Nanofibrous ε-Polycaprolactone Matrices Containing Nano-Hydroxyapatite and Humulus lupulus L. Extract: Physicochemical and Biological Characterization for Oral Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Electrospun Nanofibers
2.3. Morphological Characterization
2.4. Thermogravimetric Analysis
2.5. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.6. Mechanical Characterization
2.7. In Vitro Degradation Assays
2.8. Antibacterial Assay
2.8.1. Determination of Growth Inhibition Zones of the H. lupulus Extract
2.8.2. Determination of Growth Inhibition Zones of the Matrices
2.9. Cell Culture and Cell Viability Assay
2.10. Statistical Analysis
3. Results
3.1. Morphological Characterization
3.2. Thermogravimetric Analysis (TGA)
3.3. FTIR Analysis
3.4. In Vitro Degradation Analysis
3.5. Mechanical Characterization
3.6. Antibacterial Assay
3.6.1. Determination of Growth Inhibition Zones
3.6.2. Determination of Growth Inhibition Zones of the Matrices
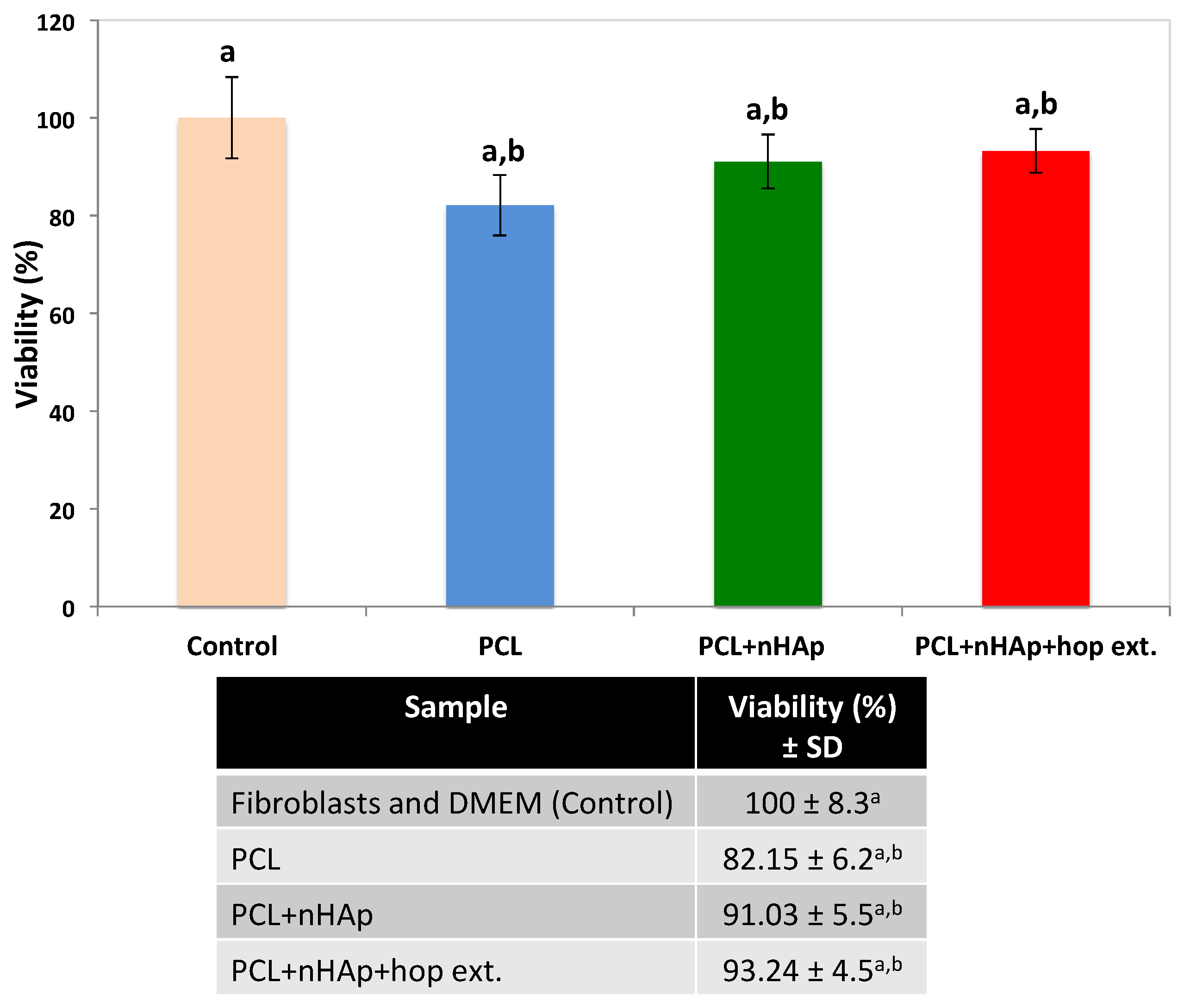
3.7. Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FTIR | Fourier-transform infrared spectroscopy |
| Hop | Humulus lupulus L. |
| nHAp | Nano-hydroxyapatite |
| PBS | Phosphate-buffered saline |
| PCL | Poly(ε-caprolactone) |
| SEM | Scanning Electron Microscope |
| TGA | Thermogravimetric Analysis (TGA) |
References
- Khademhosseini, A.; Langer, R. Nanobiotechnology—Drug delivery and tissue engineering. Chem. Eng. Prog. 2006, 102, 38–42. [Google Scholar]
- Carvalho, M.S.; Cabral, J.; da Silva, C.L.; Vashishth, D. Bone matrix non-collagenous proteins in tissue engineering: Creating new bone by mimicking the extracellular matrix. Polymers 2021, 13, 1095. [Google Scholar] [CrossRef]
- Bernard, M.; Jubeli, E.; Pungente, M.D.; Yagoubi, N. Biocompatibility of polymer-based biomaterials and medical devices—Regulations, in vitro screening and risk-management. Biomater. Sci. 2018, 6, 2025–2053. [Google Scholar] [CrossRef]
- Rezaei Kolarijani, N.; Cheraghali, D.; Khastar, H.; Ehterami, A.; Alizade, M.; Vaez, A.; Amini, S.M.; Salehi, M. Nanofibrous polycaprolactone/gelatin scaffold containing gold nanoparticles: Physicochemical and biological characterization for wound healing. Wound Repair Regen. 2023, 31, 804–815. [Google Scholar] [CrossRef]
- Lykins, W.R.; Bernards, D.A.; Schlesinger, E.B.; Wisniewski, K.; Desai, T.A. Tuning polycaprolactone degradation for long acting implantables. Polymer 2022, 262, 125473. [Google Scholar] [CrossRef]
- Rahimkhoei, V.; Padervand, M.; Hedayat, M.; Seidi, F.; Dawi, E.A.; Akbari, A. Biomedical applications of electrospun polycaprolactone-based carbohydrate polymers: A review. Int. J. Biol. Macromol. 2023, 253 Pt 1, 126642. [Google Scholar] [CrossRef]
- Halim, N.A.A.; Hussein, M.Z.; Kandar, M.K. Nanomaterials—Upconverted hydroxyapatite for bone tissue engineering and a platform for drug delivery. Int. J. Nanomed. 2021, 16, 6477. [Google Scholar] [CrossRef]
