3.1. Lab-Scale Compounds Development and Evaluation of FR Performance
Two FR compounds were developed (FR1, FR2,
Table 1) as candidates for the manufacture of flame-retarded and ageing-resistant corrugated conduits. First of all, the thermal stability of the pure FR additives was studied (
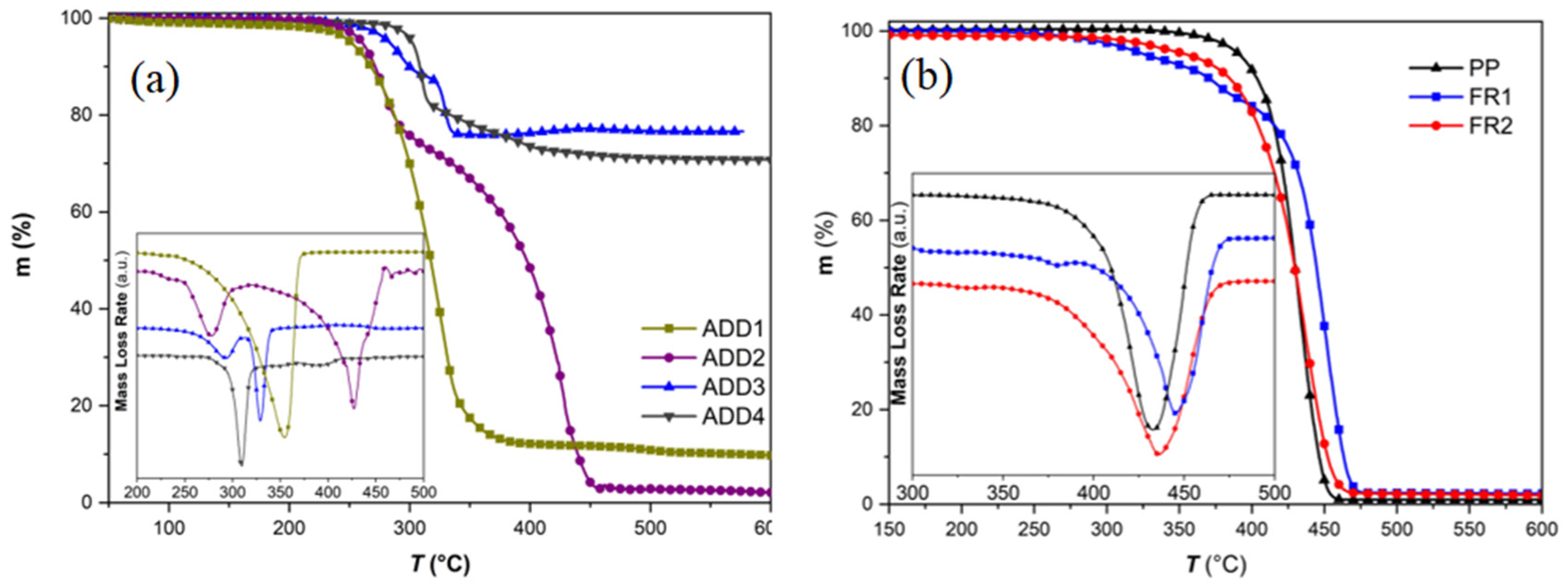
Figure 1a) in order to correlate the anticipated FR mechanism (gas and/or condensed phase) to additive type and then to formulated compounds. The cyclic phosphonate ester (ADD1) showed a single mass-loss step beginning at ca. 259 °C (
T5%), reaching a maximum decomposition rate at ca. 335 °C (
Td), and leaving a residue of ca. 9 wt.% at 600 °C, thus exhibiting a minor charring ability [
30,
31,
32]. On the contrary, the NOR-HAS compound (ADD2) shows a two-step decomposition curve, with the first step having its maximum mass-loss rate at ca. 277 °C (
Td1), corresponding to the cleavage of the N-O-R bond, and exhibiting a weight loss of ca. 25 wt.%, and the second step, which is the decomposition of the carbon lattice of the additive, occurring at ca. 426 °C (
Td2) [
27]. The residue left at 600 °C was only 2 wt.%, thus proving that the pertinent additive is released mainly in the gas phase. Regarding the phosphinates (ADD3, ADD4), they decompose above 320 °C and release gases such as PH
3 (
Td2 = 329 °C) and H
2O (
Td3 = 400 °C), meanwhile leaving aluminum pyrophosphate as a heavy inorganic char [
33,
34,
35,
36] of ca. 75 wt.% at 600 °C, rendering these additives as good candidates to act in the condensed phase when added at high contents (e.g., 20 wt.%); this stable thick layer would block oxygen penetration and/or flame propagation to the polymer matrix. Moreover, ADD3 shows a three-step decomposition since, apart from the phosphinate fraction, which is the main ingredient of the pertinent additive, it contains also a NOR-HAS-type adjuvant. Therefore, the first step (
Td1), occurring at ca. 292 °C, corresponds to the decomposition of the NOR-HAS adjuvant, which also lies within the decomposition range of the pure NOR-HAS additive (ADD2). On the contrary, ADD4, which is cited as a hypophosphite adjuvant, contains only mixtures of phosphinates, thus only two decomposition steps are clearly observed, with the step corresponding to water (300–400 °C) formation being more intense.
