Preparation of Two-Dimensional Polyaniline Sheets with High Crystallinity via Surfactant Interface Self-Assembly and Their Encryption Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
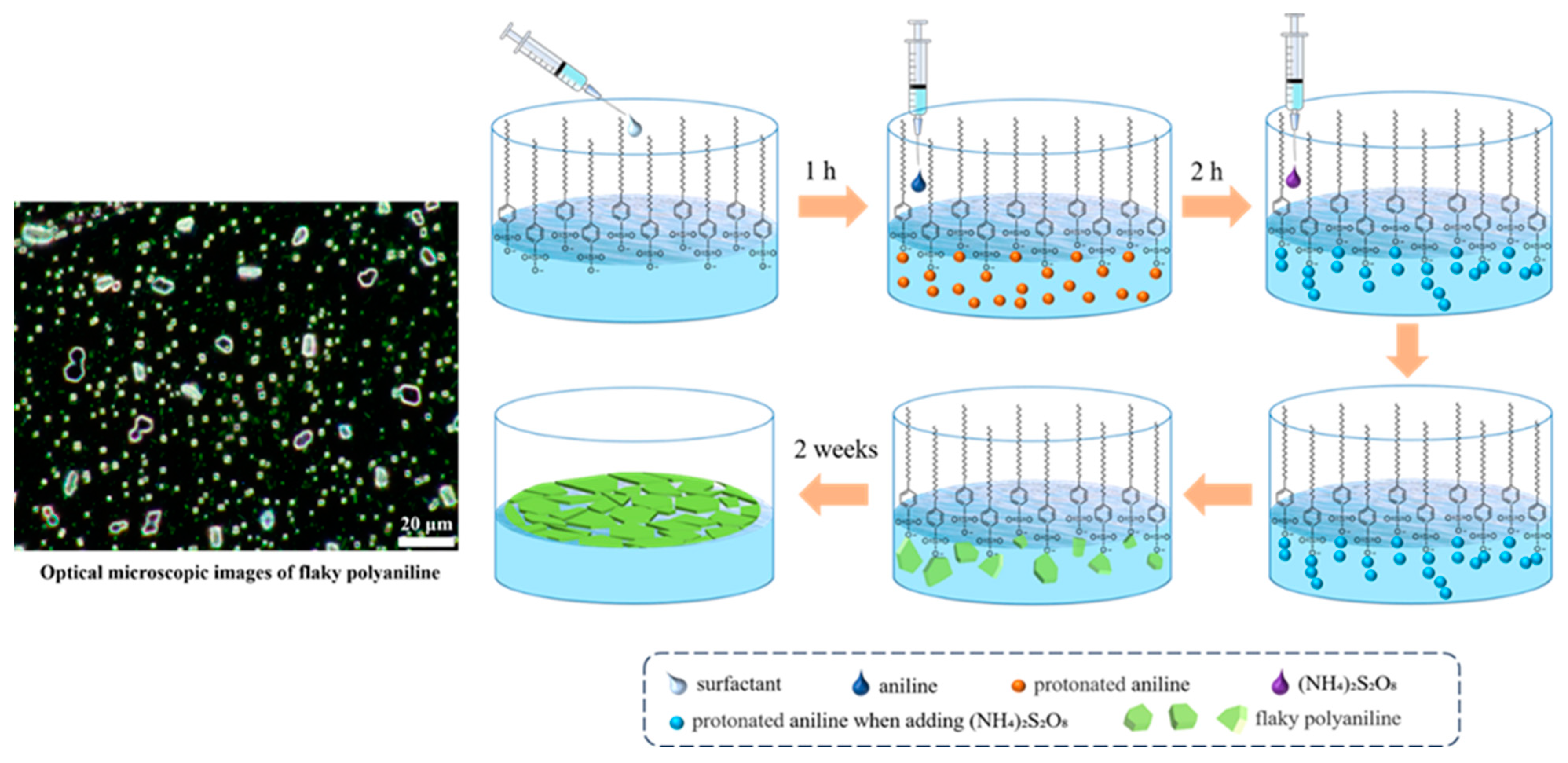
2.2. Synthesis of Flaky Polyaniline (F-PANI) and Amorphous Polyaniline (A-PANI)
2.3. Characterization
3. Results and Discussion
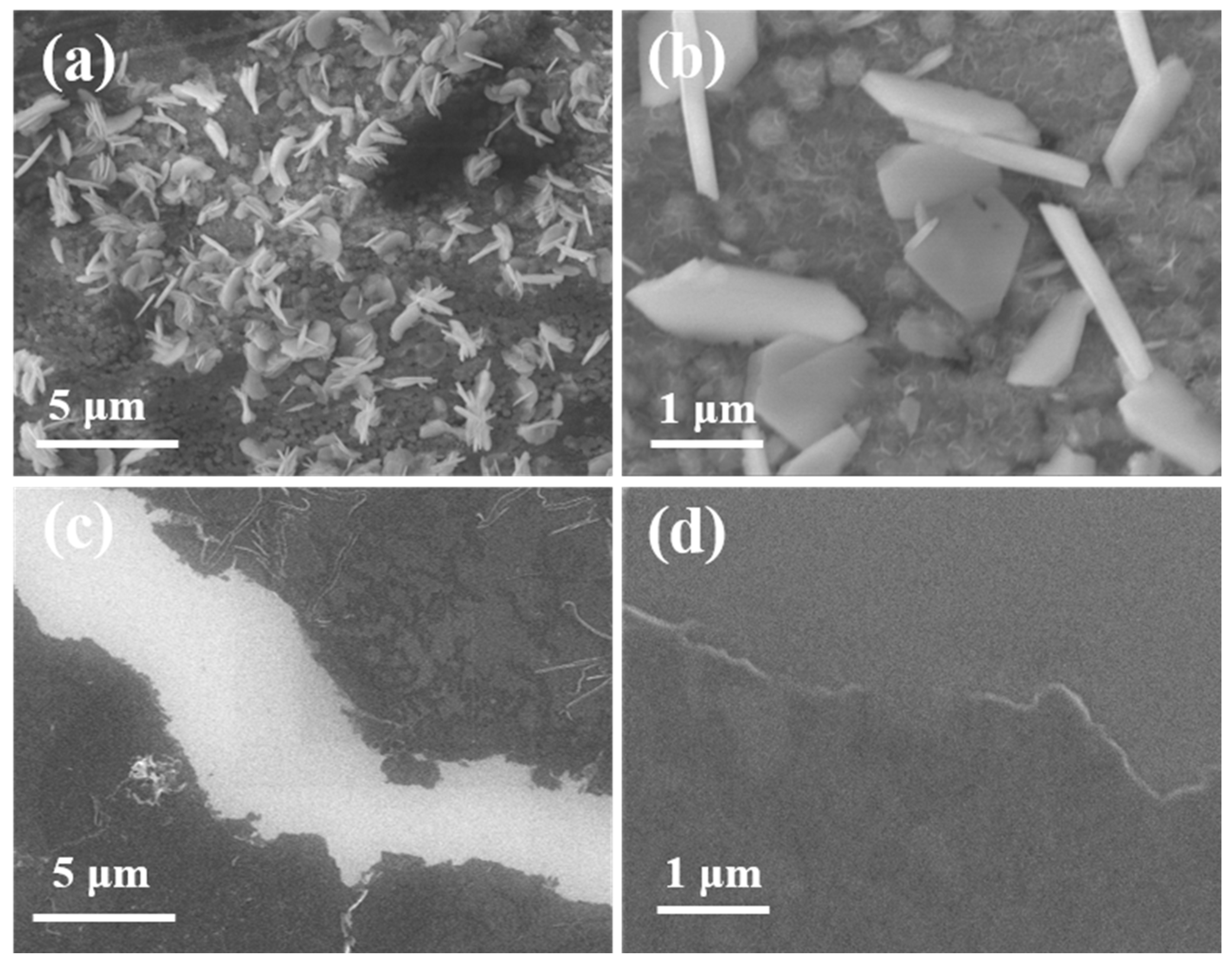
3.1. Synthesis and Morphology of F-PANI and A-PANI
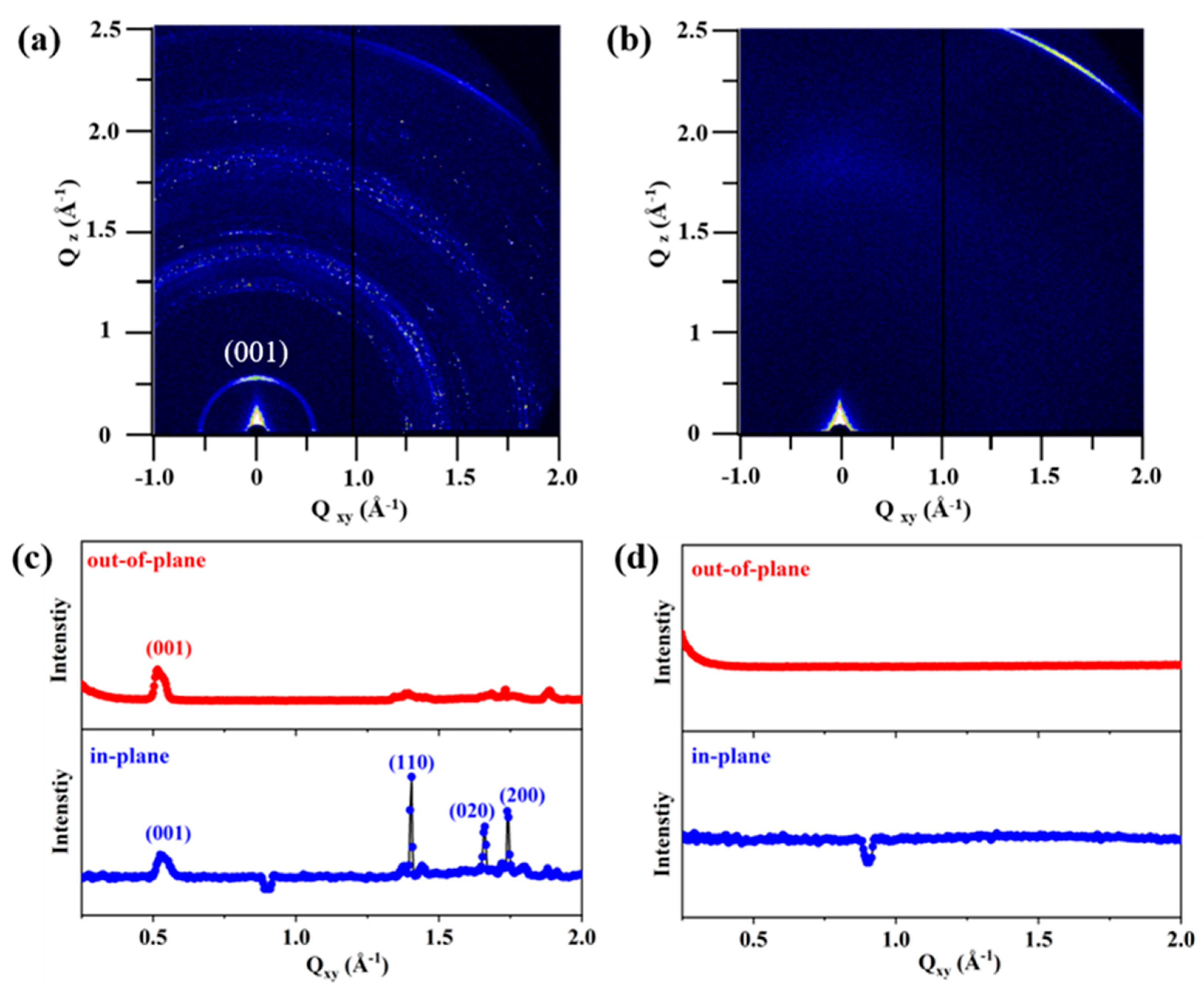
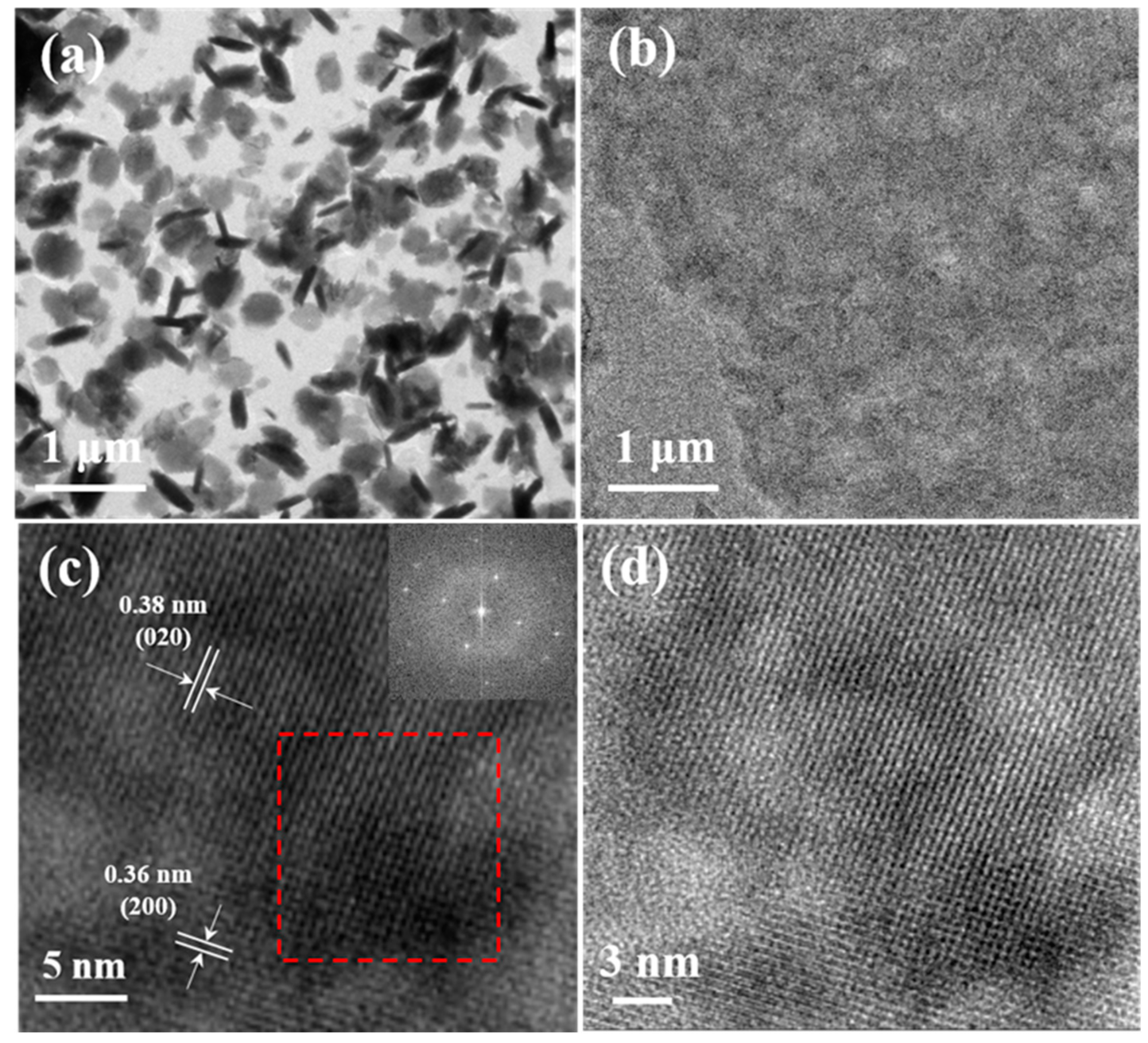
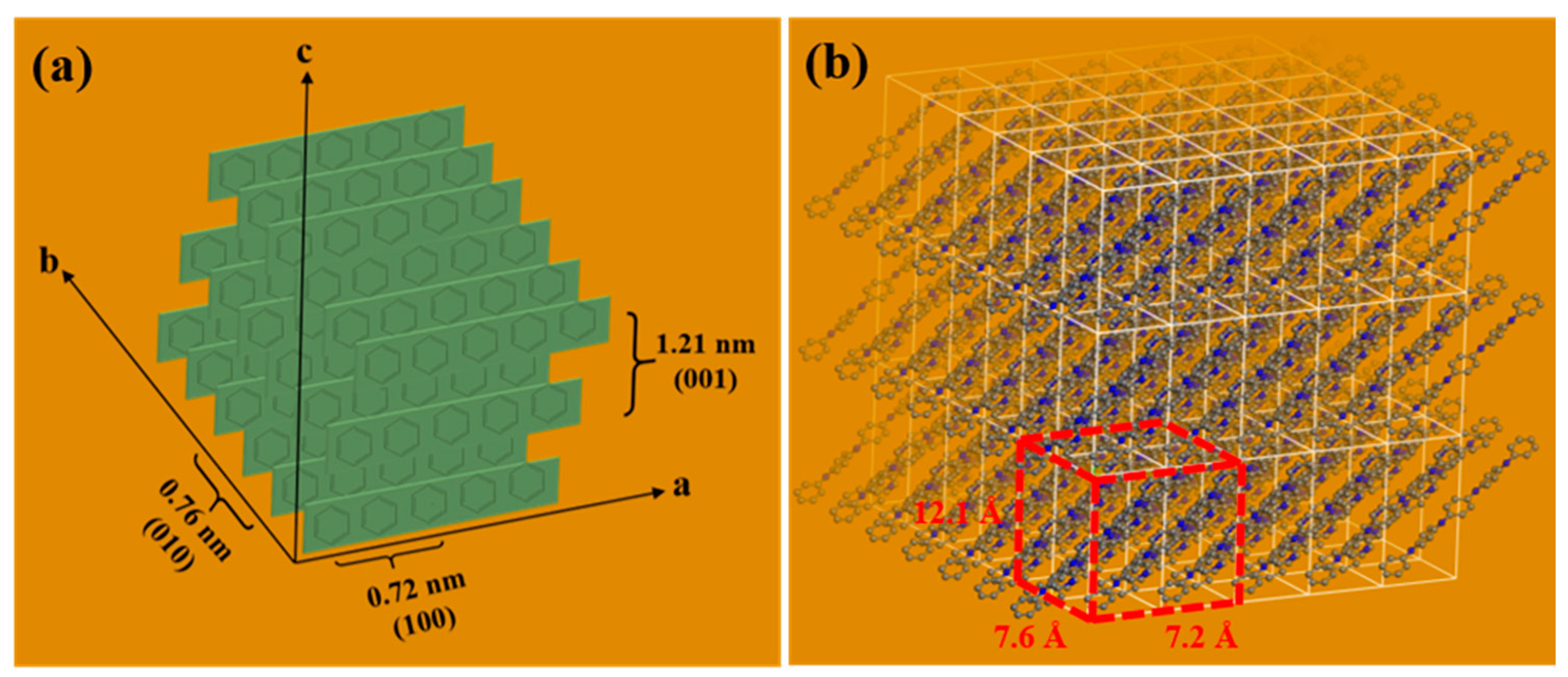
3.2. Structural Characterization of F-PANI and A-PANI
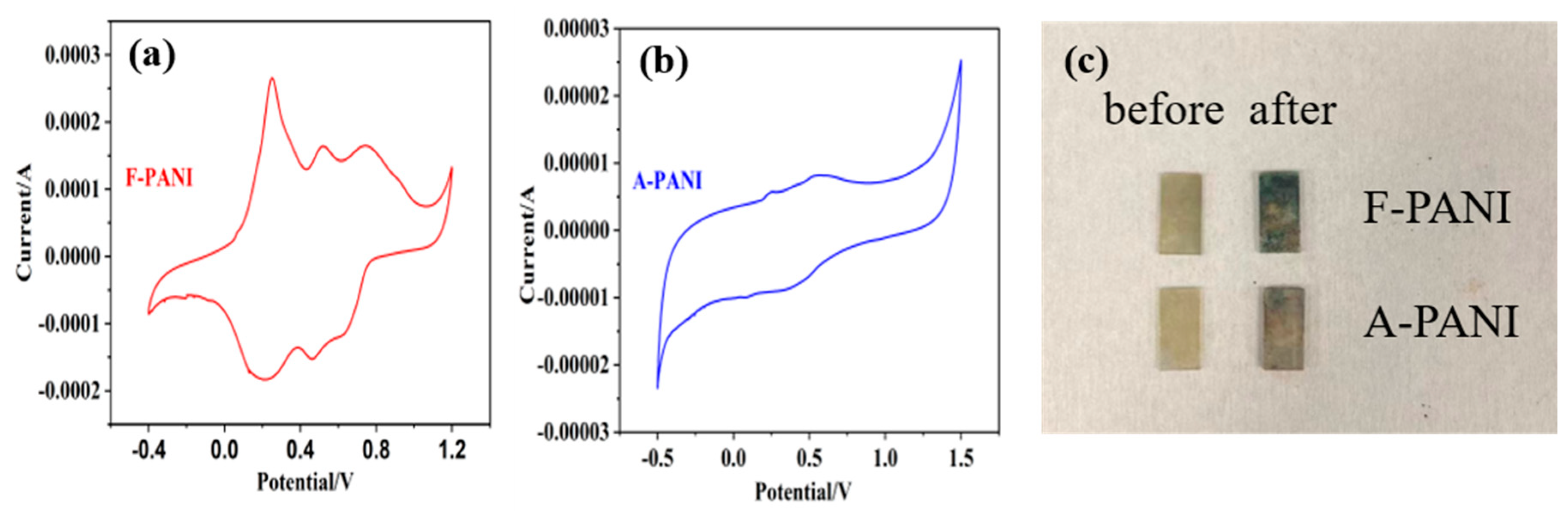
3.3. Electrochromic Properties and Anti-Counterfeiting Label
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, A.M.; Strauss, M.J.; Corcos, A.R.; Hirani, Z.; Ji, W.; Hamachi, L.S.; Aguilar-Enriquez, X.; Chavez, A.D.; Smith, B.J.; Dichtel, W.R. Two-Dimensional Polymers and Polymerizations. Chem. Rev. 2022, 122, 442–564. [Google Scholar] [CrossRef] [PubMed]
- Colson, J.W.; Dichtel, W.R. Rationally synthesized two-dimensional polymers. Nat. Chem. 2013, 5, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Kissel, P.; Murray, D.J.; Wulftange, W.J.; Catalano, V.J.; King, B.T. A nanoporous two-dimensional polymer by single-crystal-to-single-crystal photopolymerization. Nat. Chem. 2014, 6, 774–778. [Google Scholar] [CrossRef]
- Li, J.L.; Liu, X.X.; Feng, Y.; Yin, J.H. Recent progress in polymer/two-dimensional nanosheets composites with novel performances. Prog. Polym. Sci. 2022, 126, 51. [Google Scholar] [CrossRef]