- Munir, M.U.; Salman, S.; Javed, I.; Bukhari, S.N.A.; Ahmad, N.; Shad, N.A.; Aziz, F. Nano-hydroxyapatite as a delivery system: Overview and advancements. Artif. Cells Nanomed. Biotechnol. 2021, 49, 717–727. [Google Scholar] [CrossRef]
- Du, M.; Chen, J.; Liu, K.; Xing, H.; Song, C. Recent advances in biomedical engineering of nano-hydroxyapatite including dentistry, cancer treatment and bone repair. Compos. B Eng. 2021, 215, 108790. [Google Scholar] [CrossRef]
- Haider, M.K.; Kharaghani, D.; Sun, L.; Ullah, S.; Sarwar, M.N.; Ullah, A.; Khatri, M.; Yoshiko, Y.; Gopiraman, M.; Kim, I.S. Synthesized bioactive lignin nanoparticles/polycaprolactone nanofibers: A novel nanobiocomposite for bone tissue engineering. Biomater. Adv. 2023, 144, 213203. [Google Scholar] [CrossRef]
- Hussain, Z.; Mehmood, S.; Liu, X.; Liu, Y.; Wang, G.; Pei, R. Decoding bone-inspired and cell-instructive cues of scaffolds for bone tissue engineering. Eng. Regen. 2023, 5, 21–44. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, T.; Chen, J.; Su, J.; Zhi, X.; Pan, P.; Zou, L.; Zhang, Q. Magnetic bioinspired micro/nanostructured composite scaffold for bone regeneration. Colloids Surf. B Biointerfaces 2019, 174, 70–79. [Google Scholar] [CrossRef]
- Hoveidaei, A.H.; Sadat-Shojai, M.; Mosalamiaghili, S.; Salarikia, S.R.; Roghani-Shahraki, H.; Ghaderpanah, R.; Ersi, M.H.; Conway, J.D. Nano-hydroxyapatite structures for bone regenerative medicine: Cell-material interaction. Bone 2023, 179, 116956. [Google Scholar] [CrossRef]
- Awad, H.A.; O’Keefe, R.J.; Mao, J.J. Bone tissue engineering. In Principles of Tissue Engineering; Elsevier: Oxford, UK, 2020; pp. 1511–1519. [Google Scholar]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontol. 2000 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Hamada, S.; Slade, H.D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 1980, 44, 331–384. [Google Scholar] [CrossRef]
- He, M.; Wang, Q.; Xie, L.; Wu, H.; Zhao, W.; Tian, W. Hierarchically multi-functionalized graded membrane with enhanced bone regeneration and self-defensive antibacterial characteristics for guided bone regeneration. Chem. Eng. J. 2020, 398, 125542. [Google Scholar] [CrossRef]
- Li, J.; Lai, Y.; Li, M.; Chen, X.; Zhou, M.; Wang, W.; Li, J.; Cui, W.; Zhang, G.; Liu, L.; et al. Repair of infected bone defect with clindamycin-tetrahedral DNA nanostructure complex-loaded 3D bioprinted hybrid scaffold. Chem. Eng. J. 2022, 435 Pt 1, 134855. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Li, T.; Liu, X.; Hao, Z.; Ding, S.; Panichayupakaranan, P.; Zhu, K.; Shen, J. Plant natural flavonoids against multidrug resistant pathogens. Adv. Sci. 2021, 8, 2100749. [Google Scholar] [CrossRef]
- Abiko, Y.; Paudel, D.; Uehara, O. Hops components and oral health. J. Funct. Foods 2022, 92, 105035. [Google Scholar] [CrossRef]
