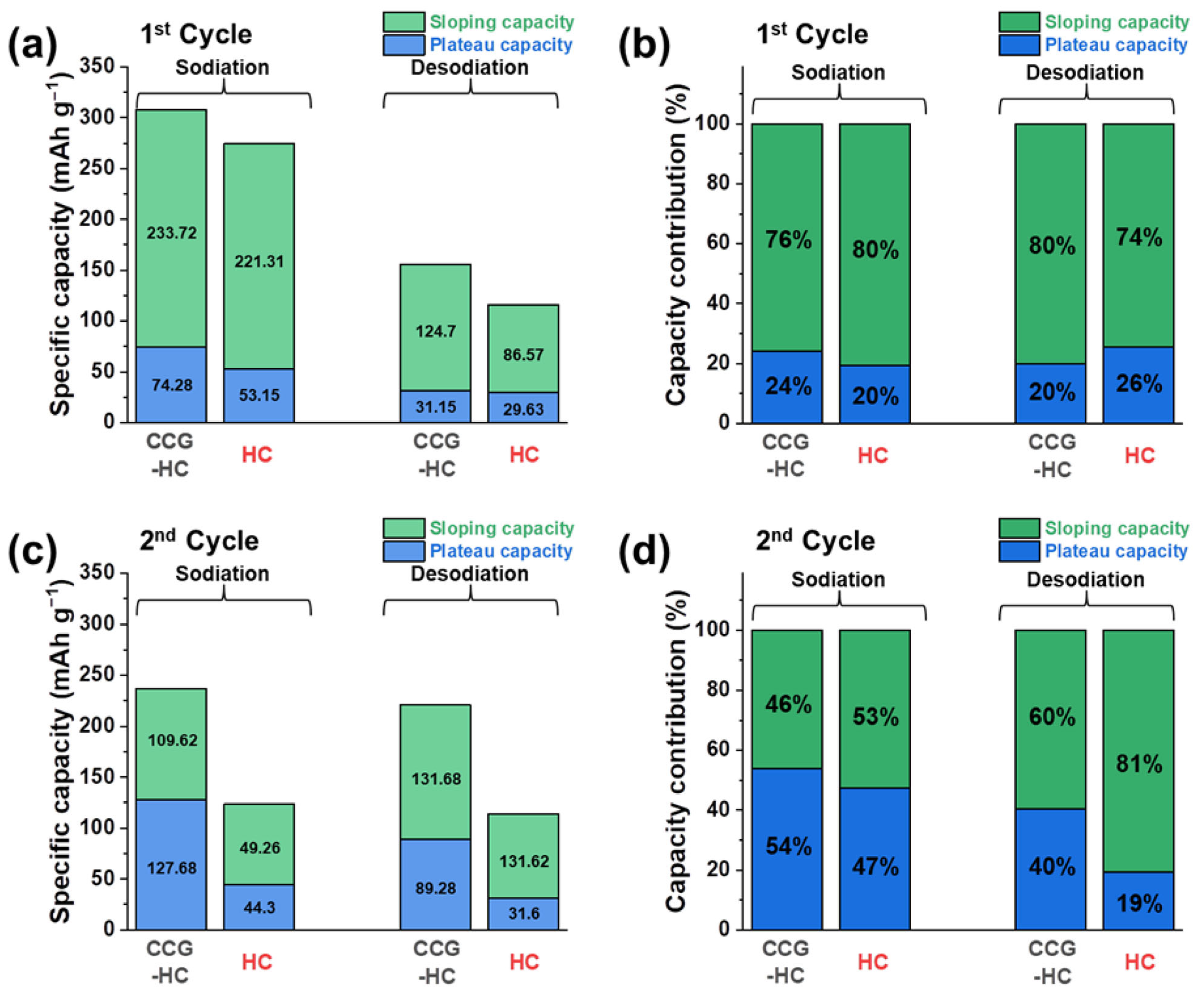
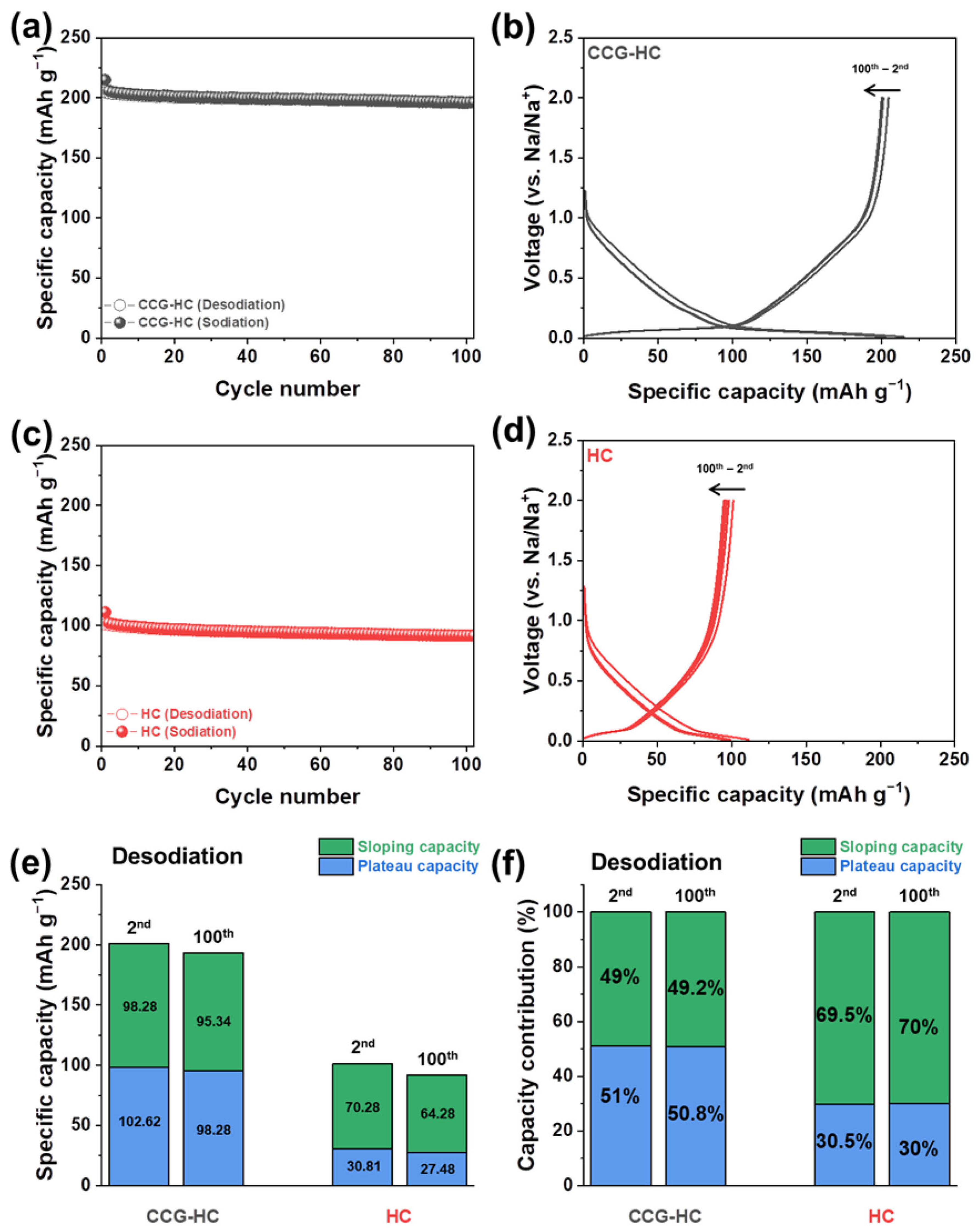
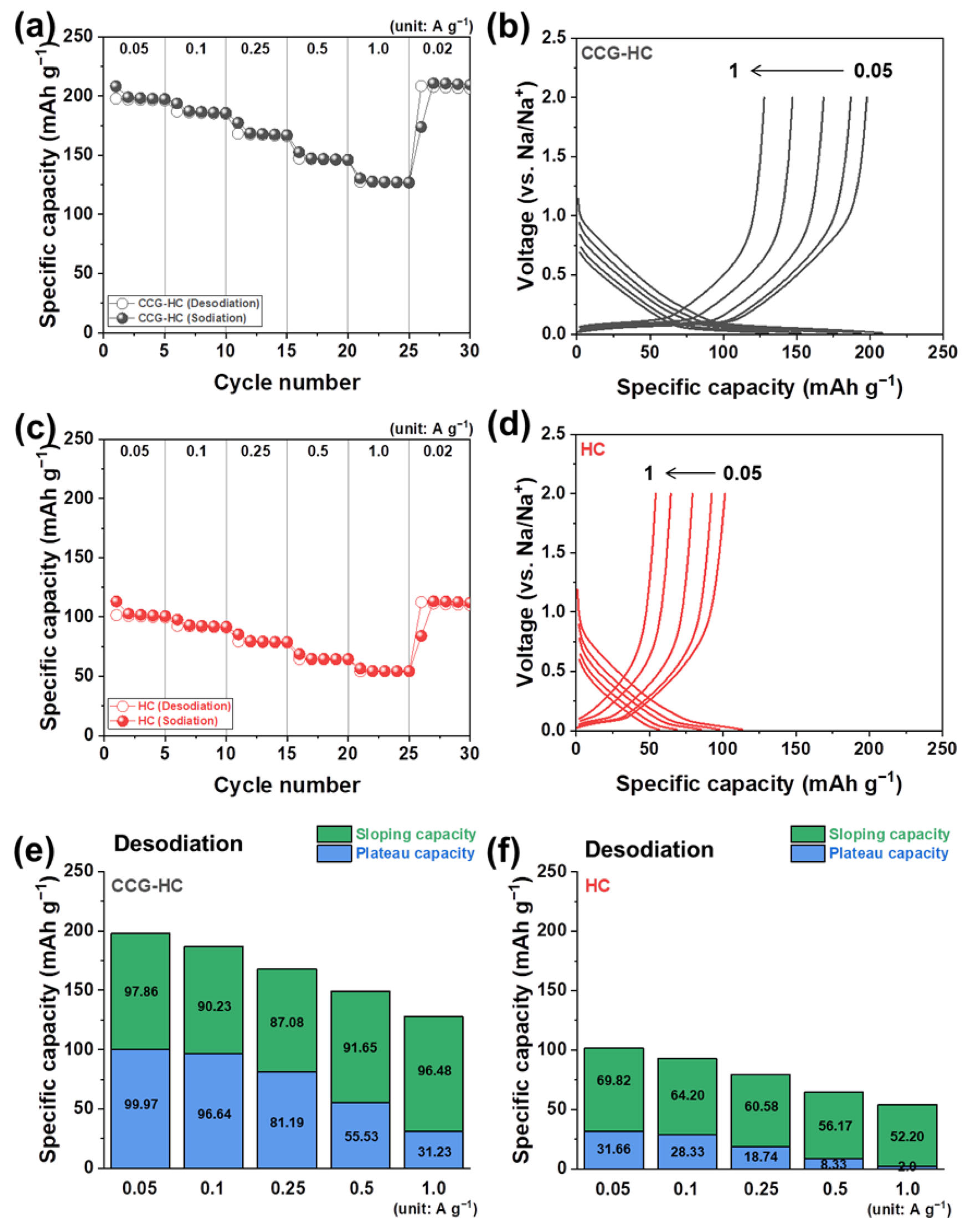
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Pasta, M.; Wessells, C.D.; Huggins, R.A.; Cui, Y. A high-rate and long cycle life aqueous electrolyte battery for grid-scale energy storage. Nat. Commun. 2012, 3, 1149. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Muhammad, S.; Jo, M.R.; Kim, H.; Song, K.; Agyeman, D.A.; Kim, Y.-I.; Yoon, W.-S.; Kang, Y.-M. In situ analyses for ion storage materials. Chem. Soc. Rev. 2016, 45, 5717–5770. [Google Scholar] [CrossRef] [PubMed]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-ion batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Vaalma, C.; Buchholz, D.; Weil, M.; Passerini, S. A cost and resource analysis of sodium-ion batteries. Nat. Rev. Mater. 2018, 3, 18013. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Seo, D.-H.; Ma, X.; Ceder, G.; Kang, K. Electrode materials for rechargeable sodium-ion batteries: Potential alternative to current lithium-ion batteries. Adv. Energy Mater. 2012, 2, 710–721. [Google Scholar] [CrossRef]
- Xiang, X.; Zhang, K.; Chen, J. Recent advances and prospects of cathode materials for sodium-ion batteries. Adv. Mater. 2015, 27, 5343–5364. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Maughan, A.E.; Teeter, G.; Tremolet de Villers, B.J.; Bak, S.-M.; Han, S.-D. Structural stabilization of P2-type sodium iron manganese oxides by electrochemically inactive Mg-substitution: Insights of redox behavior and voltage decay. ChemSusChem 2020, 13, 5972–5982. [Google Scholar] [CrossRef]
- Berthelot, R.; Cartlier, D.; Delmas, C. Electrochemical investigation of the P2-NaxCoO2 phase diagram. Nat. Mater. 2010, 10, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.R.; Kim, Y.; Yang, J.; Jeong, M.; Song, K.; Kim, Y.-I.; Lim, J.-M.; Cho, M.; Shin, J.-H.; Kim, Y.-M. Triggered reversible phase transformation between layered and spinel structure in manganese-based layered compounds. Nat. Commun. 2019, 10, 3385. [Google Scholar] [CrossRef]