Regarding flame retardancy, FR1 compound (ADD1, ADD2, 11 wt.%) is anticipated to operate simultaneously in both the gas and condensed phase. The phosphonate (ADD1) and the NOR-HAS (ADD2), upon heating (high temperature, thermolysis), release phosphorous- (PO
·), nitrogen- (NO
·, N
·), and alkyl-type (RO
·, R
·) radicals, which are very reactive and, on one hand, they cause rapid degradation of PP in the solid phase, leading to carbonization, while on the other hand, in the gas phase, they quench active radicals from the flame zone (O
·, OH
·, CH
3·), thus terminating the fire [
27,
29,
30]. The latter was verified by TGA (
Figure 1b), where a ca. 75 °C lower
T5% value vs. reference PP was recorded (
Table S1), underlining the rapid degradation of PP in the presence of NOR-HAS and the gas phase side of the FR mechanism. On the other hand, the condensed phase part can be correlated to the
Td increase (by ca. 14 °C), with no significant residue increase, since the sample ended up at a residue of ca. 2 wt.%, similar to the PP value. This TGA-based FR1 function in the gas and condensed phase was macroscopically confirmed during the UL94V test: the specimens, after ignition, immediately extinguished, presenting a low char formation in their bottom edge with a simultaneous dripping that did not cause ignition of the cotton indicator (non-flaming dripping). The dripping and the gaseous byproducts resulted in an average mass loss of ca. 9.1 wt.%. The intense radical decomposition of PP in the solid phase is mainly induced by the thermolysis of the NOR-HAS component, resulting in chain scission and promoting non-flaming dripping, as observed by the increased melt flow [
27,
36]. V0 classification was reached for FR1, with a total burning time recorded at 13.7 s, while a very low smoke formation was evidenced, thus rendering the particular compound as promising for developing Halogen Free and Low Smoke (HFLS) conduits. The latter feature will be quantifiably evaluated in the following Smoke Density tests in samples of corrugated conduits, according to EN IEC 61034-2 standard [
4].
On the other hand, in FR2, the flame retardancy mechanism is anticipated to operate mainly in the gas phase. In our case, the phosphinates (ADD3 and ADD4) are applied at a total loading of only 4 wt.%; thus, charring is not anticipated to be the dominant mechanism. Apart from the produced PH
3 and H
2O gases, which cool and dilute the flame zone, bromine radicals (Br
·) are also released in the gas phase by the decomposition of the phosphorous bromine salt contained in ADD3, which in turn quenches the radicals that enhance flame propagation [
34,
35,
36,
37,
38]. TGA verifies the latter, since a reduction of the
T5% by ca. 40 °C was evidenced (
Figure 1b,
Table S1) due to the formed gases and radicals. On the other hand,
Td and final residue were found to be similar to reference PP. The absence of FR condensed phase mechanism can also be correlated to the UL94V test, where FR2 samples ignited faster and intense flaming dripping was directly observed. The total burning time was determined at higher levels, i.e., 25.4 s, compared to FR1, which is laid within the V0 specifications; however, the ignition of the cotton indicator directly classifies FR2 in V2 category. In addition, a much higher average mass loss was determined, i.e., ca. 29.2 wt.%, reflecting the predominance of the gas phase mechanism, which in turn yielded intense flaming dripping. It is worthwhile mentioning that FR2, due to ADD3, which is a halogenated additive, contains ca. 980 ppm of bromine. Nevertheless, it complies with the current low-halogen standard (EN50642, [
5]) for cable protection systems, which permits bromine content up to 1500 ppm and also complies with the stricter DINVDE V 0604-2-100 [
46], which permits halogen content up to 1000 ppm. Furthermore, the smoke emissions during the UL94 test were qualitatively observed to be slightly more intense compared to FR1, which is expected since more gases are produced. However, the FR2 compound can still be promising for the manufacture of HFLS conduits and will also be checked in the following Smoke Density tests in samples of corrugated conduits, according to EN IEC 61034-2 standard [
4].
3.2. Characterization of Lab-Scale Compounds
The FRs influence on PP thermal properties was evaluated by typical DSC analysis under nitrogen atmosphere (
Figure 2a,b,
Table S1). The phosphonate (ADD1) and NOR-HAS presence at a loading of 11 wt.% in FR1 caused slower melt crystallization rates, i.e.,
Tc was found to be significantly decreased by ca. 7 °C, inducing higher mass fraction crystallinity (
Xc = 36%) and
Tm2 was increased by 3 °C compared to PP. In the case of FR2, thermal properties more similar to PP were determined, i.e., a ca. 0.5 °C increase in
Tc and
Tm, and an identical crystallinity of 32%, as a consequence of the lower loading of 4 wt.% of the phosphinates ADD3 and ADD4. Moreover, a broad shoulder on the melting endotherm of FR2 is observed, possibly attributed to the melting of smaller crystals. Overall, it seems that the effect of the FR additives’ incorporation on the thermal properties is more pronounced in FR1 compared to FR2.
The NOR-HAS additive (ADD2, component of ADD3) offers a versatile functionality, acting as a stabilizer against oxidative degradation at low temperatures (<150 °C) or as a radical generator at high temperatures. This behavior is attributed to the production of nitroxyl-type radicals (NO
·), caused by the thermolysis of the nitroxylether, that quench the formed peroxyl radicals (ROO
·) during the oxidation cycle [
20,
24,
26,
30,
47,
48]. The latter was evaluated in the FR compounds through the determination of Onset Oxidation Temperature (OOT) (
Figure 2c) [
30,
49]. Accordingly, the OOT of reference PP was determined at ca. 246 °C, while it was found significantly increased by ca. 11 °C for FR1 (1 wt.% ADD2,
Table S1). Therefore, for FR1, oxidation resistance is anticipated. As can be seen from the OOT-DSC curve of FR1 (
Figure 2c), there is a small endotherm at ca. 245 °C, which corresponds to the melting point of the phosphonate (ADD1,
Figure S2). Ιn the case of FR2, OOT was not significantly increased (only by ca. 3 °C), obviously due to the lower amount of NOR-HAS component: the total FRs loading 4 wt.% in FR2 is lower compared to 11 wt.% in FR1, while the precise NOR-HAS content in ADD3 is not revealed by the manufacturer, but can be assumed to be lower than 1 wt.% in the final FR2 compound.
