Polypropylene Modified with Polyethylene Through Reactive Melt Blending: Fabrication and Characterizations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Characterization
3. Results and Discussion
3.1. FTIR Analysis and Solubility Test
3.2. Dynamic Mechanical Properties
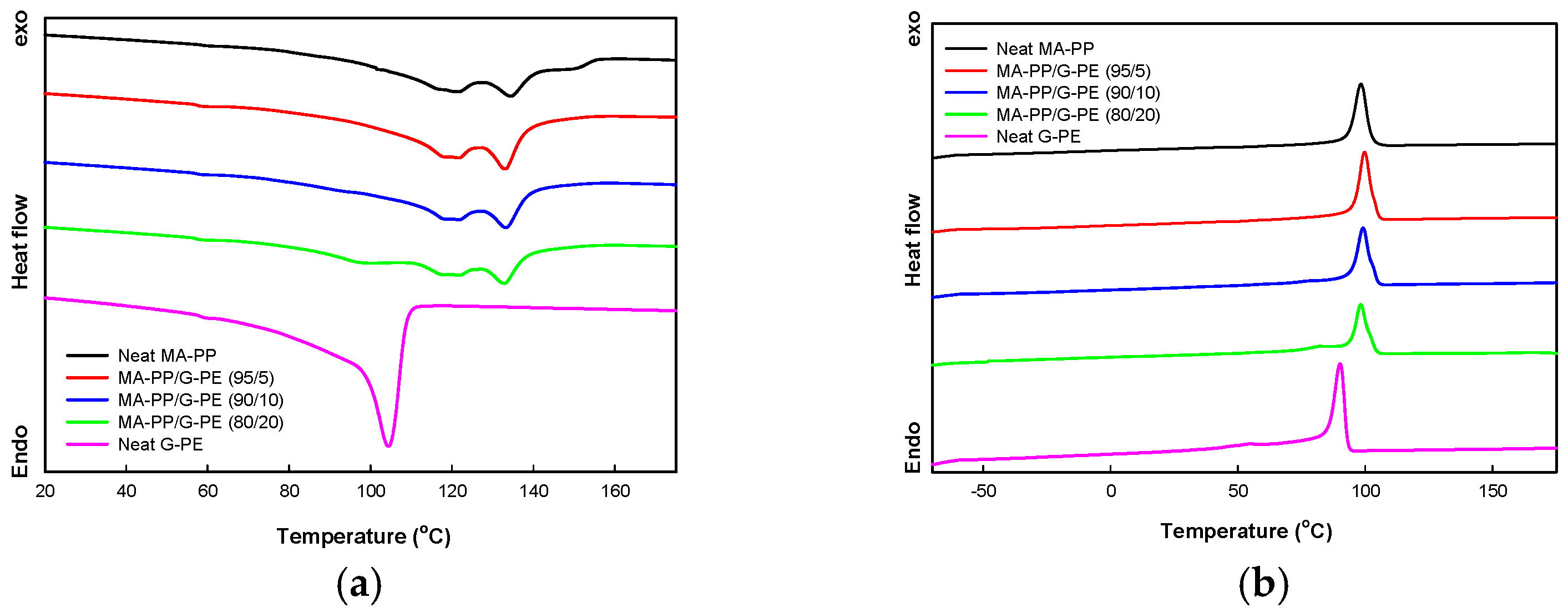
3.3. Melting and Crystallization Behavior
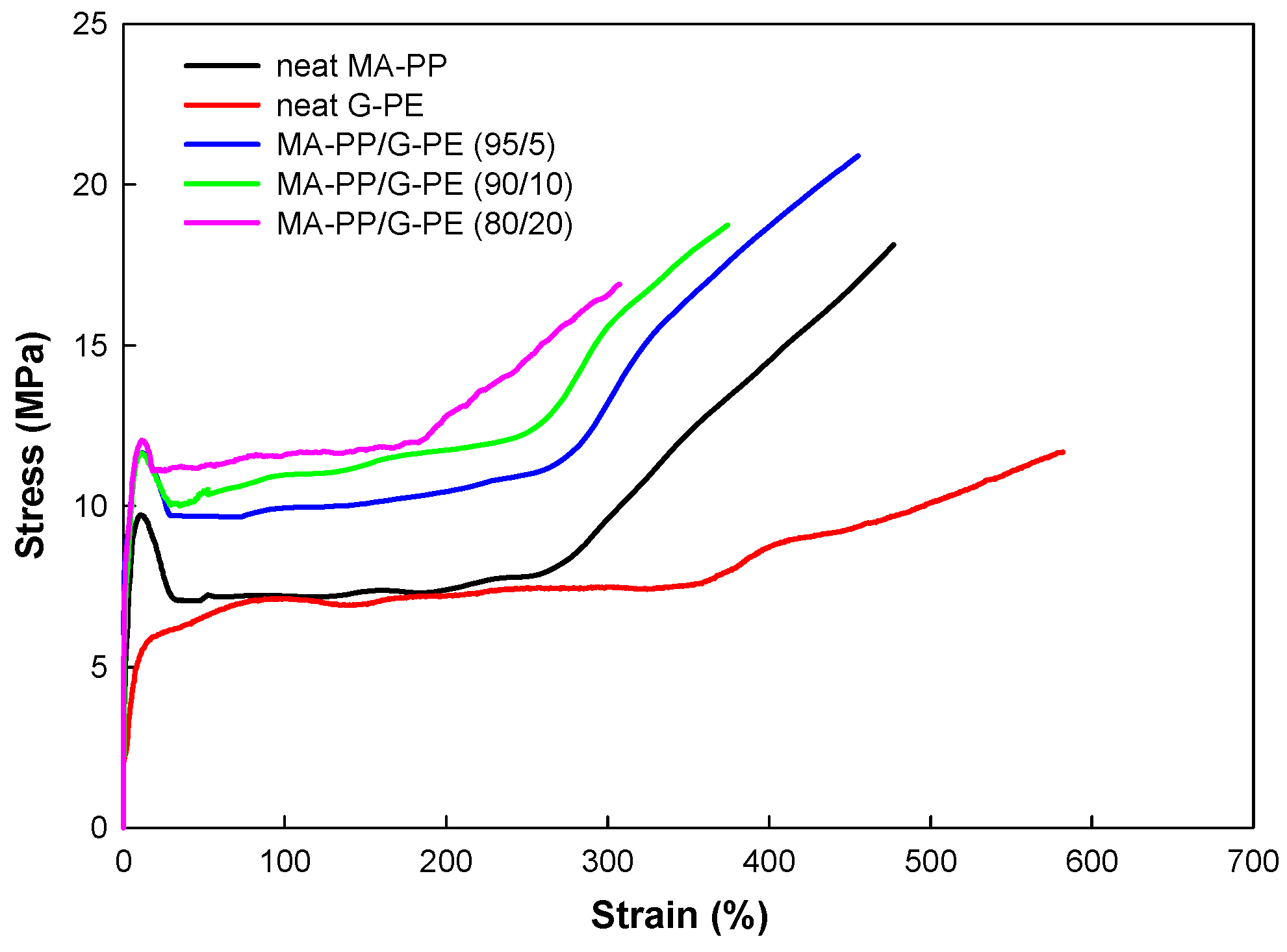
3.4. Tensile Properties
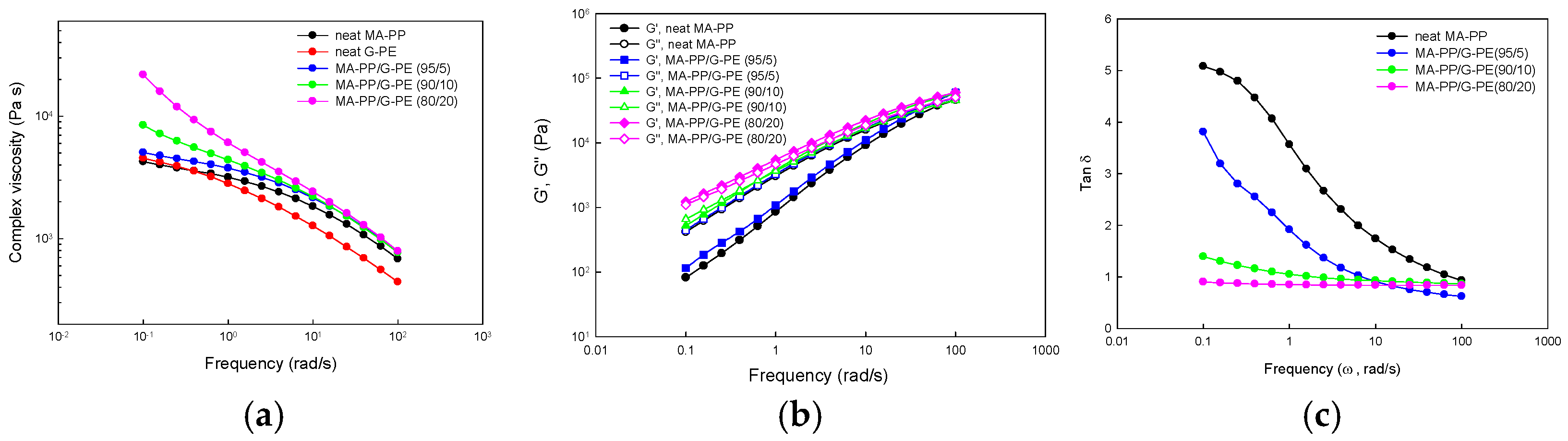
3.5. Rheological Properties
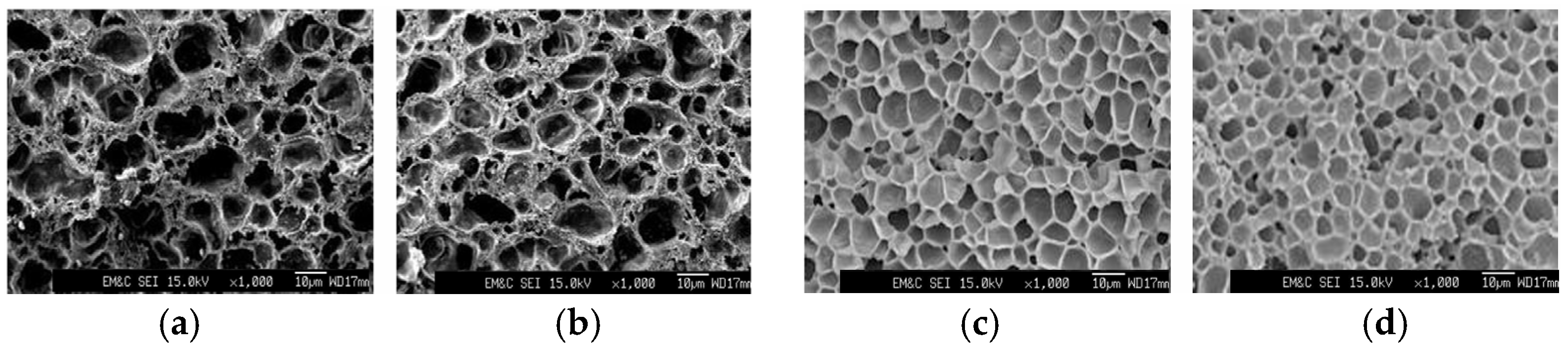
3.6. Foam Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Maddah, H.A. Polypropylene as a promising plastic: A review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar]
- Himma, N.F.; Anisah, S.; Prasetya, N.; Wenten, I.G. Advances in preparation, modification, and application of polypropylene membrane. J. Polym. Eng. 2016, 36, 329–362. [Google Scholar] [CrossRef]
- Yuan, X.; Chung, T.C.M. Cross-linking effect on dielectric properties of polypropylene thin films and applications in electric energy storage. Appl. Phys. Lett. 2011, 98, 062901. [Google Scholar] [CrossRef]
- Cheng, L.; Chi, X.; Yan, C.; Xie, D.; Liu, X.; Wen, Y.; Liu, W.; Li, S. Polypropylene nanocomposite for power equipment: A review. IET Nanodielectr. 2018, 1, 92–103. [Google Scholar] [CrossRef]
- Du, B.; Li, Z.; Zhou, S.; Fan, M. Research progress and perspective of polypropylene-based insulation for HVDC cables. J. Electr. Eng. 2021, 16, 2–11. [Google Scholar]
- Hossain, M.T.; Shahid, M.A.; Mahmud, N.; Habib, A.; Rana, M.S.; Khan, S.A.; Hossain, M.D. Research and applications of polypropylene: A review. Discov. Nano 2024, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi, A.; Mighri, F.; Ajji, A.; Rodrigue, D. Current issues and challenges in polypropylene foaming: A review. Cell. Polym. 2015, 34, 299–338. [Google Scholar] [CrossRef]
- Stange, J.; Münstedt, H. Rheological properties and foaming behavior of polypropylenes with different molecular structures. J. Rheol. 2006, 50, 907–923. [Google Scholar] [CrossRef]
