Synthesis and Oxidative Degradation of Leucine-Based Poly(diacylhydrazine)
Abstract
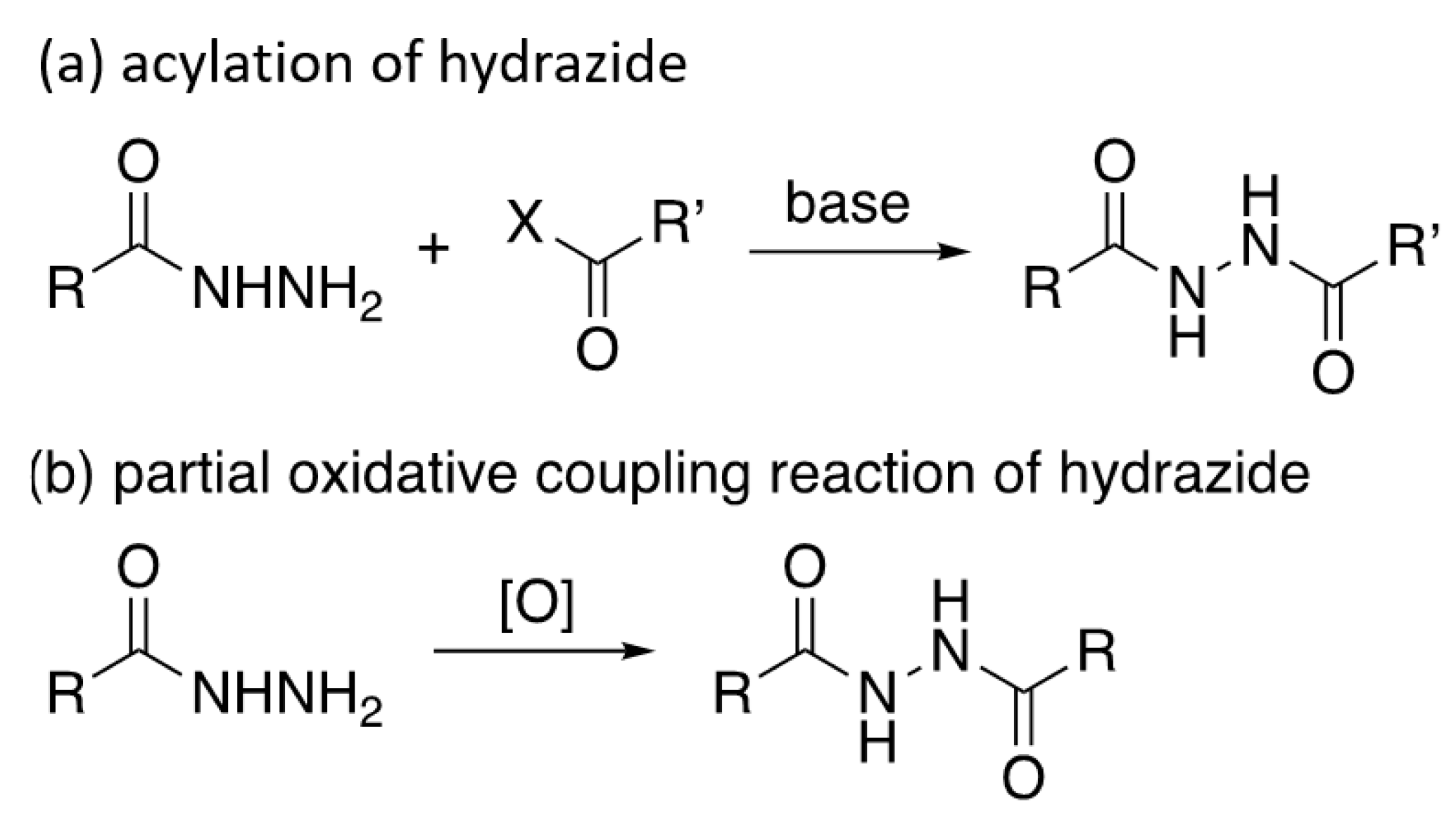
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
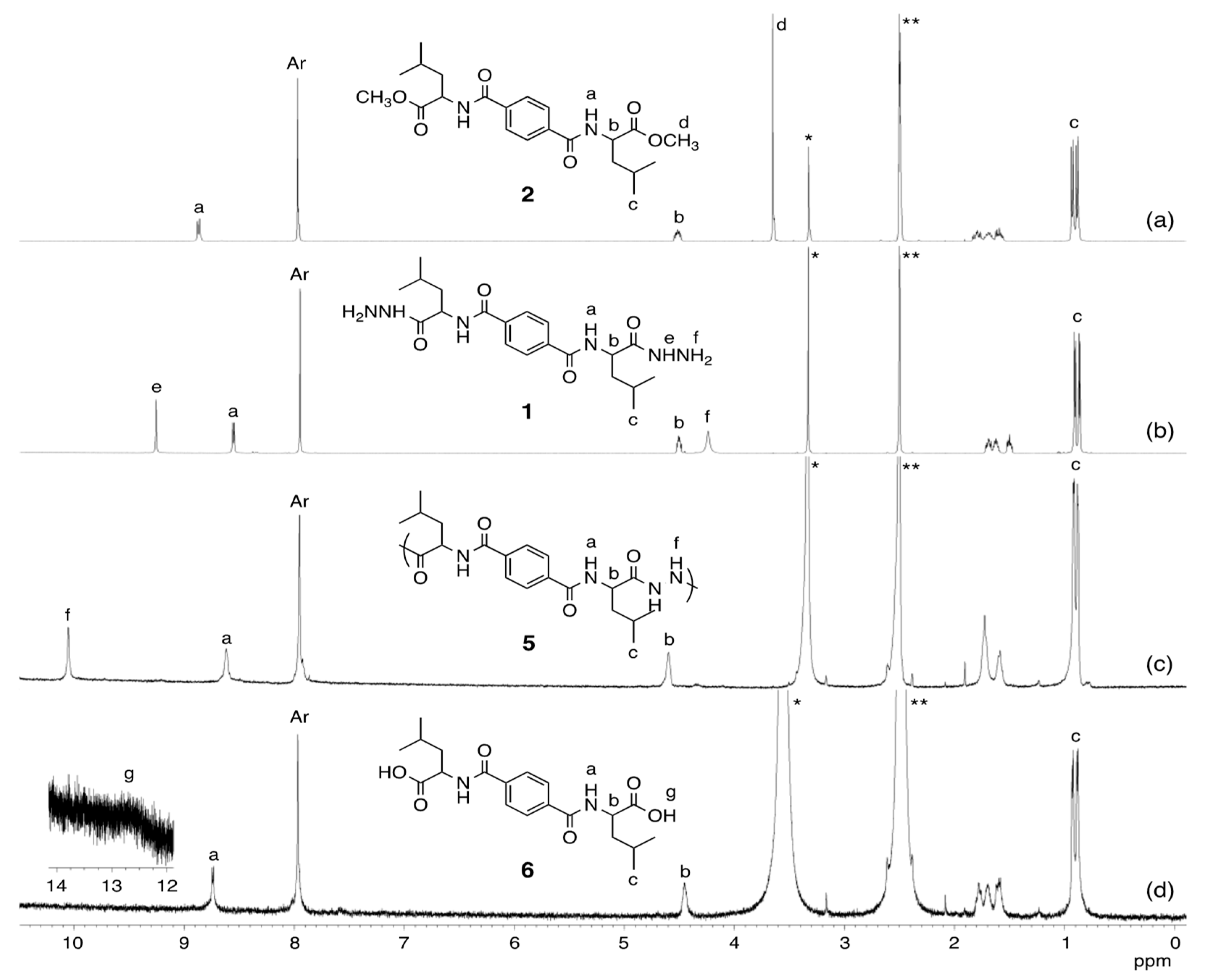
2.2. Synthesis of Terephthaloyl Bis(L-Leucine Methyl Ester) 2
- 1H NMR (DMSO-d6, 400 MHz): d 8.87 (d, J = 7.6 Hz, 2H, NH), 7.97 (s, 4H, Ar), 4.56–4.47 (m, 2H, CH), 3.65 (s, 6H, CH3), 1.85–1.54 (m, 6H, CH2 and CH), 0.93 (d, J = 6.5 Hz, 6H, CH3), and 0.89 (d, J = 6.5 Hz, 6H, CH3) ppm.
