Predicting Diffusion Coefficients in Nafion Membranes during the Soaking Process Using a Machine Learning Approach
Abstract
1. Introduction
1.1. Nafion in Salt Solutions
1.2. Nafion and ML
1.3. Aim of this Study
2. Materials and Methods
3. Results
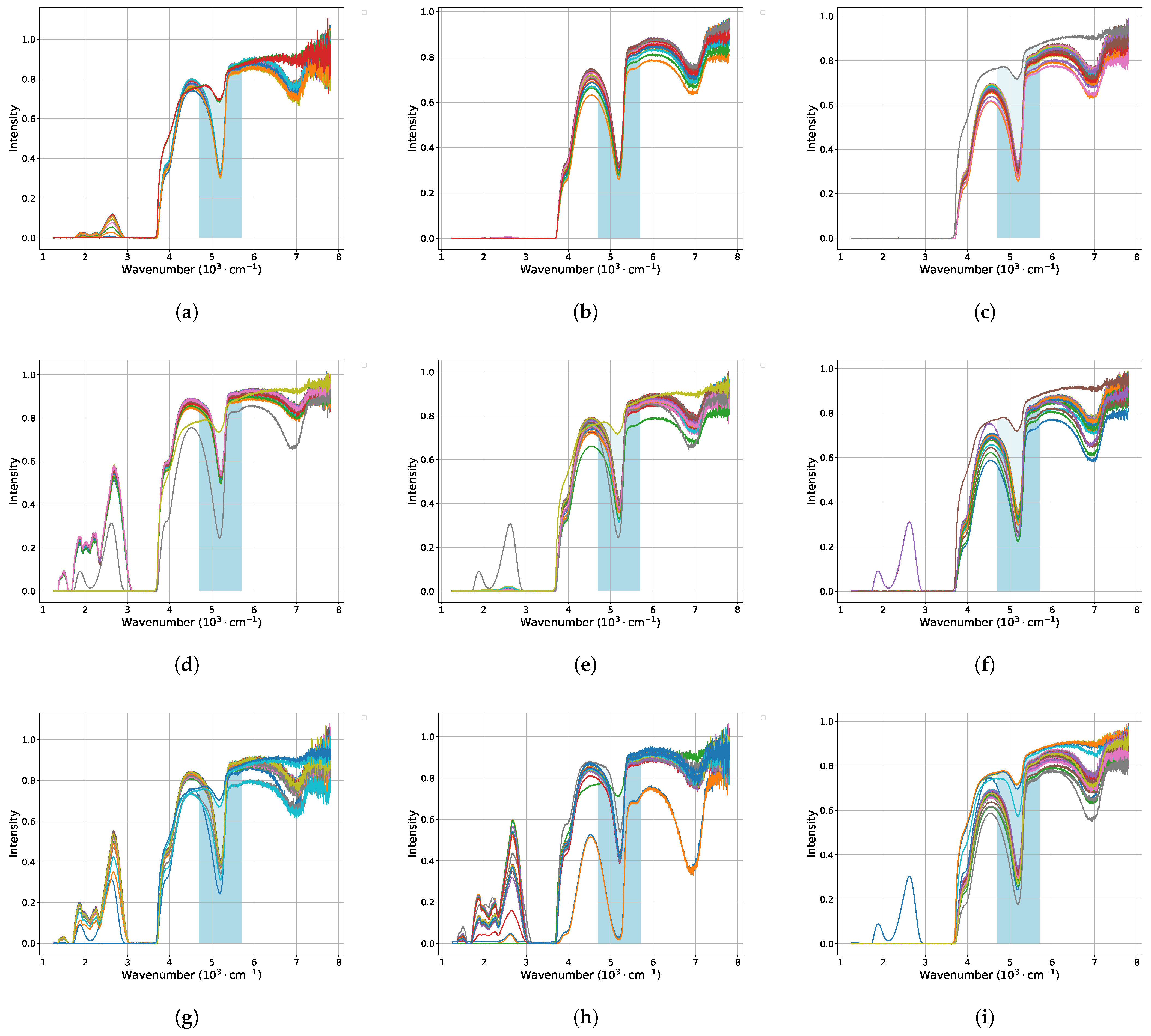
3.1. IR Spectra and Fitting
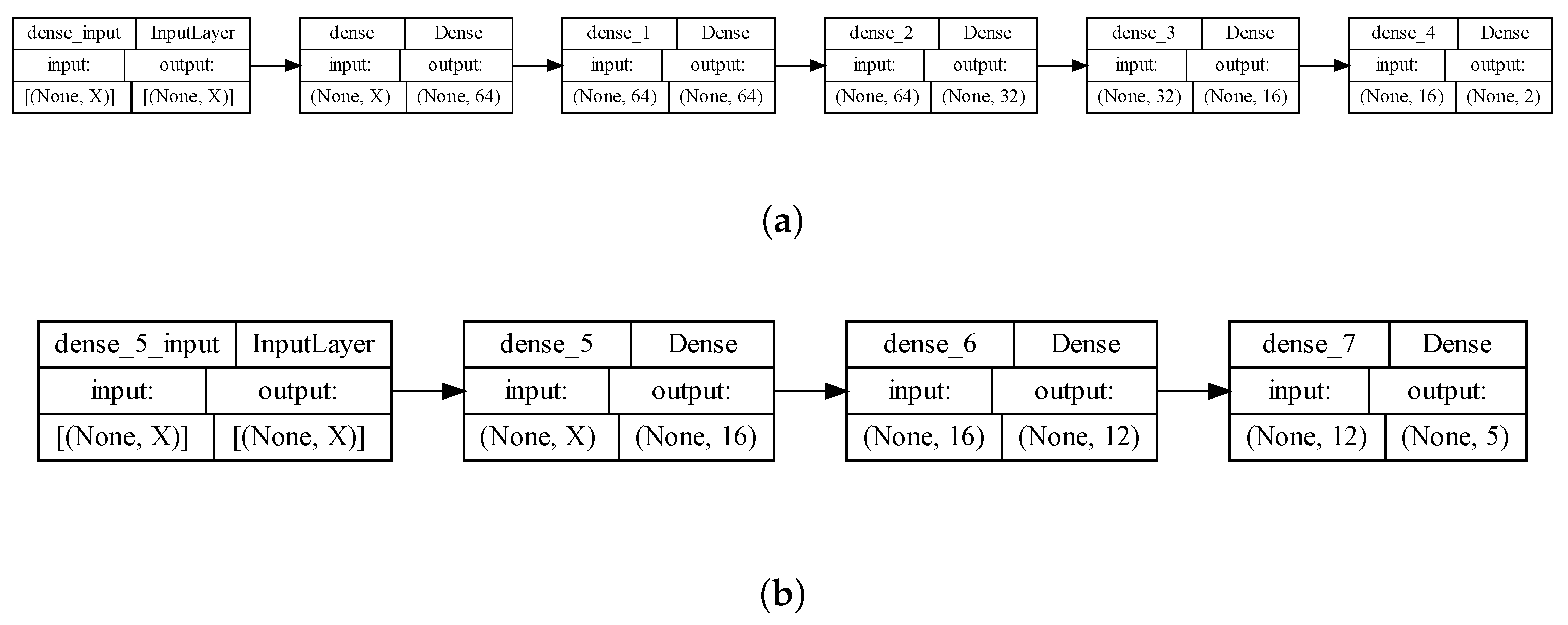
3.2. Neural Network (NN)-Based Model for Predicting Diffusion Coefficients
- Definition of a multi-layer NN architecture using the TensorFlow framework. The architecture comprised multiple dense layers with varying numbers of neurons and activation functions, which were treated as parameters to be optimized by the genetic algorithm.
- GA implementation using the DEAP (Distributed Evolutionary Algorithms in Python) library. We defined the individuals as a combination of integers representing the number of neurons in each dense layer and a categorical variable representing the activation function. The GA aimed to optimize these parameters to minimize the mean squared error (MSE) loss function of the NN model.
- GA iteratively evolved a population of candidate solutions (NN architectures) over multiple generations. Each candidate solution was evaluated by training the corresponding NN model on the training dataset and computing its MSE loss on the validation dataset. The fitness of each solution was determined by its validation MSE.
- After a predefined number of generations, the GA selected the best-performing individual (NN architecture) based on its validation MSE. We split the data into training and testing sets in an 80/20 ratio. This architecture was then used to train a final NN model on the entire training dataset.
- The performance of the final NN model was evaluated on an independent testing dataset to assess its generalization ability and predictive accuracy. The MSE loss on the test dataset was calculated as a measure of model performance.
4. Discussion
- Improving deep learning models: Research on developing more efficient and accurate deep learning models for predicting diffusion coefficients. This may involve using more complex network architectures and optimizing training parameters [80].
- Using recurrent neural networks (RNNs): Research on applying recurrent neural networks to analyze time series data, such as changes in substance concentration over time, to predict diffusion coefficients [81].
- Training on diverse datasets: Research aimed at training models on diverse and larger datasets of diffusion data [82]. This can help improve the models’ generalization ability and make them more applicable to various conditions and materials.
- Investigating the influence of material structure: Research on analyzing the influence of the material’s structure on its diffusion properties using neural networks [83]. This may involve analyzing the microstructure of the material or its chemical composition.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, G.A.; Oke, A.F.; Nagy, G.; Moghaddam, B.; Adams, R.N. Nafion-coated electrodes with high selectivity for CNS electrochemistry. Brain Res. 1984, 290, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Recent approaches to improve Nafion performance for fuel cell applications: A review. Int. J. Hydrogen Energy 2019, 44, 28919–28938. [Google Scholar] [CrossRef]