- González-Salitre, L.; González-Olivares, L.G.; Basilio-Cortes, U.A. Humulus lupulus L. a potential precursor to human health: High hops craft beer. Food Chem. 2023, 405, 134959. [Google Scholar] [CrossRef]
- Betancur, M.; López, J.; Salazar, F. Antimicrobial activity of compounds from hop (Humulus lupulus L.) following supercritical fluid extraction: An overview. Chil. J. Agric. Res. 2023, 83, 499–509. [Google Scholar] [CrossRef]
- Khaliullina, A.; Kolesnikova, A.; Khairullina, L.; Morgatskaya, O.; Shakirova, D.; Patov, S.; Nekrasova, P.; Bogachev, M.; Kurkin, V.; Trizna, E.; et al. The antimicrobial potential of the hop (Humulus lupulus L.) extract against Staphylococcus aureus and oral Streptococci. Pharmaceuticals 2024, 17, 162. [Google Scholar] [CrossRef]
- Bolton, J.L.; Dunlap, T.L.; Hajirahkimkhan, A.; Mbachu, O.; Chen, S.-N.; Chadwick, L.; Nikolic, D.; van Breemen, R.B.; Pauli, G.F.; Dietz, B.M. The multiple biological targets of hops and bioactive compounds. Chem. Res. Toxicol. 2019, 32, 222–233. [Google Scholar] [CrossRef]
- Hiraki, D.; Uehara, O.; Harada, F.; Takai, R.; Takahasi, S.; Taraya, S.; Abiko, Y. Effect of xanthohumol on Porphyromonas gingivalis. Jpn. J. Conserv. Dent. 2019, 62, 271–278. [Google Scholar]
- Harish, V.; Haque, E.; Śmiech, M.; Taniguchi, H.; Jamieson, S.; Tewari, D.; Bishayee, A. Xanthohumol for human malignancies: Chemistry, pharmacokinetics and molecular targets. Int. J. Mol. Sci. 2021, 22, 4478. [Google Scholar] [CrossRef]
- Jeong, H.M.; Han, E.H.; Jin, Y.H.; Choi, Y.H.; Lee, K.Y.; Jeong, H.G. Xanthohumol from the hop plant stimulates osteoblast differentiation by RUNX2 activation. Biochem. Biophys. Res. Commun. 2011, 409, 82–89. [Google Scholar] [CrossRef]
- Abdul Hameed, M.M.; Mohamed Khan, S.A.P.; Thamer, B.M.; Rajkumar, N.; El-Hamshary, H.; El-Newehy, M. Electrospun nanofibers for drug delivery applications: Methods and mechanism. Polym. Adv. Technol. 2023, 34, 6–23. [Google Scholar] [CrossRef]
- Liu, H.; Bai, Y.; Huang, C.; Wang, Y.; Ji, Y.; Du, Y.; Bligh, S.W.A. Recent progress of electrospun herbal medicine nanofibers. Biomolecules 2023, 13, 184. [Google Scholar] [CrossRef]
- Liu, J.; Zou, Q.; Wang, C.; Lin, M.; Li, Y.; Zhang, R.; Li, Y. Electrospinning and 3D printed hybrid bi-layer scaffold for guided bone regeneration. Mater. Des. 2021, 210, 110047. [Google Scholar] [CrossRef]
- ISO 10993-13; Biological Evaluation of Medical Devices—Part 13: Identification and Quantification of Degradation Products from Polymeric Medical Devices. ISO: Geneva, Switzerland, 2010.
- Bauer, A.W. Antibiotic susceptibility testing by a standardized single disc method. Am. J. Clin. Pathol. 1966, 45, 149–158. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices. Part 5: Tests for Cytotoxicity: In Vitro Methods. ISO: Geneva, Switzerland, 1993.