- Dong, R.H.; Pfeffermann, M.; Liang, H.W.; Zheng, Z.K.; Zhu, X.; Zhang, J.; Feng, X.L. Large-Area, Free-Standing, Two-Dimensional Supramolecular Polymer Single-Layer Sheets for Highly Efficient Electrocatalytic Hydrogen Evolution. Angew. Chem.-Int. Edit. 2015, 54, 12058–12063. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.J.; Liao, C.; Hafez, A.M.; Zhu, H.L.; Wu, S.P. Two-dimensional MXenes for energy storage. Chem. Eng. J. 2018, 338, 27–45. [Google Scholar] [CrossRef]
- Shehzad, K.; Xu, Y.; Gao, C.; Duan, X.F. Three-dimensional macro-structures of two-dimensional nanomaterials. Chem. Soc. Rev. 2016, 45, 5541–5588. [Google Scholar] [CrossRef]
- Pinto, G.M.; Cremonezzi, J.M.O.; Ribeiro, H.; Andrade, R.J.E.; Demarquette, N.R.; Fechine, G.J.M. From two-dimensional materials to polymer nanocomposites with emerging multifunctional applications: A critical review. Polym. Compos. 2023, 44, 1438–1470. [Google Scholar] [CrossRef]
- Lv, Q.; Si, W.Y.; He, J.J.; Sun, L.; Zhang, C.F.; Wang, N.; Yang, Z.; Li, X.D.; Wang, X.; Deng, W.Q.; et al. Selectively nitrogen-doped carbon materials as superior metal-free catalysts for oxygen reduction. Nat. Commun. 2018, 9, 11. [Google Scholar] [CrossRef]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Metal nanoparticles supported on two-dimensional graphenes as heterogeneous catalysts. Coord. Chem. Rev. 2016, 312, 99–148. [Google Scholar] [CrossRef]
- Pang, J.B.; Mendes, R.G.; Bachmatiuk, A.; Zhao, L.; Ta, H.Q.; Gemming, T.; Liu, H.; Liu, Z.F.; Rummeli, M.H. Applications of 2D MXenes in energy conversion and storage systems. Chem. Soc. Rev. 2019, 48, 72–133. [Google Scholar] [CrossRef]
- Dong, R.H.; Zhang, T.; Feng, X.L. Interface-Assisted Synthesis of 2D Materials: Trend and Challenges. Chem. Rev. 2018, 118, 6189–6235. [Google Scholar] [CrossRef]
