Poly(dl-lactide) Polymer Blended with Mineral Phases for Extrusion 3D Printing—Studies on Degradation and Biocompatibility
Abstract
1. Introduction
2. Materials and Methods
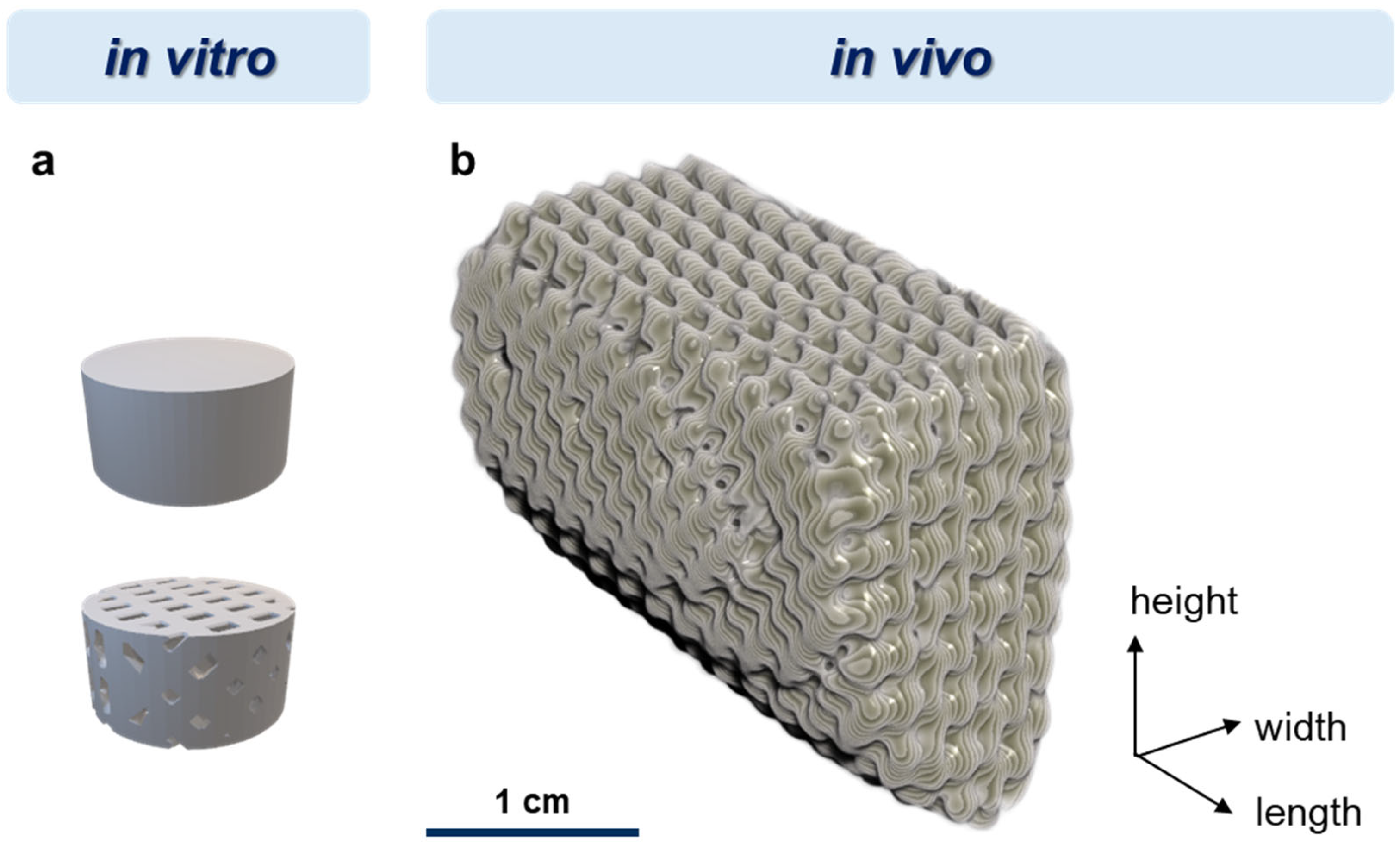
2.1. Materials and Scaffold Fabrication
2.2. Aging Experiment and Analysis of Degradation
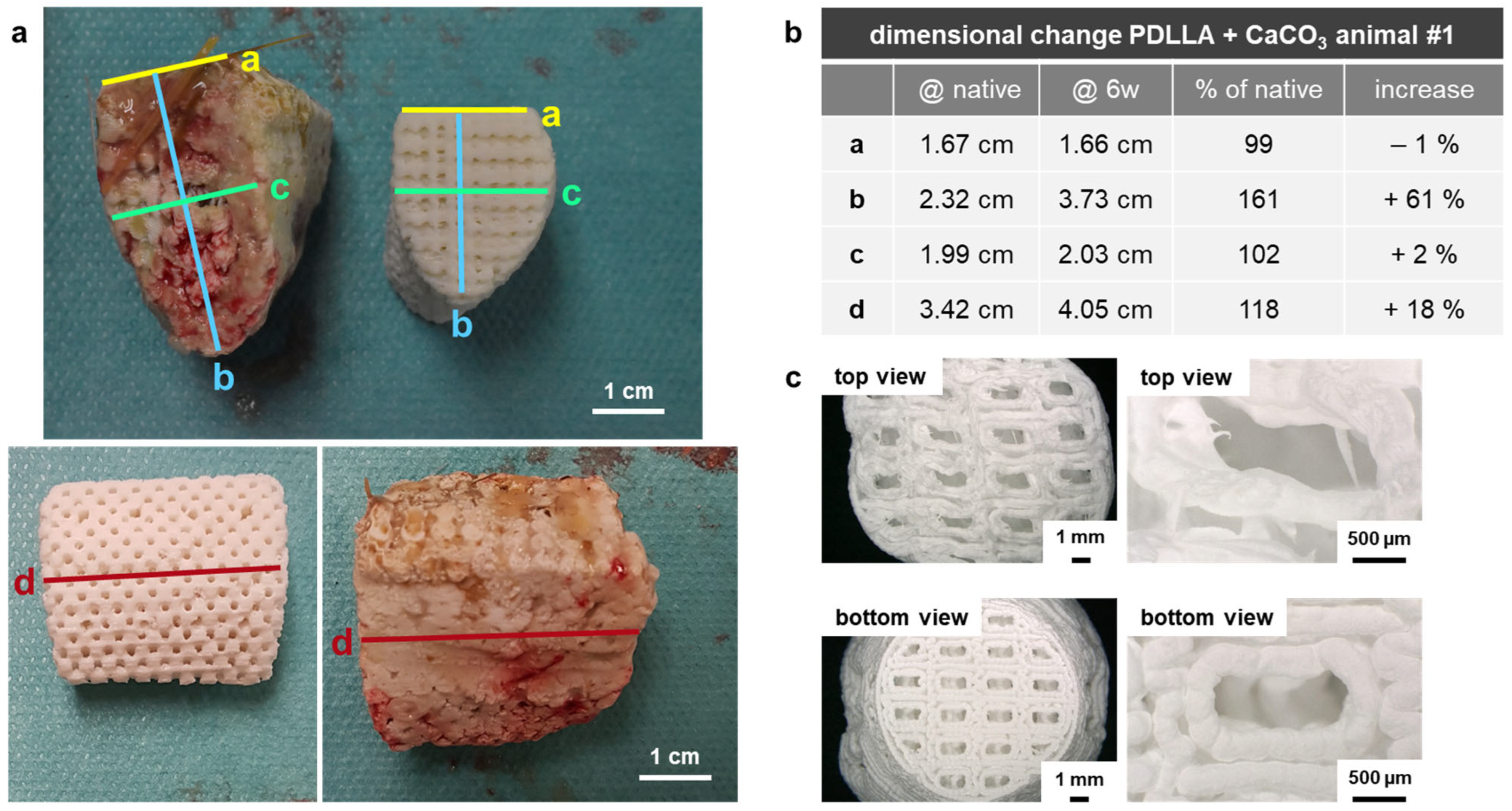
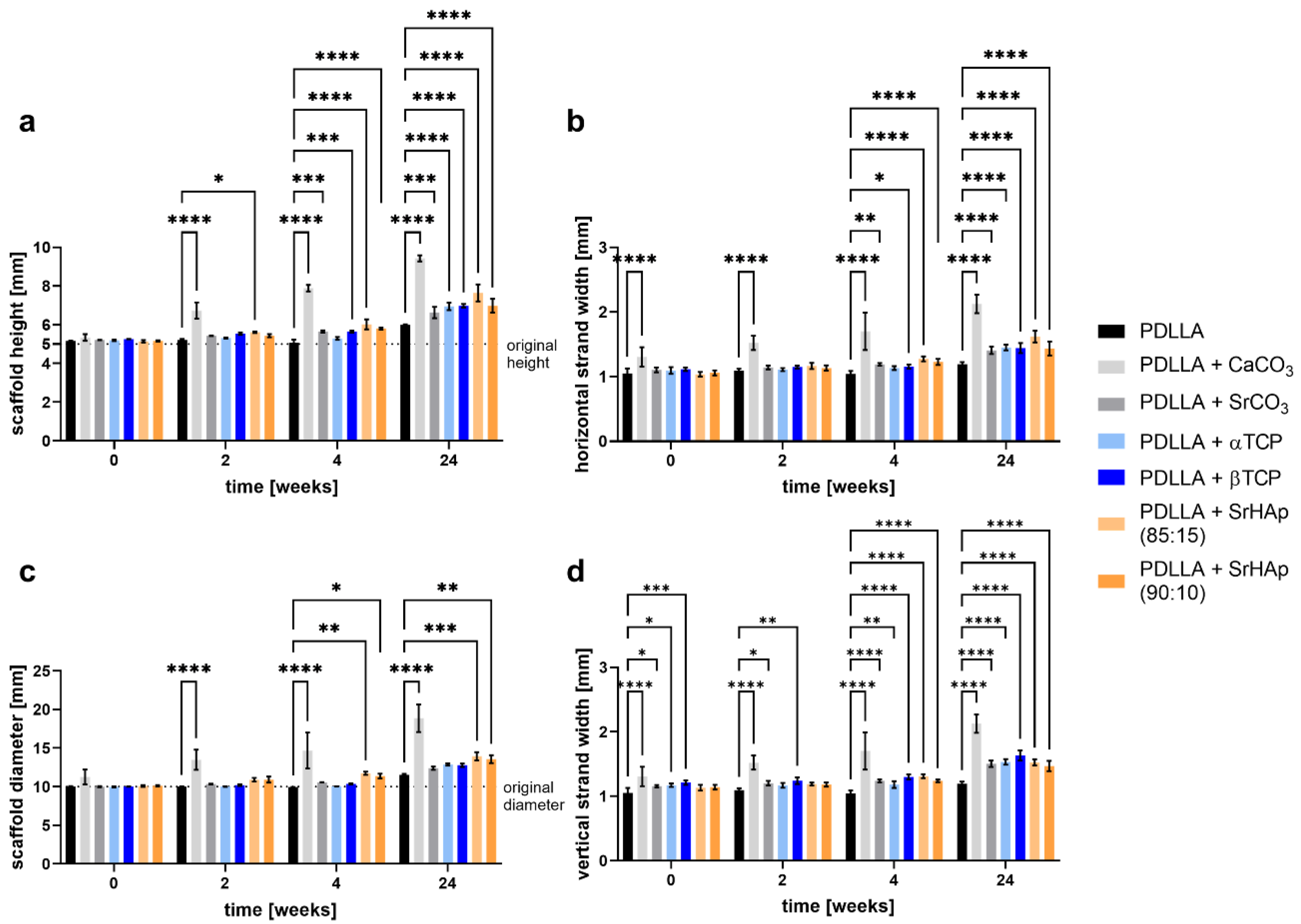
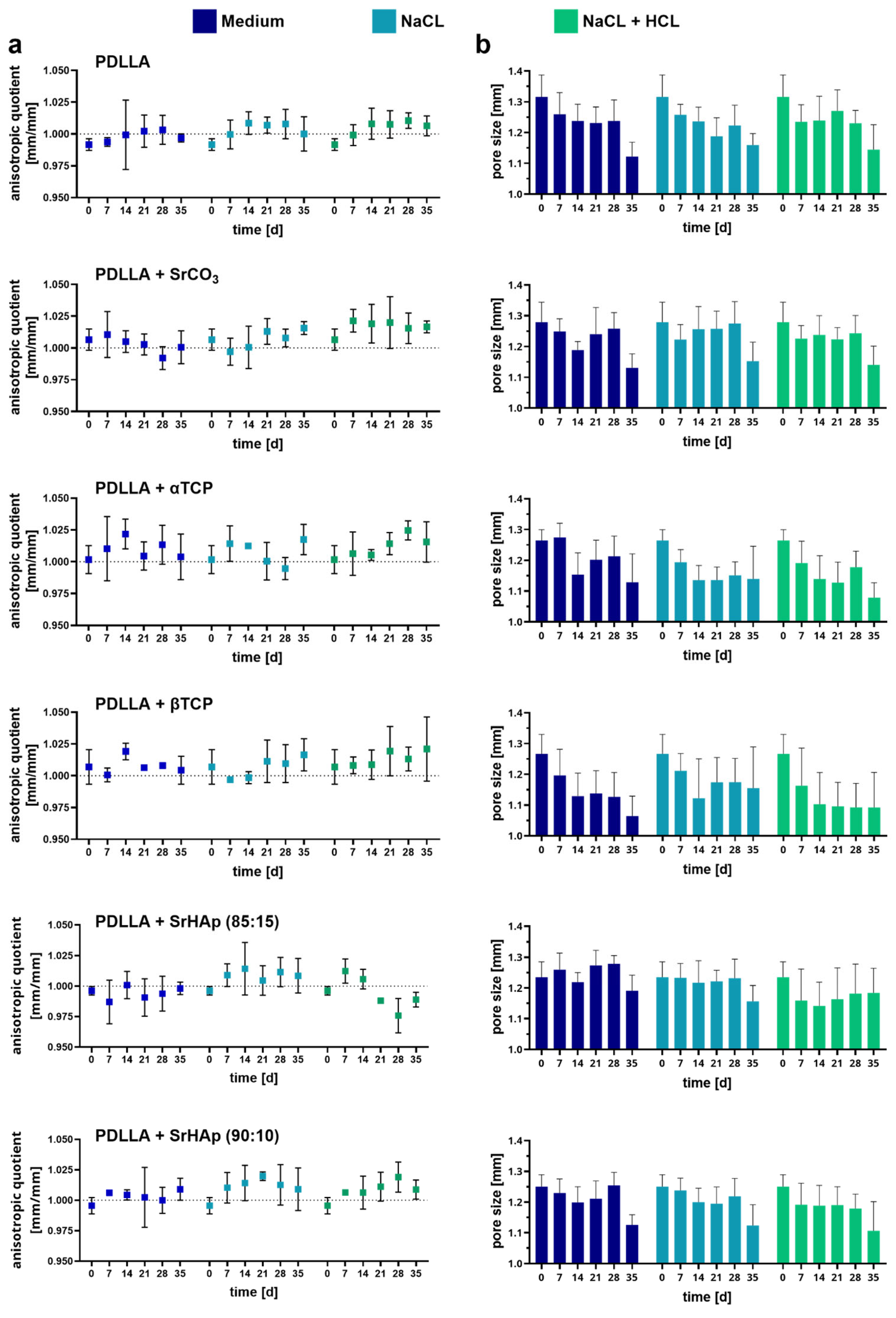
2.2.1. Measurement of Dimensional Change
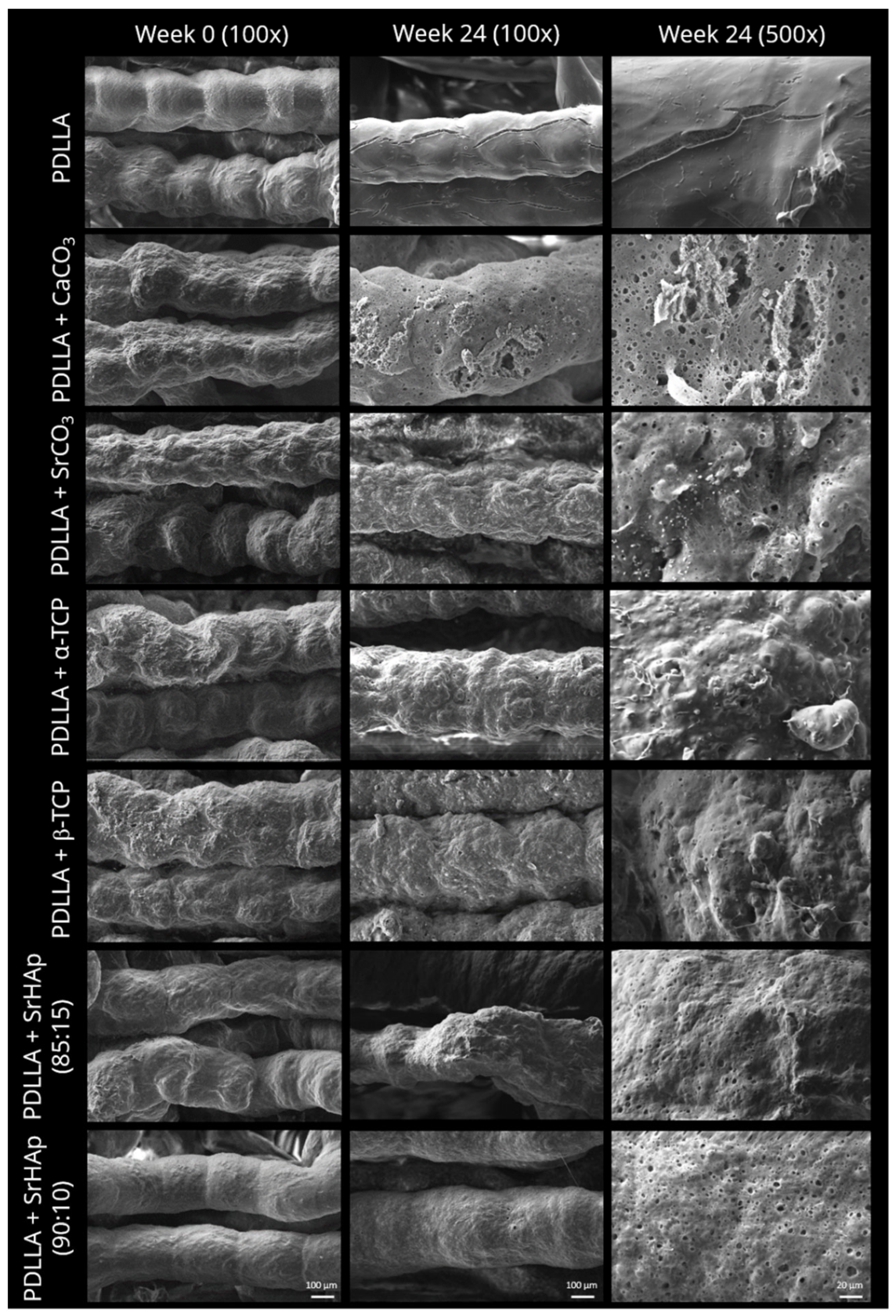
2.2.2. Scanning Electron Microscopy
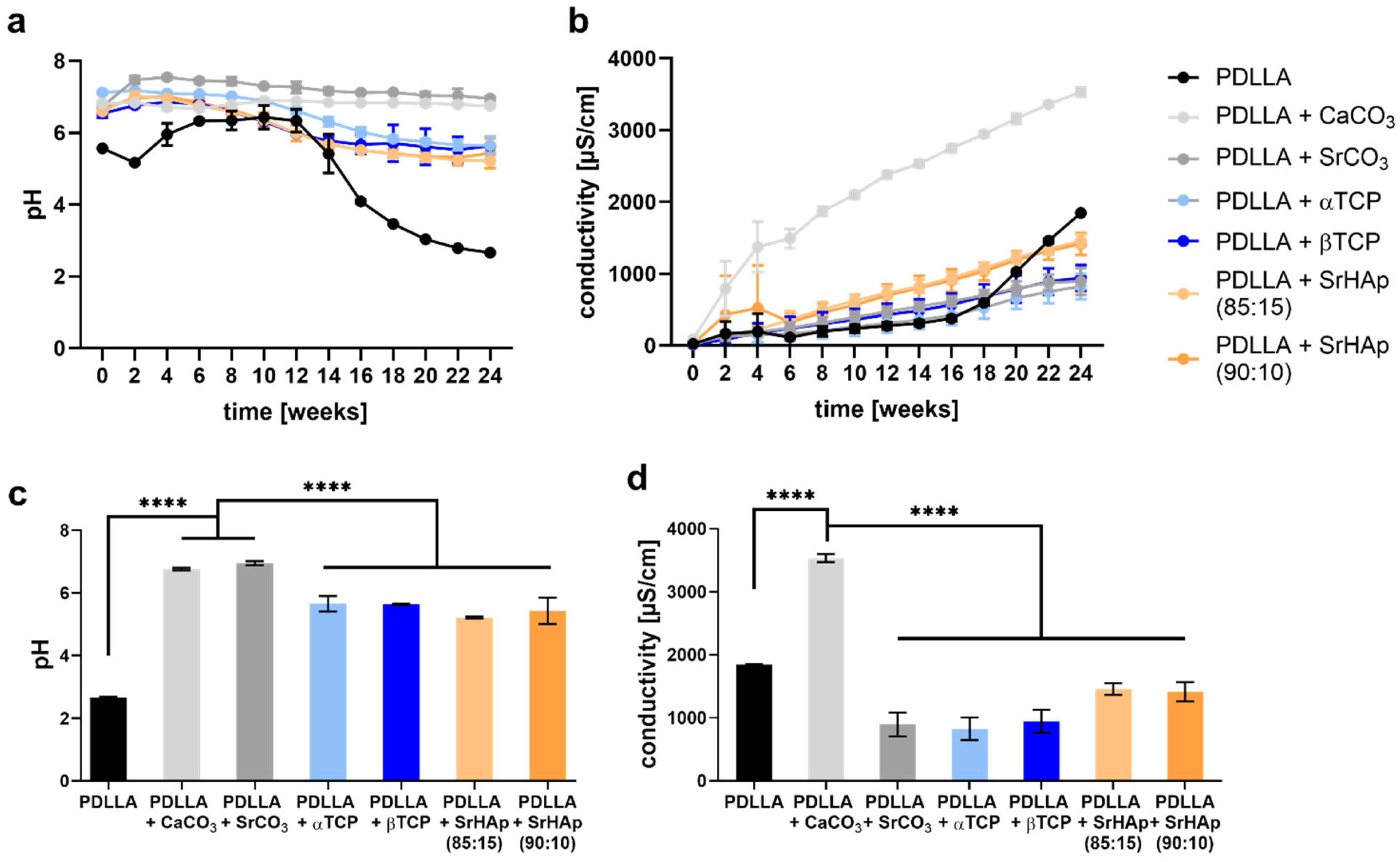
2.2.3. Measurement of pH Value and Conductivity
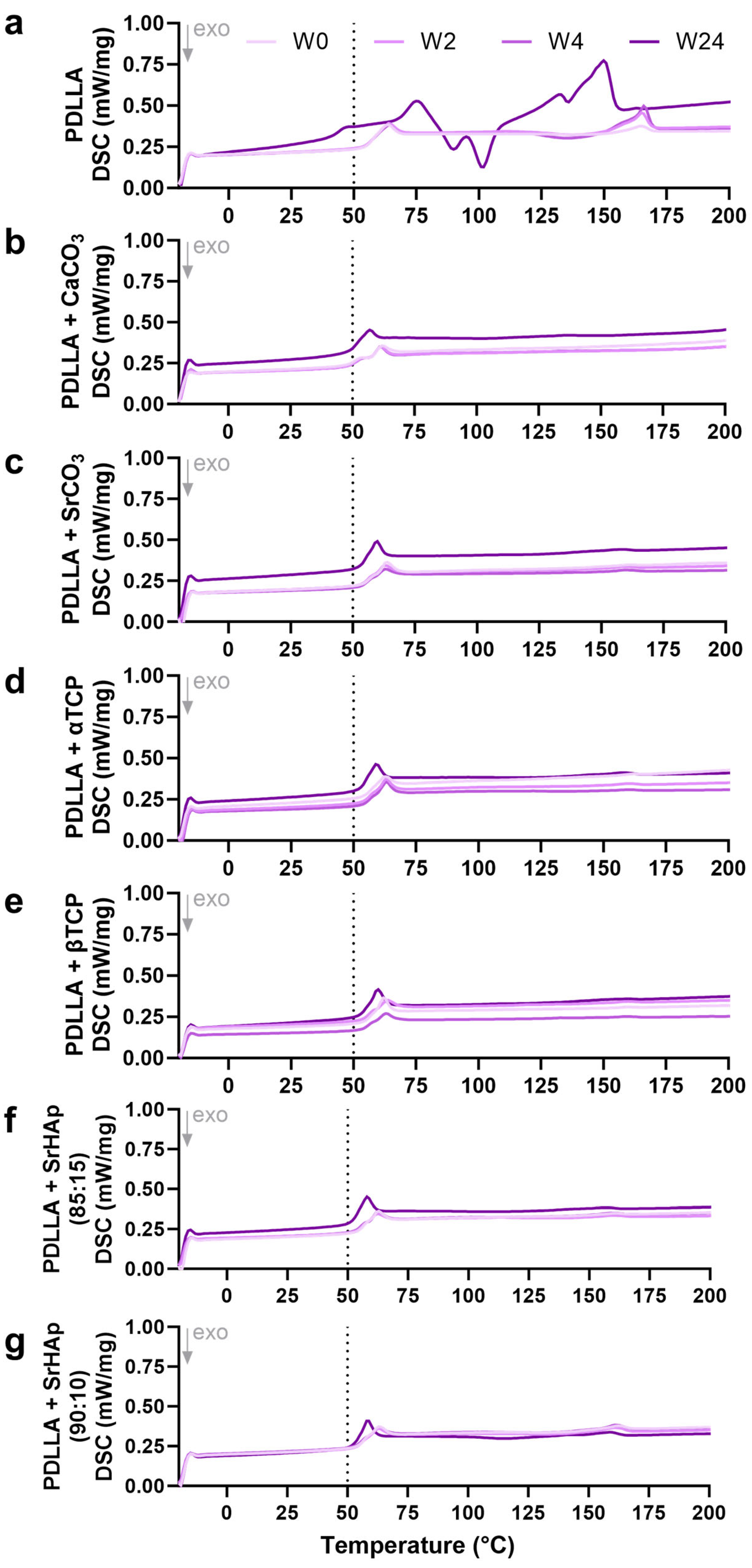
2.2.4. Differential Scanning Calorimetry
2.2.5. Measurement of Viscosity
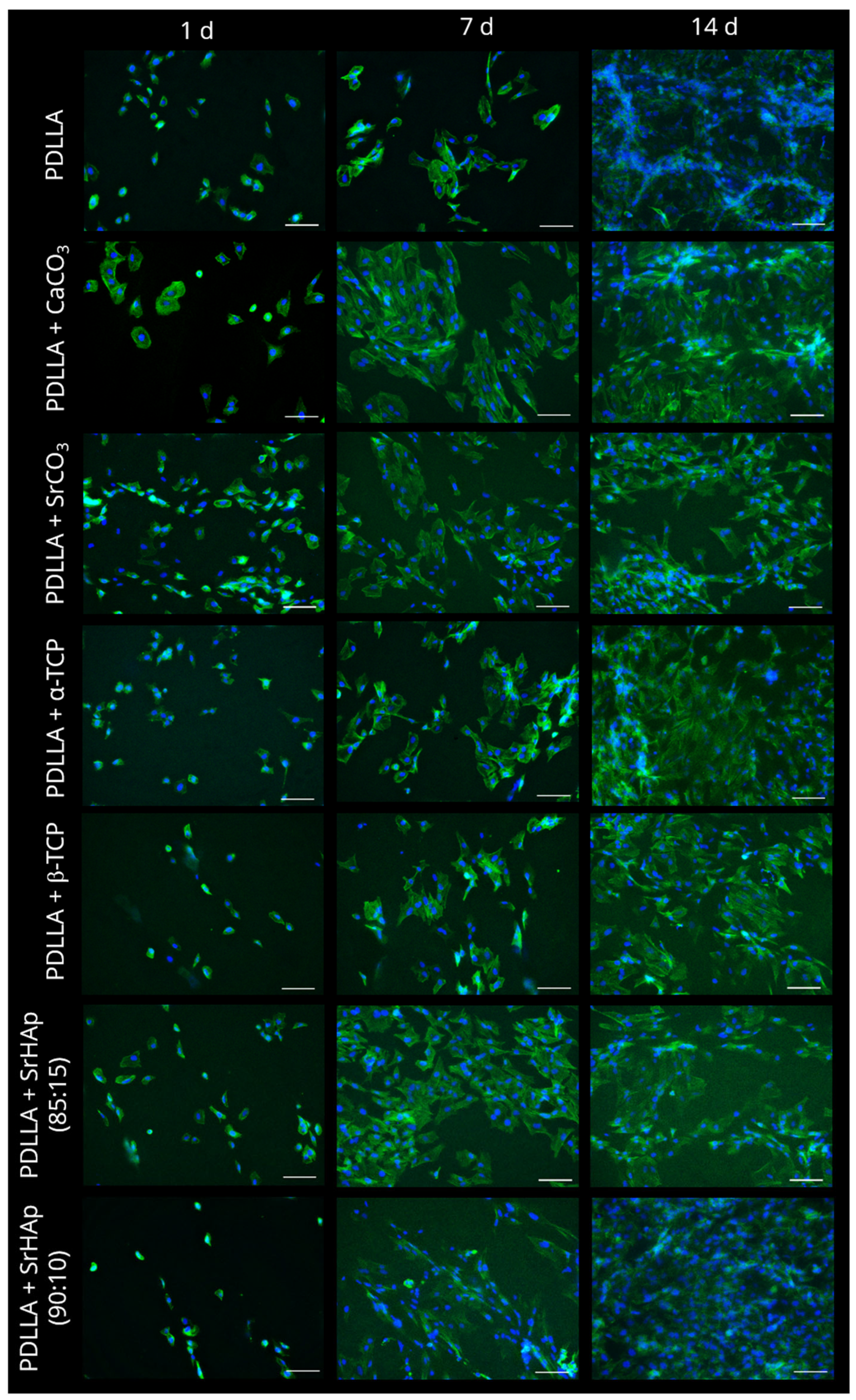
2.3. Cell Culture Experiments
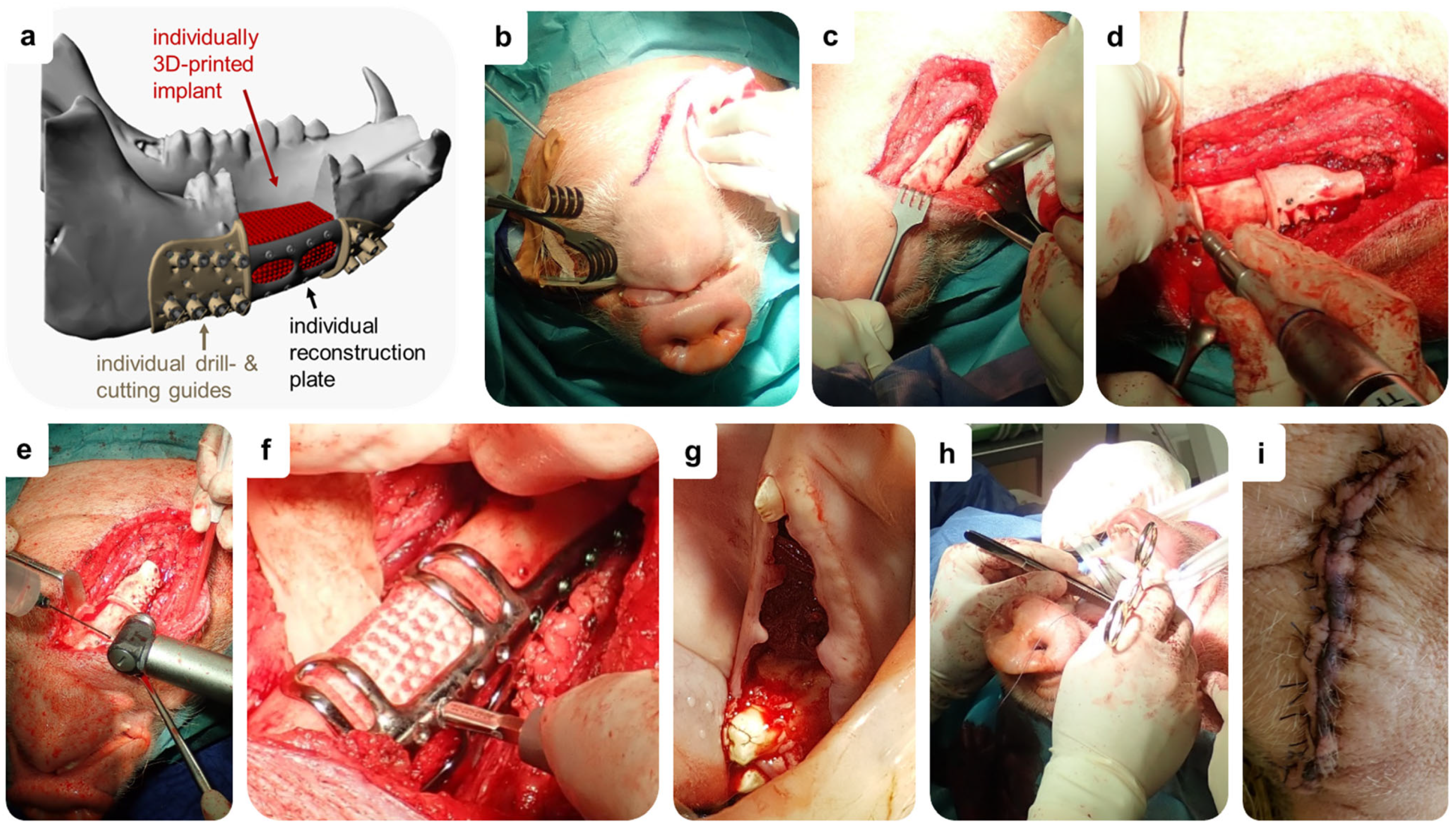
2.4. Pilot In Vivo Study
2.4.1. Study Design
2.4.2. Animals and Ethics Statement
2.4.3. DVT Measurements
2.4.4. Surgical Procedure for Implantation of Individual Implants
2.5. In Vitro Swelling Model for Prediction
2.6. Statistics
3. Results
3.1. Influence of the Mineral-Phase Microparticles on Degradation
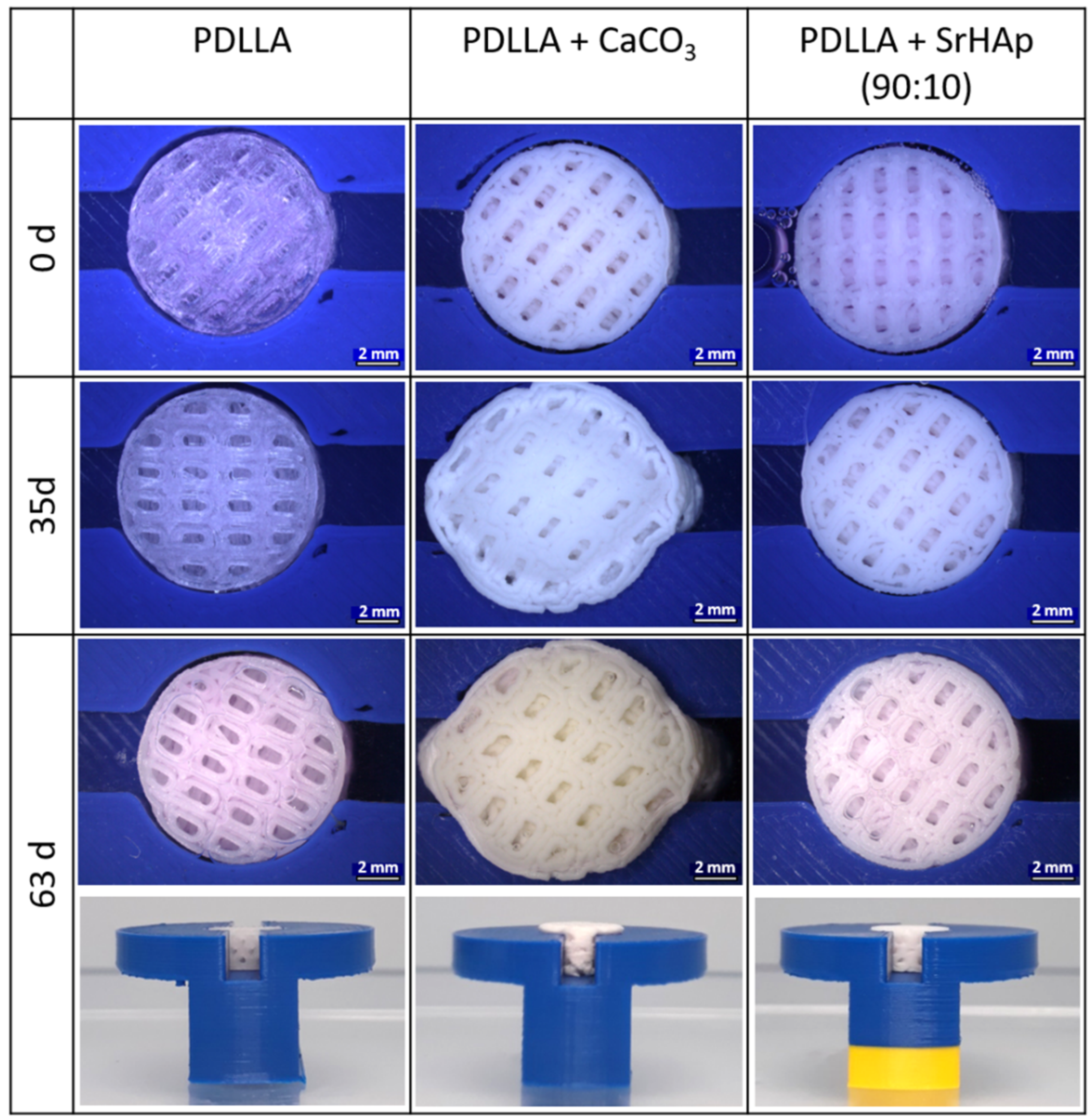
3.1.1. Dimensional Change
3.1.2. Morphology of Strand Surface
3.1.3. Development of pH and Conductivity
3.1.4. Differential Scanning Calorimetry
3.1.5. Changes in Viscosity
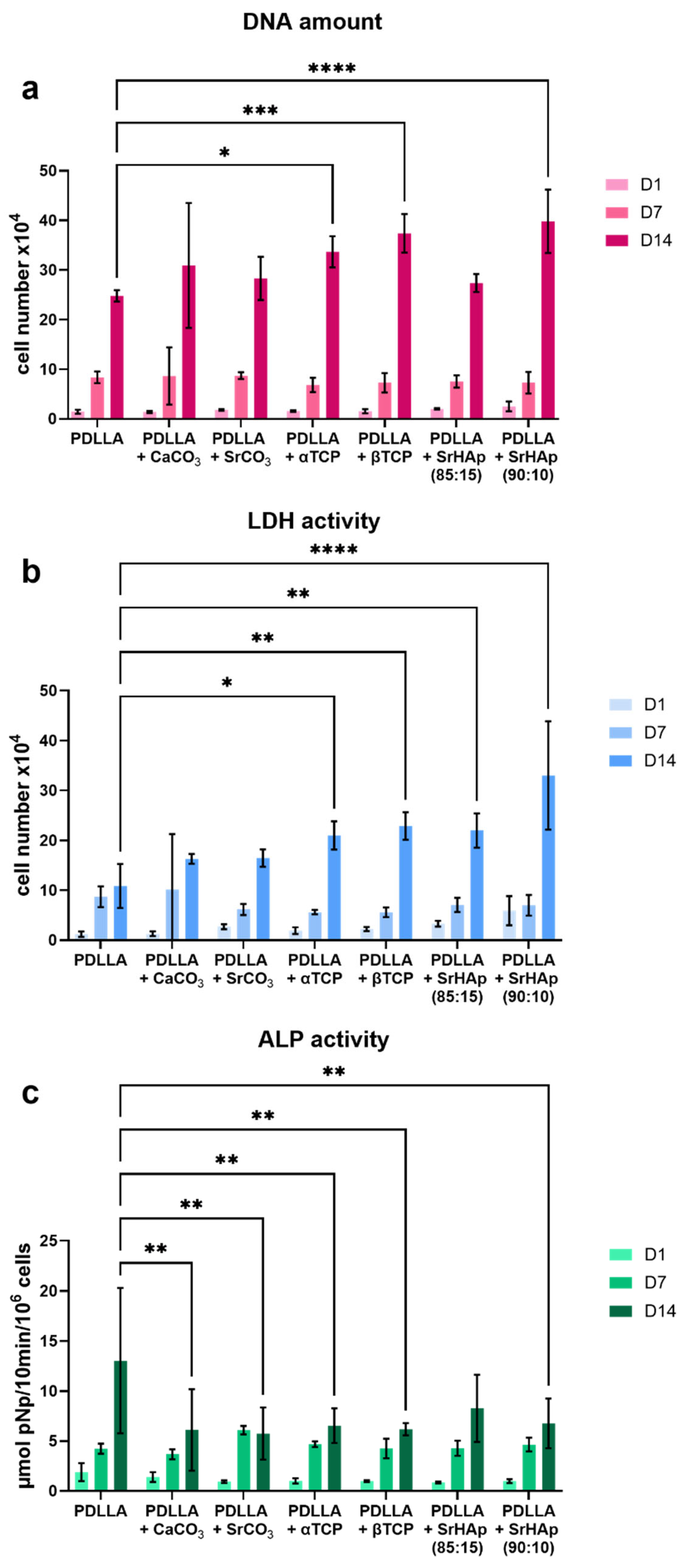
3.2. Analysis of Cytocompatibility
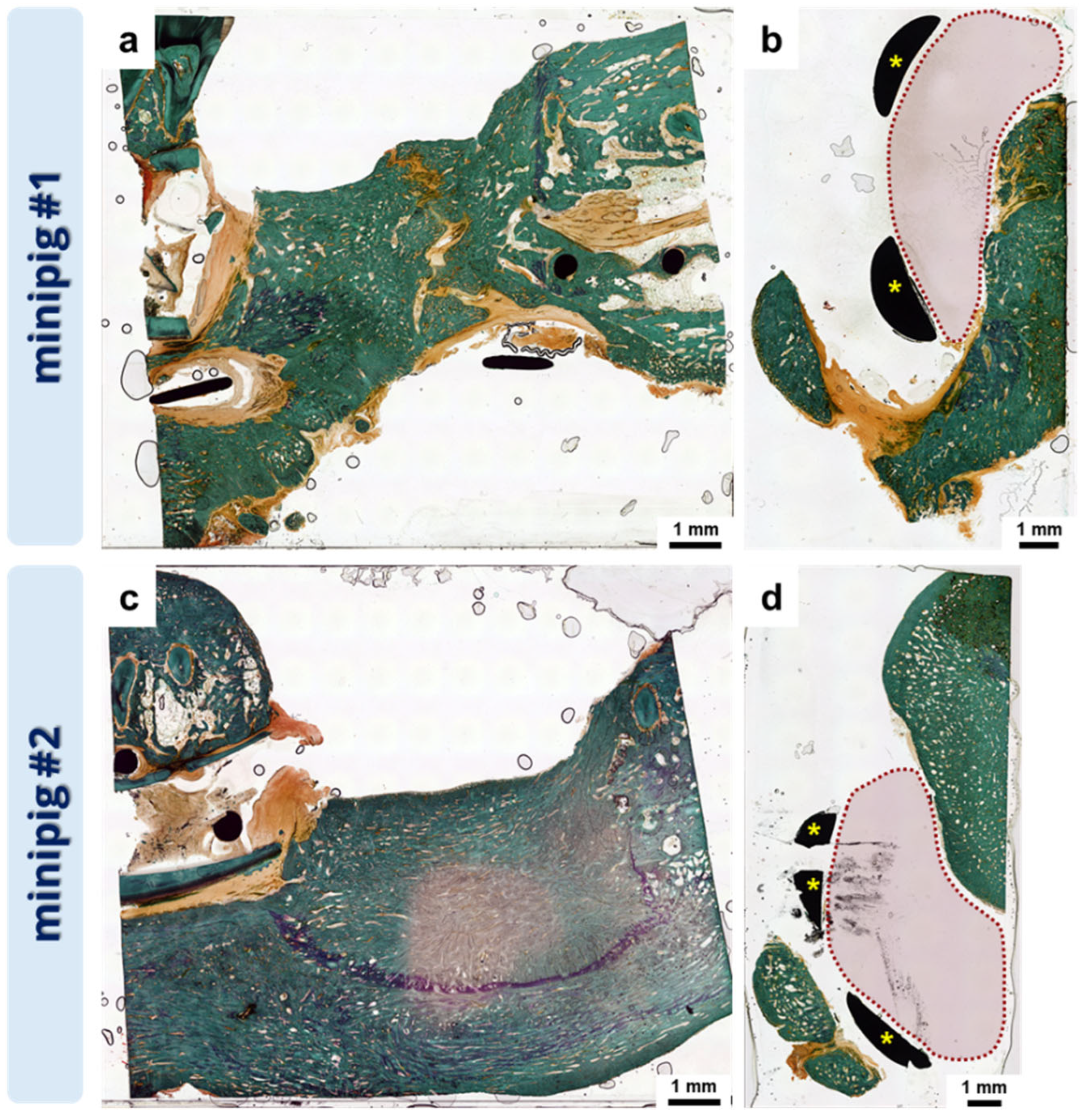
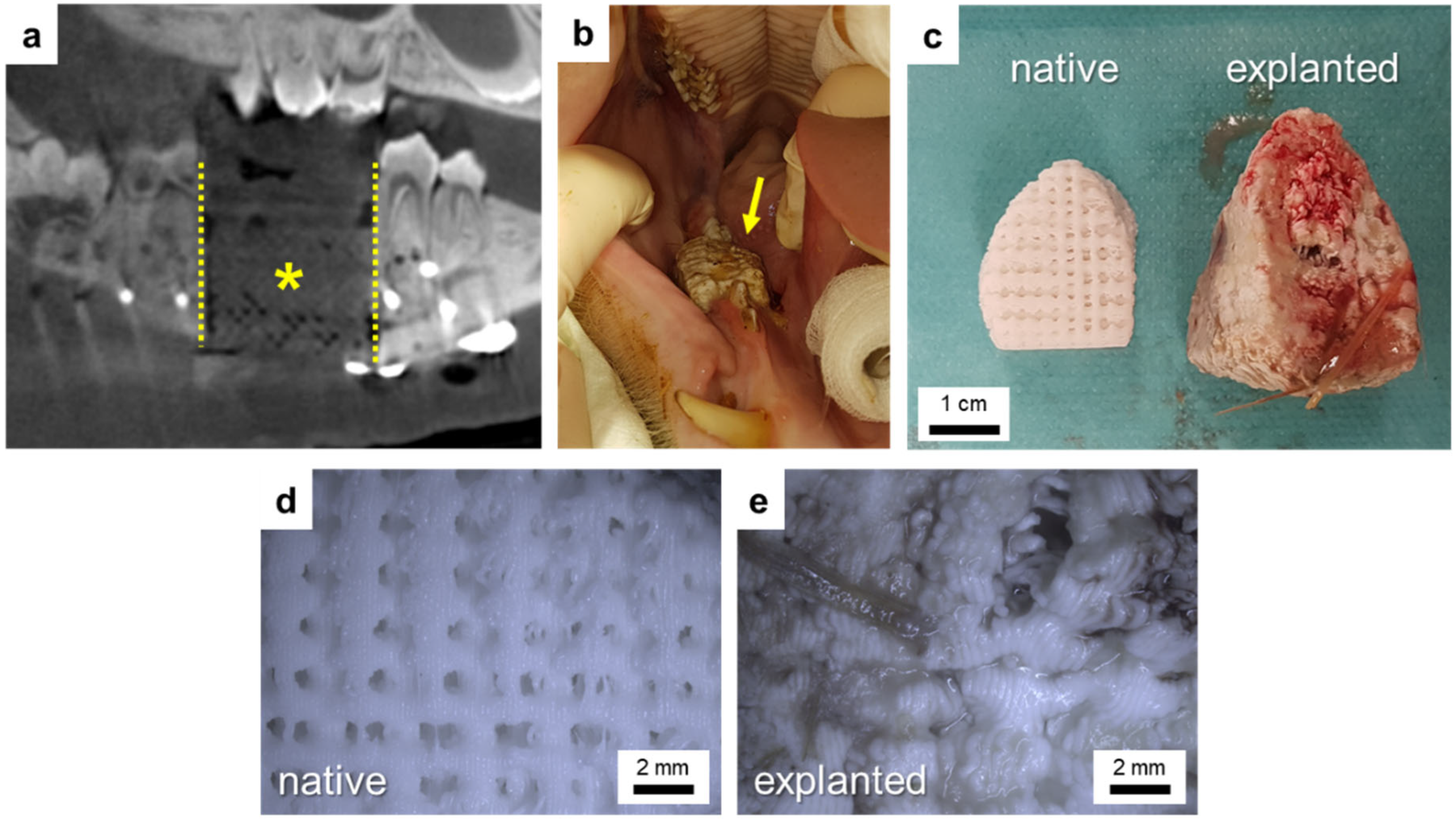
3.3. Pilot In Vivo Experiment
3.4. Development of an In Vitro Degradation Model for Swelling Prediction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


| PDLLA | PDLLA + CaCO3 | PDLLA + SrHAp (90:10) | |
|---|---|---|---|
| 0 d | 79.2 ± 0.9 | 78.3 ± 1.6 | 79.5 ± 0.9 |
| 7 d | 80.8 ± 0.3 | 91.3 ± 0.4 | 83.2 ± 0.9 |
| 14 d | 81.8 ± 0.3 | 96.4 ± 2.6 | 83.7 ± 0.9 |