- He, C.; Costeux, S.; Wood-Adams, P.; Dealy, J. Molecular structure of high melt strength polypropylene and its application to polymer design. Polymer 2003, 44, 7181–7188. [Google Scholar] [CrossRef]
- Graebling, D. Synthesis of branched polypropylene by a reactive extrusion process. Macromolecules 2002, 35, 4602–4610. [Google Scholar] [CrossRef]
- Lin, W.; Shao, Z.; Dong, J.; Chung, T.C.M. Crosslinked polypropylene prepared by PP copolymers containing flexible styrene groups. Macromolecules 2009, 42, 3750–3754. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, K.J. Crosslinking of polypropylene by peroxide and multifunctional monomer during reactive extrusion. Adv. Polym. Technol. 1993, 12, 263–269. [Google Scholar] [CrossRef]
- Tian, J.; Yu, W.; Zhou, C. The preparation and rheology characterization of long chain branching polypropylene. Polymer 2006, 47, 7962–7969. [Google Scholar] [CrossRef]
- Su, F.; Huang, H.X. Rheology and melt strength of long chain branching polypropylene prepared by reactive extrusion with various peroxides. Polym. Eng. Sci. 2010, 50, 342–350. [Google Scholar] [CrossRef]
- Stanic, S.; Gottlieb, G.; Koch, T.; Gopperl, L.; Schmid, K.; Knaus, S.; Archodoulaki, V.M. Influence of different type of peroxides on the long-chain branching of PP via reactive extrusion. Polymers 2020, 12, 886. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Lu, Y.; Wei, G.; Zhang, X.; Liu, Y.; Qiao, J. Effect of radiation on the crosslinking and branching of polypropylene. J. Appl. Polym. Sci. 2002, 85, 1758–1764. [Google Scholar] [CrossRef]
- Lugao, A.B.; Artel, B.W.H.; Yoshiga, A.; Lima, L.F.C.P.; Parra, D.F.; Bueno, J.R.; Liberman, S.; Farrah, M.; Tercariol, W.R.; Otaguro, T.H. Production of high melt strength polypropylene by gamma irradiation. Rad. Phys. Chem. 2007, 76, 1691–1695. [Google Scholar] [CrossRef]
- Yoshii, F.; Makuuchi, K.; Kikukawa, S.; Tanaka, T.; Saitoh, J.; Koyama, K. High- melt-strength polypropylene with electron beam irradiation in the presence of polyfunctional monomers. J. Appl. Polym. Sci. 1996, 60, 617–623. [Google Scholar] [CrossRef]