2.3. Synthesis of Dihydrazide Monomer 1
- 1H-NMR (DMSO-d6, 600 MHz): d 9.25 (s, 2H, NH), 8.55 (d, J = 8.1 Hz, 2H, NH), 7.95 (s, 4H, Ar), 4.54–4.47 (m, 2H, CH), 4.24 (br, 4H, NH2), 1.74–1.47 (m, 6H, CH2 and CH), 0.91 (d, J = 6.5 Hz, 6H, CH3), and 0.87 (d, J = 6.5 Hz, 6H, CH3) ppm.
2.4. Synthesis of Poly(diacylhydrazine) 5: Oxidative Coupling Polymerization of Dihydrazide Monomer 1
- 1H NMR (DMSO-d6, 600 MHz): d 10.05 (br, 2H, NH), 8.62 (br, 2H, NH), 7.95 (br, 4H, Ar), 4.60 (br, 2H, CH), 1.79 (br, 4H, CH2), 1.59 (br, 2H, CH), 0.92 (d, J = 4.6 Hz, 6H, CH3), and 0.88 (d, J = 4.0 Hz, 6H, CH3) ppm.
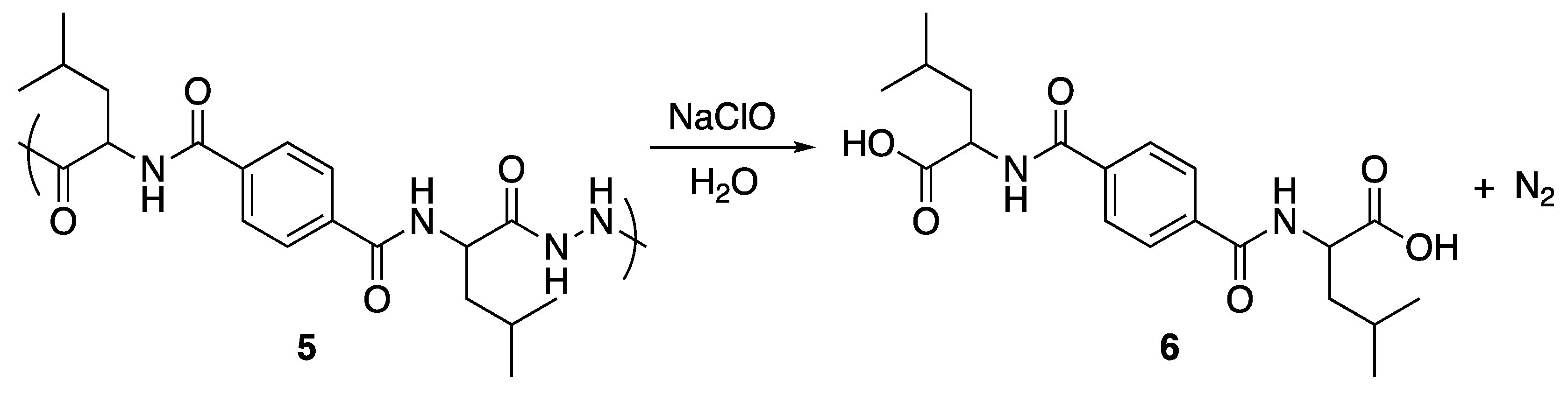
2.5. Oxidative Degradation of Poly(diacylhydrazine) 5
- 1H NMR (DMSO-d6, 600 MHz): d 12.7 (br, 2H, OH), 8.74 (d, J = 7.1 Hz, 2H, NH), 7.97 (s, 4H, Ar), 4.49–4.41 (m, 2H, CH), 1.83–1.52 (m, 6H, CH2 and CH), 0.93 (d, J = 6.4 Hz, 6H, CH3), and 0.88 (d, J = 6.3 Hz, 6H, CH3) ppm.