| 21 d | 80.0 ± 0.3 | 100.3 ± 2.6 | 82.6 ± 0.9 |
| 28 d | 80.0 ± 0.3 | 105.2 ± 3.1 | 83.3 ± 1.4 |
| 35 d | 79.6 ± 0.5 | 109.4 ± 5.1 | 83.4 ± 1.5 |
| 42 d | 79.5 ± 0.6 | 109.3 ± 2.7 | 84.3 ± 1.3 |
| 49 d | 82.2 ± 0.3 | 115.9 ± 2.8 | 87.1 ± 1.3 |
| 56 d | 82.9 ± 0.5 | 118.9 ± 3.5 | 88.7 ± 1.0 |
| 63 d | 82.5 ± 0.1 | 120.2 ± 3.8 | 89.7 ± 0.4 |
| PDLLA | PDLLA + CaCO3 | PDLLA + SrHAp (90:10) | |
|---|---|---|---|
| 0 d | 78.9 ± 0.4 | 79.1 ± 1.0 | 80.6 ± 0.2 |
| 7 d | 81.1 ± 1.0 | 89.6 ± 2.3 | 82.4 ± 0.7 |
| 14 d | 80.9 ± 0.8 | 94.8 ± 2.5 | 83.0 ± 0.6 |
| 21 d | 79.9 ± 0.7 | 99.4 ± 2.5 | 82.5 ± 1.2 |
| 28 d | 79.9 ± 0.3 | 107.0 ± 4.1 | 83.3 ± 1.1 |
| 35 d | 78.8 ± 0.7 | 105.8 ± 3.9 | 83.6 ± 1.2 |
| 42 d | 78.5 ± 0.3 | 107.7 ± 6.1 | 83.8 ± 1.5 |
| 49 d | 81.8 ± 0.8 | 113.6 ± 6.9 | 86.8 ± 1.6 |
| 56 d | 82.6 ± 0.6 | 117.1 ± 10.9 | 88.9 ± 1.1 |
| 63 d | 82.1 ± 0.7 | 119.2 ± 11.0 | 89.7 ± 1.8 |
References
- Memon, A.R.; Wang, E.; Hu, J.; Egger, J.; Chen, X. A Review on Computer-Aided Design and Manufacturing of Patient-Specific Maxillofacial Implants. Expert Rev. Med. Devices 2020, 17, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly (Lactic Acid)-Based Biomaterials for Orthopaedic Regenerative Engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef]
- Grémare, A.; Guduric, V.; Bareille, R.; Heroguez, V.; Latour, S.; L’heureux, N.; Fricain, J.; Catros, S.; Le Nihouannen, D. Characterization of Printed PLA Scaffolds for Bone Tissue Engineering. J. Biomed. Mater. Res. 2018, 106, 887–894. [Google Scholar] [CrossRef]
- Madhavan Nampoothiri, K.; Nair, N.R.; John, R.P. An Overview of the Recent Developments in Polylactide (PLA) Research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef]