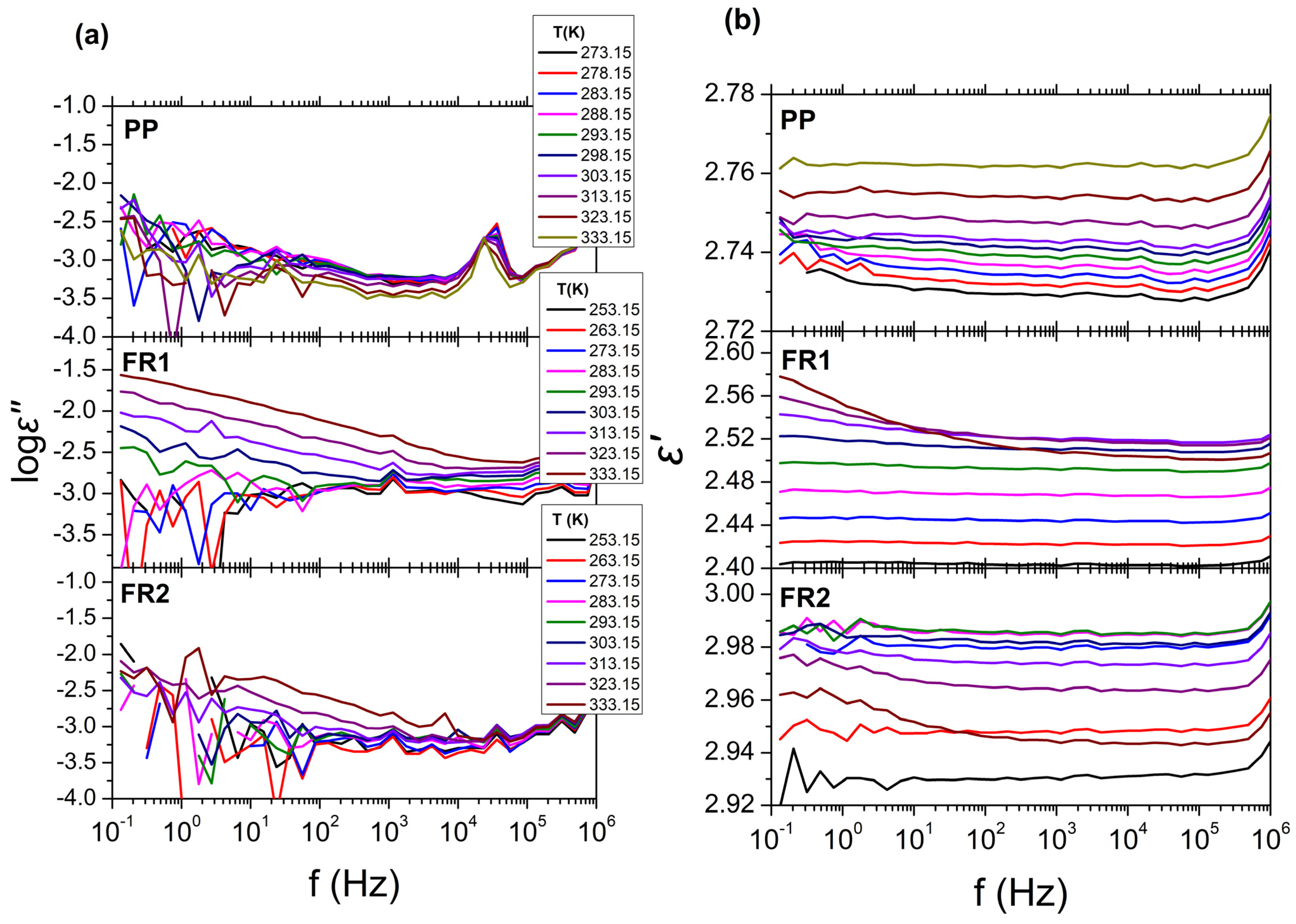
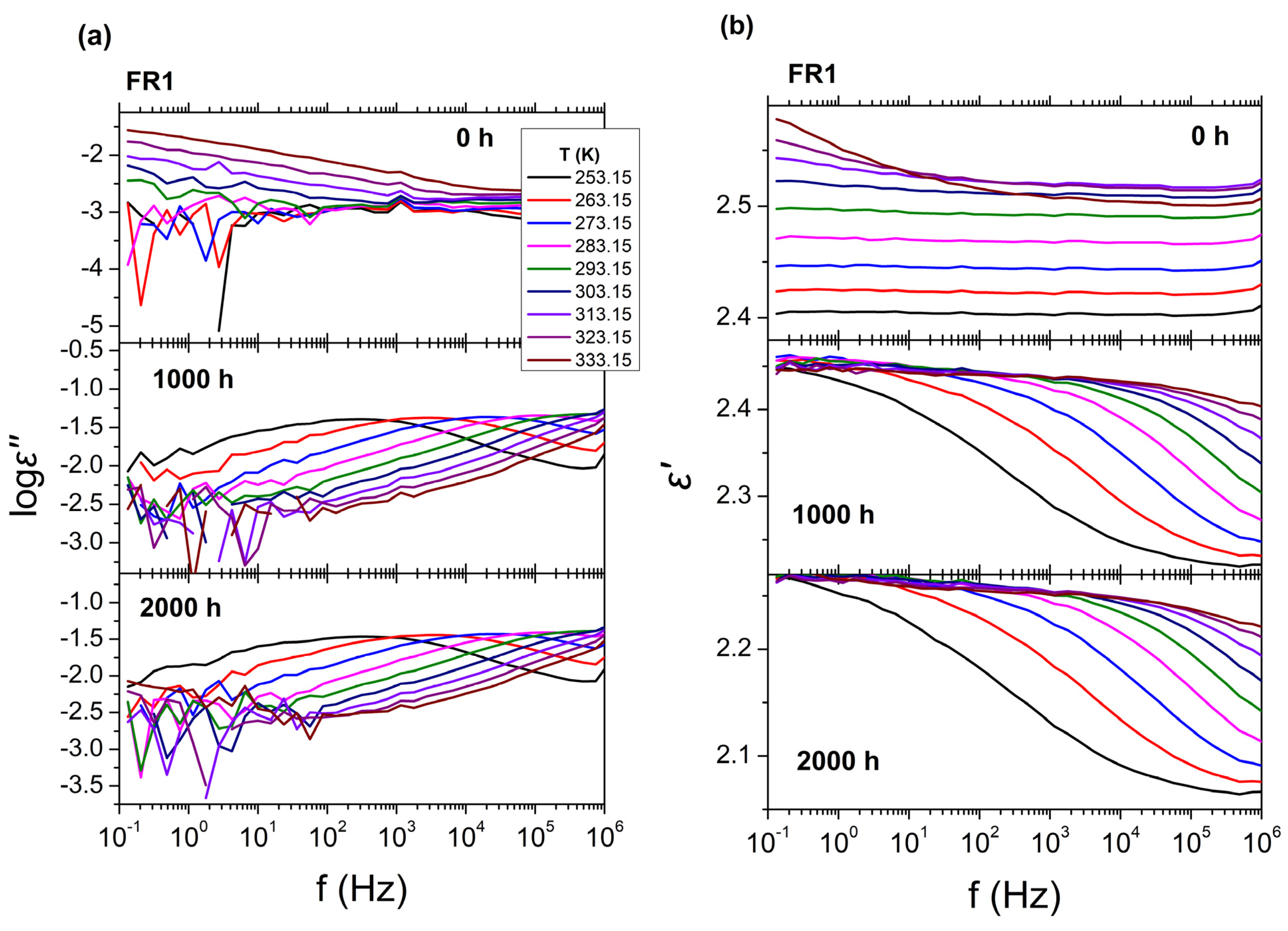
Since the FR compounds are intended for the manufacture of cable conduits, the influence of the FRs presence on the dielectric behavior of PP was also assessed. The results from BDS for FR1, FR2, and reference PP are presented in
Figure 3, in the form of isothermal curves of the dielectric permittivity (
ε′), dielectric losses (log
ε″) over frequency (
f), for all temperatures examined. As can be seen, no relaxation processes can be observed in the dielectric spectra for all systems. This is expected for reference PP due to the non-polar segments of the polymeric chains, leading to extremely low dielectric losses, near the experimental limit of BDS (
ε″~10
−3). The dielectric behavior of FR1 and FR2, is similar with that of reference PP, exhibiting low dielectric loss values (
ε″~10
−3) and dielectric permittivity values of
ε′~2.5 and
ε′~2.9, respectively (
ε′
PP~2.7). This result can be related with the relatively small percentage of additives (<12 wt.%) that is contained in FR1 and FR2. In the case of FR1, an increase in dielectric losses can be observed at low frequencies (
f < 10
2 Hz), which can be related to dc-conductivity (
σdc) or the EPE phenomenon. Finally, for all systems, the extremely low values of the dielectric losses could not permit the analysis of the dielectric spectra with the use of HN equations (Equations (3) and (4)).
The compounds’ processability was compared to reference PP (MFR = 1.34 g/10 min) via MFR measurements. The 11 wt.% content of ADD1 and NOR-HAS resulted in an MFR increase up to 2.16 g/10 min, which can be attributed to the simultaneous melting of ADD1 (
Tm = 230–245 °C) and ADD2 (
Tm~120 °C), as shown in
Figure S2. In FR2, no significant change on the MFR was observed, since the contained additives are infusible.
Regarding the mechanical performance of the FR compounds, which is also a critical property for the application of corrugated conduits, tensile and impact properties were studied and compared to the PP reference (
Table S2). Mechanical testing is strongly affected by the additives’ compatibility with the polymer matrix and the additives’ content as well as by the degree of homogeneity achieved during compounding. In general, the relative standard deviations (RSD, %) for the impact strength measurements were found to be higher for the compounds (ca. 14–21%) vs. reference PP (5.6%), indicating poor additives dispersion, especially for FR1. Moreover, all PP specimens remained unbroken during the impact test, which was expected, since they were unnotched; on the contrary, all FR1 specimens completely broke. Based on the melting characteristics of FR1 additives (
