Enhanced Bone Healing in Critical-Sized Rabbit Femoral Defects: Impact of Helical and Alternate Scaffold Architectures
Abstract
1. Introduction
2. Materials and Methods
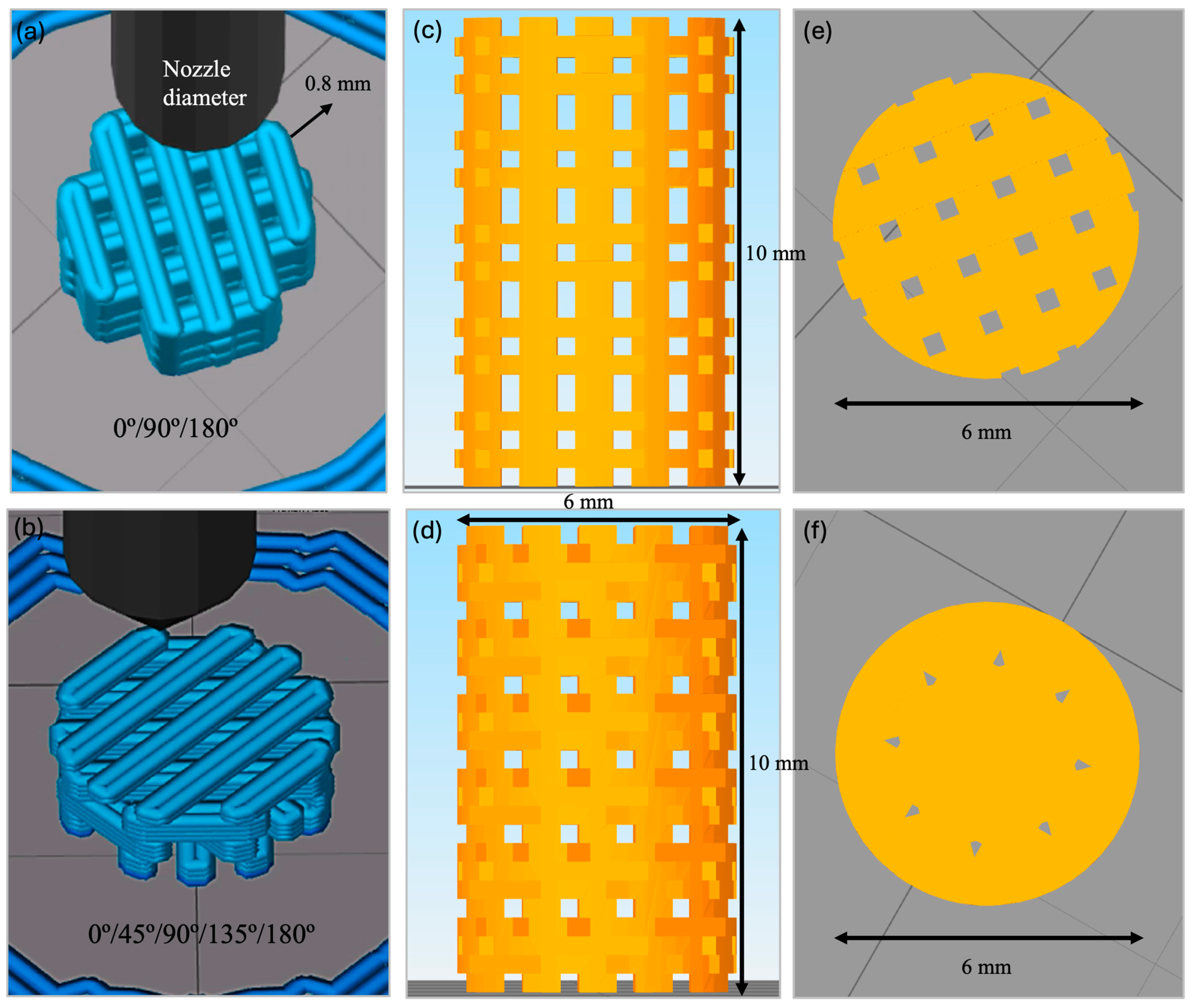
2.1. Fabrication of 3D-Printed PLA-bioCaP Scaffold
2.2. Characterization of the 3D-Printed Scaffolds
2.2.1. Pore Morphology
2.2.2. Mechanical Test
2.3. Animal Model
2.4. Micro-CT Analysis
2.5. Histologic and Histomorphometric Analysis
2.6. Statistical Analysis
3. Results
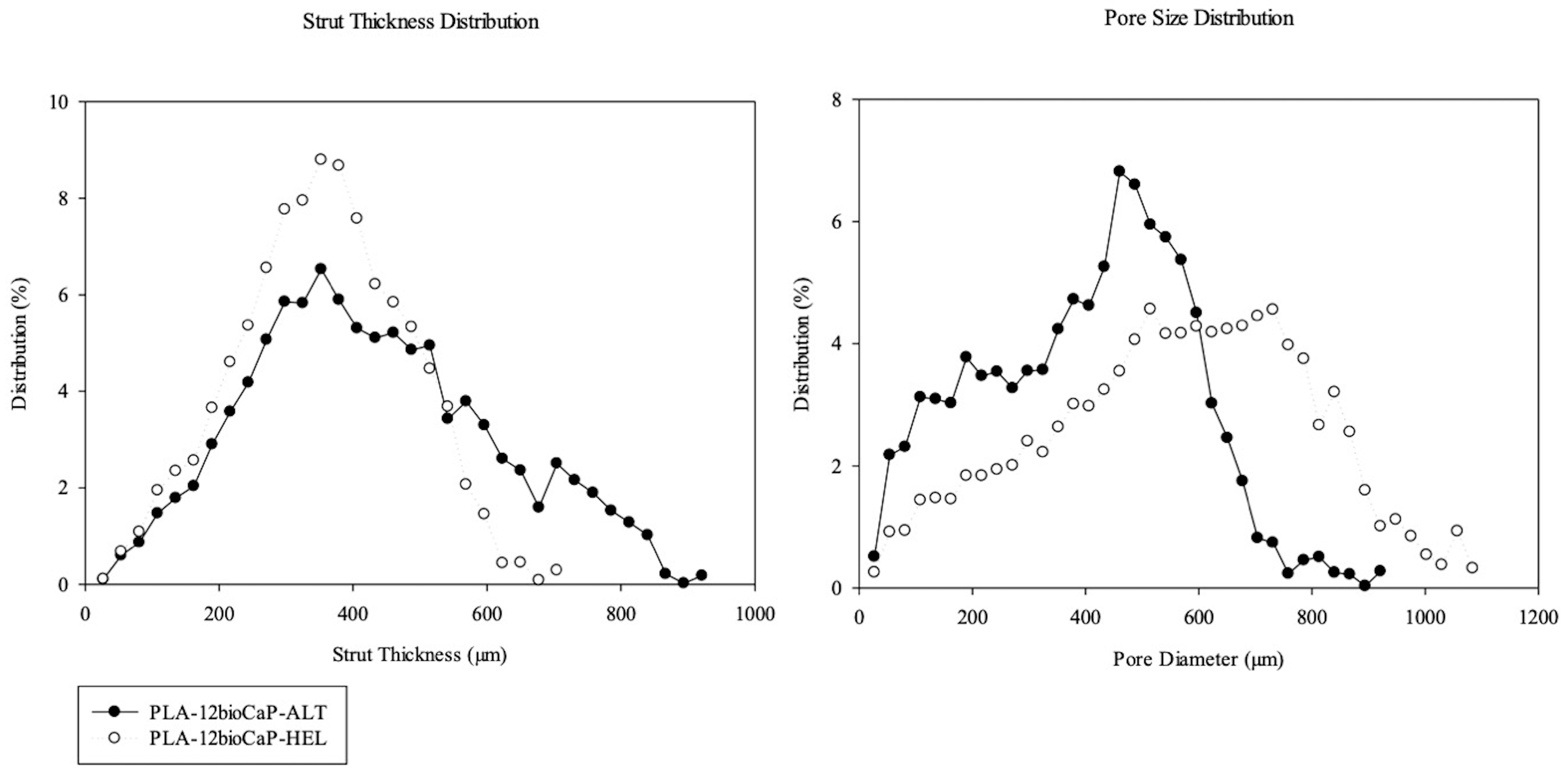
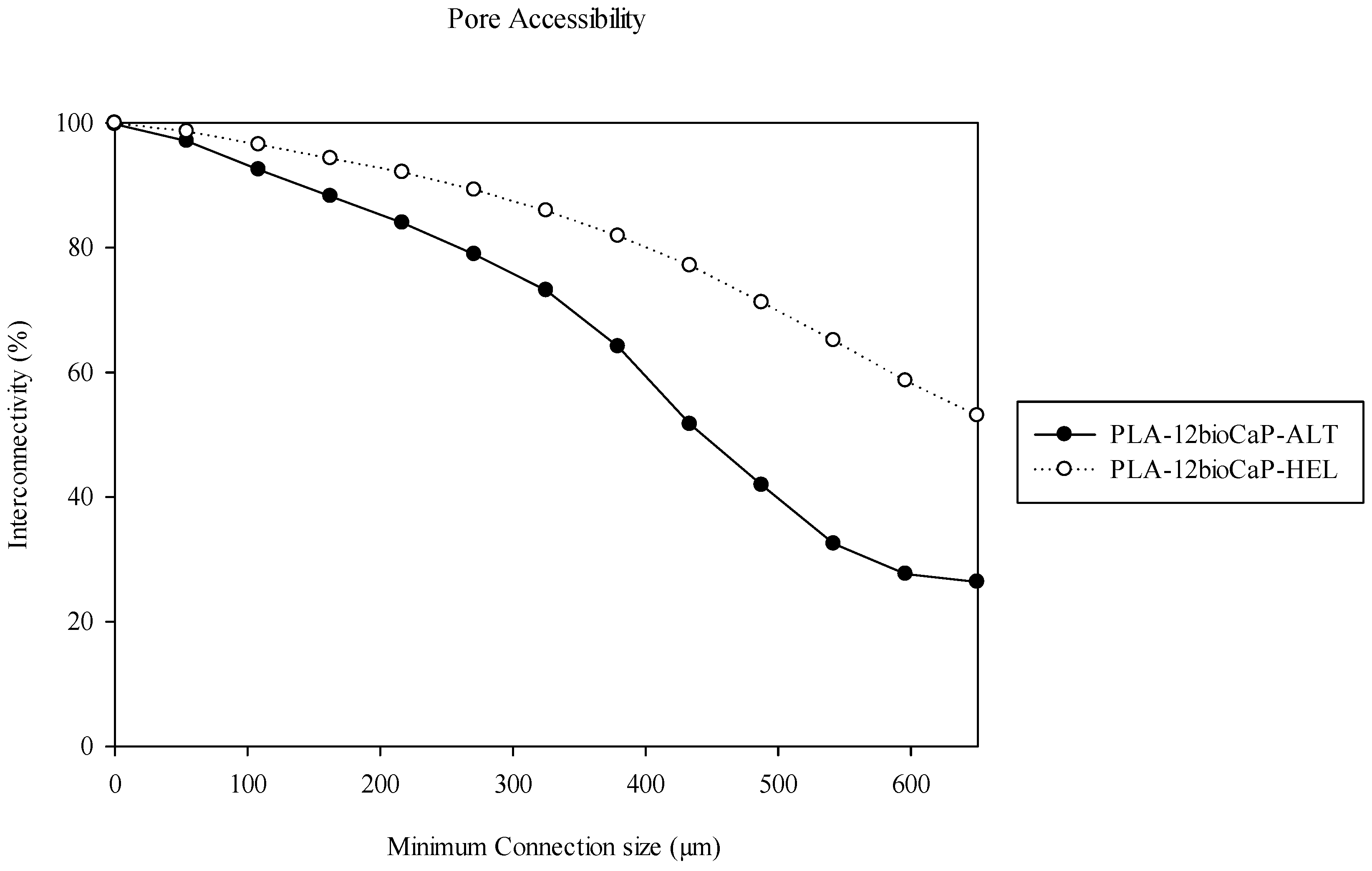
3.1. Pore Morphology
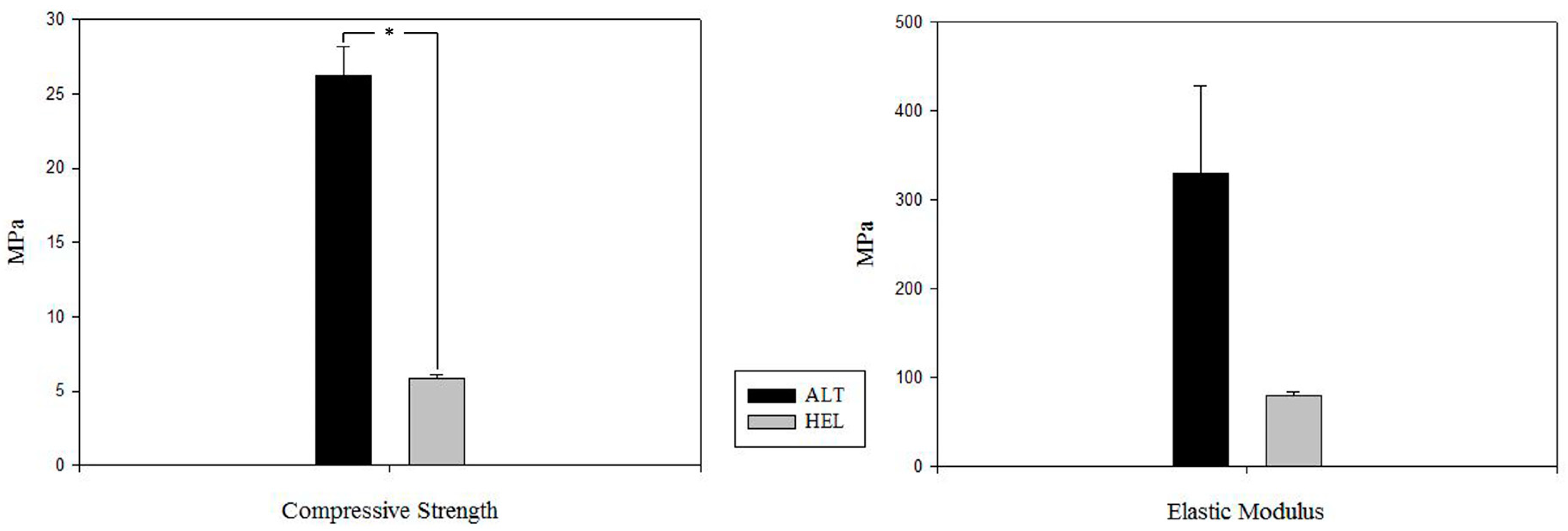
3.2. Mechanical Test
3.3. Animal Model
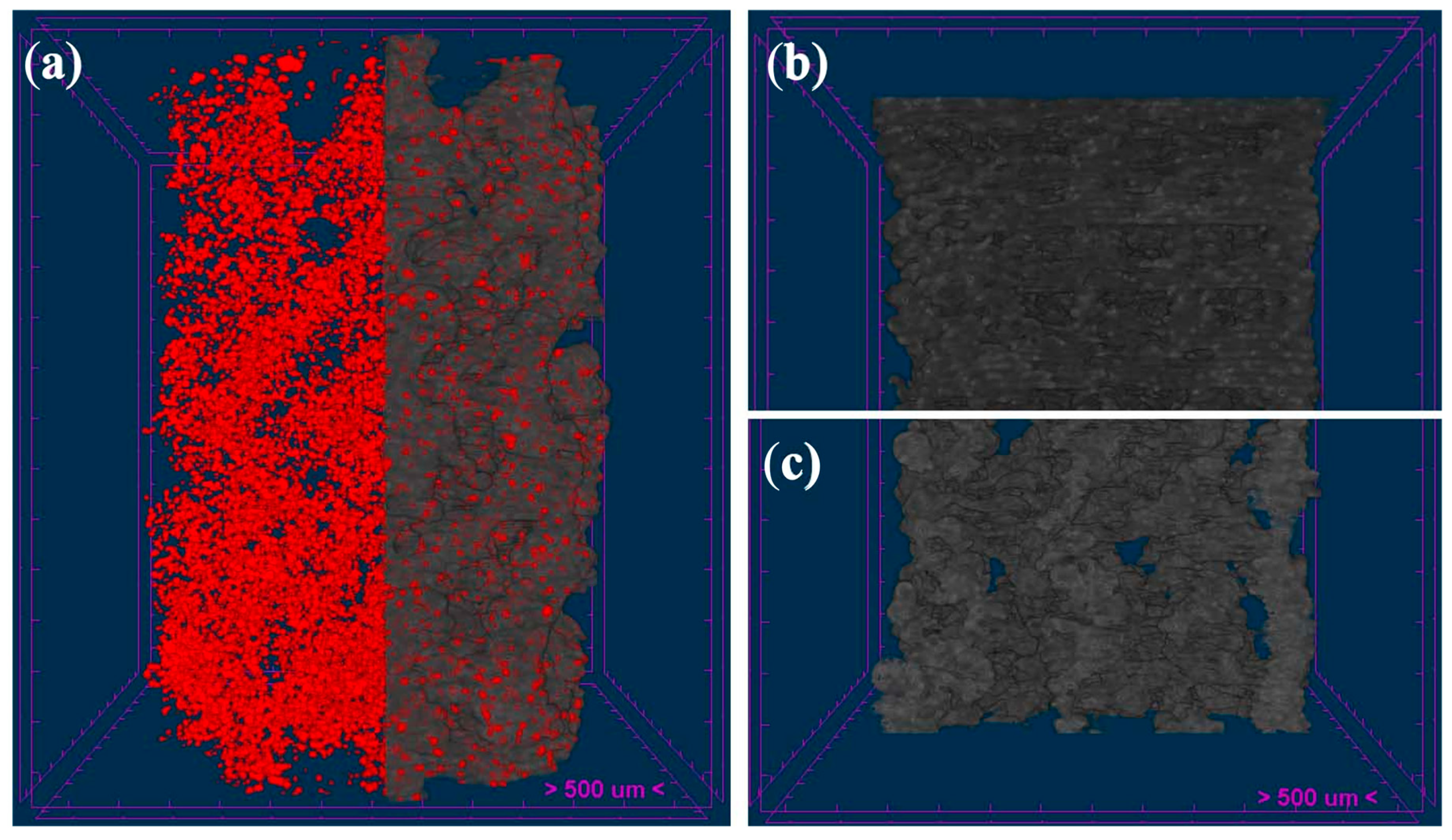
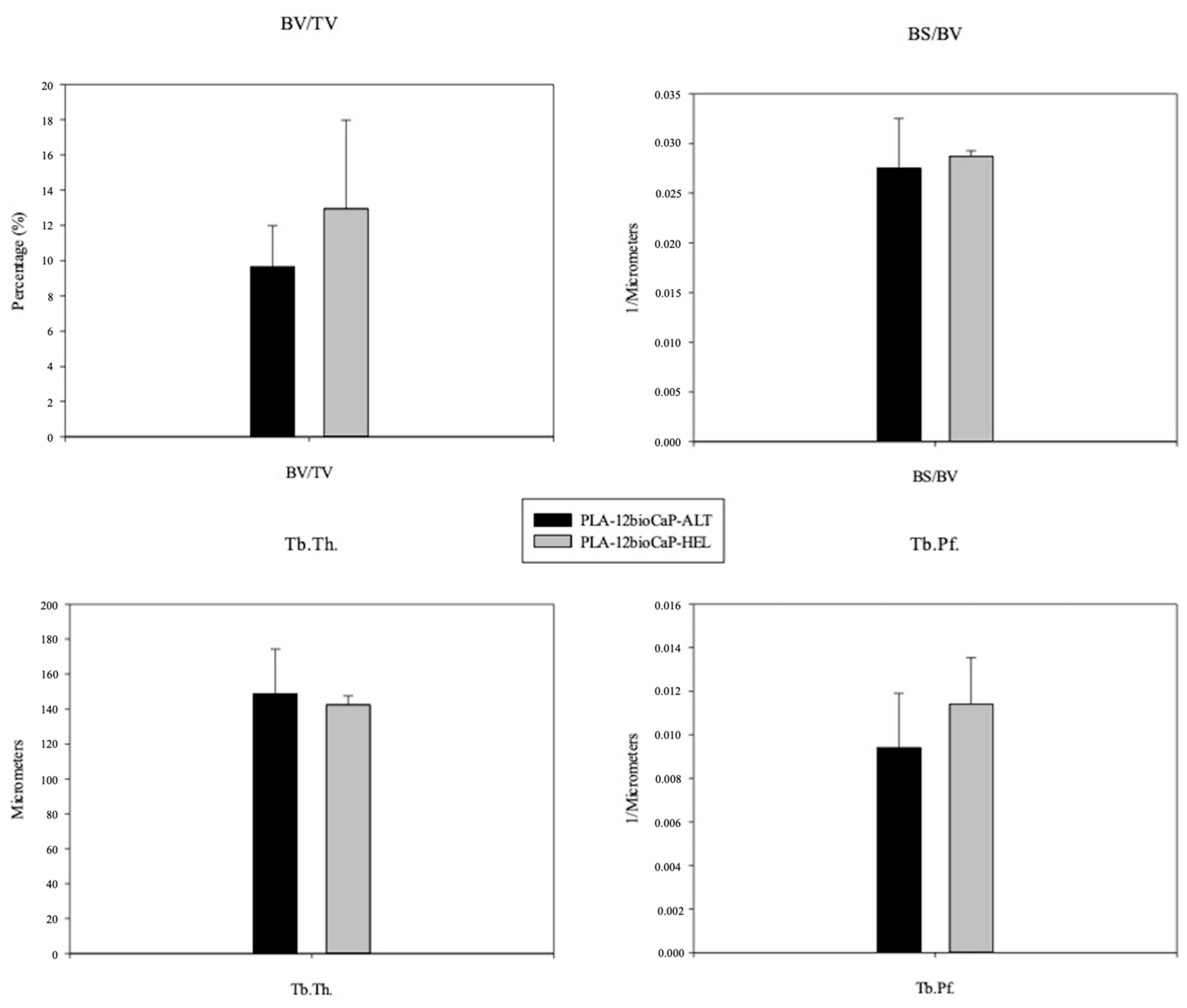
3.4. Micro-CT
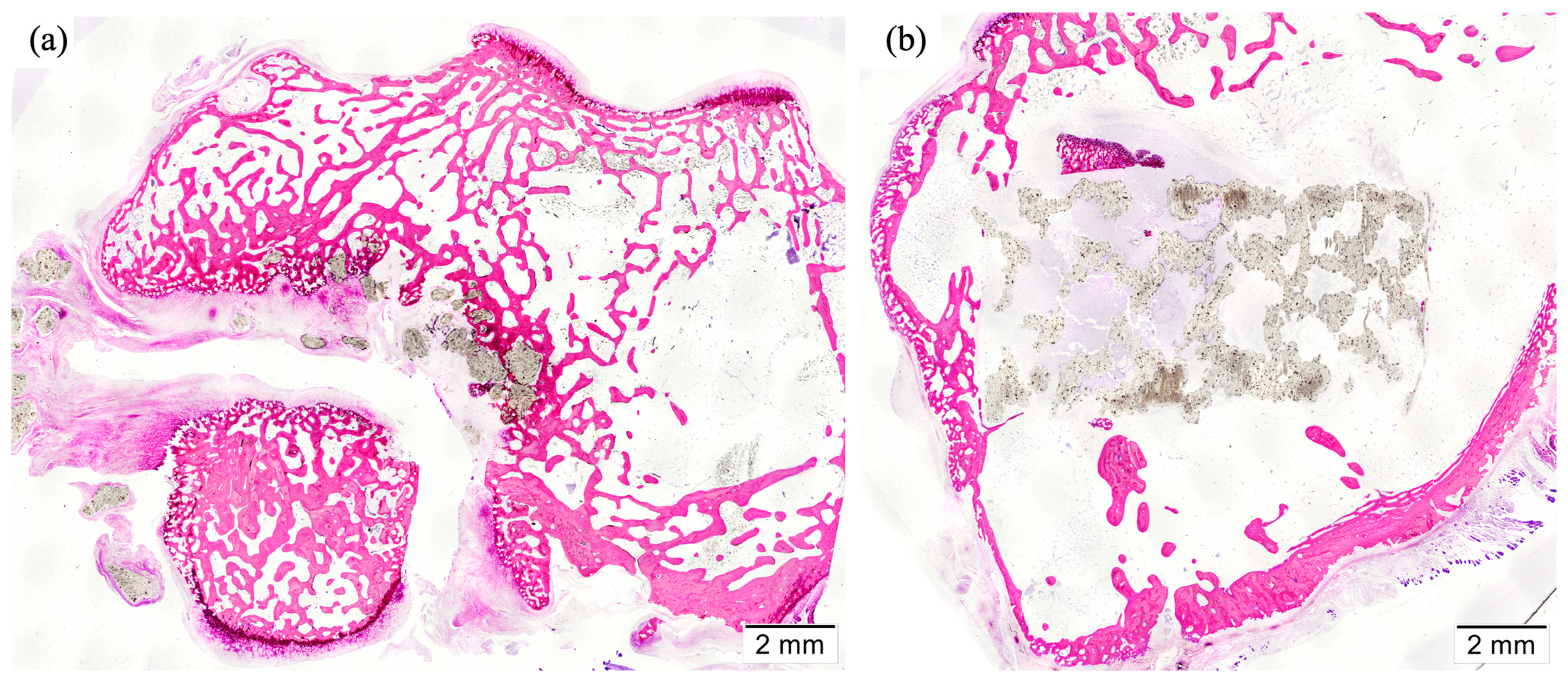
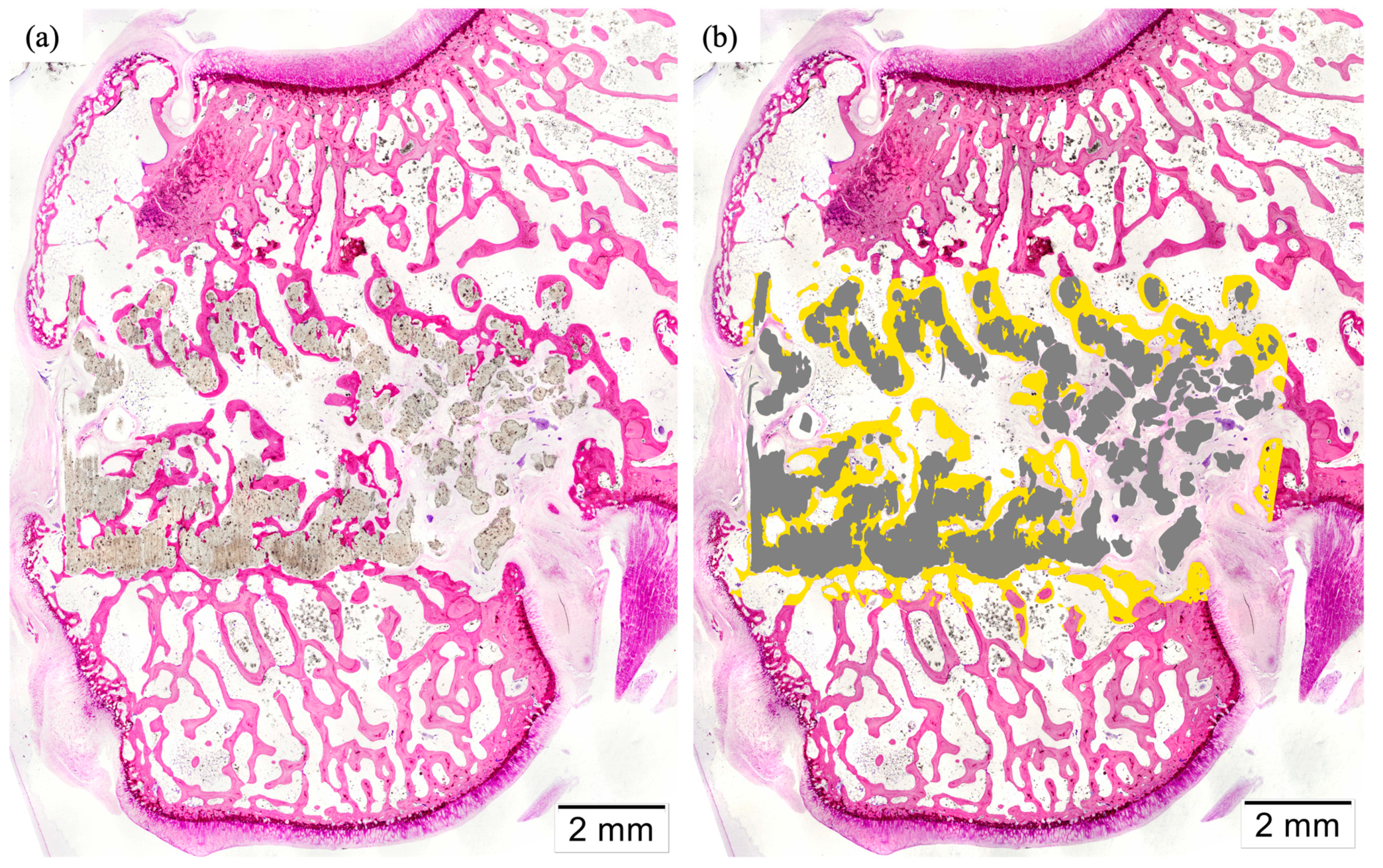
3.5. Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, D.; Tare, R.S.; Yang, L.-Y.; Williams, D.F.; Ou, K.-L.; Oreffo, R.O.C. Biofabrication of Bone Tissue: Approaches, Challenges and Translation for Bone Regeneration. Biomaterials 2016, 83, 363–382. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the Art and New Perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2013, 40, 363–408. [Google Scholar] [CrossRef]
- Xue, N.; Ding, X.; Huang, R.; Jiang, R.; Huang, H.; Pan, X.; Min, W.; Chen, J.; Duan, J.-A.; Liu, P.; et al. Bone Tissue Engineering in the Treatment of Bone Defects. Pharmaceuticals 2022, 15, 879. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, T.; Chen, M.; Yao, K.; Huang, X.; Zhang, B.; Li, Y.; Liu, J.; Wang, Y.; Zhao, Z. Bone Physiological Microenvironment and Healing Mechanism: Basis for Future Bone-Tissue Engineering Scaffolds. Bioact. Mater. 2021, 6, 4110–4140. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.K.; Li, L.; Qin, L.; Wang, X.L.; Lai, Y.X. Bone Defect Animal Models for Testing Efficacy of Bone Substitute Biomaterials. J. Orthop. Transl. 2015, 3, 95–104. [Google Scholar] [CrossRef]
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The Roles of Signaling Pathways in Bone Repair and Regeneration. J. Cell. Physiol. 2018, 233, 2937–2948. [Google Scholar] [CrossRef]