- Liu, W.; Ulaganathan, M.; Abdelwahab, I.; Luo, X.; Chen, Z.X.; Tan, S.J.R.; Wang, X.W.; Liu, Y.P.; Geng, D.C.; Bao, Y.; et al. Two-Dimensional Polymer Synthesized via Solid-State Polymerization for High-Performance Supercapacitors. ACS Nano 2018, 12, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, S.; Pitriana, P.; Hidayat, R.; Fitrilawati; Bahtiar, A.; Siregar, R.E.; Ozaki, M. Application of Hybrid Polymer as a Two Dimensional Grating and It’s Lasing Characteristic. Sains Malays. 2011, 40, 39–42. [Google Scholar]
- Schuster, J.; Köhn, R.; Keilbach, A.; Döblinger, M.; Amenitsch, H.; Bein, T. Two-Dimensional-Hexagonal Periodic Mesoporous Polymer Resin Thin Films by Soft Templating. Chem. Mat. 2009, 21, 5754–5762. [Google Scholar] [CrossRef]
- Duan, C.H.; Chen, K.S.; Huang, F.; Yip, H.L.; Liu, S.J.; Zhang, J.; Jen, A.K.Y.; Cao, Y. Synthesis, Characterization, and Photovoltaic Properties of Carbazole-Based Two-Dimensional Conjugated Polymers with Donor-π-Bridge-Acceptor Side Chains. Chem. Mat. 2010, 22, 6444–6452. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, S.Q.; Huo, L.J.; Zhang, M.J.; Hou, J.H. Molecular Design toward Highly Efficient Photovoltaic Polymers Based on Two-Dimensional Conjugated Benzodithiophene. Accounts Chem. Res. 2014, 47, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.M.; Raghunath, P.; Lin, Y.C.; Lin, H.K.; Ko, C.L.; Su, Y.W.; Lin, M.C.; Wei, K.H. Linear solubilizing side chain substituents enhance the photovoltaic properties of two-dimensional conjugated benzodithiophene-based polymers. Polymer 2015, 79, 262–270. [Google Scholar] [CrossRef]
- Jiang, X.L.; van der Horst, A.; Lima, V.; Schoenmakers, P.J. Comprehensive two-dimensional liquid chromatography for the characterization of functional acrylate polymers. J. Chromatogr. A 2005, 1076, 51–61. [Google Scholar] [CrossRef]