- Banerjee, S.; Curtin, D.E. Nafion® perfluorinated membranes in fuel cells. J. Fluor. Chem. 2004, 125, 1211–1216. [Google Scholar] [CrossRef]
- Takamatsu, T.; Hashiyama, M.; Eisenberg, A. Sorption phenomena in Nafion membranes. J. Appl. Polym. Sci. 1979, 24, 2199–2220. [Google Scholar] [CrossRef]
- Takamatsu, T.; Eisenberg, A. Densities and expansion coefficients of nafion polymers. J. Appl. Polym. Sci. 1979, 24, 2221–2235. [Google Scholar] [CrossRef]
- Roche, E.; Pineri, M.; Duplessix, R.; Levelut, A. Small-angle scattering studies of nafion membranes. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1–11. [Google Scholar] [CrossRef]
- Lindheimer, A.; Molenat, J.; Gavach, C. A study of the superselectivity of Nafion perfluorosulfonic membranes. J. Electroanal. Chem. Interfacial Electrochem. 1987, 216, 71–88. [Google Scholar] [CrossRef]
- Capeci, S.W.; Pintauro, P.N.; Bennion, D.N. The Molecular-Level Interpretation of Salt Uptake and Anion Transport in Nafion Membranes. J. Electrochem. Soc. 1989, 136, 2876. [Google Scholar] [CrossRef]
- Bontha, J.; Pintauro, P.N. Water orientation and ion solvation effects during multicomponent salt partitioning in a Nafion cation exchange membrane. Chem. Eng. Sci. 1994, 49, 3835–3851. [Google Scholar] [CrossRef]
- Lehmani, A.; Turq, P.; Périé, M.; Périé, J.; Simonin, J.P. Ion transport in Nafion® 117 membrane. J. Electroanal. Chem. 1997, 428, 81–89. [Google Scholar] [CrossRef]
- Samec, Z.; Trojanek, A.; Langmaier, J.; Samcova, E. Diffusion Coefficients of Alkali Metal Cations in Nafion® from Ion-Exchange Measurements: An Advanced Kinetic Model. J. Electrochem. Soc. 1997, 144, 4236. [Google Scholar] [CrossRef]
- Kawano, Y.; Wang, Y.; Palmer, R.A.; Aubuchon, S.R. Stress-strain curves of Nafion membranes in acid and salt forms. Polímeros 2002, 12, 96–101. [Google Scholar] [CrossRef]
- Lee, S.J.; Yu, T.L.; Lin, H.L.; Liu, W.H.; Lai, C.L. Solution properties of nafion in methanol/water mixture solvent. Polymer 2004, 45, 2853–2862. [Google Scholar] [CrossRef]
- Villaluenga, J.; Barragan, V.; Seoane, B.; Ruiz-Bauza, C. Sorption and permeation of solutions of chloride salts, water and methanol in a Nafion membrane. Electrochim. Acta 2006, 51, 6297–6303. [Google Scholar] [CrossRef]
- Randová, A.; Hovorka, Š.; Izák, P.; Bartovská, L. Swelling of Nafion in methanol–water–inorganic salt ternary mixtures. J. Electroanal. Chem. 2008, 616, 117–121. [Google Scholar] [CrossRef]
- Hallinan, D.T., Jr.; De Angelis, M.G.; Giacinti Baschetti, M.; Sarti, G.C.; Elabd, Y.A. Non-fickian diffusion of water in nafion. Macromolecules 2010, 43, 4667–4678. [Google Scholar] [CrossRef]
- Zhao, Q.; Majsztrik, P.; Benziger, J. Diffusion and interfacial transport of water in Nafion. J. Phys. Chem. B 2011, 115, 2717–2727. [Google Scholar] [CrossRef] [PubMed]