- Berner, A.; Woodruff, M.A.; Lam, C.X.F.; Arafat, M.T.; Saifzadeh, S.; Steck, R.; Ren, J.; Nerlich, M.; Ekaputra, A.K.; Gibson, I.; et al. Effects of Scaffold Architecture on Cranial Bone Healing. Int. J. Oral Maxillofac. Surg. 2014, 43, 506–513. [Google Scholar] [CrossRef]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone Substitutes: A Review of Their Characteristics, Clinical Use, and Perspectives for Large Bone Defects Management. J. Tissue Eng. 2018, 9, 204173141877681. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone Grafts: Which Is the Ideal Biomaterial? J. Clin. Periodontol. 2019, 46, 92–102. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone Regeneration Strategies: Engineered Scaffolds, Bioactive Molecules and Stem Cells Current Stage and Future Perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Garot, C.; Bettega, G.; Picart, C. Additive Manufacturing of Material Scaffolds for Bone Regeneration: Toward Application in the Clinics. Adv. Funct. Mater. 2021, 31, 2006967. [Google Scholar] [CrossRef]
- Milazzo, M.; Contessi Negrini, N.; Scialla, S.; Marelli, B.; Farè, S.; Danti, S.; Buehler, M.J. Additive Manufacturing Approaches for Hydroxyapatite-Reinforced Composites. Adv. Funct. Mater. 2019, 29, 1903055. [Google Scholar] [CrossRef]
- Ostrowska, B.; Di Luca, A.; Moroni, L.; Swieszkowski, W. Influence of Internal Pore Architecture on Biological and Mechanical Properties of Three-dimensional Fiber Deposited Scaffolds for Bone Regeneration. J. Biomed. Mater. Res. 2016, 104, 991–1001. [Google Scholar] [CrossRef]
- Liang, X.; Gao, J.; Xu, W.; Wang, X.; Shen, Y.; Tang, J.; Cui, S.; Yang, X.; Liu, Q.; Yu, L.; et al. Structural Mechanics of 3D-Printed Poly (Lactic Acid) Scaffolds with Tetragonal, Hexagonal and Wheel-like Designs. Biofabrication 2019, 11, 035009. [Google Scholar] [CrossRef]
- Gleadall, A.; Visscher, D.; Yang, J.; Thomas, D.; Segal, J. Review of Additive Manufactured Tissue Engineering Scaffolds: Relationship between Geometry and Performance. Burn. Trauma 2018, 6, 19. [Google Scholar] [CrossRef]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.; Shao, Z.; Sun, M.; Ao, Y.; Boyan, B.D.; Chen, H. Advances in Porous Scaffold Design for Bone and Cartilage Tissue Engineering and Regeneration. Tissue Eng. Part B Rev. 2019, 25, 14–29. [Google Scholar] [CrossRef]
- Rajasekharan, A.K.; Bordes, R.; Sandström, C.; Ekh, M.; Andersson, M. Hierarchical and Heterogeneous Bioinspired Composites-Merging Molecular Self-Assembly with Additive Manufacturing. Small 2017, 13, 1700550. [Google Scholar] [CrossRef]