3. Results
3.1. Synthesis of Monomer 1
3.2. Synthesis of Polymer
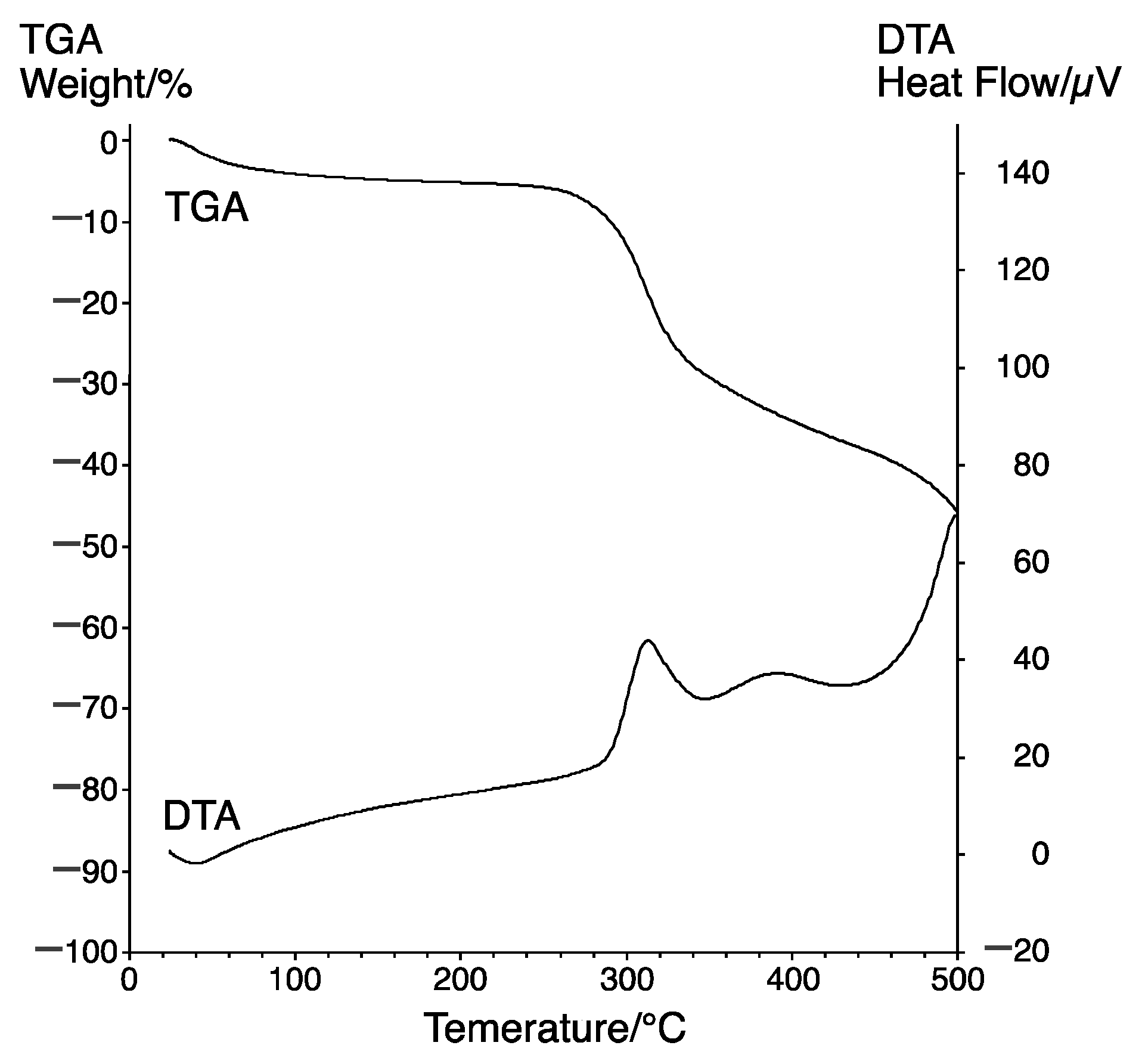
3.3. Thermal Properties of Poly(diacylhydrazine) 5
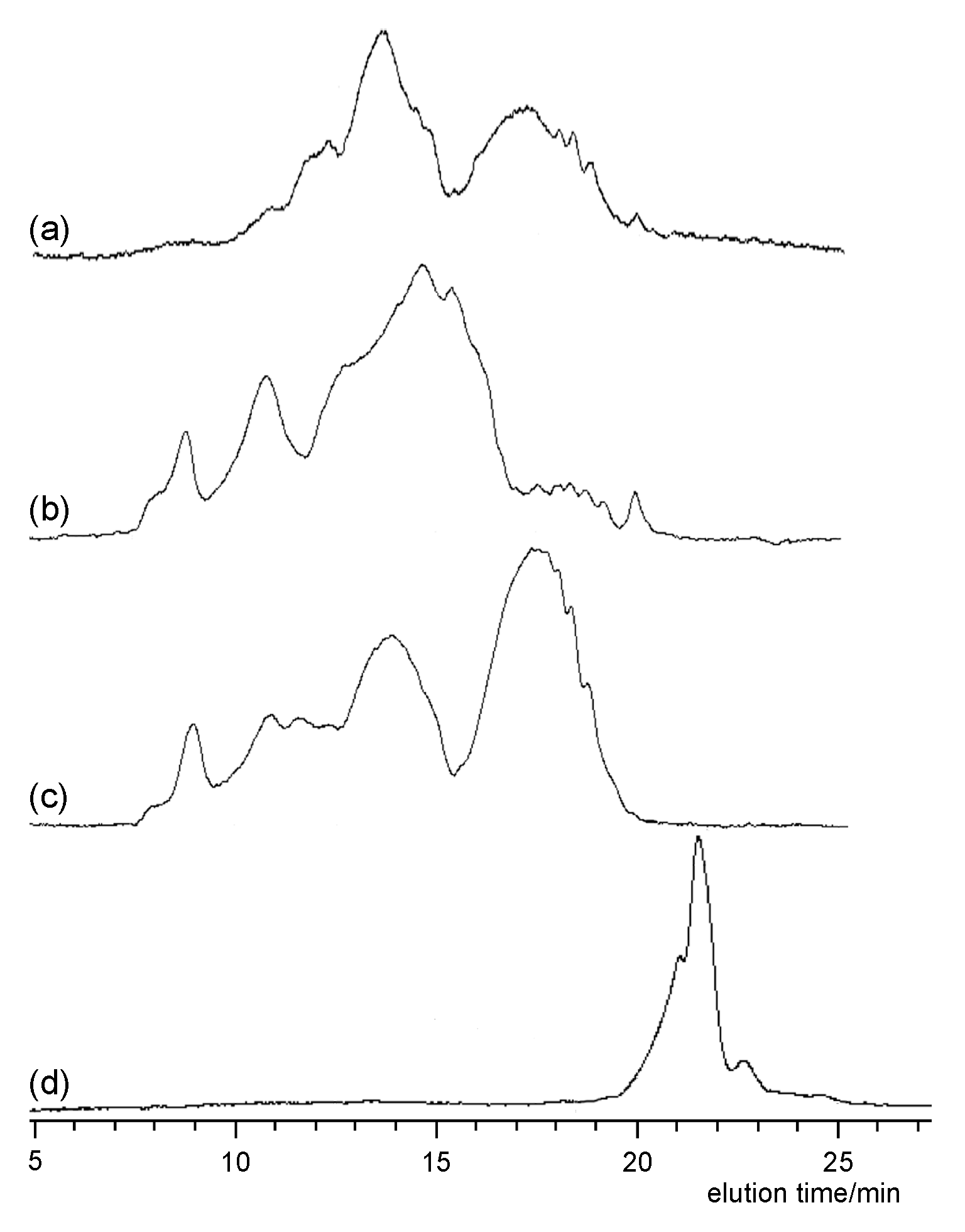
3.4. Oxidative Degradation of Poly(diacylhydrazine) 5
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhandari, N.; Bhandari, G.; Bista, S.; Pokhrel, B.; Bist, K.; Dhakal, K. Degradation of fundamental polymers/plastics used in daily life: A review. Bibechana 2021, 18, 240–243. [Google Scholar] [CrossRef]
- Psarrou, M.; Kothri, M.G.; Vamvakaki, M. Photo- and Acid-Degradable Polyacylhydrazone–Doxorubicin Conjugates. Polymers 2021, 13, 2461. [Google Scholar] [CrossRef] [PubMed]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. Npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Martinez, M.R.; Matyjaszewski, K. Degradable and Recyclable Polymers by Reversible Deactivation Radical Polymerization. CCS Chem. 2022, 4, 2176–2211. [Google Scholar] [CrossRef]
- Șucu, T.; Shaver, M.P. Inherently degradable cross-linked polyesters and polycarbonates: Resins to be cheerful. Polym. Chem. 2020, 11, 6397–6412. [Google Scholar] [CrossRef]
- Yeon, H.; Kumar, A.; Song, J.; Lee, J.; Wang, S.J.; Kim, S.Y.; Cho, J.; Cho, D.; Kim, T.A. On-Demand Degradable and Acid-Generating Polymers Using Phenacyl Ester Derivatives. Macromolecules 2024, 57, 2928–2936. [Google Scholar] [CrossRef]
- Feist, J.D.; Lee, D.C.; Xia, Y. A versatile approach for the synthesis of degradable polymers via controlled ring-opening metathesis copolymerization. Nat. Chem. 2022, 14, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.; Johnson, P.S.; Nortcliffe, A.; Wheeler, S. Improved Method for the Preparation of Oxadiazoles from Diacyl Hydrazines. Synth. Commun. 2010, 40, 3021–3026. [Google Scholar] [CrossRef]