- Chaabane, L.; Dammak, L.; Grande, D.; Larchet, C.; Huguet, P.; Nikonenko, S.; Nikonenko, V. Swelling and permeability of Nafion® 117 in water–methanol solutions: An experimental and modelling investigation. J. Membr. Sci. 2011, 377, 54–64. [Google Scholar] [CrossRef]
- Gebel, G.; Lyonnard, S.; Mendil-Jakani, H.; Morin, A. The kinetics of water sorption in Nafion membranes: A small-angle neutron scattering study. J. Phys. Condens. Matter 2011, 23, 234107. [Google Scholar] [CrossRef]
- Izquierdo-Gil, M.A.; Barragán, V.; Villaluenga, J.; Godino, M. Water uptake and salt transport through Nafion cation-exchange membranes with different thicknesses. Chem. Eng. Sci. 2012, 72, 1–9. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Matsunaga, T.; Amemiya, K.; Ohira, A.; Hasegawa, N.; Shinohara, K.; Ando, M.; Yoshida, T. Dispersion of rod-like particles of nafion in salt-free water/1-propanol and water/ethanol solutions. J. Phys. Chem. B 2014, 118, 14922–14928. [Google Scholar] [CrossRef] [PubMed]
- Soniat, M.; Houle, F.A. Swelling and diffusion during methanol sorption into hydrated nafion. J. Phys. Chem. B 2018, 122, 8255–8268. [Google Scholar] [CrossRef]
- Mabuchi, T.; Huang, S.F.; Tokumasu, T. Dispersion of Nafion ionomer aggregates in 1-propanol/water solutions: Effects of ionomer concentration, alcohol content, and salt addition. Macromolecules 2020, 53, 3273–3283. [Google Scholar] [CrossRef]
- Wang, Z.F.; You, Y.L.; Li, F.F.; Kong, W.R.; Wang, S.Q. Research progress of NMR in natural product quantification. Molecules 2021, 26, 6308. [Google Scholar] [CrossRef]
- Volkov, V.I.; Chernyak, A.V.; Gnezdilov, O.I.; Skirda, V.D. Hydration, self-diffusion and ionic conductivity of Li+, Na+ and Cs+ cations in Nafion membrane studied by NMR. Solid State Ion. 2021, 364, 115627. [Google Scholar] [CrossRef]
- Ninham, B.W.; Bolotskova, P.N.; Gudkov, S.V.; Baranova, E.N.; Kozlov, V.A.; Shkirin, A.V.; Vu, M.T.; Bunkin, N.F. Nafion swelling in salt solutions in a finite sized cell: Curious phenomena dependent on sample preparation protocol. Polymers 2022, 14, 1511. [Google Scholar] [CrossRef] [PubMed]
- Van Meeteren, U.; Van Gelder, H.; Van Ieperen, W. Reconsideration of the use of deionized water as vase water in postharvest experiments on cut flowers. Postharvest Biol. Technol. 1999, 17, 175–187. [Google Scholar] [CrossRef]
- Khajeh-Hosseini-Dalasm, N.; Ahadian, S.; Fushinobu, K.; Okazaki, K.; Kawazoe, Y. Prediction and analysis of the cathode catalyst layer performance of proton exchange membrane fuel cells using artificial neural network and statistical methods. J. Power Sources 2011, 196, 3750–3756. [Google Scholar] [CrossRef]
- Pan, M.; Li, C.; Pan, C.; Lei, H.; Huang, H. A novel predicting method on degree of catalytic reaction in fuel cells. Int. J. Energy Res. 2020, 44, 6860–6872. [Google Scholar] [CrossRef]
- Khajeh-Hosseini-Dalasm, N.; Fesanghary, M.; Fushinobu, K.; Okazaki, K. A study of the agglomerate catalyst layer for the cathode side of a proton exchange membrane fuel cell: Modeling and optimization. Electrochim. Acta 2012, 60, 55–65. [Google Scholar] [CrossRef]