- Zhu, P.; Yin, H.; Wei, J.; Wu, J.; Ping, D.; Zhang, X. A bilayer biocompatible polycaprolactone/zinc oxide/Capparis spinosa L. ethyl acetate extract/polylactic acid nanofibrous composite scaffold for novel wound dressing applications. Int. J. Biol. Macromol. 2023, 242 Pt 3, 125093. [Google Scholar] [CrossRef]
- Irani, M.; Abadi, P.G.; Ahmadian-Attari, M.M.; Rezaee, A.; Kordbacheh, H.; Goleij, P. In vitro and in vivo studies of Dragon’s blood plant (D. cinnabari)-loaded electrospun chitosan/PCL nanofibers: Cytotoxicity, antibacterial, and wound healing activities. Int. J. Biol. Macromol. 2024, 257, 128634. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, P.K.; Stachewicz, U. The impact of relative humidity on electrospun polymer fibers: From structural changes to fiber morphology. Adv. Colloid Interfac. Sci. 2020, 286, 102315. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Bonakdar, M.; Rodrigue, D. Electrospinning: Processes, structures, and materials. Macromolecules 2024, 4, 58–103. [Google Scholar] [CrossRef]
- Chong, L.H.; Hassan, M.I.; Sultana, N. Electrospun polycaprolactone (PCL) and PCL/nano-hydroxyapatite (PCL/nHA)-based nanofibers for bone tissue engineering application. In Proceedings of the IEEE 10th Asian Control Conference (ASCC), Sabah, Malaysia, 31 May–3 June 2015; pp. 1–4. [Google Scholar]
- Hassan, M.I.; Sultana, N. Characterization, drug loading and antibacterial activity of nanohydroxyapatite/polycaprolactone (nHA/PCL) electrospun membrane. 3 Biotech. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fortună, M.E.; Ungureanu, E.; Rotaru, R.; Bargan, A.; Ungureanu, O.C.; Brezuleanu, C.O.; Harabagiu, V. Synthesis and properties of modified biodegradable polymers based on caprolactone. Polymers 2023, 15, 4731. [Google Scholar] [CrossRef] [PubMed]
- Emadi, H.; Karevan, M.; Masoudi Rad, M.; Sadeghzade, S.; Pahlevanzadeh, F.; Khodaei, M.; Khayatzadeh, S.; Lotfian, S. Bioactive and biodegradable polycaprolactone-based nanocomposite for bone repair applications. Polymers 2023, 15, 3617. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Huang, S.; Yang, Z.; Liu, T.; Qilin, Z.; Feng, J.; Zeng, B. Biomechanical properties of a customizable TPU/PCL blended esophageal stent fabricated by 3D printing. Mater. Today Commun. 2023, 34, 105196. [Google Scholar] [CrossRef]