- Hasegawa, M. Polyhydrazides and polyoxadiazoles. Encycl. Polym. Sci. Tech. 1969, 11, 169–187. [Google Scholar]
- Bock, H.; Rudolph, G.; Baltin, E. Chemistry of hydrazine. VI. Oxidation of 1,2-disubstituted hydrazines with N-bromosuccinimide. Chem. Ber. 1965, 98, 2054–2055. [Google Scholar] [CrossRef]
- Stolle, R.; Reichert, W. Thermal decomposition of azodibenzoyl and dimethyl azodicarboxylate. J. Prakt. Chem. 1929, 123, 82–84. [Google Scholar] [CrossRef]
- Stolle, R. Preparation and Reactions of Azoacyl Compounds. Ber. 1912, 45, 273–289. [Google Scholar]
- Kihara, N.; Il, R.; Ogawa, A. Synthesis and properties of nylon-0,2-oxidatively degradable polymer that is stable in air. J. Polym. Sci. A Polym. Chem. 2007, 45, 963–967. [Google Scholar] [CrossRef]
- Kihara, N.; Iino, Y.; Misawa, T. Oxidative degradation of poly(isophthaloylhydrazine-1,2-diyl)s. J. Polym. Sci. A Polym. Chem. 2008, 46, 6255–6262. [Google Scholar] [CrossRef]
- Yanaze, K.; Kihara, N. Superabsorbent polymer solubilized instantly by decrosslinking with sodium hypochlorite. Polym. J. 2021, 53, 1153–1155. [Google Scholar] [CrossRef]
- Horner, L.; Fernekess, H. Reaction of hydrazine derivatives and hydrazones with peracetic acid. Chem. Ber. 1961, 94, 712–724. [Google Scholar] [CrossRef]
- Prakash, O.; Sharma, V.; Sadana, A. Hypervalent iodine oxidation of acid hydrazides: A new synthesis of N,N′-diacylhydrazines. Synth. Commun. 1997, 27, 3371–3377. [Google Scholar] [CrossRef]
- Kulkarni, P.P.; Kadam, A.J.; Desai, U.V.; Mane, R.B.; Wadgaonkar, P.P. A simple and efficient oxidation of hydrazides to N,N′-diacylhydrazines using Oxone in an aqueous medium. J. Chem. Res. Synop. 2000, 4, 184–185. [Google Scholar] [CrossRef]