- Saravanan, K.; Mason, C.W.; Rudola, S.; Wong, K.H.; Balaya, P. The first report on excellent cycling stability and superior rate capability of Na3V2(PO4)3 for sodium ion batteries. Adv. Energy Mater. 2013, 3, 444–450. [Google Scholar] [CrossRef]
- Yang, J.; Han, D.-W.; Jo, M.R.; Song, K.; Kim, Y.-I.; Chou, S.-L.; Liu, H.-K.; Kang, Y.-M. Na3V2(PO4)3 particles partly embedded in carbon nanofibers with superb kinetics for ultra-high power sodium ion batteries. J. Mater. Chem. A 2015, 3, 1005–1009. [Google Scholar] [CrossRef]
- Yang, J.; Choi, D.; Kim, K.-S.; Kim, D.U.; Kim, J. Poly(vinylalcohol) (PVA) assisted sol-gel fabrication of porous carbon network-Na3V2(PO4)3 composites cathode for enhanced kinetics in sodium ion batteries. Polymers 2022, 14, 149. [Google Scholar] [CrossRef]
- Wessells, C.D.; Huggins, R.A.; Cui, Y. Copper hexacyanoferrate battery electrodes with long cycle life and high power. Nat. Commun. 2011, 2, 550. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Cheng, J.; Goodenough, J.B. Prussian blue: A new framework of electrode materials for sodium batteries. Chem. Commun. 2012, 48, 6544–6546. [Google Scholar] [CrossRef]
- Pasta, M.; Wang, R.Y.; Ruffo, R.; Qiao, R.; Lee, H.-W.; Shyam, B.; Guo, M.; Wang, Y.; Wray, L.A.; Yang, W.; et al. Manganese-cobalt hexacyanoferrate cathodes for sodium-ion batteries. J. Mater. Chem. A 2016, 4, 4211–4223. [Google Scholar] [CrossRef]
- Lu, Y.C.; Ma, C.; Alvarado, J.; Kidera, T.; Dimov, N.; Meng, Y.S.; Okada, S. Electrochemical properties of tin oxide anodes for sodium-ion batteries. J. Power Sources 2015, 284, 287–295. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, K.; Wing-hei Lau, V.; Lee, G.-H.; Park, M.; Kang, Y.-M. Engineering solid electrolyte interphase on red phosphorus for long-term and high-capacity sodium storage. Chem. Mater. 2019, 32, 448–458. [Google Scholar] [CrossRef]
- Li, W.J.; Chou, S.L.; Wang, J.Z.; Liu, H.K.; Dou, S.X. Simply mixed commercial red phosphorus and carbon nanotube composite with exceptionally reversible sodium-ion storage. Nano Lett. 2013, 13, 5480–5484. [Google Scholar] [CrossRef]