- Su, T.T.; Jiang, H.; Gong, H. Thermal stabilities and the thermal degradation kinetics of poly(ε-caprolactone). Polymer-Plast. Technol. Eng. 2008, 47, 398–403. [Google Scholar] [CrossRef]
- Motloung, M.P.; Mofokeng, T.G.; Ray, S.S. Viscoelastic, thermal, and mechanical properties of melt-processed poly(ε-caprolactone) (PCL)/hydroxyapatite (HAP) composites. Materials 2021, 15, 104. [Google Scholar] [CrossRef]
- Dias, D.; Vale, A.C.; Cunha, E.P.; C Paiva, M.; Reis, R.L.; Vaquette, C.; Alves, N.M. 3D-printed cryomilled poly(ε-caprolactone)/graphene composite scaffolds for bone tissue regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 961–972. [Google Scholar] [CrossRef]
- Loyo, C.; Cordoba, A.; Palza, H.; Canales, D.; Melo, F.; Vivanco, J.F.; Vallejos, R.; Millán, C.; Corrales, T.; Zapata, P.A. Effect of Gelatin Coating and GO incorporation on the properties and degradability of electrospun PCL scaffolds for bone tissue regeneration. Polymers 2023, 16, 129. [Google Scholar] [CrossRef]
- Alexeev, D.; Tschopp, M.; Helgason, B.; Ferguson, S.J. Electrospun biodegradable poly(ε-caprolactone) membranes for annulus fibrosus repair: Long-term material stability and mechanical competence. JOR Spine 2021, 4, e1130. [Google Scholar] [CrossRef]
- Swetha, S.; Balagangadharan, K.; Lavanya, K.; Selvamurugan, N. Three-dimensional-poly(lactic acid) scaffolds coated with gelatin/magnesium-doped nano-hydroxyapatite for bone tissue engineering. Biotechnol. J. 2021, 16, 2100282. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Kosmalska, A.; Zaborski, M. Characteristics of compounds in hops using cyclic voltammetry, UV–VIS, FTIR and GC–MS analysis. Food Chem. 2014, 156, 353–361. [Google Scholar] [CrossRef]
- Pawar, R.; Pathan, A.; Nagaraj, S.; Kapare, H.; Giram, P.; Wavhale, R. Polycaprolactone and its derivatives for drug delivery. Polym. Adv. Technol. 2023, 34, 3296–3316. [Google Scholar] [CrossRef]
- Yaseri, R.; Fadaie, M.; Mirzaei, E.; Samadian, H.; Ebrahiminezhad, A. Surface modification of polycaprolactone nanofibers through hydrolysis and aminolysis: A comparative study on structural characteristics, mechanical properties, and cellular performance. Sci. Rep. 2023, 13, 9434. [Google Scholar] [CrossRef]
- Rashid, T.U.; Gorga, R.E.; Krause, W.E. Mechanical properties of electrospun fibers—A critical review. Adv. Eng. Mater. 2021, 23, 2100153. [Google Scholar] [CrossRef]
