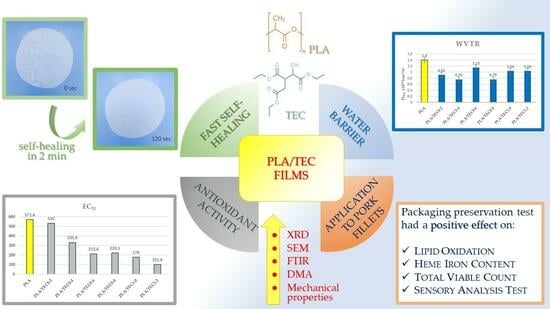
Νovel Polylactic Acid/Tetraethyl Citrate Self-Healable Active Packaging Films Applied to Pork Fillets’ Shelf-Life Extension
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experimental Design—Preparation of Extruded PLA/TEC Composite Pellets
2.3. Films Formation
2.4. Physicochemical Characterization of PLA/TECx Composite Films
2.5. TEC Migration Test
2.6. Characterization of Active Packaging Properties of Obtained PLA/TEC Composite Films
2.6.1. Water/Oxygen Barrier Properties
2.6.2. In Vitro Antioxidant Activity Determination of the Obtained PLA/TECx Composite Films
Preparation of [DPPH•] Free Radical Standard Solutions
Preparation of [DPPH•] Free Radical Calibration Curve
2.6.3. Determination of EC50 (Concentration Required to Obtain 50% Antioxidant Effect) Antioxidant Activity of PLA/TECx Films
2.6.4. Antibacterial Activity of Obtained PLA/TEC Composite Films
2.7. Packaging Test of Fresh Pork Fillets Wrapped with PLA and PLA/TEC0.6 Films
2.7.1. Packaging Preservation Test of Pork Meat Fillets
2.7.2. Lipid Oxidation of Pork Fillets with Thiobarbituric Acid Reactive Substances
2.7.3. Heme Iron Content
2.7.4. Total Viable Count (TVC) of Pork Fillets
2.7.5. Sensory Analysis of Pork Fillets
2.8. Statistical Analysis
3. Results
