3.1. Morphological Analysis of Sn-MWCNT Composites
The impact of MWCNTs with distinct functional groups on the morphology of Ag-MWCNT composites was assessed by comparing the microstructures of Ag-MWCNT composites derived from untreated MWCNTs, carboxylated MWCNTs, and hydroxylated MWCNTs. SDS served as a dispersant while sodium borohydride ethanol solution acted as a reducing agent. As shown in
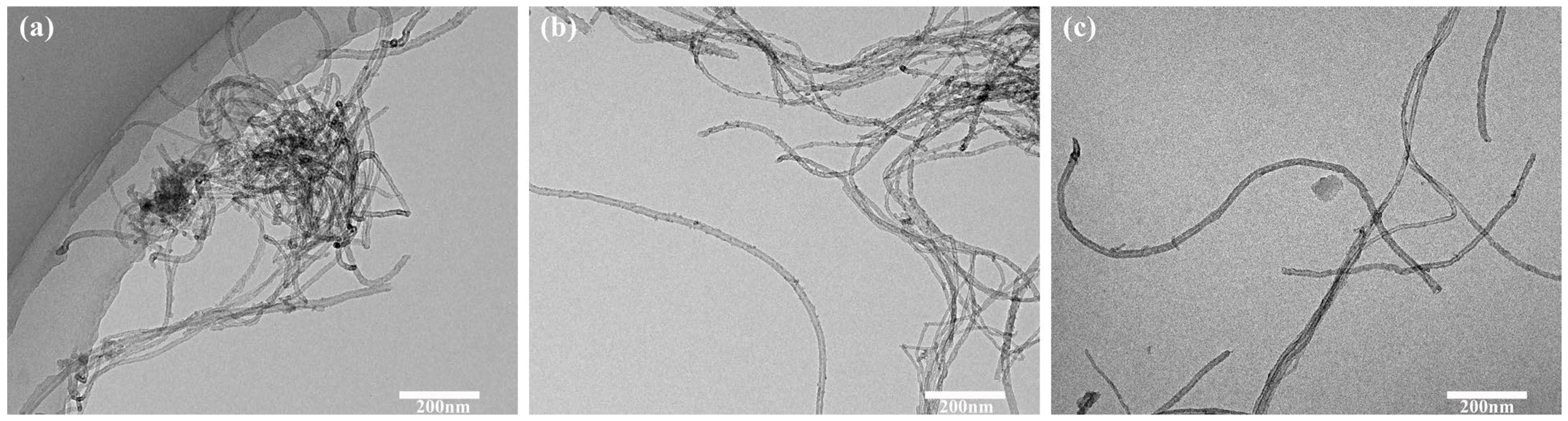
Figure 1a, Sn-MWCNT composites obtained from untreated MWCNTs exhibited lower surface loading of tin nanoparticles, presenting a smooth state primarily, and numerous carbon nanotubes were aggregated together. Due to the inherent stability of the surface of non-functionalized carbon nanotubes, it is difficult for metal ions to attach to the surface without active sites, which affects the loading efficiency of silver nanoparticles. The successful loading of tin nanoparticles onto the surface of functionalized MWCNTs is evident from
Figure 1b,c. However, the carboxylated MWCNTs exhibited a higher loading rate of tin nanoparticles compared to hydroxylated MWCNTs, with a reduced amount of free tin. The functionalization process rendered the surface of MWCNTs more reactive, facilitating the effective attachment of other atoms or groups. Although both carboxyl and hydroxyl groups on the surface of carbon nanotubes could form stable complexes with metal ions, carboxyl groups possess an additional adjacent double-bond oxygen compared to hydroxyl groups. Therefore, one oxygen atom is utilized when forming a complex between hydroxyl groups and metal ions, while carboxyl groups can provide two oxygen atoms. Moreover, in general, complexes formed by carboxyl groups may be more stable, resulting in denser silver deposition on the MWCNT surface during subsequent reduction and directly affecting the loading rate of silver nanoparticles on carbon nanotubes.
