Computational Fluid Dynamics Analysis and Empirical Evaluation of Carboxymethylcellulose/Alginate 3D Bioprinting Inks for Screw-Based Microextrusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials for Bioprinting
2.2. Configuration of Screw-Based Dispenser System
2.3. Preparation of Bioinks
2.4. Rheological Characterization
2.5. Model Design for CFD
2.6. Governing Equations
2.7. Analysis Conditions
2.8. Empirical Evaluation of Mass Flow Rate
2.9. Cell Viability Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Rheological Properties
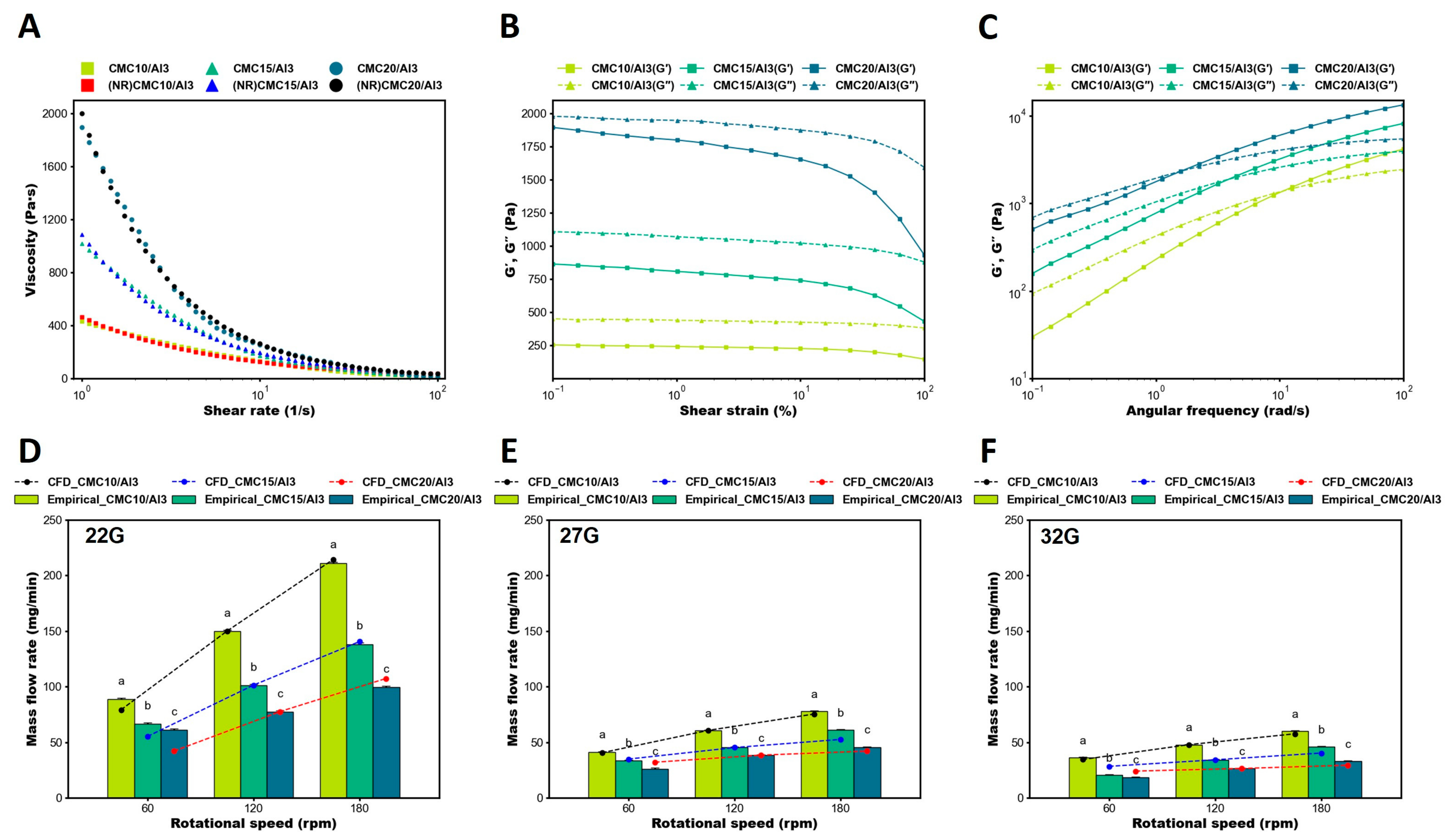
3.2. Comparison of Empirical Evaluation and CFD Analysis in Mass Flow Rate
3.3. Flow Field of CFD Analysis
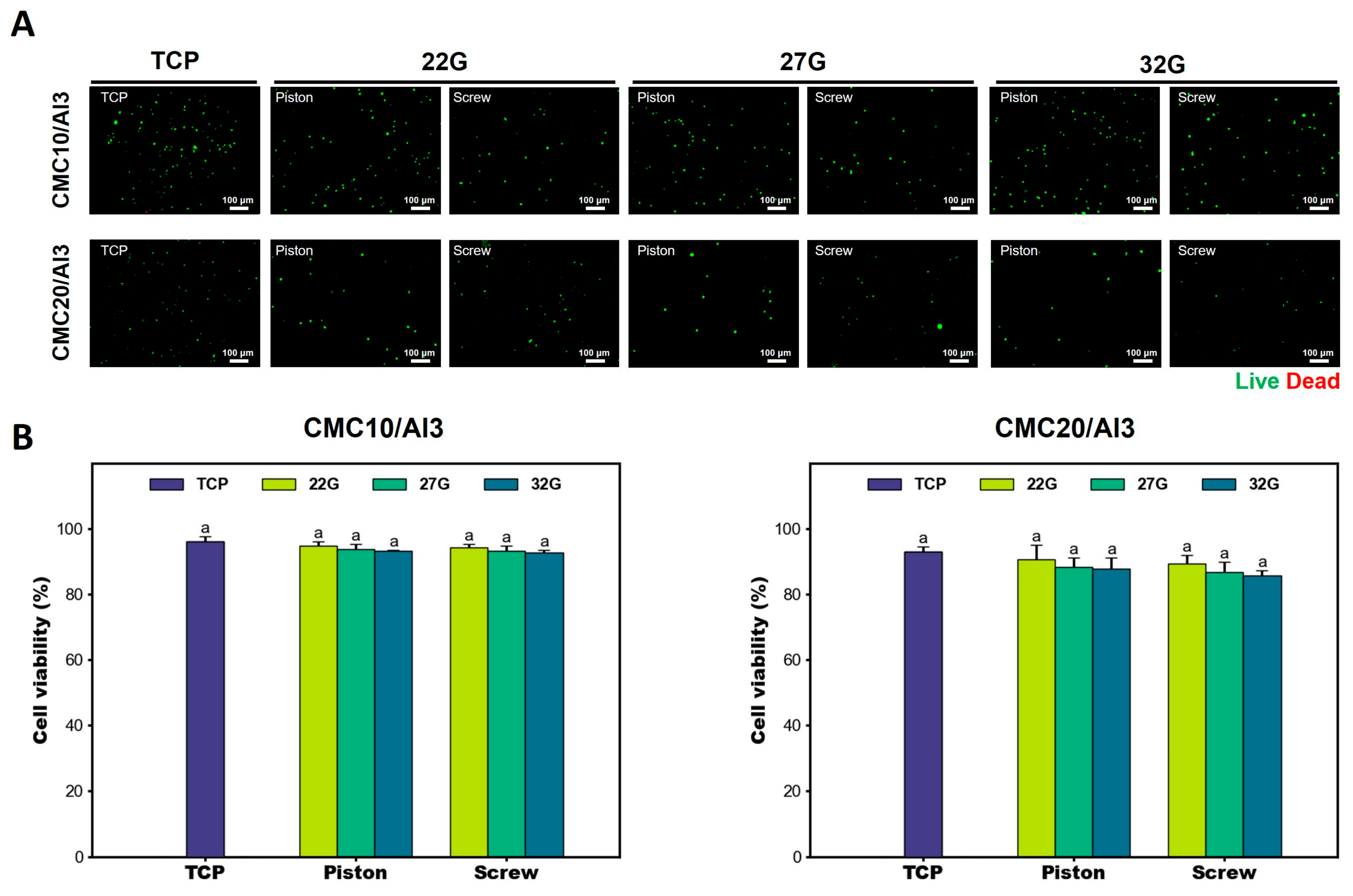
3.4. Cell Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panda, S.; Hajra, S.; Mistewicz, K.; Nowacki, B.; In-Na, P.; Krushynska, A.; Mishra, Y.K.; Kim, H.J. A focused review on three-dimensional bioprinting technology for artificial organ fabrication. Biomater. Sci. 2022, 10, 5054–5080. [Google Scholar] [CrossRef]
- Ferris, C.J.; Gilmore, K.J.; Beirne, S.; McCallum, D.; Wallace, G.G. Bio-ink for on-demand printing of living cells. Biomater. Sci. 2013, 1, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.; Kim, J.; Ji, M.; Cho, E.; Sim, H.B.; Chang, Y.; Chung, J.H.; Paik, M.; Kim, J.; et al. Osseointegrative and immunomodulative 3D-Printing Ti6Al4V-based implants embedded with biogenic hydroxyapatite. Mater. Des. 2024, 240, 112822. [Google Scholar] [CrossRef]
- De Mori, A.; Peña Fernández, M.; Blunn, G.; Tozzi, G.; Roldo, M. 3D printing and electrospinning of composite hydrogels for cartilage and bone tissue engineering. Polymers 2018, 10, 285. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Liu, Y.; Hamid, Q.; Snyder, J.; Wang, C.; Sun, W. Evaluating fabrication feasibility and biomedical application potential of in situ 3D printing technology. Rapid Prototyp. J. 2016, 22, 947–955. [Google Scholar] [CrossRef]