- Kang, S.-W.; Bae, J.-H.; Park, S.-A.; Kim, W.-D.; Park, M.-S.; Ko, Y.-J.; Jang, H.-S.; Park, J.-H. Combination Therapy with BMP-2 and BMSCs Enhances Bone Healing Efficacy of PCL Scaffold Fabricated Using the 3D Plotting System in a Large Segmental Defect Model. Biotechnol. Lett. 2012, 34, 1375–1384. [Google Scholar] [CrossRef]
- Do, A.V.; Smith, R.; Acri, T.M.; Geary, S.M.; Salem, A.K. 3D Printing Technologies for 3D Scaffold Engineering; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-08-100980-2. [Google Scholar]
- Saijo, H.; Igawa, K.; Kanno, Y.; Mori, Y.; Kondo, K.; Shimizu, K.; Suzuki, S.; Chikazu, D.; Iino, M.; Anzai, M.; et al. Maxillofacial Reconstruction Using Custom-Made Artificial Bones Fabricated by Inkjet Printing Technology. J. Artif. Organs 2009, 12, 200–205. [Google Scholar] [CrossRef]
- Gul, M.; Arif, A.; Ghafoor, R. Role of Three-Dimensional Printing in Periodontal Regeneration and Repair: Literature Review. J. Indian. Soc. Periodontol. 2019, 23, 504. [Google Scholar] [CrossRef]
- Kermavnar, T.; Shannon, A.; O’Sullivan, K.J.; McCarthy, C.; Dunne, C.P.; O’Sullivan, L.W. Three-Dimensional Printing of Medical Devices Used Directly to Treat Patients: A Systematic Review. 3D Print. Addit. Manuf. 2021, 8, 366–408. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D Printing of Polymer Matrix Composites: A Review and Prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Bouyer, M.; Garot, C.; Machillot, P.; Vollaire, J.; Fitzpatrick, V.; Morand, S.; Boutonnat, J.; Josserand, V.; Bettega, G.; Picart, C. 3D-Printed Scaffold Combined to 2D Osteoinductive Coatings to Repair a Critical-Size Mandibular Bone Defect. Mater. Today Bio 2021, 11, 100113. [Google Scholar] [CrossRef]
- Rengier, F.; Mehndiratta, A.; Von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.-U.; Giesel, F.L. 3D Printing Based on Imaging Data: Review of Medical Applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335–341. [Google Scholar] [CrossRef]
- Tümer, E.H.; Erbil, H.Y. Extrusion-Based 3D Printing Applications of PLA Composites: A Review. Coatings 2021, 11, 390. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic Acid: Synthesis and Biomedical Applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in Modern Medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Sapuan, S.M.; Harussani, M.M.; Hakimi, M.Y.A.Y.; Haziq, M.Z.M.; Atikah, M.S.N.; Asyraf, M.R.M.; Ishak, M.R.; Razman, M.R.; Nurazzi, N.M.; et al. Polylactic Acid (Pla) Biocomposite: Processing, Additive Manufacturing and Advanced Applications. Polymers 2021, 13, 1326. [Google Scholar] [CrossRef]