Figure S2), both the cyclic phosphonate ester (ADD1) and the NOR-HAS (ADD2) might have partially or even totally melted during the extrusion and/or subsequent injection molding, thus creating different phases in the material bulk, which in turn could result in weaker spots. For the case of FR2, where the phosphinates (ADD3 and ADD4) are completely infusible additives, compounding permitted a more homogenous dispersion of the solid particles and distribution in PP matrix. During the impact tests, all specimens of FR2 remained unbroken, thus showing a similar behavior to PP. Regarding impact strength values (
Table S2), in FR1, where the FR loading is 11 wt.%, an increased
aiu compared to reference PP was determined, unlike in the case of FR2, where the low loading of 4 wt.% did not seem to significantly affect the
aiu. In terms of tensile properties, the FR compounds at both total loadings (11 and 4 wt.%) exhibited similar values for Young’s modulus and stress at yield (tensile strength) with reference PP, but they presented a more brittle and stiffer behavior based on the strong decrease in the strain at break (
εmax), with RSD values between 10% and 20% [
19,
48].
3.3. Performance of Lab-Scale FR Compounds during Ageing
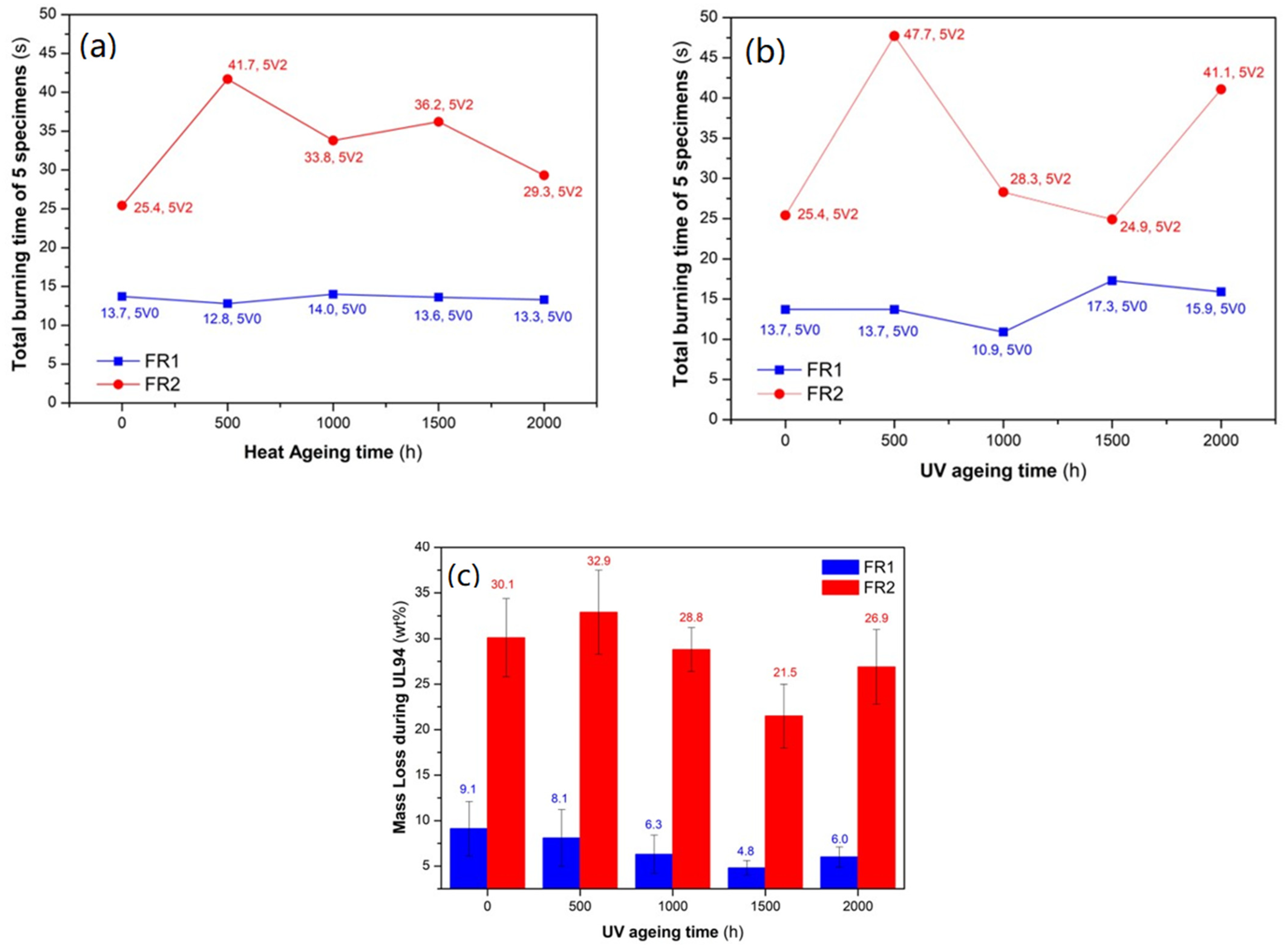
A very important aspect for long-term and durable application, such as in the case of conduits, is the retention of both flame retardancy and mechanical properties. Therefore, the developed FR compounds were subjected to separate accelerated artificial ageing experiments: one accelerated heat ageing test, performed in an air circulating oven at 110 °C for 2000 h; and one accelerated weathering test in the presence of UV radiation and humidity also for 2000 h, in a climate chamber. Regarding flame retardancy, the two FR compounds maintained their initial (prior to ageing) UL94 classification, in both sets of accelerated ageing tests. Beginning with heat ageing (
Figure 4a), FR1 was the best performing compound, with all the specimens constantly at V0 class and an excellent retention of the total burning time at ca. 14 s. FR2 also successfully remained in the V2 category but exhibited ups and downs in total burning time. Similar were the results after the UV weathering test (
Figure 4b), with FR1 constantly in the V0 class, with insignificant variations (up to ca. 3 s) in total burning time; and FR2 constantly in the V2 class, with more distinct ups and downs in terms of total burning time. However, at the sampling intervals 1000, 1500, and 2000 h of weathering, all the specimens of FR1 exhibited increased stickiness after their removal of the climate chamber; however, this did not affect their performance in the UL94 test. This can be explained by the interaction of humidity with ADD1 at the temperature of 50–60 °C at which UV ageing took place, causing partial hydrolysis of the additive and thus showing a much lower melting point [
31,
50]. Finally, especially for the UV-aged samples, the UL94 bars were weighed prior to and after the UL94 test, so as to determine the mass that was lost through the formation of gases and dripping (