- Gurlin, R.E.; Giraldo, J.A.; Latres, E. 3D bioprinting and translation of beta cell replacement therapies for type 1 diabetes. Tissue Eng. Part B Rev. 2021, 27, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Medical applications for 3D printing: Current and projected uses. Pharm. Ther. 2014, 39, 704. [Google Scholar]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Ho, C.M.B.; Ng, S.H.; Yoon, Y. A review on 3D printed bioimplants. Int. J. Precis. Eng. Manuf. 2015, 16, 1035–1046. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Ahadian, S.; Xu, C.; Montazerian, H.; Ko, H.; Nasiri, R.; Barros, N.; Khademhosseini, A. Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Mater. Today Bio 2019, 1, 100008. [Google Scholar] [CrossRef] [PubMed]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Ng, W.L.; Yeong, W.Y. Resolution and shape in bioprinting: Strategizing towards complex tissue and organ printing. Appl. Phys. Rev. 2019, 6, 011307. [Google Scholar] [CrossRef]
- Fathi, S.; Lei, I.M.; Cao, Y.; Huang, Y.Y.S. Microcapillary cell extrusion deposition with picolitre dispensing resolution. Bio-Des. Manuf. 2023, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- DeBari, M.K.; Keyser, M.N.; Bai, M.A.; Abbott, R.D. 3D printing with silk: Considerations and applications. Connect. Tissue Res. 2020, 61, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef]
- Panwar, A.; Tan, L.P. Current status of bioinks for micro-extrusion-based 3D bioprinting. Molecules 2016, 21, 685. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, W.; Yan, L.; Huang, D.; Lin, L. Extrusion-based food printing for digitalized food design and nutrition control. J. Food Eng. 2018, 220, 1–11. [Google Scholar] [CrossRef]
- Justino Netto, J.M.; Idogava, H.T.; Frezzatto Santos, L.E.; Silveira, Z.d.C.; Romio, P.; Alves, J.L. Screw-assisted 3D printing with granulated materials: A systematic review. Int. J. Adv. Manuf. Technol. 2021, 115, 2711–2727. [Google Scholar] [CrossRef] [PubMed]
- La Gala, A.; Fiorio, R.; Erkoç, M.; Cardon, L.; D’hooge, D.R. Theoretical evaluation of the melting efficiency for the single-screw micro-extrusion process: The case of 3D printing of ABS. Processes 2020, 8, 1522. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Janarthanan, G.; Tran, H.N.; Ham, H.J.; Yoon, J.; Noh, I. Bioink homogeneity control during 3D bioprinting of multicomponent micro/nanocomposite hydrogel for even tissue regeneration using novel twin screw extrusion system. Chem. Eng. J. 2021, 415, 128971. [Google Scholar] [CrossRef]