The MWCNTs exhibited hydrophobic properties, and the presence of strong van der Waals forces promoted their aggregation into clusters or polymers within the system, significantly influencing the loading behavior of tin nanoparticles and silver nanoparticles. Therefore, for the preparation of Sn-MWCNT composites, dispersants should be incorporated into the system for MWCNT treatment, while the non-covalent surface modification of carbon nanotubes was essential to facilitate their disaggregation, ensuring the homogeneous dispersion of carbon nanotubes and preserving their original aspect ratio and electronic structure. SDS and SDBS were utilized as dispersants during the preparation of Sn-MWCNT composites.
As illustrated in
Figure 2, the dispersion effect of Sn-MWCNTs prepared with SDS dispersant was significantly superior to that of the other two. SDS and SDBS were two commonly employed anionic surfactants, frequently utilized for the dispersion of carbon nanotubes. Anionic surfactants could facilitate the dispersal of carbon nanotubes by dissociating the negatively charged groups on their surface, thereby promoting effective disaggregation and uniform dispersion within the system. The SDBS dispersant exhibited superior performance compared to SDS in the synthesis of Ag-MWCNTs through the direct reduction of AgNO
3. Specifically, the benzene ring structure in SDBS could form specific π-π stacking interactions with carbon nanotubes, resulting in non-covalent bond formation, which effectively disaggregated carbon nanotubes and prevented agglomeration in the solution. However, in the experiment, the addition of stannous chloride to MWCNTs was chosen for the synthesis of Ag-MWCNTs through reduction and substitution processes. Upon adding stannous chloride, a significant amount of chloride ions were present in the solution, resulting in the sulfonic acid in SDBS reacting with these chloride ions, finally leading to a considerable decrease in the dispersion ability of SDBS. Consequently, the agglomeration of carbon nanotubes within the system also impacted the loading rate of metal on the surface of MWCNTs.
3.2. Morphology Analysis and Characterization of Ag-MWCNT Composites
The quantity and morphology of silver nanoparticles on the surface of carbon nanotubes were influenced by the addition of dispersants, functional groups, and stannous chloride. The investigation was conducted to examine the impact of stannous chloride addition on the morphology of Ag-MWCNT composites. Four experiments were conducted for comparison, with varying amounts of stannous chloride added: 0.2 g, 0.5 g, 1 g, and 1.5 g. The inclusion of other substances remained unaltered, with the exception of the incorporation of stannous chloride.
As illustrated in
Figure 3, when the addition amount of stannous chloride is 0.2 g, the silver content in the system is relatively low. Despite the small particle size of silver nanoparticles on carbon nanotube surfaces, the loading quantity of silver nanoparticles remained insufficient to effectively enhance the electrical and thermal conductivity of the paste in subsequent usage. On the other hand, with stannous chloride contents of 1 g and 1.5 g, a higher loading quantity of silver nanoparticles onto the carbon nanotubes’ surface was achieved, which resulted in larger silver nanoparticles along with increased free silver ions. Consequently, when the replacement reaction occurred, tin predominantly existed in stable +4 ionic form, and there was a rapid expansion in silver content leading to the significant agglomeration of silver nanoparticles within the system. Conversely, when stannous chloride content was too low, reduced tin concentration failed to support substantial loading of silver nanoparticles on the surface of carbon nanotubes. Especially after reducing agent addition, tin ion concentration rapidly decreased, making the reduction reaction more challenging. Based on these findings, it was concluded that Ag-MWCNTs prepared using an addition amount of stannous chloride of 0.5 g would be utilized for subsequent analysis.