- Huo, L.J.; Tan, Z.A.; Wang, X.; Zhou, Y.; Han, M.F.; Li, Y.F. Novel two-dimensional donor-acceptor conjugated polymers containing quinoxaline units: Synthesis, characterization, and photovoltaic properties. J. Polym. Sci. Pol. Chem. 2008, 46, 4038–4049. [Google Scholar] [CrossRef]
- Zakaria, M.B.; Hu, M.; Tsujimoto, Y.; Sakka, Y.; Suzuki, N.; Kamachi, Y.; Imura, M.; Ishihara, S.; Ariga, K.; Yamauchi, Y. Controlled Crystallization of Cyano-Bridged Cu-Pt Coordination Polymers with Two-Dimensional Morphology. Chem.-Asian J. 2014, 9, 1511–1514. [Google Scholar] [CrossRef]
- Liu, W.; Loh, K.P. Two-Dimensional Conjugated Polymers Based on C-C Coupling. Accounts Chem. Res. 2017, 50, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.D.; Song, M.Y.; Ju, S.; Huang, X.; Wang, X.J.; Shi, X.T.; Zhu, Y.; Wang, Z.; Chen, J.; Li, H.; et al. Wafer-Scale Ultrathin Two-Dimensional Conjugated Microporous Polymers: Preparation and Application in Heterostructure Devices. ACS Appl. Mater. Interfaces 2018, 10, 4010–4017. [Google Scholar] [CrossRef]
- Wang, K.; Qi, D.D.; Li, Y.L.; Wang, T.Y.; Liu, H.B.; Jiang, J.Z. Tetrapyrrole macrocycle based conjugated two-dimensional mesoporous polymers and covalent organic frameworks: From synthesis to material applications. Coord. Chem. Rev. 2019, 378, 188–206. [Google Scholar] [CrossRef]
- Liu, S.Y.; Wei, C.Y.; Wang, H.; Yang, W.L.; Zhang, J.; Wang, Z.P.; Zhao, W.L.; Lee, P.S.; Cai, G.F. Processable nanoarchitectonics of two-dimensional metallo-supramolecular polymer for electrochromic energy storage devices with high coloration efficiency and stability. Nano Energy 2023, 110, 10. [Google Scholar] [CrossRef]