- Xiao, Y.; Hwang, J.Y.; Sun, Y.K. Micro-intertexture carbon-free iron sulfides as advanced high tap density anodes for rechargeable batteries. ACS Appl. Mater. Interfaces 2017, 9, 39416–39426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, X.; Li, J. Facile synthesis of nanostructured MnO2 as anode materials for sodium-ion batteries. ChemNanoMat 2016, 2, 196–200. [Google Scholar] [CrossRef]
- Yu, X.Y.; David Lou, X.W. Mixed metal sulfides for electrochemical energy storage and conversion. Adv. Energy Mater. 2018, 8, 1701592. [Google Scholar] [CrossRef]
- Jian, Z.; Liu, P.; Li, F.; Chen, M.; Zhou, H. Monodispersed hierarchical Co3O4 spheres intertwined with carbon nanotubes for use as anode materials in sodium-ion batteries. J. Mater. Chem. A 2014, 2, 13805–13809. [Google Scholar] [CrossRef]
- Nobuhara, K.; Nakayama, H.; Nose, M.; Nakanishi, S.; Iba, H. First-principles study of alkali metal-graphite intercalation compounds. J. Power Sources 2013, 243, 585–587. [Google Scholar] [CrossRef]
- Moriwake, H.; Kuwabara, A.; Fisher, C.A.J.; Ikuhara, Y. Why is sodium-intercalated graphite unstable? RSC Adv. 2017, 7, 36550–36554. [Google Scholar] [CrossRef]
- Li, W.; Li, M.; Adair, K.R.; Sun, X.; Yu, Y. Carbon nanofiber-based nanostructures for lithium-ion and sodium-ion batteries. J. Mater. Chem. A 2017, 5, 13882–13906. [Google Scholar] [CrossRef]
- Tang, K.; Fu, L.; White, L.; Yu, M.-M.; Titirici, M.-M.; Antonietti, M.; Maier, J. Hollow carbon nanospheres with superior rate capability for sodium-based batteries. Adv. Energy Mater. 2012, 2, 873–877. [Google Scholar] [CrossRef]
- Wang, P.; Qiao, B.; Du, Y.; Li, Y.; Zhou, X.; Dai, Z.; Bao, J. Fluorine-doped carbon particles derived from Lotus petioles as high-performance anode materials for sodium-ion batteries. J. Phys. Chem. C 2015, 119, 21336–21344. [Google Scholar] [CrossRef]
- Hongshuai, H.; Xiaoqing, Q.; Weifeng, W.; Yun, Z.; Xiaobo, J. Carbon anode materials for advanced sodium-ion batteries. Adv. Energy Mater. 2017, 7, 1602898. [Google Scholar]
- Simone, V.; Boulineau, A.; Geyer, A.; Rouchon, D.; Simonin, L.; Martiner, S. Hard carbon derived from cellulose as anode for sodium ion batteries: Dependence of electrochemical properties on structure. J. Energy Chem. 2016, 25, 761–768. [Google Scholar] [CrossRef]