- Yang, M.; Li, J.; Guo, S. A reactive extrusion process with the aid of ultrasound for preparing crosslinked polypropylene. Polym. Eng. Sci. 2017, 57, 821–829. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, M.C.; Song, H.Y.; Hyun, K.; Hong, S.C. Preparation and characteristics of polypropylene with long chain branches utilizing the C-H insertion capability of azidoformate. Polym. Test. 2022, 116, 107792. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, M.C.; Song, H.Y.; Hyun, K.; Hong, S.C. Preparation of polypropylene with strain hardening characteristics via the noncatalytic C-H insertion of azidoformate. ACS Appl. Polym. Mater. 2023, 5, 420–428. [Google Scholar] [CrossRef]
- Tang, H.; Dai, W.; Chen, B. A new method for producing high melt strength polypropylene with reactive extrusion. Polym. Eng. Sci. 2008, 48, 1339–1344. [Google Scholar] [CrossRef]
- Guapacha, J.; Valles, E.M.; Quinzani, L.M.; Failla, M.D. Long-chain branched polypropylene obtained using an epoxy resin as crosslinking agent. Polym. Bull. 2017, 74, 2297–2318. [Google Scholar] [CrossRef]
- Létofféa, A.; García-Rodrígueza, S.M.; Hoppe, S.; Canilho, N.; Godard, O.; Pasc, A.; Royaud, I.; Ponçot, M. Switching from brittle to ductile isotactic polypropylene-g-maleic anhydride by crosslinking with capped-end polyether diamine. Polymer 2019, 164, 67–78. [Google Scholar] [CrossRef]
- Létofféa, A.; Hoppe, S.; Lainé, R.; Canilho, N.; Pasc, A.; Rouxel, D.; Jiménez Riobóo, R.J.; Hupont, S.; Royaud, I.; Ponçot, M. Resilience improvement of an isotactic polypropylene-g-maleic anhydride by crosslinking using polyether triamine agents. Polymer 2019, 179, 121655. [Google Scholar] [CrossRef]
- Li, Y.; Yao, Z.; Chen, Z.H.; Qiu, S.L.; Zeng, C.; Cao, K. High melt strength polypropylene by ionic modification: Preparation, rheological properties and foaming behaviors. Polymer 2015, 70, 207–214. [Google Scholar] [CrossRef]