- Huang, X.; Li, H.S.; Tu, Z.Y.; Liu, L.Y.; Wu, X.Y.; Chen, J.; Liang, Y.Y.; Zou, Y.; Yi, Y.P.; Sun, J.L.; et al. Highly Conducting Neutral Coordination Polymer with Infinite Two-Dimensional Silver-Sulfur Networks. J. Am. Chem. Soc. 2018, 140, 15153–15156. [Google Scholar] [CrossRef] [PubMed]
- Sahabudeen, H.; Qi, H.Y.; Ballabio, M.; Polozij, M.; Olthof, S.; Shivhare, R.; Jing, Y.; Park, S.; Liu, K.J.; Zhang, T.; et al. Highly Crystalline and Semiconducting Imine-Based Two-Dimensional Polymers Enabled by Interfacial Synthesis. Angew. Chem.-Int. Edit. 2020, 59, 6028–6036. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, T.S.; Wu, X.; Jiang, C.; Chen, F.; Bai, W.; Bai, R.K. Self-exfoliation of 2D covalent organic frameworks: Morphology transformation induced by solvent polarity. RSC Adv. 2018, 8, 3803–3808. [Google Scholar] [CrossRef]
- Yang, H.Y.; Zhang, T.; Xue, Q.J. Recent advances in single-crystalline two-dimensional polymers: Synthesis, characterization and challenges. Chin. Chem. Lett. 2022, 33, 4989–5000. [Google Scholar] [CrossRef]
- Shen, X.; Zheng, Q.B.; Kim, J.K. Rational design of two-dimensional nanofillers for polymer nanocomposites toward multifunctional applications. Prog. Mater. Sci. 2021, 115, 65. [Google Scholar] [CrossRef]
- Jin, Y.H.; Hu, Y.M.; Zhang, W. Tessellated multiporous two-dimensional covalent organic frameworks. Nat. Rev. Chem. 2017, 1, 11. [Google Scholar] [CrossRef]
- Evans, A.M.; Parent, L.R.; Flanders, N.C.; Bisbey, R.P.; Vitaku, E.; Kirschner, M.S.; Schaller, R.D.; Chen, L.X.; Gianneschi, N.C.; Dichtel, W.R. Seeded growth of single-crystal two-dimensional covalent organic frameworks. Science 2018, 361, 53–57. [Google Scholar] [CrossRef]