- Ding, R.; Ding, Y.; Zhang, H.; Wang, R.; Xu, Z.; Liu, Y.; Yin, W.; Wang, J.; Li, J.; Liu, J. Applying machine learning to boost the development of high-performance membrane electrode assembly for proton exchange membrane fuel cells. J. Mater. Chem. A 2021, 9, 6841–6850. [Google Scholar] [CrossRef]
- Parker, A.J.; Opletal, G.; Barnard, A.S. Classification of platinum nanoparticle catalysts using machine learning. J. Appl. Phys. 2020, 128, 014301. [Google Scholar]
- Niu, H.; Wan, X.; Wang, X.; Shao, C.; Robertson, J.; Zhang, Z.; Guo, Y. Single-atom rhodium on defective g-C3N4: A promising bifunctional oxygen electrocatalyst. ACS Sustain. Chem. Eng. 2021, 9, 3590–3599. [Google Scholar] [CrossRef]
- Wang, N.; Bo, X.; Zhou, M. Single-step and room-temperature synthesis of laser-induced Pt/VC nanocomposites as effective bifunctional electrocatalysts for hydrogen evolution and oxygen evolution reactions. ACS Appl. Mater. Interfaces 2022, 14, 23332–23341. [Google Scholar] [CrossRef] [PubMed]
- Seyhan, M.; Akansu, Y.E.; Murat, M.; Korkmaz, Y.; Akansu, S.O. Performance prediction of PEM fuel cell with wavy serpentine flow channel by using artificial neural network. Int. J. Hydrogen Energy 2017, 42, 25619–25629. [Google Scholar] [CrossRef]
- Ding, R.; Zhang, S.; Chen, Y.; Rui, Z.; Hua, K.; Wu, Y.; Li, X.; Duan, X.; Wang, X.; Li, J.; et al. Application of machine learning in optimizing proton exchange membrane fuel cells: A review. Energy AI 2022, 9, 100170. [Google Scholar] [CrossRef]
- Günay, M.E.; Tapan, N.A.; Akkoç, G. Analysis and modeling of high-performance polymer electrolyte membrane electrolyzers by machine learning. Int. J. Hydrogen Energy 2022, 47, 2134–2151. [Google Scholar] [CrossRef]
- Daffertshofer, A.; Lamoth, C.J.; Meijer, O.G.; Beek, P.J. PCA in studying coordination and variability: A tutorial. Clin. Biomech. 2004, 19, 415–428. [Google Scholar] [CrossRef]
- Mohamed, A.; Ibrahem, H.; Yang, R.; Kim, K. Optimization of Proton Exchange Membrane Electrolyzer Cell Design Using Machine Learning. Energies 2022, 15, 6657. [Google Scholar] [CrossRef]
- Zaveri, J.C.; Dhanushkodi, S.R.; Kumar, C.R.; Taler, J.; Majdak, M.; Węglowski, B. Predicting the Performance of PEM Fuel Cells by Determining Dehydration or Flooding in the Cell Using Machine Learning Models. Energies 2023, 16, 6968. [Google Scholar] [CrossRef]
- Bunkin, N.F.; Kozlov, V.A.; Shkirin, A.V.; Ninham, B.W.; Balashov, A.A.; Gudkov, S.V. Dynamics of Nafion membrane swelling in H2O/D2O mixtures as studied using FTIR technique. J. Chem. Phys. 2018, 148, 124901. [Google Scholar] [CrossRef] [PubMed]
- Newville, M.; Stensitzki, T.; Allen, D.B.; Rawlik, M.; Ingargiola, A.; Nelson, A. LMFIT: Non-Linear Least-Square Minimization and Curve-Fitting for Python; Astrophysics Source Code Library: Houghton, MI, USA, 2016; p. ascl-1606. [Google Scholar]
- Chen, G.; Shen, Z.; Iyer, A.; Ghumman, U.F.; Tang, S.; Bi, J.; Chen, W.; Li, Y. Machine-learning-assisted de novo design of organic molecules and polymers: Opportunities and challenges. Polymers 2020, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Malashin, I.P.; Tynchenko, V.S.; Nelyub, V.A.; Borodulin, A.S.; Gantimurov, A.P. Estimation and Prediction of the Polymers’ Physical Characteristics Using the Machine Learning Models. Polymers 2023, 16, 115. [Google Scholar] [CrossRef] [PubMed]