- Brunello, G.; Panda, S.; Schiavon, L.; Sivolella, S.; Biasetto, L.; Fabbro, M.D. The Impact of Bioceramic Scaffolds on Bone Regeneration in Preclinical in Vivo Studies: A Systematic Review. Materials 2020, 13, 1500. [Google Scholar] [CrossRef]
- Alksne, M.; Kalvaityte, M.; Simoliunas, E.; Rinkunaite, I.; Gendviliene, I.; Locs, J.; Rutkunas, V.; Bukelskiene, V. In Vitro Comparison of 3D Printed Polylactic Acid/Hydroxyapatite and Polylactic Acid/Bioglass Composite Scaffolds: Insights into Materials for Bone Regeneration. J. Mech. Behav. Biomed. Mater. 2020, 104, 103641. [Google Scholar] [CrossRef]
- Alonso-Fernández, I.; Haugen, H.J.; López-Peña, M.; González-Cantalapiedra, A.; Muñoz, F. Use of 3D-Printed Polylactic Acid/Bioceramic Composite Scaffolds for Bone Tissue Engineering in Preclinical in Vivo Studies: A Systematic Review. Acta Biomater. 2023, 168, 1–21. [Google Scholar] [CrossRef]
- Kook, M.-S.; Roh, H.-S.; Kim, B.-H. Effect of Oxygen Plasma Etching on Pore Size-Controlled 3D Polycaprolactone Scaffolds for Enhancing the Early New Bone Formation in Rabbit Calvaria. Dent. Mater. J. 2018, 37, 599–610. [Google Scholar] [CrossRef]
- Jones, A.C.; Arns, C.H.; Hutmacher, D.W.; Milthorpe, B.K.; Sheppard, A.P.; Knackstedt, M.A. The Correlation of Pore Morphology, Interconnectivity and Physical Properties of 3D Ceramic Scaffolds with Bone Ingrowth. Biomaterials 2009, 30, 1440–1451. [Google Scholar] [CrossRef]
- Mirkhalaf, M.; Wang, X.; Entezari, A.; Dunstan, C.R.; Jiang, X.; Zreiqat, H. Redefining Architectural Effects in 3D Printed Scaffolds through Rational Design for Optimal Bone Tissue Regeneration. Appl. Mater. Today 2021, 25, 101168. [Google Scholar] [CrossRef]
- Mastrogiacomo, M.; Scaglione, S.; Martinetti, R.; Dolcini, L.; Beltrame, F.; Cancedda, R.; Quarto, R. Role of Scaffold Internal Structure on in Vivo Bone Formation in Macroporous Calcium Phosphate Bioceramics. Biomaterials 2006, 27, 3230–3237. [Google Scholar] [CrossRef]
- Peng, W.; Liu, Y.; Wang, C. Definition, Measurement, and Function of Pore Structure Dimensions of Bioengineered Porous Bone Tissue Materials Based on Additive Manufacturing: A Review. Front. Bioeng. Biotechnol. 2023, 10, 1081548. [Google Scholar] [CrossRef]