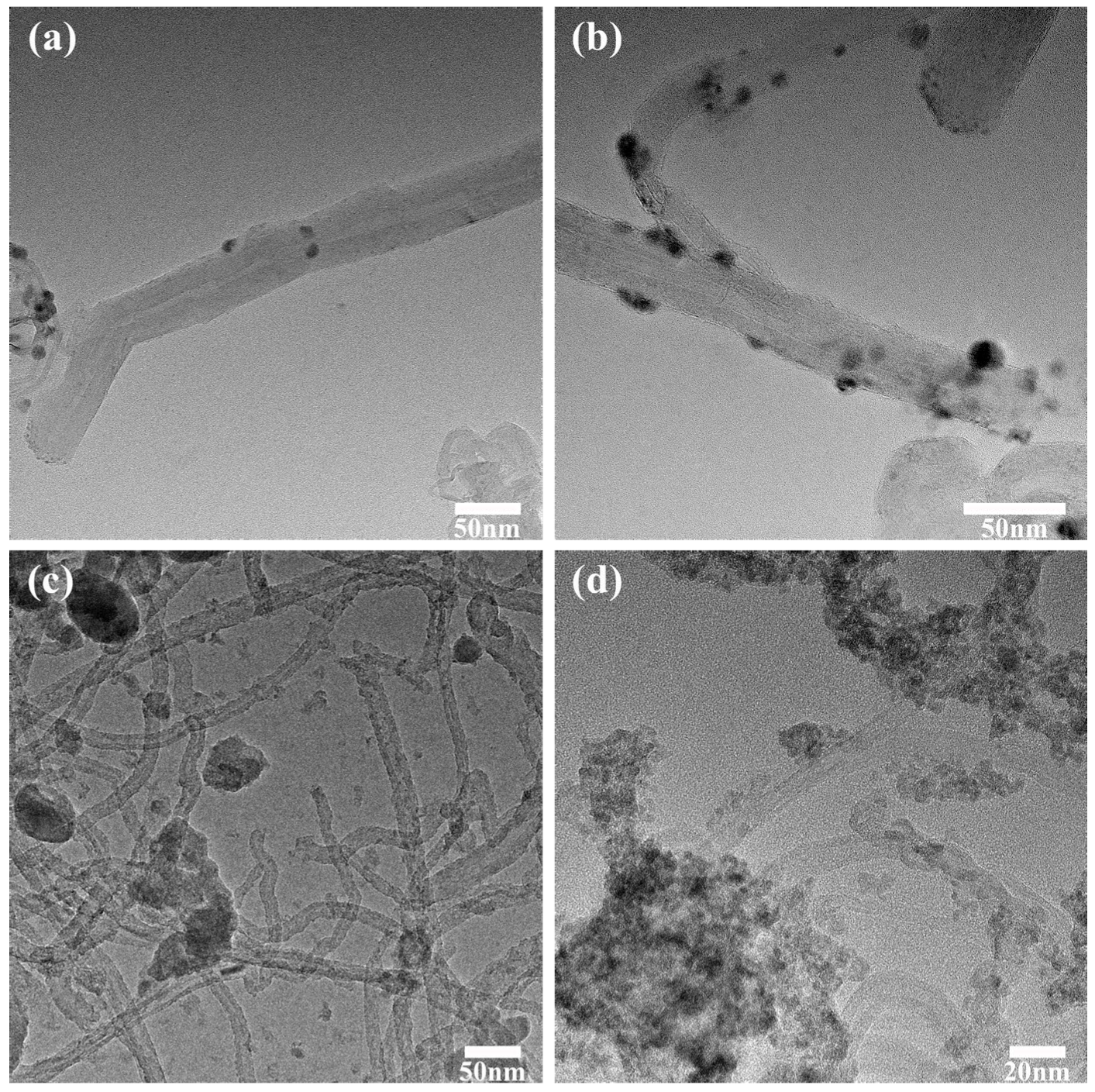
The Energy Dispersive Spectrometer (EDS) was primarily utilized for the elemental analysis of minuscule regions within materials. In this study, energy spectrum analysis was performed on the silver nanoparticles, as depicted in
Figure 4, notably, a trace amount of tin nanoparticles was detected within the silver nanoparticles loaded onto the surface of carbon nanotubes, which was attributed to the preferential reactivity of tin atoms on the surface during the replacement reaction. With the reaction progressing, silver atoms gradually loaded the tin surface, hindering further contact between internal tin atoms and the silver nitrate solution. Therefore, a small amount of tin atoms remained within the silver structure, accounting for approximately 8% of total metal atoms, the influence of which on overall performance was negligible.