- Fu, S.Y.; Feng, X.Q.; Lauke, B.; Mai, Y.W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Composites Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Croisier, F.; Duwez, A.S.; Jérôme, C.; Léonard, A.F.; Van Der Werf, K.O.; Dijkstra, P.J.; Bennink, M.L. Mechanical testing of electrospun PCL fibers. Acta Biomater. 2012, 8, 218–224. [Google Scholar] [CrossRef]
- Alharbi, N.; Daraei, A.; Lee, H.; Guthold, M. The effect of molecular weight and fiber diameter on the mechanical properties of single, electrospun PCL nanofibers. Mater. Today Commun. 2023, 35, 105773. [Google Scholar] [CrossRef]
- Modiri-Delshad, T.; Ramazani, A.; Khoobi, M.; Akbari Javar, H.; Akbari, T.; Amin, M. Fabrication of chitosan/polycaprolactone/Myrtus communis L. extract nanofibrous mats with enhanced antibacterial activities. Polym. Polym. Compos. 2023, 31, 09673911231151506. [Google Scholar] [CrossRef]
- Moraczewski, K.; Stepczyńska, M.; Malinowski, R.; Karasiewicz, T.; Jagodziński, B.; Rytlewski, P. Modification of polycaprolactone with plant extracts to improve the aging resistance. Materials 2023, 16, 5154. [Google Scholar] [CrossRef]
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Preparation and characterization of Calendula officinalis-loaded PCL/gum Arabic nanocomposite scaffolds for wound healing applications. Iran. Polym. J. 2019, 28, 51–63. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Virani, S.; Zavro, M.; Haas, G.J. Inhibition of Streptococcus mutans and other oral Streptococci by hop (Humulus lupulus L.) constituents. Econ. Bot. 2003, 57, 118–125. [Google Scholar] [CrossRef]
- Haas, G.J.; Barsoumian, R. Antimicrobial activity of hop resins. J. Food Prot. 1994, 57, 59–61. [Google Scholar] [CrossRef]
- Teuber, M. Low antibiotic potency of isohumulone. Appl. Microbiol. 1970, 19, 871. [Google Scholar] [CrossRef]
- Klimek, K.; Tyśkiewicz, K.; Miazga-Karska, M.; Dębczak, A.; Rój, E.; Ginalska, G. Bioactive compounds obtained from Polish “Marynka” hop variety using efficient two-step supercritical fluid extraction and comparison of their antibacterial, cytotoxic, and anti-proliferative activities in vitro. Molecules 2021, 26, 2366. [Google Scholar] [CrossRef]