- Pati, F.; Jang, J.; Lee, J.W.; Cho, D. Extrusion Bioprinting; Essentials of 3D Biofabrication and Translation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 123–152. [Google Scholar]
- Fisch, P.; Holub, M.; Zenobi-Wong, M. Improved accuracy and precision of bioprinting through progressive cavity pump-controlled extrusion. Biofabrication 2020, 13, 015012. [Google Scholar] [CrossRef] [PubMed]
- Paxton, N.; Smolan, W.; Böck, T.; Melchels, F.; Groll, J.; Jungst, T. Proposal to assess printability of bioinks for extrusion-based bioprinting and evaluation of rheological properties governing bioprintability. Biofabrication 2017, 9, 044107. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.; Gandhi, M.; Khalil, S.; Yan, K.C.; Marcolongo, M.; Barbee, K.; Sun, W. Characterization of cell viability during bioprinting processes. Biotechnol. J. Healthc. Nutr. Technol. 2009, 4, 1168–1177. [Google Scholar] [CrossRef]
- Gillispie, G.; Prim, P.; Copus, J.; Fisher, J.; Mikos, A.G.; Yoo, J.J.; Atala, A.; Lee, S.J. Assessment methodologies for extrusion-based bioink printability. Biofabrication 2020, 12, 022003. [Google Scholar] [CrossRef] [PubMed]
- Boularaoui, S.; Al Hussein, G.; Khan, K.A.; Christoforou, N.; Stefanini, C. An overview of extrusion-based bioprinting with a focus on induced shear stress and its effect on cell viability. Bioprinting 2020, 20, e00093. [Google Scholar] [CrossRef]
- Shi, J.; Wu, B.; Li, S.; Song, J.; Song, B.; Lu, W.F. Shear stress analysis and its effects on cell viability and cell proliferation in drop-on-demand bioprinting. Biomed. Phys. Eng. Express 2018, 4, 045028. [Google Scholar] [CrossRef]
- Malekpour, A.; Chen, X. Printability and cell viability in extrusion-based bioprinting from experimental, computational, and machine learning views. J. Funct. Biomater. 2022, 13, 40. [Google Scholar] [CrossRef]
- Mobaraki, M.; Ghaffari, M.; Yazdanpanah, A.; Luo, Y.; Mills, D.K. Bioinks and bioprinting: A focused review. Bioprinting 2020, 18, e00080. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Mao, S.; Sun, W.; Yao, R. The influence of printing parameters on cell survival rate and printability in microextrusion-based 3D cell printing technology. Biofabrication 2015, 7, 045002. [Google Scholar] [CrossRef] [PubMed]