The successful formation of silver and tin nanoparticles on the surface of carbon nanotubes was characterized using X-ray diffraction (XRD). XRD images of carboxylated MWCNTs, MWCNTs loaded with tin nanoparticles, and MWCNTs loaded with silver nanoparticles are depicted in
Figure 5. The XRD results of carboxylated MWCNTs are depicted in curve (a), wherein the dominant peak corresponds to the crystal plane (002) of the carbon nanotubes. The XRD patterns reveal that the peaks observed at 2θ = 30.64°, 32.00°, 43.84°, and 44.06° in curve (b) correspond to the crystal planes of Sn indexed as (200), (101), (220), and (211), respectively, confirming the presence of Sn-MWCNTs. The peak of tin exhibits sharpness without any additional impurity peaks, indicating the excellent crystallinity of tin and the absence of the oxidation of tin nanoparticles on the surface of carbon nanotubes. The aforementioned favorable condition facilitates subsequent silver replacement reactions occurring on the carbon tube surface. The XRD patterns of Ag-MWCNTs are presented in curve (c). Upon comparing the XRD cards, it can be observed that the peaks at 2θ = 38.08°, 44.24°, 64.44°, and 77.38° correspond to the crystallographic planes (111), (200), (220), and (311), respectively. Notably, no distinct peak of tin is detected in the spectrum, indicating the successful replacement of tin and the absence of silver oxidation. The sharp peak indicates enhanced silver crystallinity, and the grain crystallinity was estimated by fitting the half-peak width. The results showed that the final crystallinity of silver nanotubes on carbon nanotube surfaces reached 72.31%. By comparing the XRD results of Ag-MWCNTs and Sn-MWCNTs, it was evident that the peak of silver significantly surpassed that of tin due to a valence state issue. Tin tended to replace more silver, resulting in a higher content of silver than tin and an elevated silver peak.
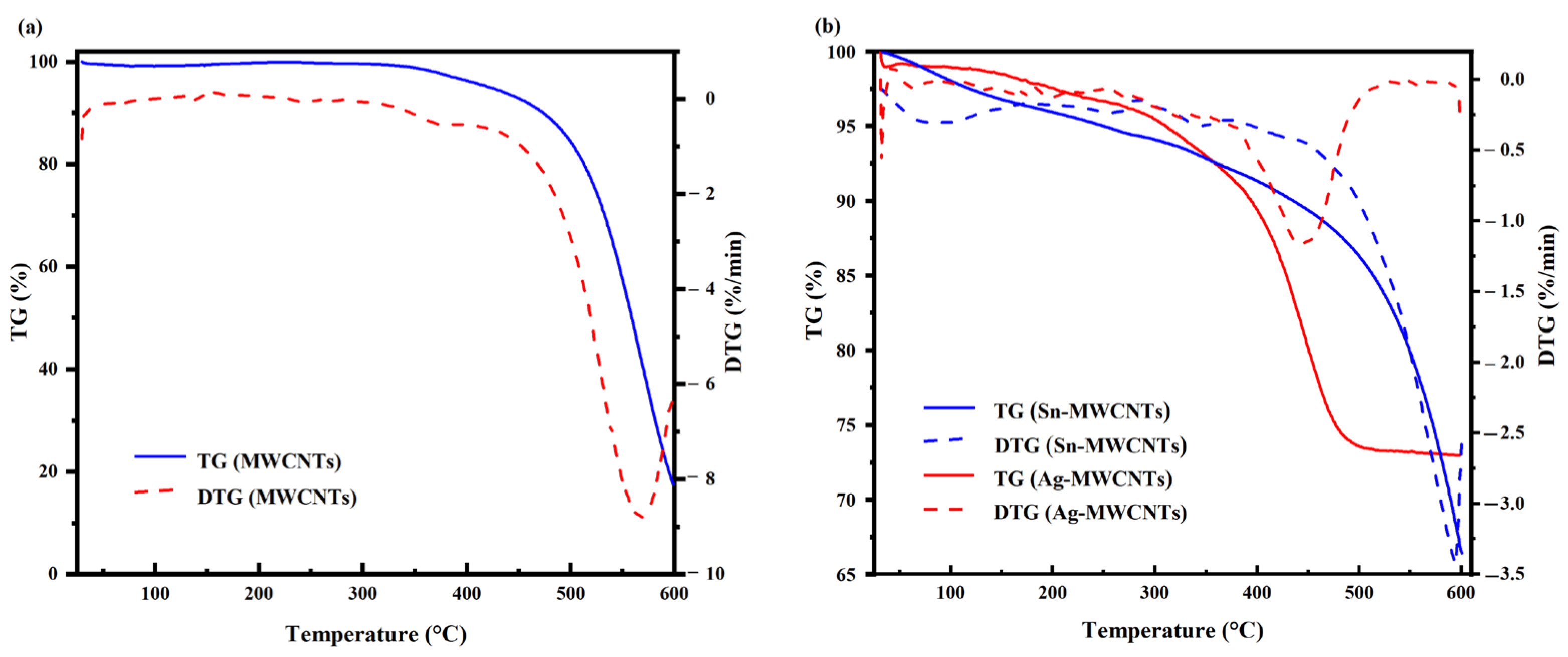
The decomposition temperature of carbon nanotubes was characterized by thermogravimetric (TG) analysis. TG analysis was performed separately on carboxylated MWCNTs, Sn-MWCNTs, and Ag-MWCNTs. As the TG results show in