- Annunziata, M.; Nastri, L.; Cecoro, G.; Guida, L. The Use of Poly-d,l-Lactic Acid (PDLLA) Devices for Bone Augmentation Techniques: A Systematic Review. Molecules 2017, 22, 2214. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Slamovich, E.B.; Webster, T.J. Less Harmful Acidic Degradation of Poly(Lactic-Co-Glycolic Acid) Bone Tissue Engineering Scaffolds through Titania Nanoparticle Addition. Int. J. Nanomed. 2006, 1, 541–545. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Lode, A.; Placht, A.-M.; Fecht, T.; Wolfram, T.; Grom, S.; Hoess, A.; Vater, C.; Bräuer, C.; Heinemann, S.; et al. A Comparative Analysis of 3D Printed Scaffolds Consisting of Poly(Lactic-Co-Glycolic) Acid and Different Bioactive Mineral Fillers: Aspects of Degradation and Cytocompatibility. Biomater. Sci. 2023, 11, 5590–5604. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, M. Degradation of Poly (DL-Lactic Acid-Co-Glycolic Acid) Containing Calcium Carbonate and Hydroxyapatite Fillers-Effect of Size and Shape of the Fillers. Dent. Mater. J. 2003, 22, 371–382. [Google Scholar] [CrossRef]
- Agrawal, C.M.; Athanasiou, K.A. Technique to Control pH in Vicinity of Biodegrading PLA-PGA Implants. J. Biomed. Mater. Res. 1997, 38, 105–114. [Google Scholar] [CrossRef]
- Hentschel, L.; Petersmann, S.; Gonzalez-Gutierrez, J.; Kynast, F.; Schäfer, U.; Arbeiter, F.; Holzer, C. Parameter Optimization of the ARBURG Plastic Freeforming Process by Means of a Design of Experiments Approach. Adv. Eng. Mater. 2023, 25, 2200279. [Google Scholar] [CrossRef]
- Lode, A.; Heiss, C.; Knapp, G.; Thomas, J.; Nies, B.; Gelinsky, M.; Schumacher, M. Strontium-Modified Premixed Calcium Phosphate Cements for the Therapy of Osteoporotic Bone Defects. Acta Biomater. 2018, 65, 475–485. [Google Scholar] [CrossRef] [PubMed]
- DIN EN ISO 10993-13:2010-11; Biological Evaluation of Medical Devices—Part 13: Identification and Quantification of Degradation Products from Polymeric Medical Devices (ISO 10993-13:2010). German Version EN ISO 10993-13:2010; German Institute for Standardization: Berlin, Germany, 2010. [CrossRef]