- Zhao, L.-F.; Hu, Z.; Lai, W.-H.; Tao, Y.; Peng, J.; Miao, Z.-C.; Wang, Y.-X.; Chou, S.-L.; Liu, H.-K.; Dou, S.-X. Hard carbon anodes: Fundamental understanding and commercial perspectives for Na-ion batteries beyond Li-ion and K-ion counterparts. Adv. Energy Mater. 2021, 11, 2002704. [Google Scholar] [CrossRef]
- Kim, J.-B.; Lee, G.-H.; Wing-hei Lau, V.; Zhang, J.; Zou, F.; Chen, M.; Zhou, L.; Nam, K.-Y.; Kang, Y.-M. Microstructural investigation into Na-ion storage behaviors of cellulose-based hard carbons for Na-ion batteries. J. Phys. Chem. C 2021, 125, 14559–14566. [Google Scholar] [CrossRef]
- Alvin, S.; Yoon, D.; Chandra, C.; Cahyadi, H.S.; Park, J.-H.; Chang, W.; Chung, K.Y.; Kim, J. Revealing sodium ion storage mechanism in hard carbon. Carbon 2019, 145, 67–81. [Google Scholar] [CrossRef]
- Lee, G.-H.; Hwang, T.; Kim, J.-B.; Yang, J.; Zou, F.; Cho, M.; Kang, Y.-M. Origin of enhanced reversible Na ion storage in hard carbon anodes through p-type molecular doping. J. Mater. Chem. A 2022, 10, 16506–16513. [Google Scholar] [CrossRef]
- Luo, W.; Bommier, C.; Jian, Z.; Li, X.; Carter, R.; Vail, S.; Lu, Y.; Lee, J.-J.; Ji, X. Low-Surface-Area Hard Carbon Anode for Na-Ion Batteries via Graphene Oxide as a Dehydration Agent. ACS Appl. Mater. Interfaces 2015, 7, 2626–2631. [Google Scholar] [CrossRef] [PubMed]
- Lotfabad, E.M.; Ding, J.; Cui, K.; Kohandehghan, A.; Kalisvaart, W.P.; Hazelton, M.; Mitlin, D. High-density sodium and lithium ion battery anodes from banana peels. ACS Nano 2014, 8, 7115–7129. [Google Scholar] [CrossRef]
- Hong, J.; Qie, L.; Zeng, R.; Yi, Z.; Zhang, W.; Wang, D.; Yin, W.; Wu, C.; Fan, Q.-J.; Zhang, W.-X.; et al. Biomass derived hard carbon used as a high performance anode material for sodium ion batteries. J. Mater. Chem. A 2014, 2, 12733–12738. [Google Scholar] [CrossRef]
- Wu, L.; Buchholz, D.; Vaalma, C.; Giffin, G.A.; Passerini, S. Apple biowaste derived hard carbon as a powerful anode material for Na ion batteries. ChemElectroChem 2016, 3, 292–298. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, F. Naturally derived nanostructured materials from biomass for rechargeable lithium/sodium batteries. Nano Energy 2015, 17, 91–103. [Google Scholar] [CrossRef]