- Rutz, A.L.; Lewis, P.L.; Shah, R.N. Toward next-generation bioinks: Tuning material properties pre-and post-printing to optimize cell viability. MRS Bull 2017, 42, 563–570. [Google Scholar] [CrossRef]
- Gudapati, H.; Yan, J.; Huang, Y.; Chrisey, D.B. Alginate gelation-induced cell death during laser-assisted cell printing. Biofabrication 2014, 6, 035022. [Google Scholar] [CrossRef] [PubMed]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Highley, C.B.; Rodell, C.B.; Burdick, J.A. Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels. Adv. Mater. 2015, 27, 5075–5079. [Google Scholar] [CrossRef] [PubMed]
- Schwab, A.; Levato, R.; D’Este, M.; Piluso, S.; Eglin, D.; Malda, J. Printability and shape fidelity of bioinks in 3D bioprinting. Chem. Rev. 2020, 120, 11028–11055. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Kim, J.E.; Lee, J.; Park, S.; Lee, S.; Chung, J.H.; Kim, J.; Seonwoo, H. Development of 3D Printable Calcium Phosphate Cement Scaffolds with Cockle Shell Powders. Materials 2023, 16, 6154. [Google Scholar] [CrossRef]
- Rodriguez, M.J.; Brown, J.; Giordano, J.; Lin, S.J.; Omenetto, F.G.; Kaplan, D.L. Silk based bioinks for soft tissue reconstruction using 3-dimensional (3D) printing with in vitro and in vivo assessments. Biomaterials 2017, 117, 105–115. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Lee, S.; Kweon, H.Y.; Jo, Y.; Kim, J.; Chung, J.H.; Seonwoo, H. Development of Silk Fibroin-Based Non-Crosslinking Thermosensitive Bioinks for 3D Bioprinting. Polymers 2023, 15, 3567. [Google Scholar] [CrossRef] [PubMed]
- Kamel, S.; Ali, N.; Jahangir, K.; Shah, S.M.; El-Gendy, A.A. Pharmaceutical significance of cellulose: A review. Express. Polym. Lett 2008, 2, 758–778. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Lim, J.W.; Byun, I.; Jang, K.; Kim, J.; Chung, J.H.; Kim, J.; Seonwoo, H. Development of Plum Seed-Derived Carboxymethylcellulose Bioink for 3D Bioprinting. Polymers 2023, 15, 4473. [Google Scholar] [CrossRef] [PubMed]