- Maroofy, H.N.; Hafezi, M.J. Ionic cross-linking of polypropylene: Effect of maleic anhydride grafting temperature and ionic plasticizer. J. Polym. Res. 2024, 31, 243. [Google Scholar] [CrossRef]
- Muljana, H.; Arends, S.; Remerie, K.; Boven, G.; Picchioni, F. Crosslinking of polypropylene via the Diels-Alder reaction. Polymers 2022, 14, 1176. [Google Scholar] [CrossRef]
- Muljana, H.; Remerie, K.; Boven, G.; Picchioni, F.; Bpse, R.K. Crosslinking of polypropylene with thiophene and imidazole. Polymers 2022, 14, 2198. [Google Scholar] [CrossRef]
- Ali, M.A.; Okamoto, K.; Yamaguchi, M.; Kasai, T.; Koshirai, A. Rheological properties for polypropylene modified by polytetrafluoroethylene. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 2008–2014. [Google Scholar] [CrossRef]
- Fujii, Y.; Nishikawa, R.; Phulkerd, P.; Yamaguchi, M. Modifying the rheological properties of polypropylene under elongational flow by adding polyethylene. J. Rheol. 2019, 63, 11–18. [Google Scholar] [CrossRef]
- Lopez-Barron, C.R.; Tsou, A.H. Strain hardening of polyethylene/polypropylene blends via interfacial reinforcement with poly(ethylene-cb-propylene) comb block copolymers. Macromolecules 2017, 50, 2986–2995. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, B.; Liu, X. Preparation of polypropylene blends with the long chain branched behavior through reactive blending induced by pre-irradiation. Polymer 2020, 177, 109188. [Google Scholar] [CrossRef]
- Wu, M.H.; Wang, C.C.; Chen, C.Y. Preparation of high melt strength polypropylene by addition of an ionically modified polypropylene. Polymer 2020, 202, 122743. [Google Scholar] [CrossRef]
- Han, X.; Zeng, C.; Lee, L.J.; Koelling, K.W.; Tomasko, D.L. Extrusion of polystyrene nanocomposite foams with supercritical CO2. Polym. Eng. Sci. 2003, 43, 1261. [Google Scholar] [CrossRef]
- Xu, Z.M.; Jiang, X.L.; Liu, T.; Hu, G.H.; Zhao, L.; Zhu, Z.N.; Yuan, W.K. Foaming of polypropylene with supercritical carbon dioxide. J. Supercrit. Fluids 2007, 41, 299–310. [Google Scholar] [CrossRef]