- Chepurnenko, A. Determining the Rheological Parameters of Polymers Using Artificial Neural Networks. Polymers 2022, 14, 3977. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi, A.; Morovati, V.; Dargazany, R. A physics-informed assembly of feed-forward neural network engines to predict inelasticity in cross-linked polymers. Polymers 2020, 12, 2628. [Google Scholar] [CrossRef] [PubMed]
- Abdolrasol, M.G.; Hussain, S.S.; Ustun, T.S.; Sarker, M.R.; Hannan, M.A.; Mohamed, R.; Ali, J.A.; Mekhilef, S.; Milad, A. Artificial neural networks based optimization techniques: A review. Electronics 2021, 10, 2689. [Google Scholar] [CrossRef]
- Kosarac, A.; Mladjenovic, C.; Zeljkovic, M.; Tabakovic, S.; Knezev, M. Neural-network-based approaches for optimization of machining parameters using small dataset. Materials 2022, 15, 700. [Google Scholar] [CrossRef] [PubMed]
- Smithson, M. Confidence Intervals; Number 140; Sage: Thousand Oaks, CA, USA, 2003. [Google Scholar]
- Goswami, A.; Acharya, A.; Pandey, A. Study of self-diffusion of monovalent and divalent cations in Nafion-117 ion-exchange membrane. J. Phys. Chem. B 2001, 105, 9196–9201. [Google Scholar] [CrossRef]
- Okada, T.; Møller-Holst, S.; Gorseth, O.; Kjelstrup, S. Transport and equilibrium properties of Nafion® membranes with H+ and Na+ ions. J. Electroanal. Chem. 1998, 442, 137–145. [Google Scholar] [CrossRef]
- Suresh, G.; Scindia, Y.; Pandey, A.; Goswami, A. Isotopic and ion-exchange kinetics in the Nafion-117 membrane. J. Phys. Chem. B 2004, 108, 4104–4110. [Google Scholar] [CrossRef]
- Fick, A.V. On liquid diffusion. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1855, 10, 30–39. [Google Scholar] [CrossRef]
- Suresh, G.; Scindia, Y.; Pandey, A.; Goswami, A. Self-diffusion coefficient of water in Nafion-117 membrane with different monovalent counterions: A radiotracer study. J. Membr. Sci. 2005, 250, 39–45. [Google Scholar] [CrossRef]
- Suresh, G.; Pandey, A.; Goswami, A. Self-diffusion coefficients of water in Nafion-117 membrane with multivalent counterions. J. Membr. Sci. 2006, 284, 193–197. [Google Scholar] [CrossRef]
- Al-Shehari, T.; Alsowail, R.A. An insider data leakage detection using one-hot encoding, synthetic minority oversampling and machine learning techniques. Entropy 2021, 23, 1258. [Google Scholar] [CrossRef] [PubMed]
- Protić, D.; Stanković, M.; Prodanović, R.; Vulić, I.; Stojanović, G.M.; Simić, M.; Ostojić, G.; Stankovski, S. Numerical feature selection and hyperbolic tangent feature scaling in machine learning-based detection of anomalies in the computer network behavior. Electronics 2023, 12, 4158. [Google Scholar] [CrossRef]
- Ali, Y.A.; Awwad, E.M.; Al-Razgan, M.; Maarouf, A. Hyperparameter search for machine learning algorithms for optimizing the computational complexity. Processes 2023, 11, 349. [Google Scholar] [CrossRef]