- DIN EN ISO 11357-3:2018-07; Plastics—Differential Scanning Calorimetry (DSC)—Part 3: Determination of Temperature and Enthalpy of Melting and Crystallization (ISO 11357-3:2018). German Version EN ISO 11357-3:2018; German Institute for Standardization: Berlin, Germany, 2018. [CrossRef]
- DIN EN ISO 1628-1:2021-06; Plastics—Determination of the Viscosity of Polymers in Dilute Solution Using Capillary Viscometers—Part 1: General Principles (ISO 1628-1:2021). German Version EN ISO 1628-1:2021; German Institute for Standardization: Berlin, Germany, 2021. [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cao, B.; Jiang, N. The Mechanical Properties and Degradation Behavior of 3D-Printed Cellulose Nanofiber/Polylactic Acid Composites. Materials 2023, 16, 6197. [Google Scholar] [CrossRef] [PubMed]
- Hurtel-Lemaire, A.S.; Mentaverri, R.; Caudrillier, A.; Cournarie, F.; Wattel, A.; Kamel, S.; Terwilliger, E.F.; Brown, E.M.; Brazier, M. The Calcium-Sensing Receptor Is Involved in Strontium Ranelate-Induced Osteoclast Apoptosis. J. Biol. Chem. 2009, 284, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J. Strontium Ranelate: New Insights into Its Dual Mode of Action. Bone 2007, 40, S5–S8. [Google Scholar] [CrossRef]
- Ara, M.; Watanabe, M.; Imai, Y. Effect of Blending Calcium Compounds on Hydrolytic Degradation of Poly(Dl-Lactic Acid-Co-Glycolic Acid). Biomaterials 2002, 23, 2479–2483. [Google Scholar] [CrossRef] [PubMed]
- Antheunis, H.; Van Der Meer, J.-C.; De Geus, M.; Heise, A.; Koning, C.E. Autocatalytic Equation Describing the Change in Molecular Weight during Hydrolytic Degradation of Aliphatic Polyesters. Biomacromolecules 2010, 11, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Jia, J.; Liu, M.; Peng, S.; Zhao, Z.; Shuai, C. Degradation Mechanisms and Acceleration Strategies of Poly (Lactic Acid) Scaffold for Bone Regeneration. Mater. Des. 2021, 210, 110066. [Google Scholar] [CrossRef]