Figure 6a for carboxylated MWCNTs, the decomposition of carbon nanotubes initiated at approximately 330 °C, with an accelerated rate at 420 °C and reaching its maximum value at 567 °C. According to the TG result comparison presented in
Figure 6b, it can be observed that the carbon nanotubes loaded with silver exhibited an onset of decomposition at a temperature of 256 °C, followed by a subsequent increase in decomposition rate, reaching its maximum at 445 °C. When the temperature reached 290 °C, the tin-loaded carbon nanotubes exhibited decomposition, with the decomposition rate peaking at 595 °C. By comparing the initial decomposition temperature, it can be inferred that metal loading on the surface of carbon nanotubes induced structural damage. Although metal loading reduced the decomposition temperature, Ag-MWCNTs exhibited a significantly higher decomposition temperature than the paste curing temperature and did not cause any damage to the carbon nanotubes during curing.
The DSC method was carried out to analyze the silver melting point on the surface of carbon nanotubes, which can be utilized for creating an electrical and thermal conductive pathway by combining carbon nanotubes with silver powder in paste. As shown in
Figure 7, there is a significant endothermic peak at 36 °C, which may be related to the volatilization of the solvent. With increasing temperature, subsequent smaller sawtooth-shaped heat absorption peaks emerged, corresponding to varying particle sizes of nanosilver within the system.
3.3. Analysis of Modified Paste
TG analysis was also performed on the pastes containing Ag-MWCNTs, and the results are presented in
Figure 8. As the temperature increased, a slight decrease in weight was observed initially, which can be attributed to water evaporation. Upon reaching the curing temperature of 180 °C, no further change in weight occurred, indicating that the structure of paste remained intact within this temperature range. However, as the temperature reached 210 °C, a continuous decrease in weight was observed, suggesting decomposition of a portion of the epoxy resin. When the temperature reached 355 °C, the decomposition rate of the paste peaked. As the temperature continued to rise, the epoxy resin underwent substantial decomposition at around 400 °C, and its decomposition rate gradually approached zero. Ultimately, at 500 °C, a residual mass of 73.78% remained.