- Kiliçarslan, S. PSO+ GWO: A hybrid particle swarm optimization and Grey Wolf optimization based Algorithm for fine-tuning hyper-parameters of convolutional neural networks for Cardiovascular Disease Detection. J. Ambient Intell. Humaniz. Comput. 2023, 14, 87–97. [Google Scholar] [CrossRef]
- Novac, O.C.; Chirodea, M.C.; Novac, C.M.; Bizon, N.; Oproescu, M.; Stan, O.P.; Gordan, C.E. Analysis of the application efficiency of TensorFlow and PyTorch in Convolutional Neural Network. Sensors 2022, 22, 8872. [Google Scholar] [CrossRef]
- Hanin, B. Universal function approximation by deep neural nets with bounded width and relu activations. Mathematics 2019, 7, 992. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, Y.; Ge, Z.; Mu, H.; Qi, D.; Ni, H. Transfer learning for leaf small dataset using improved ResNet50 network with mixed activation functions. Forests 2022, 13, 2072. [Google Scholar] [CrossRef]
- Wang, X.; Ren, H.; Wang, A. Smish: A novel activation function for deep learning methods. Electronics 2022, 11, 540. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Song, Y.; Rong, X. The influence of the activation function in a convolution neural network model of facial expression recognition. Appl. Sci. 2020, 10, 1897. [Google Scholar] [CrossRef]
- Truong, T.X.; Nhu, V.H.; Phuong, D.T.N.; Nghi, L.T.; Hung, N.N.; Hoa, P.V.; Bui, D.T. A New Approach Based on TensorFlow Deep Neural Networks with ADAM Optimizer and GIS for Spatial Prediction of Forest Fire Danger in Tropical Areas. Remote Sens. 2023, 15, 3458. [Google Scholar] [CrossRef]
- Hayatbini, N.; Kong, B.; Hsu, K.l.; Nguyen, P.; Sorooshian, S.; Stephens, G.; Fowlkes, C.; Nemani, R.; Ganguly, S. Conditional generative adversarial networks (cGANs) for near real-time precipitation estimation from multispectral GOES-16 satellite imageries—PERSIANN-cGAN. Remote Sens. 2019, 11, 2193. [Google Scholar] [CrossRef]
- Tran, T.H.G.; Ressl, C.; Pfeifer, N. Integrated change detection and classification in urban areas based on airborne laser scanning point clouds. Sensors 2018, 18, 448. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Hu, F.; Nian, G.; Yang, T. Deep residual learning for nonlinear regression. Entropy 2020, 22, 193. [Google Scholar] [CrossRef] [PubMed]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R.; Taylor, J. Linear regression. In An Introduction to Statistical Learning: With Applications in Python; Springer: London, UK, 2023; pp. 69–134. [Google Scholar]
- Barootchi, S.; Tavelli, L.; Majzoub, J.; Stefanini, M.; Wang, H.L.; Avila-Ortiz, G. Alveolar ridge preservation: Complications and cost-effectiveness. Periodontol. 2000 2023, 92, 235–262. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huo, X. A survey of numerical algorithms that can solve the Lasso problems. Wiley Interdiscip. Rev. Comput. Stat. 2023, 15, e1602. [Google Scholar] [CrossRef]
- Reddy, P.S.M. Decision tree regressor compared with random forest regressor for house price prediction in mumbai. J. Surv. Fish. Sci. 2023, 10, 2323–2332. [Google Scholar]
- Setiawan, A.; Utami, E.; Ariatmanto, D. Cattle Weight Estimation Using Linear Regression and Random Forest Regressor. J. RESTI (Rekayasa Sist. Dan Teknol. Inf.) 2024, 8, 72–79. [Google Scholar] [CrossRef]