- Shen, F.; Luo, W.; Dai, J.; Yao, Y.; Zhu, M.; Hitz, E.; Tang, Y.; Chen, Y.; Sprenkle, V.L.; Li, X.; et al. Ultra-thick, low-totuosity, and mesoporous wood carbon anode for high-performance sodium-ion batteries. Adv. Energy Mater. 2016, 6, 1600377. [Google Scholar] [CrossRef]
- Sagues, W.J.; Yang, J.; Moroe, N.; Han, S.-D.; Vinzant, T.; Yung, M.; Jameel, H.; Nimlos, M.; Park, S. A simple method for producing bio-based anode materials for lithium-ion batteries. Green Chem. 2020, 22, 7093–7108. [Google Scholar] [CrossRef]
- Kim, J.; Han, S.D.; Koo, B.; Lee, S.H.; Yang, J. Structure Dependent Electrochemical Behaviors of Hard Carbon Anode Materials Derived from Natural Polymer for Next-Generation Sodium Ion Battery. Polymers 2023, 15, 4373. [Google Scholar] [CrossRef]
- Yoon, D.; Hwang, J.; Chang, W.; Kim, J. Carbon with expanded and well-developed graphene planes derived directly from condensed lignin as a high-performance anode for sodium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 569–581. [Google Scholar] [CrossRef]
- Brown, T.R.; Brown, R.C. A review of cellulosic biofuel commercial-scale projects in the United States. Biofuel Bioprod. Biorefin. 2013, 7, 235–245. [Google Scholar] [CrossRef]
- Madhu, R.; Periasamy, A.P.; Schlee, P.; Herou, S.; Titirici, M.-M. Lignin: A sustainable precursor for nanostructured carbon materials for supercapacitos. Carbon 2023, 207, 172–197. [Google Scholar] [CrossRef]
- Xi, Y.; Liu, X.; Xiong, W.; Wang, H.; Ji, X.; Kong, F.; Yang, G.; Xu, J. Converting amorphous kraft lignin to hollow carbon shell frameworks as electrode materials for lithium-ion batteries and supercapacitors. Ind. Crops Prod. 2021, 174, 114184. [Google Scholar] [CrossRef]
- Wang, T.; Shi, Z.; Zhong, Y.; Ma, Y.; He, J.; Zhu, Z.; Cheng, X.; Lu, B.; Wu, Y. Biomass-Derived Materials for Advanced Rechargeable Batteries. Small 2024, 20, 2310907. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Lee, S.; Choi, J.; Chung, Y.S.; Kim, J.; Moon, S.; Lee, S. Understanding the catalytic mechanism of calcium compounds for enhancing crystallinity in carbon fiber. Chem. Eng. J. 2024, 479, 147728. [Google Scholar] [CrossRef]