- Gregory, E.R.; Bakhaider, R.F.; Gomez, G.F.; Huang, R.; Moser, E.A.; Gregory, R.L. Evaluating hop extract concentrations found in commercial beer to inhibit Streptococcus mutans biofilm formation. J. Appl. Microbiol. 2022, 133, 1333–1340. [Google Scholar] [CrossRef]
- Stompor, M.; Żarowska, B. Antimicrobial activity of xanthohumol and its selected structural analogues. Molecules 2016, 21, 608. [Google Scholar] [CrossRef]
- Schmalreck, A.F.; Teuber, M.; Reininger, W.; Hartl, A. Structural features determining the antibiotic potencies of natural and synthetic hop bitter resins, their precursors and derivatives. Can. J. Microbiol. 1975, 21, 205–212. [Google Scholar] [CrossRef]
- Inaba, H.; Tagashira, M.; Kanda, T.; Ohno, T.; Kawai, S.; Amano, A. Apple- and hop-polyphenols protect periodontal ligament cells stimulated with enamel matrix derivative from Porphyromonas gingivalis. J. Periodontol. 2005, 76, 2223–2229. [Google Scholar] [CrossRef]
- Shinada, K.; Tagashira, M.; Watanabe, H. Hop bract polyphenols reduced three-day dental plaque regrowth. J. Dent. Res. 2007, 86, 848–851. [Google Scholar] [CrossRef]
- DeSimone, E.; Aigner, T.B.; Humenik, M.; Lang, G.; Scheibel, T. Aqueous electrospinning of recombinant spider silk proteins. Mater. Sci. Eng. C 2020, 106, 110145. [Google Scholar] [CrossRef]
- Dalton, P.D.; Klinkhammer, K.; Salber, J.; Klee, D.; Möller, M. Direct in vitro electrospinning with polymer melts. Biomacromolecules 2006, 13, 686–690. [Google Scholar] [CrossRef]
- Lim, M.M.; Sun, T.; Sultana, N. In vitro biological evaluation of electrospun polycaprolactone/gelatine nanofibrous scaffold for tissue engineering. J. Nanomater. 2015, 16, 416. [Google Scholar]
- Philips, N.; Samuel, P.; Lozano, T.; Gvaladze, A.; Guzman, B.; Siomyk, H.; Haas, G. Effects of Humulus lupulus extract or its components on viability, lipid peroxidation, and expression of vascular endothelial growth factor in melanoma cells and fibroblasts. J. Clin. Res. 2017, 1, 15–19. [Google Scholar] [CrossRef][Green Version]
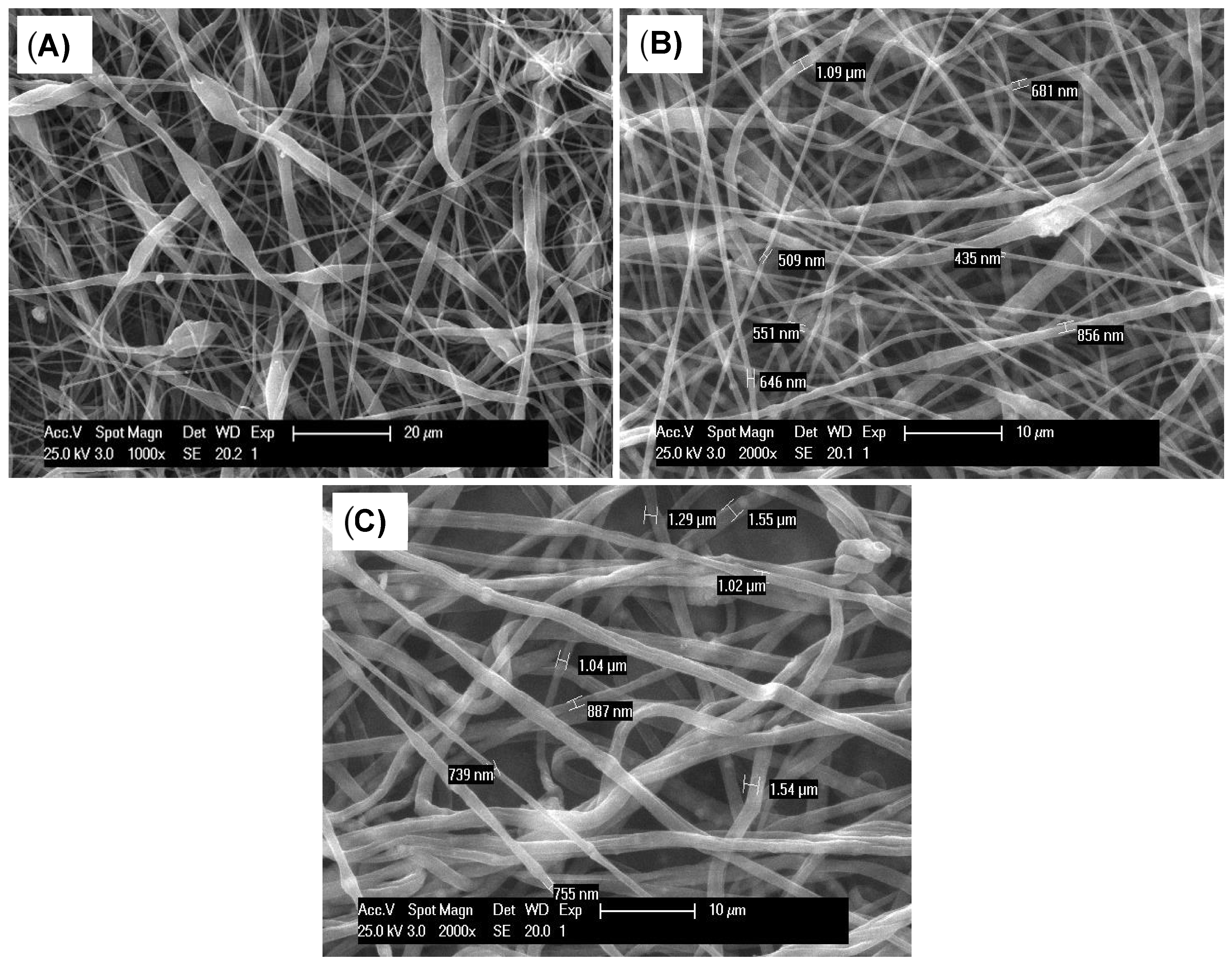
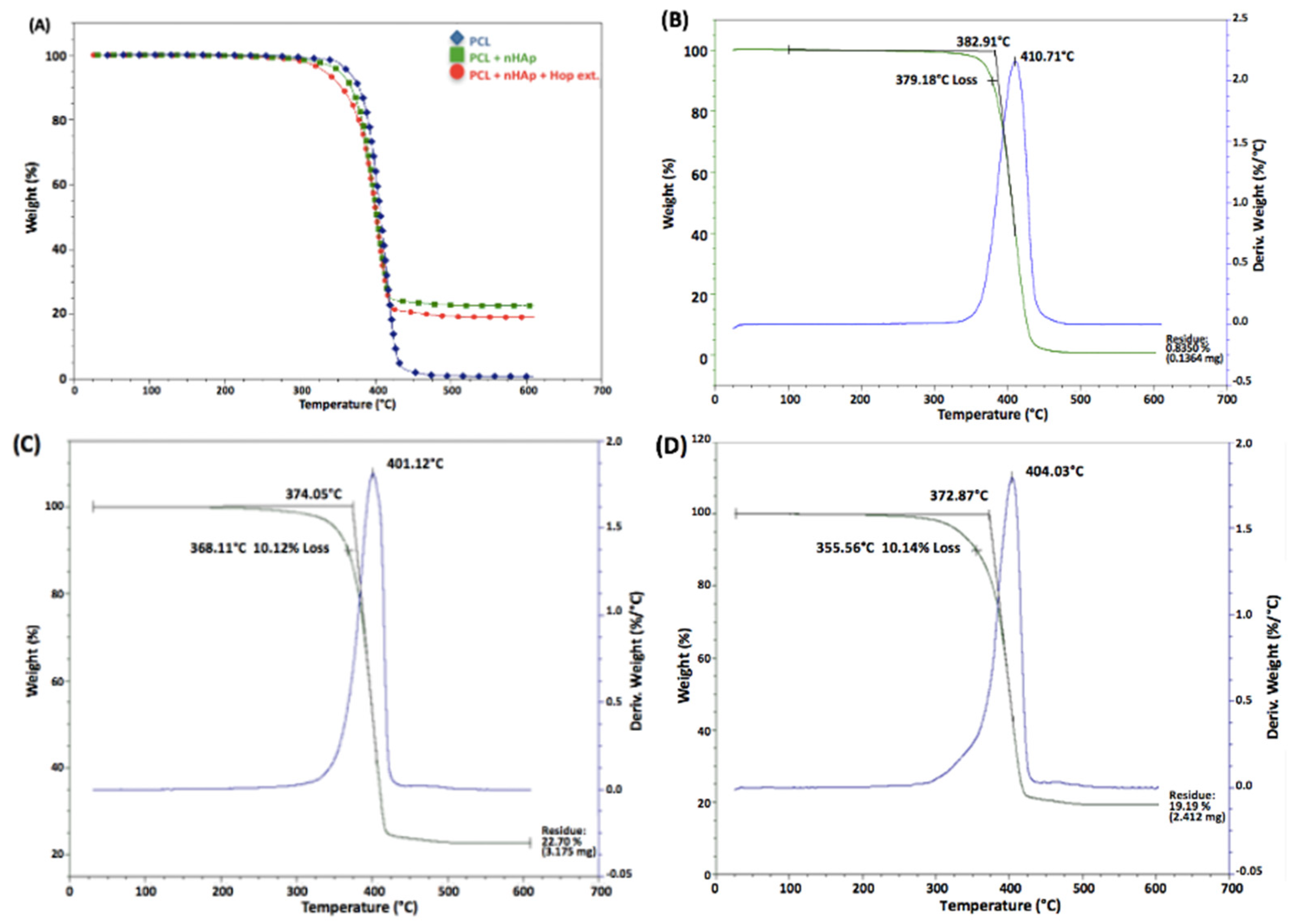
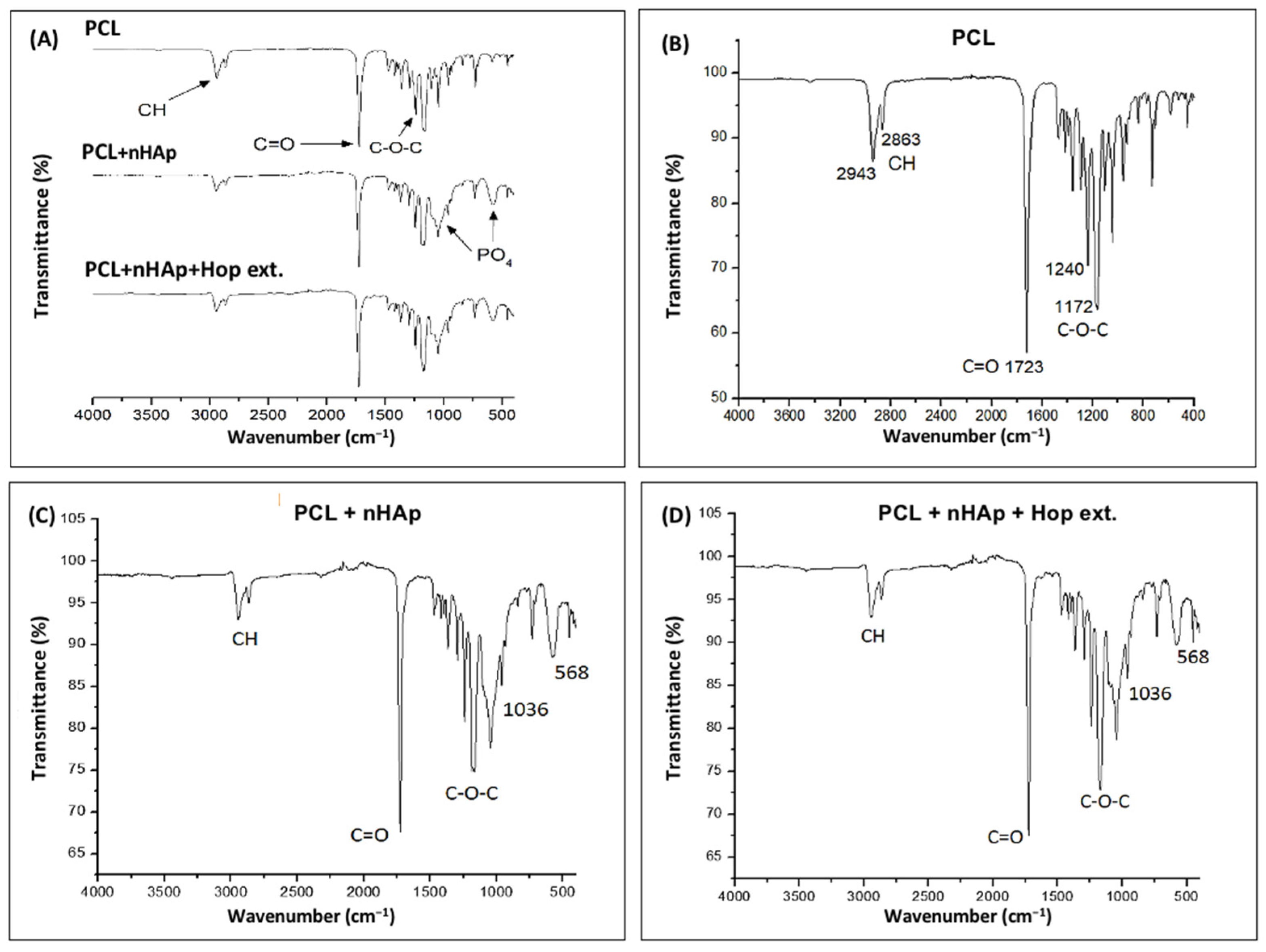
| Sample | Fiber Diameter (nm ± SD) | p Value |
|---|---|---|
| PCL | 549 ± 60 c | 0.70 |
| PCL + nHAp | 681 ± 112 b | 0.99 |
| PCL + nHAp + hop ext. | 1102 ± 162 a | 0.01 |
| Sample | Onset (°C) | Max. Weight Loss Temp (°C) | Residue (%) |
|---|---|---|---|