- Lee, K.; Chang, Y.W.; Kim, S.W. Ethylene–propylene-diene terpolymer/halloysite nanocomposites: Thermal, mechanical properties, and foam processing. J. Appl. Polym. Sci. 2014, 131, 40307. [Google Scholar] [CrossRef]
- Bettini, S.H.P.; Agnelli, J.A.M. Grafting of maleic anhydride onto polypropylene by reactive extrusion. J. Appl. Polym. Sci. 2002, 85, 2706–2717. [Google Scholar] [CrossRef]
- Shi, D.; Yang, J.; Yao, Z.; Wang, Y.; Huang, H.; Jing, W.; Yin, J.; Costa, G. Functionalization of isotactic polypropylene with maleic anhydride by reactive extrusion. Polymer 2001, 42, 5549–5557. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeon, H.B.; Hwang, G.H.; Kwon, Y.S.; Lee, J.S.; Park, G.T.; Kim, S.Y.; Kang, H.E.; Choi, E.J.; Jang, S.H.; et al. Effects of poly(ethylene-co-glycidyl methacrylate) on the microstructure, thermal, rheological, and mechanical properties of thermotropic liquid crystalline polyester blends. Polymers 2020, 12, 2124. [Google Scholar] [CrossRef] [PubMed]
- Mauri, M.; Tran, N.; Prieto, O.; Hjertberg, T.; Müller, C. Crosslinking of an ethylene-glycidyl methacrylate copolymer with amine click chemistry. Polymer 2017, 111, 24–35. [Google Scholar] [CrossRef]
- Sung, Y.T.; Kim, C.K.; Lee, H.S.; Kim, J.S.; Yoon, H.G.; Kim, W.N. Effects of crystallinity and crosslinking on the thermal and rheological properties of ethylene vinyl acetate copolymer. Polymer 2005, 46, 11844–11848. [Google Scholar] [CrossRef]
- Mishra, J.K.; Chang, Y.W.; Kim, W. The effect of peroxide crosslinking on thermal, mechanical, and rheological properties of polycaprolactone/epoxidized natural rubber blends. Polym. Bull. 2011, 66, 673–681. [Google Scholar] [CrossRef]
- Yan, D.; Wang, W.J.; Zhu, S. Effect of long chain branching on rheological properties of metallocene polyethylene. Polymer 1999, 40, 1737–1744. [Google Scholar] [CrossRef]
- Jahani, Y.; Ghetmiri, M.; Vaseghi, M.R. The effects of long-chain branching of polypropylene and chain-extension of poly (ethylene terephthalate) on the thermal behavior, rheology and morphology of their blends. RSC Adv. 2015, 28, 21620–21628. [Google Scholar] [CrossRef]