- Lim, H.K.; Hong, S.J.; Byeon, S.J.; Chung, S.M.; On, S.W.; Yang, B.E.; Lee, J.H.; Byun, S.H. 3D-Printed Ceramic Bone Scaffolds with Variable Pore Architectures. Int. J. Mol. Sci. 2020, 21, 6942. [Google Scholar] [CrossRef]
- Entezari, A.; Roohani, I.; Li, G.; Dunstan, C.R.; Rognon, P.; Li, Q.; Jiang, X.; Zreiqat, H. Architectural Design of 3D Printed Scaffolds Controls the Volume and Functionality of Newly Formed Bone. Adv. Healthc. Mater. 2019, 8, 1801353. [Google Scholar] [CrossRef]
- López-Álvarez, M.; Vigo, E.; Rodríguez-Valencia, C.; Outeiriño-Iglesias, V.; González, P.; Serra, J. In Vivo Evaluation of Shark Teeth-Derived Bioapatites. Clin. Oral Impl. Res. 2017, 28, e91–e100. [Google Scholar] [CrossRef]
- Aguiar, H.; Chiussi, S.; López-Álvarez, M.; González, P.; Serra, J. Structural Characterization of Bioceramics and Mineralized Tissues Based on Raman and XRD Techniques. Ceram. Int. 2018, 44, 495–504. [Google Scholar] [CrossRef]
- Rojas-Lozano, J.; Días-Rodriguez, P.; Barreiro, P.; López-Senraa, E.; Rodríguez-Valencia, C.; López-Álvarez, M.; Landín, M.; González, P.; Serra, P. Desarrollo de Nuevos Filamentos Para Impresión 3D Basados En Cerámicas Bioinspiradoras. Mater. Compuestos 2019, 3, 65–69. [Google Scholar]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical Cone-Beam Algorithm. J. Opt. Soc. Am. A 1984, 1, 612. [Google Scholar] [CrossRef]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Tiainen, H.; Lyngstadaas, S.P.; Ellingsen, J.E.; Haugen, H.J. Ultra-Porous Titanium Oxide Scaffold with High Compressive Strength. J. Mater. Sci Mater. Med. 2010, 21, 2783–2792. [Google Scholar] [CrossRef]
- Wu, H.-B.; Haugen, H.J.; Wintermantel, E. Supercritical CO2 in Injection Molding Can Produce Open Porous Polyurethane Scaffolds—A Parameter Study. J. Cell. Plast. 2012, 48, 141–159. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Allen, M.R. Preclinical Models for Skeletal Research: How Commonly Used Species Mimic (or Don’t) Aspects of Human Bone. Toxicol. Pathol. 2017, 45, 851–854. [Google Scholar] [CrossRef]
- Wancket, L.M. Animal Models for Evaluation of Bone Implants and Devices: Comparative Bone Structure and Common Model Uses. Vet. Pathol. 2015, 52, 842–850. [Google Scholar] [CrossRef]
- Pearce, A.; Richards, R.; Milz, S.; Schneider, E.; Pearce, S. Animal Models for Implant Biomaterial Research in Bone: A Review. Eur. Cells Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef]