- Xing, S.X.; Zhao, C.; Jing, S.Y.; Wang, Z.C. Preparation of polyaniline dispersions with different assembly structure. J. Mater. Sci. 2006, 41, 2761–2766. [Google Scholar] [CrossRef]
- Fan, H.S.; Wang, H.; Guo, J.; Zhao, N.; Xu, J. SDBS-assisted preparation of novel polyaniline planar-structure: Morphology, mechanism and hydrophobicity. J. Colloid Interface Sci. 2014, 414, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.K.; Song, D.M.; Im, S.S. Synthesis of Villi-Structured Polyaniline Sheets Using Organic Single Crystal Surface-Induced Polymerization. ACS Omega 2018, 3, 4181–4186. [Google Scholar] [CrossRef] [PubMed]
- Trchová, M.; Stejskal, J.; Prokes, J. Infrared spectroscopic study of solid-state protonation and oxidation of polyaniline. Synth. Met. 1999, 101, 840–841. [Google Scholar] [CrossRef]
- Dai, T.Y.; Lu, Y. Polycrystalline polyaniline with strong infrared absorption. Eur. Polym. J. 2008, 44, 3417–3422. [Google Scholar] [CrossRef]
- Liu, X.R.; Gao, F.Y.; Dong, G.B.; Wang, C.; Diao, X.G. Infrared Emission Characteristics of Electrochromic Thin Films Based on Polyaniline. Acta Polym. Sin. 2014, 9, 1244–1250. [Google Scholar]
- Bang, D.; Chang, Y.W.; Park, J.; Lee, J.; Yoo, K.H.; Huh, Y.M.; Haam, S. Fabrication of a near-infrared sensor using a polyaniline conducting polymer thin film. Thin Solid Films 2012, 520, 6818–6821. [Google Scholar] [CrossRef]
- da Silva, J.E.P.; Temperini, M.L.A.; de Torresi, S.I.C. Relation between structure and homogeneity of polyaniline blends by infrared and Raman spectroscopies. Synth. Met. 2003, 135, 133–134. [Google Scholar] [CrossRef]