Figure 4c). Accordingly, FR1 maintained the low (<10 wt.%) average mass loss during the flame test (although a mildly decreasing trend was observed), indicative for the gas and condensed-phase FR mechanism. FR2 also, in terms of mass loss during the UL94 test, exhibited a similar trend of ups and downs with total burning time (ca. 22–33 wt.%) as a consequence of the intense flame dripping. All aged specimens exhibited a macroscopically small increase in the smoke emission during the UL94 test, probably due to some minor ageing of the FR additives (as proved by the retention of UL94 classification); nevertheless, the emission levels are still considered very low. Summarizing, the developed FR compounds ideally retained their flammability against ageing under our experimental conditions; thus, they are considered as good candidates for upscaling to an industrial level.
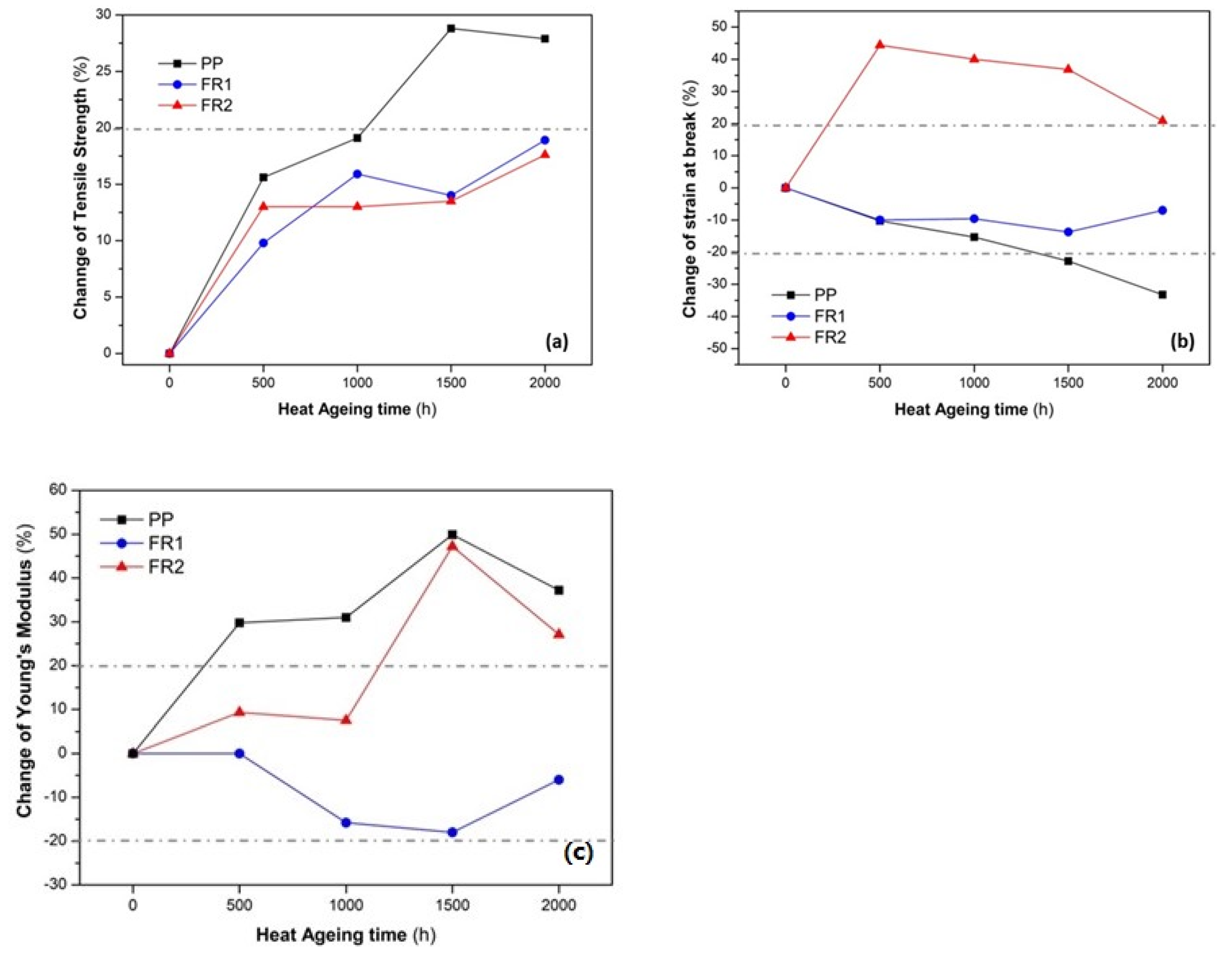
Turning to the mechanical performance during ageing, first, we examined the retention of tensile strength in the heat ageing experiments in order to assess any thermo-oxidative stabilization effect of the FR additives. Typically, polyolefins with increasing ageing time exhibit oxidative degradation triggered by heat and/or UV radiation in the presence of oxygen [
15,
39,
40]. In terms of tensile properties, PP, upon ageing, typically exhibits an increase in the Young’s modulus (
E), with a strong reduction in the elongation at break, a behavior well-known as embrittlement [
19,
48,
51,
52,
53,
54]. This was observed clearly for reference PP (
Figure 5). Therefore, a tolerance level of ±20% in the change of all three tensile values was arbitrarily selected so as to evaluate the mechanical performance of the FR compounds against heat ageing. Accordingly, the two FR compounds exhibited a more slowly increasing trend of tensile strength (
σmax,
Figure 5a), ending up at 2000 h at a total deviation in the range of 17–19%. Especially for the initial 500 h, the relative increase in tensile strength can be due to annealing of the polymer [
19]. Regarding the strain at break (
εmax,
Figure 5b) and Young’s modulus (
Figure 5c), no clear trend was observed, as anticipated, due to embrittlement: FR1 was quite stable throughout the ageing time, ending up with an insignificant reduction of
εmax in the range of ca. 7% and a reduction of 10% for
E at 2000 h. FR2, surprisingly, exhibited an increasing trend of
εmax from as soon as 500 h, reaching 20% change and a constant increase in
E even reaching 47%. It can be concluded that the developed FR compounds exhibited embrittlement during heat ageing, but at much lower extent than reference PP.
Turning to UV weathering, Izod impact strength was monitored (