- Li, H.; Chang, J. Preparation and Characterization of Bioactive and Biodegradable Wollastonite/Poly(D,L-Lactic Acid) Composite Scaffolds. J. Mater. Sci. Mater. Med. 2004, 15, 1089–1095. [Google Scholar] [CrossRef]
- Yi, J.; Xiong, F.; Li, B.; Chen, H.; Yin, Y.; Dai, H.; Li, S. Degradation Characteristics, Cell Viability and Host Tissue Responses of PDLLA-Based Scaffold with PRGD and β-TCP Nanoparticles Incorporation. Regen. Biomater. 2016, 3, 159–166. [Google Scholar] [CrossRef]
- Cheng, D.; Cao, X.; Gao, H.; Wang, Y. Superficially Porous Poly(Lactic-Co-Glycolic Acid)/Calcium Carbonate Microsphere Developed by Spontaneous Pore-Forming Method for Bone Repair. RSC Adv. 2013, 3, 6871–6878. [Google Scholar] [CrossRef]
- Bayart, M.; Dubus, M.; Charlon, S.; Kerdjoudj, H.; Baleine, N.; Benali, S.; Raquez, J.-M.; Soulestin, J. Pellet-Based Fused Filament Fabrication (FFF)-Derived Process for the Development of Polylactic Acid/Hydroxyapatite Scaffolds Dedicated to Bone Regeneration. Materials 2022, 15, 5615. [Google Scholar] [CrossRef]
- Li, S.; Vert, M. Hydrolytic Degradation of Coral/Poly(DL-Lactic Acid) Bioresorbable Material. J. Biomater. Sci. Polym. Ed. 1996, 7, 817–827. [Google Scholar] [CrossRef]
- Cotton, N.J.; Egan, M.J.; Brunelle, J.E. Composites of Poly(DL-lactide-Co-glycolide) and Calcium Carbonate: In Vitro Evaluation for Use in Orthopedic Applications. J. Biomed. Mater. Res. 2008, 85A, 195–205. [Google Scholar] [CrossRef]
- Simpson, R.L.; Nazhat, S.N.; Blaker, J.J.; Bismarck, A.; Hill, R.; Boccaccini, A.R.; Hansen, U.N.; Amis, A.A. A Comparative Study of the Effects of Different Bioactive Fillers in PLGA Matrix Composites and Their Suitability as Bone Substitute Materials: A Thermo-Mechanical and in Vitro Investigation. J. Mech. Behav. Biomed. Mater. 2015, 50, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Regenerative Medicine Institute, National Centre for Biomedical Engineering Science, National University of Ireland, Galway; Czekanska, E.; Stoddart, M.; Richards, R.; Hayes, J. In Search of an Osteoblast Cell Model for in Vitro Research. Eur. Cells Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef]