- Beguerie, T.; Weiss-Hortala, E.; Lyczko, N.; Nzihou, A. The mechanisms of calcium-catalyzed graphenization of cellulose and lignin biochars uncovered. Sci. Rep. 2023, 13, 11390. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; An, B.; Lahiri, A.; Wang, P.; Fang, Y. Doublet of D and 2D bands in graphene deposited with Ag nanoparticles by surface enhanced Raman spectroscopy. Carbon 2013, 65, 359–364. [Google Scholar] [CrossRef]
- Park, S.; Jeong, H.; Kim, B.J.; Lee, Y.K.; Yang, J.; Kim, J. Structural distinction of zigzag-edge coronoids analyzed by spectroscopies. Carbon 2023, 213, 118248. [Google Scholar] [CrossRef]
- Jeong, H.; Park, S.; Yang, J.; Lee, H.M.; An, S.; Yamada, Y.; Kim, J. Spectroscopic distinction of carbon nanobelts and nanohoops. Carbon 2023, 201, 829–836. [Google Scholar] [CrossRef]
- Kim, J.; Lee, N.; Min, Y.H.; Noh, S.; Kim, N.K.; Jung, S.; Joo, M.; Yamada, Y. Distinguishing zigzag and armchair edges on graphene nanoribbons by X-ray photoelectron and Raman spectroscopies. ACS Omega 2018, 3, 17789–17796. [Google Scholar] [CrossRef] [PubMed]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Kim, J.; Yamada, Y.; Suzuki, Y.; Ciston, J.; Sato, S. Pyrolysis of Epoxidized Fullerenes Analyzed by Spectroscopies. J. Phys. Chem. C 2014, 118, 7076–7084. [Google Scholar] [CrossRef]
- Yamada, Y.; Ysuda, H.; Murota, K.; Nakamura, M.; Sodesawa, T.; Sato, S. Analysis of heat-treated graphite oxide by X-ray photoelectron spectroscopy. J. Mater. Sci. 2013, 48, 8171. [Google Scholar] [CrossRef]
- Beda, A.; Taberna, P.-L.; Simon, P.; Ghimbeu, C.M. Hard carbons derived from green phenolic resins for Na-ion batteries. Carbon 2018, 139, 248–257. [Google Scholar] [CrossRef]
- Kim, J.; Yu, D.; Oh, E.; Jang, J.; Kim, J.; Yang, J. Carbonization temperature dependent structural modifications of waste coffee grounds derived hard carbons and their electrochemical behaviors as anode materials for sodium ion batteries. Carbon Lett. 2024. [Google Scholar] [CrossRef]