- Emmermacher, J.; Spura, D.; Cziommer, J.; Kilian, D.; Wollborn, T.; Fritsching, U.; Steingroewer, J.; Walther, T.; Gelinsky, M.; Lode, A. Engineering considerations on extrusion-based bioprinting: Interactions of material behavior, mechanical forces and cells in the printing needle. Biofabrication 2020, 12, 025022. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M. Development of 3D Bioprinter System Using the Screw-Based Dispenser for High-Precision Bioprinting. Master’s Thesis, Sunchon National University, Suncheon, Republic of Korea, 2023. [Google Scholar]
- Chand, R.; Muhire, B.S.; Vijayavenkataraman, S. Computational fluid dynamics assessment of the effect of bioprinting parameters in extrusion bioprinting. Int. J. Bioprinting 2022, 8, 545. [Google Scholar] [CrossRef] [PubMed]
- Ates, G.; Bartolo, P. Computational fluid dynamics for the optimization of internal bioprinting parameters and mixing conditions. Int. J. Bioprinting 2023, 9, 0219. [Google Scholar] [CrossRef]
- Sathish, P.B.; Gayathri, S.; Priyanka, J.; Muthusamy, S.; Narmadha, R.; Krishnakumar, G.S.; Selvakumar, R. Tricomposite gelatin-carboxymethylcellulose-alginate bioink for direct and indirect 3D printing of human knee meniscal scaffold. Int. J. Biol. Macromol. 2022, 195, 179–189. [Google Scholar]
- Berker, A. Rheology for adhesion science and technology. In Adhesion Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2002; pp. 443–498. [Google Scholar]
- McMahon, D.K.; Anderson, P.A.; Nassar, R.; Bunting, J.B.; Saba, Z.; Oakeley, A.E.; Malouf, N.N. C2C12 cells: Biophysical, biochemical, and immunocytochemical properties. Am. J. Physiol.-Cell Physiol. 1994, 266, C1795–C1802. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef]
- Stern, F.; Wilson, R.V.; Coleman, H.W.; Paterson, E.G. Verification and Validation of CFD Simulations; Iowa Institute of Hydraulic Research IIHR: Iowa City, IA, USA, 1999; p. 407. [Google Scholar]
- Lemarié, L.; Anandan, A.; Petiot, E.; Marquette, C.; Courtial, E. Rheology, simulation and data analysis toward bioprinting cell viability awareness. Bioprinting 2021, 21, e00119. [Google Scholar] [CrossRef]