- Rao, N.S.S.V.S.; Thangaraj, S.J.J.; Kumari, V.S. Flight Ticket Prediction Using Gradient Boosting Regressor Compared with Linear Regression. In Proceedings of the 2023 Eighth International Conference on Science Technology Engineering and Mathematics (ICONSTEM), Chennai, India, 6–7 April 2023; IEEE: New York, NY, USA, 2023; pp. 1–6. [Google Scholar]
- Luo, C.; Zhu, S.P.; Keshtegar, B.; Niu, X.; Taylan, O. An enhanced uniform simulation approach coupled with SVR for efficient structural reliability analysis. Reliab. Eng. Syst. Saf. 2023, 237, 109377. [Google Scholar] [CrossRef]
- Maqbool, J.; Aggarwal, P.; Kaur, R.; Mittal, A.; Ganaie, I.A. Stock prediction by integrating sentiment scores of financial news and MLP-regressor: A machine learning approach. Procedia Comput. Sci. 2023, 218, 1067–1078. [Google Scholar] [CrossRef]
- Fang, X.; Vitrac, O. Predicting diffusion coefficients of chemicals in and through packaging materials. Crit. Rev. Food Sci. Nutr. 2017, 57, 275–312. [Google Scholar] [CrossRef] [PubMed]
- Tyn, M.T.; Gusek, T.W. Prediction of diffusion coefficients of proteins. Biotechnol. Bioeng. 1990, 35, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jin, S.; Bai, D.; Fan, Y.; Shi, H.; Fernandez, C. A critical review of improved deep learning methods for the remaining useful life prediction of lithium-ion batteries. Energy Rep. 2021, 7, 5562–5574. [Google Scholar] [CrossRef]
- Medsker, L.R.; Jain, L. Recurrent neural networks. Des. Appl. 2001, 5, 2. [Google Scholar]
- Donyavi, Z.; Asadi, S. Diverse training dataset generation based on a multi-objective optimization for semi-supervised classification. Pattern Recognit. 2020, 108, 107543. [Google Scholar] [CrossRef]
- Bennemann, C.; Paul, W.; Baschnagel, J.; Binder, K. Investigating the influence of different thermodynamic paths on the structural relaxation in a glass-forming polymer melt. J. Phys. Condens. Matter 1999, 11, 2179. [Google Scholar] [CrossRef]
- Deng, J.; Wu, J.; Zhang, S.; Li, W.; Wang, Y.G. Physical informed neural networks with soft and hard boundary constraints for solving advection-diffusion equations using Fourier expansions. Comput. Math. Appl. 2024, 159, 60–75. [Google Scholar]
- Pajot, A. Incorporating Physical Knowledge into Deep Neural Network. Ph.D. Thesis, Sorbonne Université, Paris, France, 2019. [Google Scholar]
- Wang, R.; Xu, X.; Zhang, Y. Multiscale Information Diffusion Prediction with Minimal Substitution Neural Network. IEEE Trans. Neural Netw. Learn. Syst. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Croitoru, F.A.; Hondru, V.; Ionescu, R.T.; Shah, M. Diffusion models in vision: A survey. IEEE Trans. Pattern Anal. Mach. Intell. 2023, 45, 10850–10869. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, P.; Takyi-Aninakwa, P.; Jin, S.; Fernandez, C. A critical review of improved deep convolutional neural network for multi-timescale state prediction of lithium-ion batteries. Energies 2022, 15, 5053. [Google Scholar] [CrossRef]
- Krapivin, M.; Autaeu, A.; Marchese, M. Large Dataset for Keyphrases Extraction; University of Trento: Trento, Italy, 2009. [Google Scholar]