3.1. Self-Healing Properties
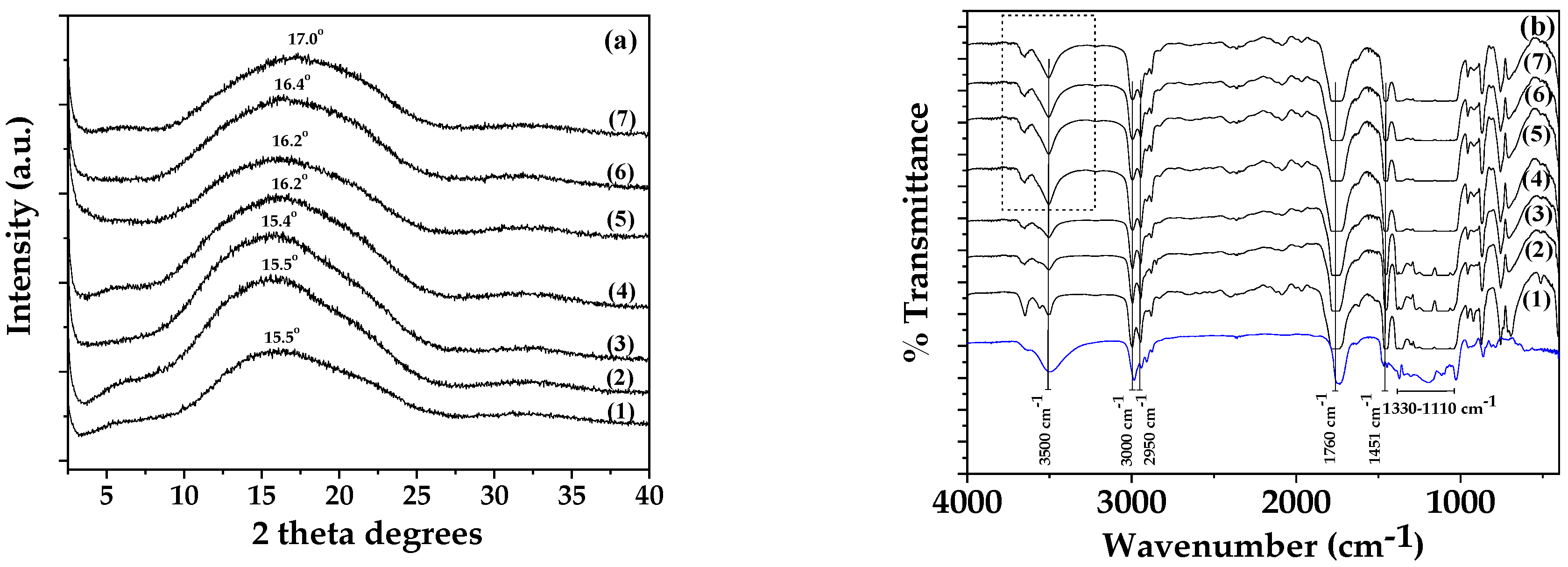
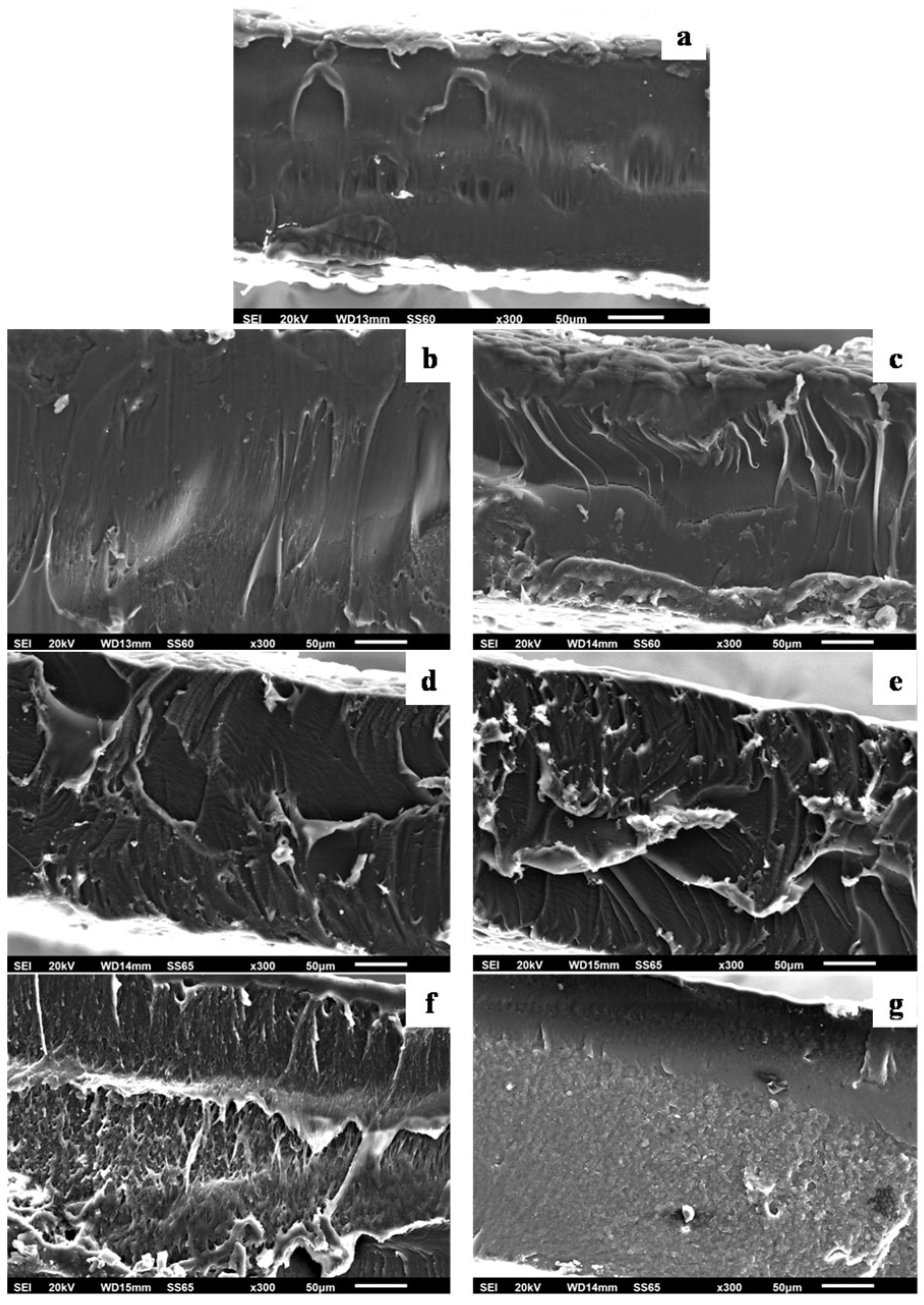
3.2. Physicochemical Characterization of PLA/TEC Composite Films with XRD Analysis, FTIR Spectroscopy and SEM Images
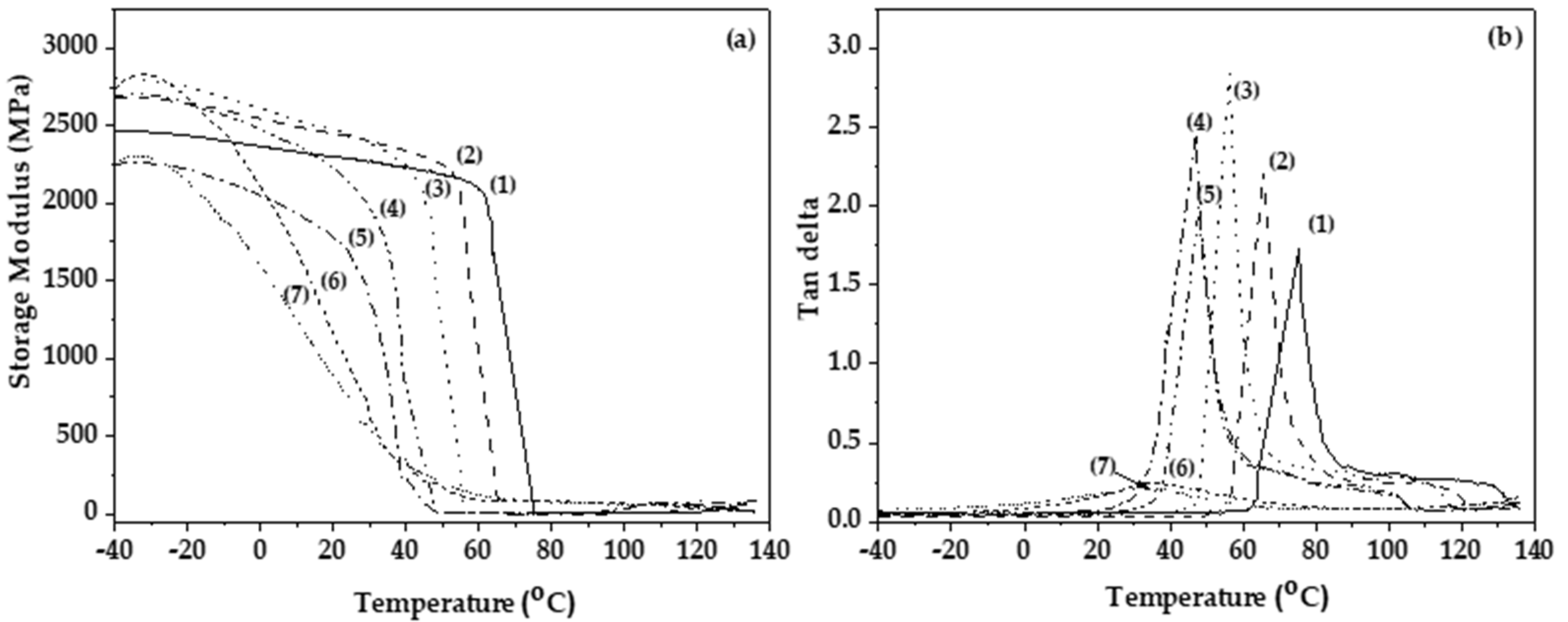
3.3. Mechanical and Thermomechanical Properties of PLA/TECx Composite Films
3.4. Water/Oxygen Barrier Properties of PLA/TECx Composite Films
3.5. Migration of Plasticizer
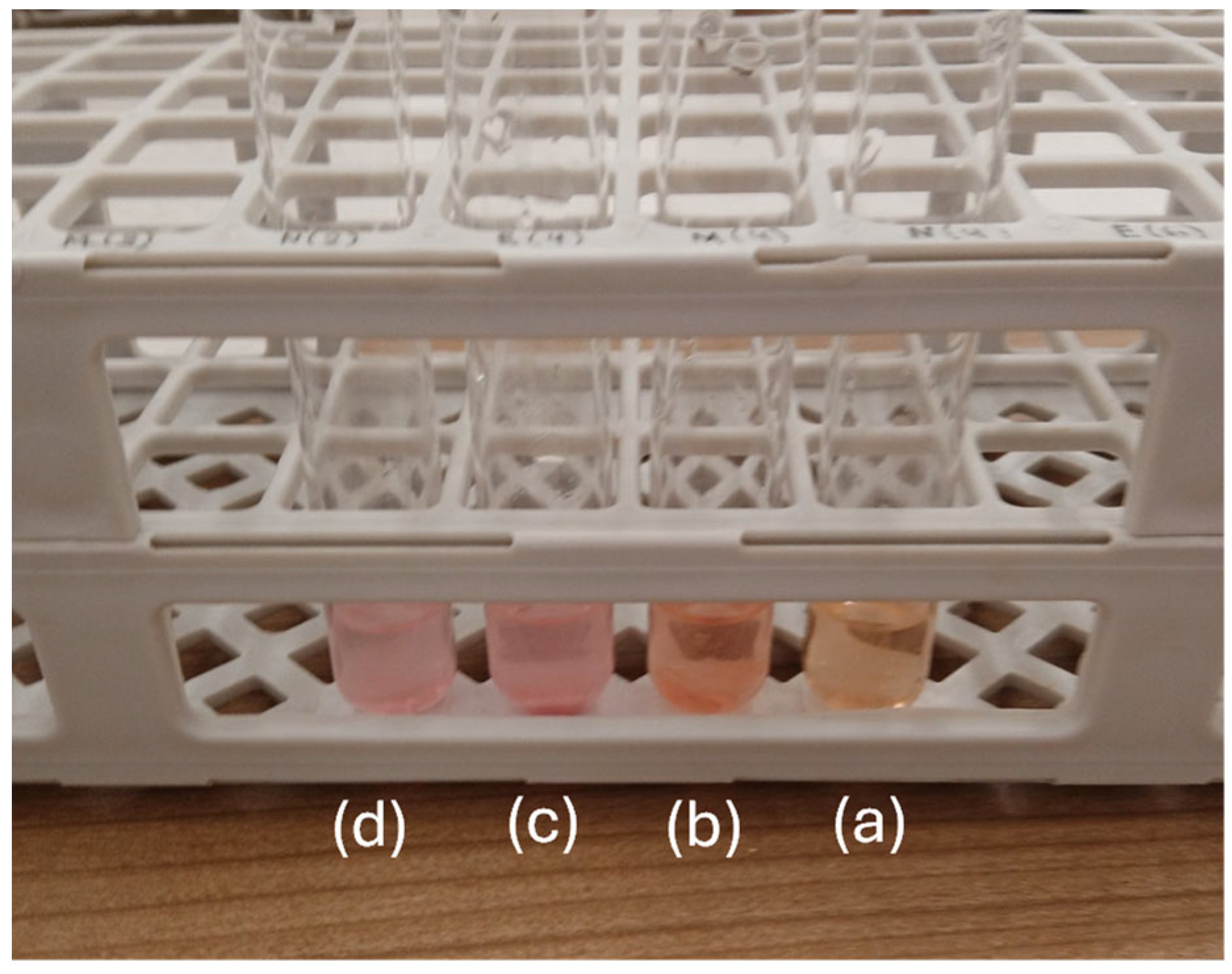
3.6. Antioxidant Activity of PLA/TECx Composite Films
3.7. Antibacterial Activity of PLA/TECx Composite Active Films
3.8. Lipid Oxidation and Heme Iron Content of Pork Fillets
3.9. Correlation of TBARS and Heme Iron
3.10. Microbiological Changes of Pork Fillets
3.11. Sensory Evaluation of Pork Fillets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cooney, R.; de Sousa, D.B.; Fernández-Ríos, A.; Mellett, S.; Rowan, N.; Morse, A.P.; Hayes, M.; Laso, J.; Regueiro, L.; Wan, A.H.L.; et al. A Circular Economy Framework for Seafood Waste Valorisation to Meet Challenges and Opportunities for Intensive Production and Sustainability. J. Clean. Prod. 2023, 392, 136283. [Google Scholar] [CrossRef]
- Esposito, B.; Sessa, M.R.; Sica, D.; Malandrino, O. Towards Circular Economy in the Agri-Food Sector. A Systematic Literature Review. Sustainability 2020, 12, 7401. [Google Scholar] [CrossRef]