| Sample | E’ at 150 °C (MPa) | Tg of MA-PP (°C) | Tg of G-PE (°C) |
|---|---|---|---|
| Neat MA-PP | 0 | −0.04 | |
| Neat G-PE | 0 | - | −47.2 |
| MA-PP/G-PE | |||
| 95/5 | 0.026 | 0.21 | - |
| 90/10 | 0.031 | 0.65 | - |
| 80/20 | 0.054 | 1.41 | - |
| Sample | Tm of MA-PP (°C) | Tm of G-PE (°C) | Tc of MA-PP (°C) |
|---|---|---|---|
| Neat MA-PP | 121.8, 134.4 | - | 98.1 |
| Neat G-PE | - | 104.4 | - |
| MA-PP/G-PE | |||
| 95/5 | 121.8, 133.2 | - | 99.7 |
| 90/10 | 121.8, 133.0 | - | 99.2 |
| 80/20 | 121.9, 132.8 | 100.7 | 98.3 |
| Sample | Young’s Modulus (MPa) | Yield Stress (MPa) | Tensile Strength (MPa) | Elongation-at-Break (%) |
|---|---|---|---|---|
| Neat MA-PP | 220 ± 4.2 | 9.5 ± 0.1 | 18.1 ± 0.2 | 480 ± 18 |
| Neat G-PE | 77 ± 2.1 | - | 11.7 ± 0.1 | 580 ± 22 |
| MA-PP/G-PE | ||||
| 95/5 | 215 ± 3.3 | 11.6 ± 0.1 | 20.9 ± 0.2 | 450 ± 18 |
| 90/10 | 210 ± 3.6 | 11.6 ± 0.1 | 18.7 ± 0.2 | 370 ± 12 |
| 80/20 | 215 ± 3.5 | 12.0 ± 0.1 | 16.9 ± 0.1 | 310 ± 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, C.; Jang, Y.; Chang, Y.-W. Polypropylene Modified with Polyethylene Through Reactive Melt Blending: Fabrication and Characterizations. Polymers 2025, 17, 49. https://doi.org/10.3390/polym17010049
Lim C, Jang Y, Chang Y-W. Polypropylene Modified with Polyethylene Through Reactive Melt Blending: Fabrication and Characterizations. Polymers. 2025; 17(1):49. https://doi.org/10.3390/polym17010049
Chicago/Turabian StyleLim, Changgyu, Yujin Jang, and Young-Wook Chang. 2025. "Polypropylene Modified with Polyethylene Through Reactive Melt Blending: Fabrication and Characterizations" Polymers 17, no. 1: 49. https://doi.org/10.3390/polym17010049
APA StyleLim, C., Jang, Y., & Chang, Y.-W. (2025). Polypropylene Modified with Polyethylene Through Reactive Melt Blending: Fabrication and Characterizations. Polymers, 17(1), 49. https://doi.org/10.3390/polym17010049