| PCL | 382.91 | 410.71 | 0.83 |
| PCL + nHAp | 374.05 | 401.12 | 22.70 |
| PCL + nHAp + hop ext. | 372.87 | 404.03 | 19.20 |
| Sample | Initial Weight (mg) | Final Weight (mg) | Weight Loss (%) |
|---|---|---|---|
| PCL | 10.13 | 10.02 | 1 |
| PCL + nHAp | 10.10 | 9.90 | 2 |
| PCL + nHAp + hop ext. | 10.13 | 9.70 | 4 |
| Sample | Tensile Strength (MPa) (Mean ± SD) | Elongation at Break (% ± SD) |
|---|---|---|
| PCL | 1.74 ± 0.63 a | 3.8 ± 0.66 a |
| PCL + nHAp | 1.39 ± 0.69 a | 2.2 ± 0.81 b |
| PCL + nHAp + hop ext. | 1.07 ± 0.35 a | 5.4 ± 1.90 a |
| Sample | Inhibition Zone (mm) ± SD | ||
|---|---|---|---|
| Sm | Pg | Aa | |
| Chlorhexidine 0.12% (+control) | 28 ± 0.4 a | 25 ± 2.1 a | 23 ± 0.4 a |
| Hop extract | 19 ± 0.5 b | 15 ± 0.8 b | 12 ± 4.4 b |
| PCL + nHAp + hop ext. | 4 ± 1.2 c | 2 ± 1.7 c | 0 ± 0.5 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villanueva-Lumbreras, J.; Rodriguez, C.; Aguilar, M.R.; Avilés-Arnaut, H.; Cordell, G.A.; Rodriguez-Garcia, A. Nanofibrous ε-Polycaprolactone Matrices Containing Nano-Hydroxyapatite and Humulus lupulus L. Extract: Physicochemical and Biological Characterization for Oral Applications. Polymers 2024, 16, 1258. https://doi.org/10.3390/polym16091258
Villanueva-Lumbreras J, Rodriguez C, Aguilar MR, Avilés-Arnaut H, Cordell GA, Rodriguez-Garcia A. Nanofibrous ε-Polycaprolactone Matrices Containing Nano-Hydroxyapatite and Humulus lupulus L. Extract: Physicochemical and Biological Characterization for Oral Applications. Polymers. 2024; 16(9):1258. https://doi.org/10.3390/polym16091258
Chicago/Turabian StyleVillanueva-Lumbreras, Jaime, Ciro Rodriguez, María Rosa Aguilar, Hamlet Avilés-Arnaut, Geoffrey A. Cordell, and Aida Rodriguez-Garcia. 2024. "Nanofibrous ε-Polycaprolactone Matrices Containing Nano-Hydroxyapatite and Humulus lupulus L. Extract: Physicochemical and Biological Characterization for Oral Applications" Polymers 16, no. 9: 1258. https://doi.org/10.3390/polym16091258
APA StyleVillanueva-Lumbreras, J., Rodriguez, C., Aguilar, M. R., Avilés-Arnaut, H., Cordell, G. A., & Rodriguez-Garcia, A. (2024). Nanofibrous ε-Polycaprolactone Matrices Containing Nano-Hydroxyapatite and Humulus lupulus L. Extract: Physicochemical and Biological Characterization for Oral Applications. Polymers, 16(9), 1258. https://doi.org/10.3390/polym16091258