- Chen, X.; Liu, C.; Fang, Y.; Ai, X.; Zhong, F.; Yang, H.; Cao, Y. Understanding of the sodium storage mechanism in hard carbon anodes. Carbon Energy 2022, 4, 1133–1150. [Google Scholar] [CrossRef]
- Gnanaraj, J.S.; Thompson, R.W.; Iaconatti, S.N.; Dicarlo, J.F.; Abraham, K.M. Formation and Growth of Surface Films on Graphitic Anode Materials for Li-Ion Batteries. Electrochem. Solid State Lett. 2005, 8, A128–A132. [Google Scholar] [CrossRef]
- Levi, M.D.; Aurbach, D. The mechanism of lithium intercalation in graphite film electrodes in aprotic media. Part 1. High resolution slow scan rate cyclic voltammetric studies and modeling. J. Electroanal. Chem. 1997, 421, 79–88. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, H.; Oh, E.; Jang, J.; Lee, S.W.; Kim, D.H.; Han, S.D.; Kim, J.; Yang, J. Negative Effect of the Calendering Process on the Interphase Formation and Electrochemical Behavior of Reduced Graphene Oxide Electrodes. ACS Appl. Mater. Inter. 2024, 16, 56271–56284. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Lee, S.-H.; Yang, J. Revealing the Calcium Assisted Partial Catalytic Graphitization of Lignin-Derived Hard Carbon Anode and Its Electrochemical Behaviors in Sodium Ion Batteries. Polymers 2025, 17, 540. https://doi.org/10.3390/polym17040540
Kim J, Lee S-H, Yang J. Revealing the Calcium Assisted Partial Catalytic Graphitization of Lignin-Derived Hard Carbon Anode and Its Electrochemical Behaviors in Sodium Ion Batteries. Polymers. 2025; 17(4):540. https://doi.org/10.3390/polym17040540
Chicago/Turabian StyleKim, Jungpil, Sang-Hyun Lee, and Junghoon Yang. 2025. "Revealing the Calcium Assisted Partial Catalytic Graphitization of Lignin-Derived Hard Carbon Anode and Its Electrochemical Behaviors in Sodium Ion Batteries" Polymers 17, no. 4: 540. https://doi.org/10.3390/polym17040540
APA StyleKim, J., Lee, S.-H., & Yang, J. (2025). Revealing the Calcium Assisted Partial Catalytic Graphitization of Lignin-Derived Hard Carbon Anode and Its Electrochemical Behaviors in Sodium Ion Batteries. Polymers, 17(4), 540. https://doi.org/10.3390/polym17040540