- Mogilaiah, K.; Prashanthi, M.; Randheer Reddy, G. An efficient oxidation of acid hydrazides to N,N′-diacylhydrazines using copper(II) acetate in solvent-free conditions under microwave irradiation. Synth. Commun. 2003, 33, 3741–3745. [Google Scholar] [CrossRef]
- Frazer, A.H.; Wallenberger, F.T. Aliphatic polyhydrazides: A new low-temperature solution polymerization. J. Polym. Sci. Part A 1964, 2, 1137–1145. [Google Scholar] [CrossRef]
- Frazer, A.H.; Wallenberger, F.T. Aromatic polyhydrazides: A new class of highly bonded, stiff polymers. J. Polym. Sci. Part A 1964, 2, 147–1156. [Google Scholar] [CrossRef]
- Frazer, A.H.; Reed, T.A. Alternating copolyhydrazide of terephthalic and isophthalic acids. Macromol. Synth. 1969, 3, 87–89. [Google Scholar]
- Nayak, K.; Ghosh, P.; Khan, M.E.H.; De, P. Side-chain amino-acid-based polymers: Self-assembly and bioapplications. Polym. Int. 2022, 71, 411–425. [Google Scholar] [CrossRef]
- Tachibana, Y.; Darbe, S.; Hayashi, S.; Kudasheva, A.; Misawa, H.; Shibata, Y.; Kasuya, K.-I. Environmental biodegradability of recombinant structural protein. Sci. Rep. 2021, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Childers, E.P.; Becker, M.L. l-Leucine-Based Poly(ester urea)s for Vascular Tissue Engineering. ACS Biomater. Sci. Eng. 2015, 1, 795–804. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Saeed Zahmatkesh, S.; Zarei, A.; Khazdooz, L.; Ruoho, A.E. Synthesis and characterization of novel optically active poly(amide–imide)s via direct amidation. Eur. Polym. J. 2005, 41, 2290–2296. [Google Scholar] [CrossRef]