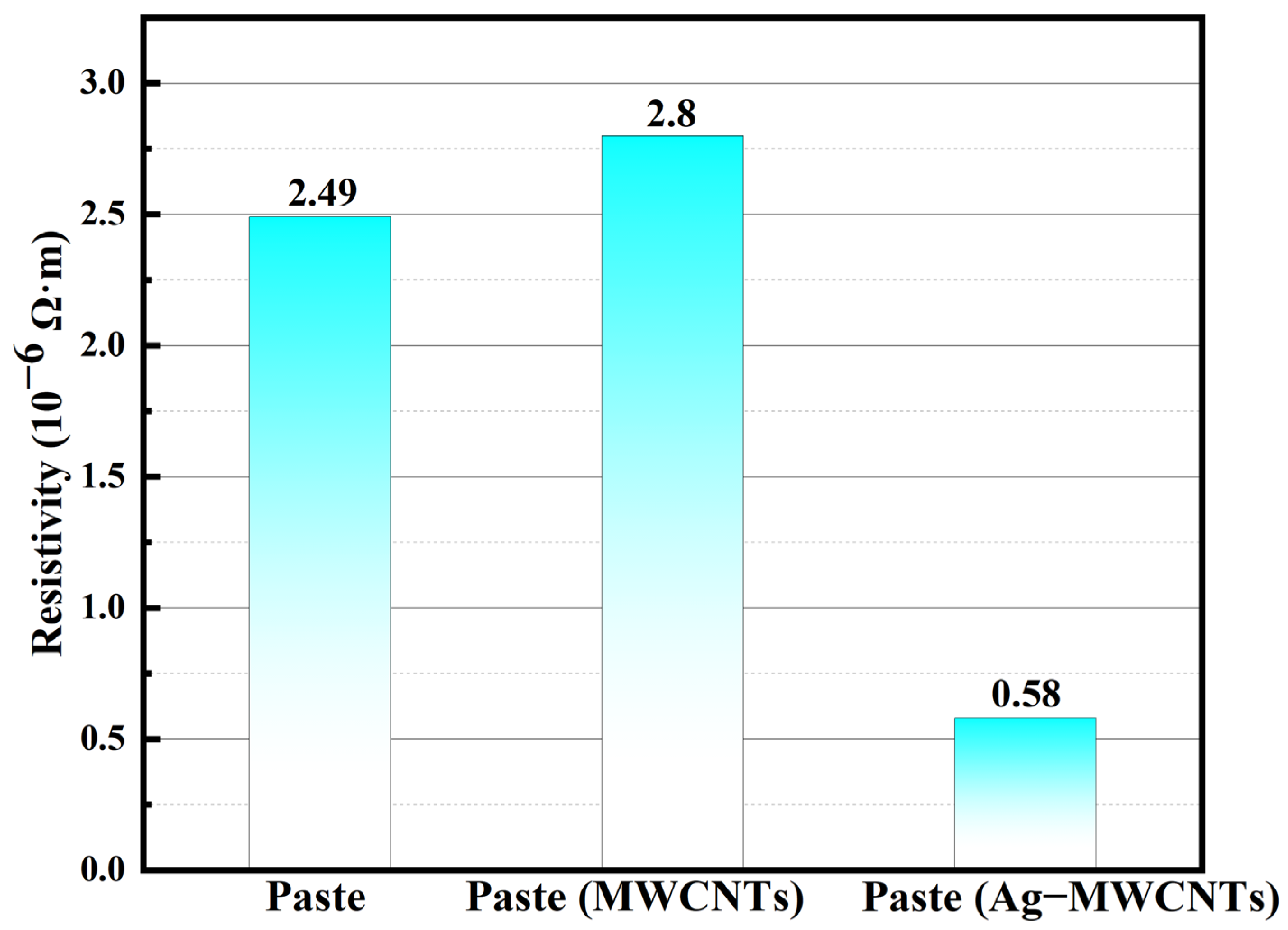
The resistivity tests were conducted on the initial paste, as well as the modified pastes containing carboxylated MWCNTs and Ag-MWCNTs, with both additives added at a concentration of 1%. As depicted in
Figure 9, the inclusion of carboxylated MWCNTs had a negligible impact on the overall resistivity of the paste, whereas the incorporation of Ag-MWCNTs significantly reduced its resistivity. This phenomenon can be attributed to the strong van der Waals forces between carbon nanotubes in paste and the high aspect ratio of carbon nanotubes. Therefore, upon addition to the system, carbon nanotubes tended to entangle and aggregate, creating substantial barriers for effective contact between flaky silver powder particles within the paste. In contrast to carboxylated MWCNTs, the presence of silver-nanoparticle-functionalized Ag-MWCNTs resulted in increased steric hindrance, thereby reducing the possibility of carbon nanotube entanglement and reaggregation. Additionally, the sintering of silver nanoparticles on the surface of carbon nanotubes with sheet-like silver powder in the paste facilitated the formation of more conductive pathways without requiring high temperatures, effectively harnessing the inherent high conductivity of carbon nanotubes.