- Hamam, M.; Chinnici, G.; Di Vita, G.; Pappalardo, G.; Pecorino, B.; Maesano, G.; D’Amico, M. Circular Economy Models in Agro-Food Systems: A Review. Sustainability 2021, 13, 3453. [Google Scholar] [CrossRef]
- Nemat, B.; Razzaghi, M.; Bolton, K.; Rousta, K. The Role of Food Packaging Design in Consumer Recycling Behavior—A Literature Review. Sustainability 2019, 11, 4350. [Google Scholar] [CrossRef]
- de Sousa, M.S.; Schlogl, A.E.; Estanislau, F.R.; Souza, V.G.L.; dos Reis Coimbra, J.S.; Santos, I.J.B. Nanotechnology in Packaging for Food Industry: Past, Present, and Future. Coatings 2023, 13, 1411. [Google Scholar] [CrossRef]
- Otto, S.; Strenger, M.; Maier-Nöth, A.; Schmid, M. Food Packaging and Sustainability–Consumer Perception vs. Correlated Scientific Facts: A Review. J. Clean. Prod. 2021, 298, 126733. [Google Scholar] [CrossRef]
- Khan, A.; Priyadarshi, R.; Bhattacharya, T.; Rhim, J.-W. Carrageenan/Alginate-Based Functional Films Incorporated with Allium Sativum Carbon Dots for UV-Barrier Food Packaging. Food Bioprocess Technol. 2023, 16, 2001–2015. [Google Scholar] [CrossRef]