- Dohle, E.; Fecht, T.; Wolfram, T.; Reinauer, F.; Wunder, A.; Heppe, K.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. In Vitro Coculture of Primary Human Cells to Analyze Angiogenesis, Osteogenesis, and the Inflammatory Response to Newly Developed Osteosynthesis Material for Pediatric Maxillofacial Traumatology: A Potential Pretesting Model before In Vivo Experiments. J. Tissue Eng. Regen. Med. 2023, 2023, 4040504. [Google Scholar] [CrossRef]
- Rezai Rad, M.; Fahimipour, F.; Dashtimoghadam, E.; Nokhbatolfoghahaei, H.; Tayebi, L.; Khojasteh, A. Osteogenic Differentiation of Adipose-Derived Mesenchymal Stem Cells Using 3D-Printed PDLLA/β-TCP Nanocomposite Scaffolds. Bioprinting 2021, 21, e00117. [Google Scholar] [CrossRef]
- Schumacher, M.; Lode, A.; Helth, A.; Gelinsky, M. A Novel Strontium(II)-Modified Calcium Phosphate Bone Cement Stimulates Human-Bone-Marrow-Derived Mesenchymal Stem Cell Proliferation and Osteogenic Differentiation in Vitro. Acta Biomater. 2013, 9, 9547–9557. [Google Scholar] [CrossRef]
- Schumacher, M.; Wagner, A.S.; Kokesch-Himmelreich, J.; Bernhardt, A.; Rohnke, M.; Wenisch, S.; Gelinsky, M. Strontium Substitution in Apatitic CaP Cements Effectively Attenuates Osteoclastic Resorption but Does Not Inhibit Osteoclastogenesis. Acta Biomater. 2016, 37, 184–194. [Google Scholar] [CrossRef]
- Thormann, U.; Ray, S.; Sommer, U.; ElKhassawna, T.; Rehling, T.; Hundgeburth, M.; Henß, A.; Rohnke, M.; Janek, J.; Lips, K.S.; et al. Bone Formation Induced by Strontium Modified Calcium Phosphate Cement in Critical-Size Metaphyseal Fracture Defects in Ovariectomized Rats. Biomaterials 2013, 34, 8589–8598. [Google Scholar] [CrossRef]