- Jain, M.; Annapoorni, S. Raman study of polyaniline nanofibers prepared by interfacial polymerization. Synth. Met. 2010, 160, 1727–1732. [Google Scholar] [CrossRef]
- Trchová, M.; Morávková, Z.; Bláha, M.; Stejskal, J. Raman spectroscopy of polyaniline and oligoaniline thin films. Electrochim. Acta 2014, 122, 28–38. [Google Scholar] [CrossRef]
- Mazeikiené, R.; Tomkuté, V.; Kuodis, Z.; Niaura, G.; Malinauskas, A. Raman spectroelectrochemical study of polyaniline and sulfonated polyaniline in solutions of different pH. Vib. Spectrosc. 2007, 44, 201–208. [Google Scholar] [CrossRef]
- Zhang, T.; Qi, H.; Liao, Z.Q.; Horev, Y.D.; Panes-Ruiz, L.A.; St Petkovf, P.; Zhang, Z.; Shivhare, R.; Zhang, P.; Liu, K.J.; et al. Engineering crystalline quasi-two-dimensional polyaniline thin film with enhanced electrical and chemiresistive sensing performances. Nat. Commun. 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.Z.; Sheng, G.; Lu, J.; Tong, X.L.; Li, S.; Jiang, X.Y.; Zhang, L.; Luo, J.R.; Shao, Y.Y.; Xia, Z.; et al. Precursor Customized Assembly of Wafer-Scale Polymerized Aniline Thin Films for Ultrasensitive NH3 Detection. Macromol. Rapid Commun. 2022, 43, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.L.; Zhu, Y.H.; Liu, L.M.; Ying, X.R.; Hsiung, C.E.; Sougrat, R.; Li, K.; Han, Y. Atomic-resolution transmission electron microscopy of electron beam-sensitive crystalline materials. Science 2018, 359, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.Y.; Lee, J.; Ahn, H.; Lee, J.; Choi, H.C.; Park, M.J. High-Conductivity Two-Dimensional Polyaniline Nanosheets Developed on Ice Surfaces. Angew. Chem.-Int. Edit. 2015, 54, 10497–10501. [Google Scholar] [CrossRef]