Furthermore, the impact of incorporating Ag-MWCNTs on the resistivity of the modified paste was investigated. The alterations in resistivity for the initial paste containing 0.5%, 1%, 1.5%, and 2% Ag-MWCNT composites are depicted in
Figure 10. The results indicate that the addition amount of Ag-MWCNT composite material did not exhibit a linear relationship with the resistivity of the modified paste. Based on a series of experiments, it was found that when 1% of Ag-MWCNT composite material was added, the conductivity of the paste reached its maximum improvement. For further investigations on the thermal conductivity and shear strength of the modified paste, a 1% addition of Ag-MWCNTs in the modified paste was utilized.
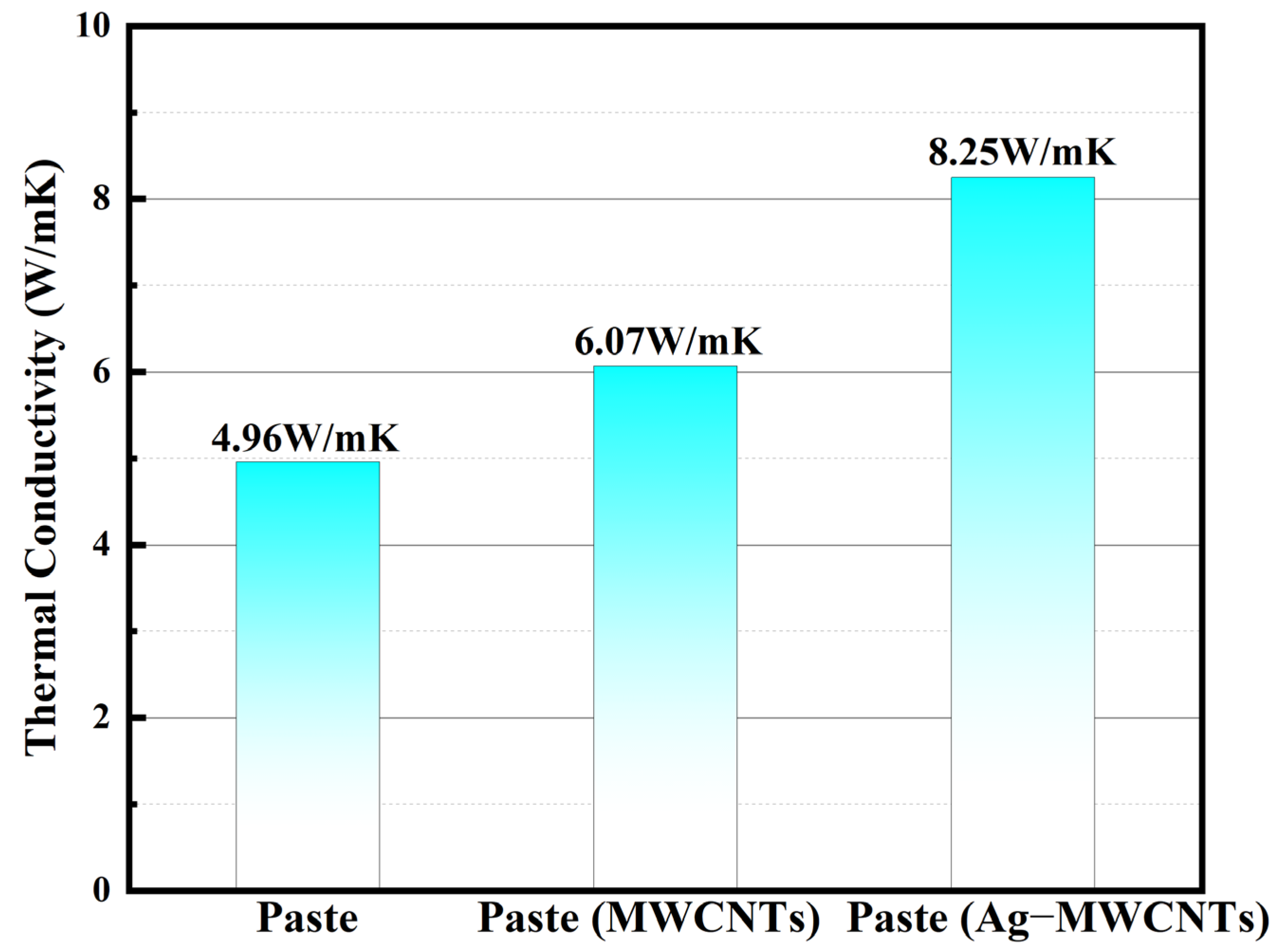
The comparative analysis of the thermal conductivity between the original paste, the modified paste incorporating carboxylated MWCNTs, and Ag-MWCNTs was conducted. As shown in
Figure 11, the thermal conductivity values for the original paste, MWCNT-enhanced paste, and Ag-MWCNT-enhanced paste were measured as 4.96 W/mK, 6.07 W/mK, and 8.25 W/mK, respectively. The observed enhancement in thermal conductivity for the Ag-MWCNT-enhanced paste can be primarily attributed to the presence of silver nanoparticles on the surface of carbon nanotubes. The thermal conductivity of conventional paste primarily relied on the heat transfer occurring between silver powders. However, when carbon nanotubes were introduced into the paste, their exceptional thermal conductivity was not fully utilized due to the inadequate contact with the sheet-like silver powders in the paste. By incorporating silver nanoparticles onto the surface of carbon tubes, a solid