- Eesa, M.; Barigou, M. CFD analysis of viscous non-Newtonian flow under the influence of a superimposed rotational vibration. Comput. Fluids 2008, 37, 24–34. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, M.; Bhandari, B. A comparative study between syringe-based and screw-based 3D food printers by computational simulation. Comput. Electron. Agric. 2019, 162, 397–404. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, M.; Bhandari, B.; Wang, Y. 3D printing: Printing precision and application in food sector. Trends Food Sci. Technol. 2017, 69, 83–94. [Google Scholar] [CrossRef]
- Nie, M.; Nagata, S.; Oda, H.; Takeuchi, S. Millimeter-thick 3D tissues constructed by densely cellularized core–shell microfluidic bioprinting. Biofabrication 2023, 15, 035010. [Google Scholar] [CrossRef] [PubMed]
- Merotto, E.; Pavan, P.G.; Piccoli, M. Three-Dimensional Bioprinting of Naturally Derived Hydrogels for the Production of Biomimetic Living Tissues: Benefits and Challenges. Biomedicines 2023, 11, 1742. [Google Scholar] [CrossRef]
- Shao, L.; Gao, Q.; Xie, C.; Fu, J.; Xiang, M.; He, Y. Directly coaxial 3D bioprinting of large-scale vascularized tissue constructs. Biofabrication 2020, 12, 035014. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Son, M.; Lee, J.; Byun, I.; Kim, J.-W.; Kim, J.; Seonwoo, H. Computational Fluid Dynamics Analysis and Empirical Evaluation of Carboxymethylcellulose/Alginate 3D Bioprinting Inks for Screw-Based Microextrusion. Polymers 2024, 16, 1137. https://doi.org/10.3390/polym16081137
Lee S, Son M, Lee J, Byun I, Kim J-W, Kim J, Seonwoo H. Computational Fluid Dynamics Analysis and Empirical Evaluation of Carboxymethylcellulose/Alginate 3D Bioprinting Inks for Screw-Based Microextrusion. Polymers. 2024; 16(8):1137. https://doi.org/10.3390/polym16081137
Chicago/Turabian StyleLee, Sungmin, Minjae Son, Juo Lee, Iksong Byun, Jin-Woo Kim, Jungsil Kim, and Hoon Seonwoo. 2024. "Computational Fluid Dynamics Analysis and Empirical Evaluation of Carboxymethylcellulose/Alginate 3D Bioprinting Inks for Screw-Based Microextrusion" Polymers 16, no. 8: 1137. https://doi.org/10.3390/polym16081137
APA StyleLee, S., Son, M., Lee, J., Byun, I., Kim, J.-W., Kim, J., & Seonwoo, H. (2024). Computational Fluid Dynamics Analysis and Empirical Evaluation of Carboxymethylcellulose/Alginate 3D Bioprinting Inks for Screw-Based Microextrusion. Polymers, 16(8), 1137. https://doi.org/10.3390/polym16081137