- Ma, J.-L.; Pan, J.-L.; Tan, B.-S.; Cui, F.-Z. Determination of Critical Size Defect of Minipig Mandible. J. Tissue Eng. Regen. Med. 2009, 3, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Heidemann, W.; Jeschkeit, S.; Ruffieux, K.; Fischer, J.H.; Wagner, M.; Krüger, G.; Wintermantel, E.; Gerlach, K.L. Degradation of Poly(d,l)Lactide Implants with or without Addition of Calciumphosphates in Vivo. Biomaterials 2001, 22, 2371–2381. [Google Scholar] [CrossRef]
- Walton, M.; Cotton, N.J. Long-Term in Vivo Degradation of Poly-L-Lactide (PLLA) in Bone. J. Biomater. Appl. 2007, 21, 395–411. [Google Scholar] [CrossRef] [PubMed]
- van Oirschot, B.A.J.A.; Geven, E.J.W.; Mikos, A.G.; Beucken, J.J.v.D.; Jansen, J.A. A Mini-Pig Mandibular Defect Model for Evaluation of Craniomaxillofacial Bone Regeneration. Tissue Eng. Part C Methods 2022, 28, 193–201. [Google Scholar] [CrossRef]
- Kauffmann, P.; Raschke, D.; Tröltzsch, M.; Santander, P.; Brockmeyer, P.; Schliephake, H. The Use of rhBMP2 for Augmentation of Established Horizontal/Vertical Defects May Require Additional Use of rhVEGF to Achieve Significant Bone Regeneration: An in Vivo Experimental Study. Clin. Oral. Implant. Res. 2021, 32, 1228–1240. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Huang, T.; Chen, X.; Li, W.; Yang, X.; Zhang, W.; Li, M.; Gao, R. Uncovering the Interplay between pH Receptors and Immune Cells: Potential Drug Targets (Review). Oncol. Rep. 2021, 46, 228. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, Q. Bacterial Infection Microenvironment-responsive Porous Microspheres by Microfluidics for Promoting Anti-infective Therapy. Smart Med. 2022, 1, e20220012. [Google Scholar] [CrossRef]


| PDLLA | PDLLA + CaCO3 | PDLLA + SrHAp (90:10) | |
|---|---|---|---|
| 0 d | 79.1 ± 1.9 | 80.3 ± 0.4 | 79.8 ± 0.6 |
| 7 d | 81.1 ± 1.1 | 90.7 ± 3.0 | 82.6 ± 0.2 |