phonon transmission junction and an efficient phonon transmission channel can be established during the curing process, facilitating the contact between carbon tubes and silver powder in the paste. This approach reduces the carrier transmission barrier between fillers and enhances carrier interface transmission efficiency. In contrast, carbon nanotubes can serve as an effective thermal conductive bridge between silver powders, facilitating the formation of multiple pathways for efficient heat transfer. With heat absorption, silver nanoparticles located on the surface of carbon nanotubes exhibited rapid migration to the carbon nanotube structure, which was attributed to the exceptional phonon average free path exhibited by carbon nanotubes, enabling swift and widespread dispersion of thermal energy.
Shear strength tests on the initial paste and the modified paste with Ag-MWCNTs were also performed. As depicted in
Figure 12, the addition of Ag-MWCNT composite material led to a significant enhancement in the shear strength of the paste, specifically increasing from 7.82 MPa to 9.01 MPa. The distinct improvement can be attributed to two primary influencing factors: firstly, through sintering, silver functionalized nanotubes adhered firmly onto the surface of carbon nanotubes and flaky silver powder in the paste, resulting in a strong bond between carbon nanotubes and the paste; secondly, owing to their inherent high strength properties, carbon nanotubes themselves contributed to enhancing the shear strength of the modified paste. On the other hand, the incorporation of carbon nanotubes can enhance epoxy resin reactivity, elevate epoxy resin crosslinking degree, and hence improve shear strength.