- Abdullahi, N. Advances in Food Packaging Technology—A Review. J. Postharvest Technol. 2018, 6, 55–64. [Google Scholar]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-Based Films and Coatings for Food Packaging: A Review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Giannakas, A.E. Plant Extracts-Based Food Packaging Films. In Natural Materials for Food Packaging Application; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2023; pp. 23–49. ISBN 978-3-527-83730-4. [Google Scholar]
- Acquavia, M.A.; Pascale, R.; Martelli, G.; Bondoni, M.; Bianco, G. Natural Polymeric Materials: A Solution to Plastic Pollution from the Agro-Food Sector. Polymers 2021, 13, 158. [Google Scholar] [CrossRef]
- European Bioplastics e.V. Available online: https://www.european-bioplastics.org/ (accessed on 12 March 2022).
- Handbook of Bioplastics and Biocomposites Engineering Applications|Wiley Online Books. Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9781118203699 (accessed on 16 August 2023).
- Biobased Materials in Food Packaging Applications—Handbook of Bioplastics and Biocomposites Engineering Applications—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/9781118203699.ch5 (accessed on 16 August 2023).
- Farah, S.; Anderson, D.G.; Langer, R. Physical and Mechanical Properties of PLA, and Their Functions in Widespread Applications—A Comprehensive Review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, N.; Majid, I.; Nayik, G.A. Bioplastics and Food Packaging: A Review. Cogent Food Agric. 2015, 1, 1117749. [Google Scholar] [CrossRef]