Figure 6a) in order to simulate the maintenance of PP conduits performance for outdoor applications, since impact is the most critical mechanical property for such products. Already, reference PP exhibited a stable behavior of impact strength during the weathering process [
19,
47,
51,
52,
53,
54], with all measured specimens again not being broken during the impact test. On the contrary, FR1, at 500 h, exhibited a strong decrease of ca. 44%, which, at 1000 and 1500 h, increased again close to the initial value and at ended up at 2000 h with a 50% total decrease. The standard deviation values are also high (18–65.5%) vs. ageing time, which is again an indication of poor additives dispersion or phase separation in the compounded material (FR1). In addition, all FR1 specimens broke during the impact tests. Therefore, it is not easy to accurately determine the effect of ageing on impact strength; nevertheless, it is obvious that FR1 shows impact strength deterioration, probably due to ADD1 partial hydrolysis as mentioned above. In contrast to FR1, FR2 exhibits a very similar behavior to reference PP (all aged specimens remained unbroken during the test) with impact strength maintenance throughout the UV weathering time. Moreover, impact specimens were weighed prior to and after each ageing interval and the mass gain due to humidity (water uptake,
Figure 6b) was calculated. Accordingly, FR2 exhibited very low water uptake, similar to PP, as expected, since PP is not considered as hygroscopic material. On the contrary, FR1 showed a much higher water uptake, which increased with UV-ageing time, proving the interaction of ADD1 with humidity and indirectly verifying the partial hydrolysis that the pertinent additive suffered.
In order to gain deeper insight regarding the UV-ageing process, FT-IR and MFR were also monitored. FT-IR ATR analysis evaluates the surface of the material, since the IR beam penetrates only a few μm in the sample, while MFR provides information about the bulk material. Regarding FT-IR analysis during UV ageing (
Figure 7a–c), FR1 at 500 h shows a peak at ca. 1700 cm
−1, which is within the carbonyl range, and another one at ca. 3300 cm
−1, which is within the hydroxyl region [
41,
42]. Both peaks exhibit increasing intensity with ageing time and suggest ageing byproducts. Moreover, significant changes in the spectra of the UV-aged FR1 samples (
Figure 7a) are noticed in the area of 900–1400 cm
−1, which can be explained by the hydrolysis that ADD1 suffered during interaction with the humidity of the climate chamber, verified macroscopically by the observed stickiness and impact strength deterioration. On the contrary, FR2 shows no change in the spectra during ageing (
Figure 7b), underlining the stability that the pertinent FR system provided. Regarding reference PP (
Figure 7c), very weak peaks only in the carbonyl range after 500 h are observed, verifying that a minor photo-degradation occurred.