| 14 d | 81.4 ± 0.1 | 95.4 ± 4.3 | 83.5 ± 0.6 |
| 21 d | 79.9 ± 1.0 | 100.4 ± 5.8 | 82.5 ± 0.6 |
| 28 d | 79.1 ± 0.8 | 102.3 ± 7.3 | 82.6 ± 0.4 |
| 35 d | 78.6 ± 0.9 | 109.8 ± 8.7 | 82.9 ± 0.7 |
| 42 d | 78.7 ± 1.0 | 111.9 ± 10.2 | 83.7 ± 0.9 |
| 49 d | 81.8 ± 1.3 | 116.4 ± 10.6 | 87.5 ± 0.7 |
| 56 d | 82.3 ± 1.4 | 118.4 ± 11.6 | 88.8 ± 0.7 |
| 63 d | 82.6 ± 1.4 | 118.7 ± 11.7 | 88.8 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vater, C.; Bräuer, C.; Grom, S.; Fecht, T.; Ahlfeld, T.; von Witzleben, M.; Placht, A.-M.; Schütz, K.; Schehl, J.M.; Wolfram, T.; et al. Poly(dl-lactide) Polymer Blended with Mineral Phases for Extrusion 3D Printing—Studies on Degradation and Biocompatibility. Polymers 2024, 16, 1254. https://doi.org/10.3390/polym16091254
Vater C, Bräuer C, Grom S, Fecht T, Ahlfeld T, von Witzleben M, Placht A-M, Schütz K, Schehl JM, Wolfram T, et al. Poly(dl-lactide) Polymer Blended with Mineral Phases for Extrusion 3D Printing—Studies on Degradation and Biocompatibility. Polymers. 2024; 16(9):1254. https://doi.org/10.3390/polym16091254
Chicago/Turabian StyleVater, Corina, Christian Bräuer, Stefanie Grom, Tatjana Fecht, Tilman Ahlfeld, Max von Witzleben, Anna-Maria Placht, Kathleen Schütz, Jan Marc Schehl, Tobias Wolfram, and et al. 2024. "Poly(dl-lactide) Polymer Blended with Mineral Phases for Extrusion 3D Printing—Studies on Degradation and Biocompatibility" Polymers 16, no. 9: 1254. https://doi.org/10.3390/polym16091254
APA StyleVater, C., Bräuer, C., Grom, S., Fecht, T., Ahlfeld, T., von Witzleben, M., Placht, A.-M., Schütz, K., Schehl, J. M., Wolfram, T., Reinauer, F., Scharffenberg, M., Wittenstein, J., Hoess, A., Heinemann, S., Gelinsky, M., Lauer, G., & Lode, A. (2024). Poly(dl-lactide) Polymer Blended with Mineral Phases for Extrusion 3D Printing—Studies on Degradation and Biocompatibility. Polymers, 16(9), 1254. https://doi.org/10.3390/polym16091254