- Sundqvist-Andberg, H.; Åkerman, M. Sustainability Governance and Contested Plastic Food Packaging–An Integrative Review. J. Clean. Prod. 2021, 306, 127111. [Google Scholar] [CrossRef]
- Siracusa, V.; Blanco, I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio-Poly(Ethylene Terephthalate) (Bio-PET): Recent Developments in Bio-Based Polymers Analogous to Petroleum-Derived Ones for Packaging and Engineering Applications. Polymers 2020, 12, 1641. [Google Scholar] [CrossRef]
- Polylactic Acid (PLA) Foams for Packaging Applications—Handbook of Bioplastics and Biocomposites Engineering Applications—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/9781118203699.ch6 (accessed on 16 August 2023).
- Walha, F.; Lamnawar, K.; Maazouz, A.; Jaziri, M. Biosourced Blends Based on Poly (Lactic Acid) and Polyamide 11: Structure–Properties Relationships and Enhancement of Film Blowing Processability. Adv. Polym. Technol. 2018, 37, 2061–2074. [Google Scholar] [CrossRef]
- Eco-Friendly Plasticizers for PLA Films—Italian Food Tech 2019. Available online: https://www.italianfoodtech.com/eco-friendly-plasticizers-for-pla-films (accessed on 16 August 2023).