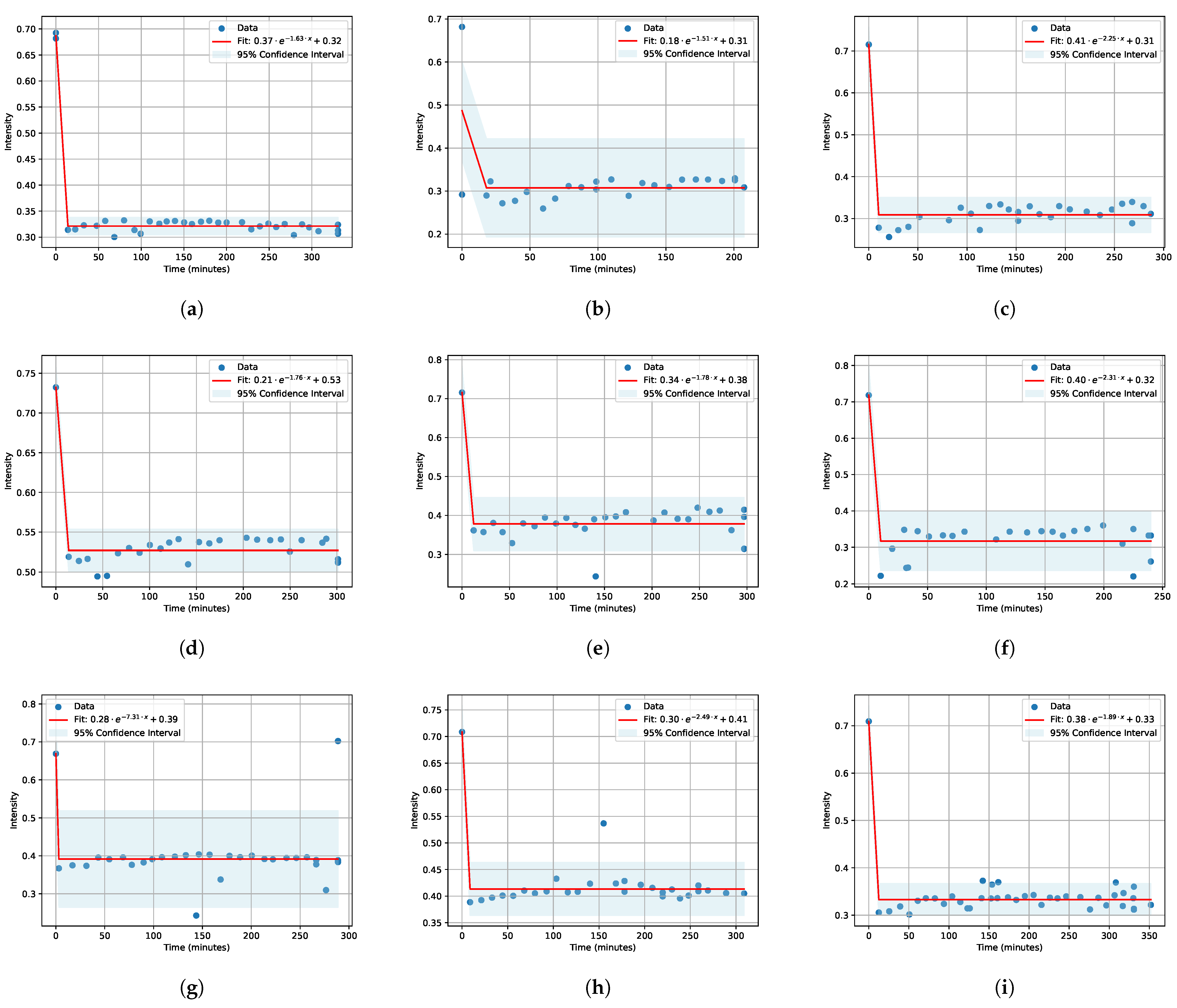
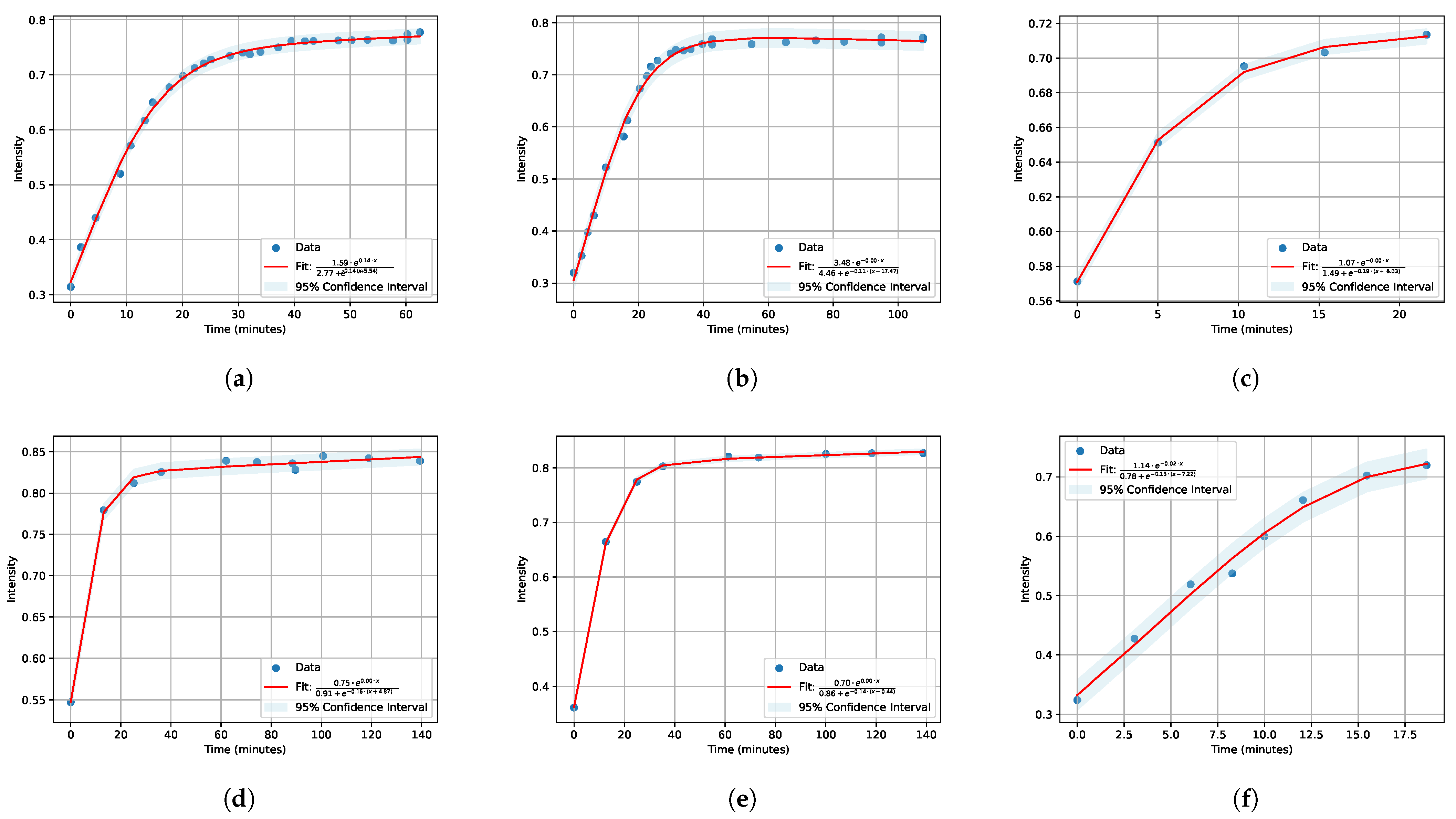


| Salt | Concentration | A | B | C |
|---|---|---|---|---|
| LiCl | 0.1 M 0.01 M 0.001 M | 0.37 0.18 0.41 | −1.63 −1.51 −2.25 | 0.32 0.31 0.31 |
| KCl | 0.1 M 0.01 M 0.001 M | 0.21 0.34 0.40 | −1.76 −1.78 −2.31 | 0.53 0.38 0.32 |
| NaCl | 0.1 M 0.01 M 0.001 M | 0.28 −7.31 0.39 | 0.3 −2.49 0.41 | 0.38 −1.89 0.33 |
| Salt | Concentration | |||||
|---|---|---|---|---|---|---|
| LiCl | 0.1 M 0.01 M 0.001 M | 1.59 3.48 1.07 | 0.14 0 0 | 2.77 4.46 1.49 | 0.14 −0.11 −0.19 | −5.54 −17.47 −5.03 |
| KCl | 0.1 M 0.01 M 0.001 M | 0.75 0.7 1.14 | 0 0 −0.02 | 0.91 0.86 0.78 | −0.16 −0.14 −0.13 | −4.87 −0.44 −7.22 |
| Real A | Real B | Predicted A | Predicted B |
|---|---|---|---|
| 0.41 | −2.25 | 0.36 | −2.32 |
| 0.34 | −1.78 | 0.35 | −1.42 |
| Regressor | A | B |
|---|---|---|
| LinearRegression | 0.825698 | 0.011197 |
| Ridge | 0.048564 | 0.127635 |
| Lasso | 0.265159 | 0.589168 |
| DecisionTreeRegressor | 0.012850 | 2.426850 |
| RandomForestRegressor | 0.138267 | 0.145108 |
| GradientBoostingRegressor | 0.002541 | 0.545394 |
| SVR | 0.012887 | 0.134859 |
| MLPRegressor | 0.056801 | 0.514195 |
| Regressor | |||||
|---|---|---|---|---|---|
| Linear Regression [70] | 0.135808 | 0.000677 | 0.272925 | 0.016502 | 17.032002 |
| Ridge [71] | 0.146546 | 0.000236 | 0.445721 | 0.009340 | 17.608812 |
| Lasso [72] | 0.526937 | 0.002125 | 1.763845 | 0.003050 | 14.911644 |
| Decision Tree Regressor [73] | 0.070900 | 0.000200 | 0.009700 | 0.000650 | 12.932100 |
| Random Forest Regressor [74] | 0.103242 | 0.002025 | 0.992413 | 0.002571 | 15.126330 |
| Gradient Boosting Regressor [75] | 0.304507 | 0.000200 | 0.207288 | 0.001429 | 11.885889 |
| SVR [76] | 0.177954 | 0.006500 | 1.016009 | 0.005524 | 13.676221 |
| MLPRegressor [77] | 0.057762 | 0.055338 | 0.031318 | 0.067580 | 9.837034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malashin, I.; Daibagya, D.; Tynchenko, V.; Gantimurov, A.; Nelyub, V.; Borodulin, A. Predicting Diffusion Coefficients in Nafion Membranes during the Soaking Process Using a Machine Learning Approach. Polymers 2024, 16, 1204. https://doi.org/10.3390/polym16091204
Malashin I, Daibagya D, Tynchenko V, Gantimurov A, Nelyub V, Borodulin A. Predicting Diffusion Coefficients in Nafion Membranes during the Soaking Process Using a Machine Learning Approach. Polymers. 2024; 16(9):1204. https://doi.org/10.3390/polym16091204
Chicago/Turabian StyleMalashin, Ivan, Daniil Daibagya, Vadim Tynchenko, Andrei Gantimurov, Vladimir Nelyub, and Aleksei Borodulin. 2024. "Predicting Diffusion Coefficients in Nafion Membranes during the Soaking Process Using a Machine Learning Approach" Polymers 16, no. 9: 1204. https://doi.org/10.3390/polym16091204
APA StyleMalashin, I., Daibagya, D., Tynchenko, V., Gantimurov, A., Nelyub, V., & Borodulin, A. (2024). Predicting Diffusion Coefficients in Nafion Membranes during the Soaking Process Using a Machine Learning Approach. Polymers, 16(9), 1204. https://doi.org/10.3390/polym16091204