- Khayyat, S.; Amr, A.E.G. Synthesis and Biological Activities of Some New (Nα-Dinicotinoyl)- bis-L-Leucyl Linear and Macrocyclic Peptides. Molecules 2014, 19, 10698–10716. [Google Scholar] [CrossRef]
- Nagashima, K.; Kihara, N.; Iino, Y. Oxidative coupling polymerization of bishydrazide for the synthesis of poly(diacylhydrazine): Oxidative preparation of oxidatively degradable polymer. J. Polym. Sci. Polym. Chem. 2012, 50, 4230–4238. [Google Scholar] [CrossRef]


| Run | 1 (g) | Oxidant (g, eq) | Solvent (mL) | Time (h) | Yield a (%) | Mn b (Mw/Mn b) |
|---|---|---|---|---|---|---|
| 1 | 1.0111 | Oxone® (2.9724, 2.0) | NMP/CH3CN/H2O (10/10/7) | 48 | 74 | 4600 (98) |
| 2 | 0.5062 | Oxone® (1.4831, 2.0) | NMP/DMAc/H2O (10/10/8) | 48 | 57 | 6700 (12,000) |
| 3 | 0.4935 | PhI(OAc)2 (0.6656, 0.88) | NMP (5) | 24 | 74 | 5500 (7300) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongwailikhit, K.; Suwannakeeree, R.; Kihara, N. Synthesis and Oxidative Degradation of Leucine-Based Poly(diacylhydrazine). Polymers 2024, 16, 1222. https://doi.org/10.3390/polym16091222
Wongwailikhit K, Suwannakeeree R, Kihara N. Synthesis and Oxidative Degradation of Leucine-Based Poly(diacylhydrazine). Polymers. 2024; 16(9):1222. https://doi.org/10.3390/polym16091222
Chicago/Turabian StyleWongwailikhit, Kanda, Ratha Suwannakeeree, and Nobuhiro Kihara. 2024. "Synthesis and Oxidative Degradation of Leucine-Based Poly(diacylhydrazine)" Polymers 16, no. 9: 1222. https://doi.org/10.3390/polym16091222
APA StyleWongwailikhit, K., Suwannakeeree, R., & Kihara, N. (2024). Synthesis and Oxidative Degradation of Leucine-Based Poly(diacylhydrazine). Polymers, 16(9), 1222. https://doi.org/10.3390/polym16091222