MFR was also measured at the sampling intervals of 1000 and 2000 h of UV ageing. Accordingly (
Figure S3), reference PP and FR2 show a remarkable MFR stability, while FR1 exhibits a ca. sevenfold increase in MFR already from 1000 h. This is in line with the observation of carbonyl peaks in the FT-IR spectra of the FR1 samples from UV ageing (
Figure 7a). This rapid increase in MFR of FR1 aged samples could be explained by the hydrolysis of ADD1, which results in much lower melting point species, thus strongly affecting the MFR and/or chain-scission of PP [
19,
54]. The latter verifies that FR1 formulation requires the addition of an acid scavenger, like zinc stearate or antioxidants, so as to inhibit the observed degradation of the ADD1 and the PP matrix [
48,
50].
The BDS results for FR1 and FR2 after heat ageing at 110 °C for 0, 1000 and 2000 h are presented in
Figure 8, in the form of isothermal curves of the dielectric permittivity (
ε′) and dielectric losses (log
ε″) over frequency (
f), for all temperatures examined. As was mentioned before, in the absence of thermal ageing (0 h), FR1 and FR2 show extremely low values of dielectric losses (log
ε″); thus, their dielectric behavior cannot be further discussed. In the case of FR1, thermal ageing leads to major changes in the dielectric spectra (
Figure 8a,b). After thermal treatment for 1000 h (40 days) at 110 °C, dielectric loss values increase at all examined temperatures (
ε″~10
−2) and two relaxation processes can be observed in the dielectric loss spectra (
Figure 8a). The fast one corresponds to
α process, related to
Tg, and the slower one corresponds to an Arrhenius-like process related with the presence of additives in the polymeric matrix of PP. After thermal treatment for 2000 h (80 days), the two processes become more distinct, with the dielectric losses increasing to even higher values (
ε″~10
−1). These changes can also be observed at the dielectric permittivity curves (
Figure 8b), where there is a gradual increase of dielectric permittivity (
ε′) with increasing ageing time, for all temperatures examined, with a value of
ε′~3.4 for 1000 h and
ε′~3.6 for 2000 h. On the other hand, in the case of FR2 (
Figure 8c,d), thermal ageing for 1000 and 2000 h does not have any significant effect on the dielectric spectra. Dielectric loss values for both ageing times remain very low at all temperatures studied, making the analysis of the dielectric loss spectra using the HN equations meaningless. For that reason, in
Figure 8c,d, the dielectric response at only two indicative temperatures is shown. In terms of dielectric permittivity, a small drop can be observed (
ε′~2.35) for both 40 and 80 days, at temperatures
T = 273 K and
T = 298 K.
Turning to BDS results after UV ageing (
Figure 9), the results for FR1 after UV ageing for 0, 1000, and 2000 h are presented, in the form of isothermal curves of the dielectric permittivity (
ε′) and dielectric losses (log
ε″) over frequency (
f), for all temperatures studied. In accordance with thermal ageing, UV ageing seems to also have a significant impact on the dielectric spectra. After treatment for 1000 h and 2000 h, one relaxation process with a broad distribution of relaxation times can be observed, whereas, in the absence of UV treatment, the dielectric spectra did not show any similar process. In terms of ageing duration effects, there is no significant difference between 1000 h and 2000 h. Dielectric loss values for both ageing times are increased (
ε″~10
−1) for all temperatures studied and the dielectric permittivity spectra show one distinct characteristic step, corresponding to the relaxation process, with a lower permittivity value of
ε′~2.3. Due to absence of any effects to dielectric behavior of FR2 after heat aging, dielectric measurements were not performed on FR2 UV aged samples. The dielectric losses of FR2 always remain very low.
Analysis of dielectric data of FR1 after heat or UV ageing with different ageing times was employed with the use of HN equations on the dielectric permittivity data (log
ε″). The relaxation times of the molecular processes obtained from this procedure were plotted over reciprocal temperature (1000/
T) (
Figure S4a). In the case of FR1 without ageing, the dielectric spectra could not be analyzed employing the HN analysis. After thermal ageing, an Arrhenius-like process can be observed in the dielectric spectra at high temperatures for both ageing times (1000 and 2000 h), corresponding to molecular mobility of charges that exist in the polymeric matrix due to the additives. In addition, a relaxation process at lower temperatures can be observed corresponding to segmental
α process, the molecular motion related to
Tg, with a characteristic VFT dependence. For
t = 100 s, the extrapolated fit of the VFT curve predicts
Tg values of −47.7 °C, for 1000 h, which is far below the
Tg of PP from other studies. In the case of FR1, thermally aged for 2000 h, both relaxation processes are shifted towards larger relaxation times, indicating slower dynamics, characterized by a higher
Tg value of −30.9 °C. Regarding the magnitude of the relaxation processes, in
Figure S4b, the normalized dielectric strength (
TΔ
ε) is plotted over reciprocal temperature. As can be observed, the dielectric strength of both the Arrhenius-like and
α process increases immensely with the increase in ageing time during thermal treatment (red line), whereas no differences are observed during UV aging.
3.4. Upscaling FR Compounds to Industrial Level
FR1 and FR2 formulations set the basis for the production of industrial-scale masterbatches (MB) at additive loadings 40 and 44 wt.%, respectively (
Table 1), i.e., 4 and 10 times the initial loading of the FR1 and FR2 compounds. The formulations of the industrial MBs were specially designed according to the expertise of our industrial compounder (PLASTIKA KRITIS) and the required MB additive range required by our end-user (KOUVIDIS SA). A high MFR homo-polymer PP (25 g/10 min) was selected as carrier, so as to facilitate the high loading of the additives during MB compounding.
The prepared masterbatches were initially characterized in terms of thermal properties in order to verify the additives’ presence and functionality based on lab-scale prior analysis. Accordingly (
Figure S5), both MBs showed two-step mass loss TGA curves, which are much more pronounced and clearly separated in comparison to the respective FR compounds (
Figure 1b), since the additives are incorporated at a much higher loading. According to
Table S1,
T5% values are up to 100 °C lower than the respective value of the PP carrier, but far below the extrusion processing window (190–220 °C); therefore, no degradation or consumption of FR additives of the MBs is anticipated during further blending with reference PP in extrusion.