- Liu, S.H.; Zhang, J.; Dong, R.H.; Gordiichuk, P.; Zhang, T.; Zhuang, X.D.; Mai, Y.Y.; Liu, F.; Herrmann, A.; Feng, X.L. Two-Dimensional Mesoscale-Ordered Conducting Polymers. Angew. Chem.-Int. Edit. 2016, 55, 12516–12521. [Google Scholar] [CrossRef]
- Argun, A.A.; Aubert, P.H.; Thompson, B.C.; Schwendeman, I.; Gaupp, C.L.; Hwang, J.; Pinto, N.J.; Tanner, D.B.; MacDiarmid, A.G.; Reynolds, J.R. Multicolored electrochromism polymers: Structures and devices. Chem. Mat. 2004, 16, 4401–4412. [Google Scholar] [CrossRef]
- Baba, A.; Tian, S.J.; Stefani, F.; Xia, C.J.; Wang, Z.H.; Advincula, R.C.; Johannsmann, D.; Knoll, W. Electropolymerization and doping/dedoping properties of polyaniline thin films as studied by electrochemical-surface plasmon spectroscopy and by the quartz crystal microbalance. J. Electroanal. Chem. 2004, 562, 95–103. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z. Preparation of Two-Dimensional Polyaniline Sheets with High Crystallinity via Surfactant Interface Self-Assembly and Their Encryption Application. Polymers 2024, 16, 1285. https://doi.org/10.3390/polym16091285
Li Z. Preparation of Two-Dimensional Polyaniline Sheets with High Crystallinity via Surfactant Interface Self-Assembly and Their Encryption Application. Polymers. 2024; 16(9):1285. https://doi.org/10.3390/polym16091285
Chicago/Turabian StyleLi, Zhiwei. 2024. "Preparation of Two-Dimensional Polyaniline Sheets with High Crystallinity via Surfactant Interface Self-Assembly and Their Encryption Application" Polymers 16, no. 9: 1285. https://doi.org/10.3390/polym16091285
APA StyleLi, Z. (2024). Preparation of Two-Dimensional Polyaniline Sheets with High Crystallinity via Surfactant Interface Self-Assembly and Their Encryption Application. Polymers, 16(9), 1285. https://doi.org/10.3390/polym16091285