- Zeiter, S.; Koschitzki, K.; Alini, M.; Jakob, F.; Rudert, M.; Herrmann, M. Evaluation of Preclinical Models for the Testing of Bone Tissue-Engineered Constructs. Tissue Eng. Part C Methods 2020, 26, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Donath, K. The Diagnostic Value of the New Method for the Study of Undecalcified Bones and Teeth with Attached Soft Tissue, (Säge-Schliff, (Sawing and Grinding) Technique). Pathol. Res. Pract. 1985, 179, 631–633. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Arns, C.; Sheppard, A.; Hutmacher, D.; Milthorpe, B.; Knackstedt, M. Assessment of Bone Ingrowth into Porous Biomaterials Using MICRO-CT. Biomaterials 2007, 28, 2491–2504. [Google Scholar] [CrossRef] [PubMed]
- Oladapo, B.I.; Zahedi, S.A.; Ismail, S.O.; Olawade, D.B. Recent Advances in Biopolymeric Composite Materials: Future Sustainability of Bone-Implant. Renew. Sustain. Energy Rev. 2021, 150, 111505. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Lee, J.; Ha, C.W.; Park, S.J.; Kim, S.; Koo, K.; Seol, Y.; Lee, Y. The Effect of 3-D Printed Polylactic Acid Scaffold with and without Hyaluronic Acid on Bone Regeneration. J. Periodontol. 2022, 93, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Yang, B.-E.; Hong, S.-J.; Choi, H.-G.; Byeon, S.-J.; Lim, H.-K.; Chung, S.-M.; Lee, J.-H.; Byun, S.-H. Bone Regeneration Capability of 3D Printed Ceramic Scaffolds. IJMS 2020, 21, 4837. [Google Scholar] [CrossRef]
- López-Álvarez, M.; Pérez-Davila, S.; Rodríguez-Valencia, C.; González, P.; Serra, J. The Improved Biological Response of Shark Tooth Bioapatites in a Comparative in Vitro Study with Synthetic and Bovine Bone Grafts. Biomed. Mater. 2016, 11, 035011. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, Y.; Li, X.; Wang, X.; Li, D.; Chung, S.; Chen, C.; Lee, I.-S. Osteogenesis of 3D Printed Macro-Pore Size Biphasic Calcium Phosphate Scaffold in Rabbit Calvaria. J. Biomater. Appl. 2019, 33, 1168–1177. [Google Scholar] [CrossRef]
- Pedrero, S.G.; Llamas-Sillero, P.; Serrano-López, J. A Multidisciplinary Journey towards Bone Tissue Engineering. Materials 2021, 14, 4896. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The Effect of Mean Pore Size on Cell Attachment, Proliferation and Migration in Collagen–Glycosaminoglycan Scaffolds for Bone Tissue Engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Wei, Y.; Han, J.; Jiang, X.; Yang, X.; Wu, Y.; Gou, Z.; Chen, L. 3D Printed Bioceramic Scaffolds: Adjusting Pore Dimension Is Beneficial for Mandibular Bone Defects Repair. J. Tissue Eng. Regen. Med. 2022, 16, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Munar, M.L.; Ishikawa, K. Effects of Macropore Size in Carbonate Apatite Honeycomb Scaffolds on Bone Regeneration. Mater. Sci. Eng. C 2020, 111, 110848. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Kirby, M.; Roy, A.; Hu, Y.; Guo, X.E.; Wang, X. Commonality in the Microarchitecture of Trabecular Bone: A Preliminary Study. Bone 2018, 111, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Domingos, M.; Intranuovo, F.; Russo, T.; Santis, R.D.; Gloria, A.; Ambrosio, L.; Ciurana, J.; Bartolo, P. The First Systematic Analysis of 3D Rapid Prototyped Poly(ε-Caprolactone) Scaffolds Manufactured through BioCell Printing: The Effect of Pore Size and Geometry on Compressive Mechanical Behaviour and in Vitro hMSC Viability. Biofabrication 2013, 5, 045004. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous Scaffolds for Bone Regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Xu, M.; Zhai, D.; Chang, J.; Wu, C. In Vitro Assessment of Three-Dimensionally Plotted Nagelschmidtite Bioceramic Scaffolds with Varied Macropore Morphologies. Acta Biomater. 2014, 10, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Domingos, M.; Chiellini, F.; Cometa, S.; Giglio, E.D.; Grillo-Fernandes, E.; Bartolo, P.; Chiellini, E. Evaluation of in Vitro Degradation of PCL Scaffolds Fabricated via BioExtrusion—Part 2: Influence of Pore Size and Geometry: The Present Study Is to Accurately Investigate the Influence of Design Parameters, Such as Filament Distance (FD) and Lay-down Pattern, on the Degradation Behaviour and Kinetics of PCL Scaffolds, Obtained via BioExtrusion. Virtual Phys. Prototyp. 2011, 6, 157–165. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; Li, M.; Li, J.; Zhang, C.; Han, Y.; Wang, L.; Wang, K.; Zhou, C.; Liu, L.; et al. 3D Printing of PLA/n-HA Composite Scaffolds with Customized Mechanical Properties and Biological Functions for Bone Tissue Engineering. Compos. Part B Eng. 2021, 224, 109192. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical Properties and Cell Cultural Response of Polycaprolactone Scaffolds Designed and Fabricated via Fused Deposition Modeling. J. Biomed. Mater. Res. 2001, 55, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Moroni, L.; De Wijn, J.R.; Van Blitterswijk, C.A. Three-dimensional Fiber-deposited PEOT/PBT Copolymer Scaffolds for Tissue Engineering: Influence of Porosity, Molecular Network Mesh Size, and Swelling in Aqueous Media on Dynamic Mechanical Properties. J. Biomed. Mater. Res. 2005, 75A, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Serra, T.; Planell, J.A.; Navarro, M. High-Resolution PLA-Based Composite Scaffolds via 3-D Printing Technology. Acta Biomater. 2013, 9, 5521–5530. [Google Scholar] [CrossRef] [PubMed]





| Scaffold Volume | Obj.S/V | Open Porosity | Strut Thickness | Pore Size | |
|---|---|---|---|---|---|
| mm3 | mm−1 | % | μm | μm | |
| ALT | 100 ± 10 | 8 ± 2 | 45 ± 6 | 430 ± 80 | 400 ± 20 |
| HEL | 70 ± 2 | 10.6 ± 0.7 | 63 ± 1 | 350 ± 20 | 560 ± 6 |
| Pearson’s Correlation Coefficient | Scaffold Volume | Porosity | Strut Thickness |
|---|---|---|---|
| Compressive Strength | 0.98 * | −0.98 * | −0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso-Fernández, I.; Haugen, H.J.; Nogueira, L.P.; López-Álvarez, M.; González, P.; López-Peña, M.; González-Cantalapiedra, A.; Muñoz-Guzón, F. Enhanced Bone Healing in Critical-Sized Rabbit Femoral Defects: Impact of Helical and Alternate Scaffold Architectures. Polymers 2024, 16, 1243. https://doi.org/10.3390/polym16091243
Alonso-Fernández I, Haugen HJ, Nogueira LP, López-Álvarez M, González P, López-Peña M, González-Cantalapiedra A, Muñoz-Guzón F. Enhanced Bone Healing in Critical-Sized Rabbit Femoral Defects: Impact of Helical and Alternate Scaffold Architectures. Polymers. 2024; 16(9):1243. https://doi.org/10.3390/polym16091243
Chicago/Turabian StyleAlonso-Fernández, Iván, Håvard Jostein Haugen, Liebert Parreiras Nogueira, Miriam López-Álvarez, Pío González, Mónica López-Peña, Antonio González-Cantalapiedra, and Fernando Muñoz-Guzón. 2024. "Enhanced Bone Healing in Critical-Sized Rabbit Femoral Defects: Impact of Helical and Alternate Scaffold Architectures" Polymers 16, no. 9: 1243. https://doi.org/10.3390/polym16091243
APA StyleAlonso-Fernández, I., Haugen, H. J., Nogueira, L. P., López-Álvarez, M., González, P., López-Peña, M., González-Cantalapiedra, A., & Muñoz-Guzón, F. (2024). Enhanced Bone Healing in Critical-Sized Rabbit Femoral Defects: Impact of Helical and Alternate Scaffold Architectures. Polymers, 16(9), 1243. https://doi.org/10.3390/polym16091243