Td1 and
Td2 values correspond to physico-chemical reactions of the FR additives, as already explained in
Figure 1a [
33,
34,
35,
36]. Finally, regarding residue, MB1 shows a value of ca. 10 wt.%, again underlining the minor charring ability of ADD1 and ADD2, while in MB2, ca. 30 wt.% was recorded as a consequence of the high charring ability of the contained phosphinates [
34].
From the received MBs, FR1 and FR2 were reproduced, but by blending with the appropriate amount of reference PP in the twin-screw extruder, resulting in FRMB1 and FRMB2 (
Table 2). This was performed in order to verify the efficiency of the produced MBs. UL94 bars were prepared by compression molding and the UL94 test was repeated. For FRMB1, the V0 class was reached, with a total burning time for five specimens determined at 13.3 s and an average mass loss per specimen due to dripping at 9.2 wt.%, very close to the respective FR1 (13.7 s and 9.1 wt.%). Similarly, in the case of FRMB2, the V2 class was reached, with a total burning time at 29.8 s and mass loss due to dripping at 23.3 wt.%, again very close to the respective initial FR2 values (25.4 s and 29.2 wt.%, respectively). Moreover, impact specimens of FRMB1 and FRMB2 were prepared so as to evaluate the homogeneity of the compounds developed via the MBs. Indeed, as observed in
Figure 10, the compounds developed from the MBs show slightly higher average impact strength, but, meanwhile, a much lower standard deviation, which verifies that the dilution from the MBs ensures a better and more homogenous dispersion of the FR additives. Indicatively, the RSD values for FRMB1 and FRMB2 are 2.7% and 4.9%, respectively, much lower than the ones given in
Table S2.
Furthermore, after securing the optimum FR dispersion with the use of the MBs, an attempt to produce FR compounds at the lowest possible MB concentration that would, meanwhile, result in similar FR performance was performed. This attempt aims mainly at a cost-effective approach for industrializing these compounds, but especially in the case of FR2, also at a reduced halogen content. Accordingly (
Table 2), six new FR compounds (FRMB3-FRMB8) were developed based on MB1 and two (FRMB9, FRMB10) based on MB2, via twin-screw extrusion of the desired amounts of reference PP and the required MB.
Regarding compounds developed from MB1 (
Table 2,
Figure 11), FRMB3 and FRMB4, where the FR loading is 9 and 8 wt.%, respectively, similar total burning times are reached and V0 class is retained, since the dripping does not cause cotton ignition. Moreover, mass loss due to dripping is minimized for FRMB4 (8 wt.% FR loading). The latter constitutes a remarkable FR performance for the case of PP, since V0 at such low loading and for bulky applications like conduits is hardly found in the literature or in commercial additives. There are, however, novel FR systems, such as oxy-imide nitrogen based radical generators, not yet commercialized, that promise V0 class in PP only at 6 wt.% loading [
55,
56,
57,
58,
59]. When reducing the FR loading further (FRMB5-FRMB7) up to 3.52 wt.%, an increase in total burning time is observed, which is still within the V0 specifications (<50 s for 5 specimens), but the most important observation is that dripping leads to cotton ignition, thus the V2 class is attained. Meanwhile, apart from the increase in total burning time, mass loss due to dripping is also significantly increased. Finally, FRMB8, which contains an extremely low FR loading of only 2.2 wt.%, FR performance is completely lost, with four out of five specimens tested failing completely in the UL94 test and being burnt up to the clamp, as indicated by the average mass loss of 89.6 wt.%. Therefore, the particular compound is non-classified (NC). Turning to the compounds developed from MB2 (FRMB9, FRMB10,
Table 2), the FR loading is already very low in FR2 and FRMB2, i.e., 4 wt.%, and with V2 class already attained for those, the optimization margins are very narrow. Nevertheless, in FRMB9 (
Figure 11), where the FR loading was reduced to 3.2 wt.%, mildly increased total burning times and mass loss due to dripping were determined. However, a significant reduction in the bromine content from 980 ppm in FR2 and FRMB2 to 784 ppm in FRMB9 was achieved, thus FRMB9 complies even with the strict DIN VDE V 0604-2-100 standard [
46] that admits bromine up to 1000 ppm. Finally, a further reduction of the FR loading to 2 wt.% leads to a non-classified compound, i.e., FRMB10; however, two out of five samples were still categorized as V2.
From the lab-scale compounds and for cost-efficiency reasons, FRMB7 based on MB1 and FRMB9 based on MB2 were selected and industrial-scale production tests were performed according to these formulations, producing two (2) respective types of corrugated conduits (C1, C2) of ø20 mm outer nominal diameter. The produced conduits were characterized prior to and after 2000 h of accelerated UV ageing according to EN IEC 61386-22:2021 standard for their resistance to flame propagation and impact [
2]. In addition, their smoke density was determined according to EN IEC 61034-2 standard [
4] only prior to ageing (
Table 3).
Accordingly, conduit C1/0h is considered as non-flame propagating, according to IEC 61386-22 [
2], since self-extinguishing behavior was evidenced in all six measured samples, with burning times (average burning time 1.84 s) much lower than 30 s that the standard permits. Non-flame propagating behavior was maintained after 2000 h (C1/2000h) and, although the burning time was found increased, it is still far below the limit of 30 s. Similarly, both the C2/0h and C2/2000h conduit samples are considered as non-flame propagating. The latter verify the results from the lab-scale and the UL94 tests, and underline the stability of FRs performance against aging. Turning to impact resistance, the produced C1/0h and C2/0h conduits are considered as medium-type conduits, according to IEC 61386-22 standard [
2], since they can endure impact energy higher than 2 J. The impact behavior of the conduits is fully in line with the lab-scale tests of the FR compounds, and, even though a decrease ranging from 16 to 25% was observed after 2000 h, samples C1/2000h and C2/2000h still retain the medium-type impact category. Last but not least, the smoke density of the C1/0h and C2/0h was determined, according to EN IEC 61034-2 standard [
4]. In both cases, the light transmittance was much higher than the limit of the standard (60%), thus both conduits are truly low smoke (LS), verifying the macroscopical observation from the UL94 tests in the lab scale. The result, when considered alongside the totally halogen-free ADD1 and ADD2, or the designed halogen level at 784 ppm, is the creation of real halogen-free and low-smoke (HFLS) conduit products.