- Chieng, B.W.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Epoxidized Vegetable Oils Plasticized Poly(Lactic Acid) Biocomposites: Mechanical, Thermal and Morphology Properties. Molecules 2014, 19, 16024–16038. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Li, H.; Liu, Q.; Dong, H.; Duan, Y.; Zhang, J. Plasticization of Poly (Lactic) Acid Film as a Potential Coating Material. IOP Conf. Ser. Earth Environ. Sci. 2018, 108, 022062. [Google Scholar] [CrossRef]
- Mele, G.; Bloise, E.; Cosentino, F.; Lomonaco, D.; Avelino, F.; Marcianò, T.; Massaro, C.; Mazzetto, S.E.; Tammaro, L.; Scalone, A.G.; et al. Influence of Cardanol Oil on the Properties of Poly(Lactic Acid) Films Produced by Melt Extrusion. ACS Omega 2019, 4, 718–726. [Google Scholar] [CrossRef]
- Kang, H.; Li, Y.; Gong, M.; Guo, Y.; Guo, Z.; Fang, Q.; Li, X. An Environmentally Sustainable Plasticizer Toughened Polylactide. RSC Adv. 2018, 8, 11643–11651. [Google Scholar] [CrossRef]
- Maiza, M.; Benaniba, M.T.; Massardier-Nageotte, V. Plasticizing Effects of Citrate Esters on Properties of Poly(Lactic Acid). J. Polym. Eng. 2016, 36, 371–380. [Google Scholar] [CrossRef]
- Stoll, L.; Domenek, S.; Hickmann Flôres, S.; Nachtigall, S.M.B.; de Oliveira Rios, A. Polylactide Films Produced with Bixin and Acetyl Tributyl Citrate: Functional Properties for Active Packaging. J. Appl. Polym. Sci. 2021, 138, 50302. [Google Scholar] [CrossRef]
- SciELO—Brazil—Biobased Additive Plasticizing Polylactic Acid (PLA) Biobased Additive Plasticizing Polylactic Acid (PLA). Available online: https://www.scielo.br/j/po/a/HcnbBHn63pPbkLwmKtmfwpb/ (accessed on 16 August 2023).
- Herrera, N.; Singh, A.A.; Salaberria, A.M.; Labidi, J.; Mathew, A.P.; Oksman, K. Triethyl Citrate (TEC) as a Dispersing Aid in Polylactic Acid/Chitin Nanocomposites Prepared via Liquid-Assisted Extrusion. Polymers 2017, 9, 406. Available online: https://www.mdpi.com/2073-4360/9/9/406 (accessed on 16 August 2023). [CrossRef] [PubMed]
- PubChem Triethyl Citrate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6506 (accessed on 25 January 2024).
- Salmas, C.E.; Giannakas, A.E.; Karabagias, V.K.; Moschovas, D.; Karabagias, I.K.; Gioti, C.; Georgopoulos, S.; Leontiou, A.; Kehayias, G.; Avgeropoulos, A.; et al. Development and Evaluation of a Novel-Thymol@Natural-Zeolite/Low-Density-Polyethylene Active Packaging Film: Applications for Pork Fillets Preservation. Antioxidants 2023, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Karydis-Messinis, A.; Moschovas, D.; Markou, M.; Gkantzou, E.; Vasileiadis, A.; Tsirka, K.; Gioti, C.; Vasilopoulos, K.C.; Bagli, E.; Murphy, C.; et al. Development, Physicochemical Characterization and in Vitro Evaluation of Chitosan-Fish Gelatin-Glycerol Hydrogel Membranes for Wound Treatment Applications. Carbohydr. Polym. Technol. Appl. 2023, 6, 100338. [Google Scholar] [CrossRef]
- Fellows, P.J. 12—Pasteurisation. In Food Processing Technology, 3rd ed.; Fellows, P.J., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston/Cambridge, UK, 2009; pp. 381–395. ISBN 978-1-84569-216-2. [Google Scholar]
- Giannakas, A.E.; Salmas, C.E.; Leontiou, A.; Moschovas, D.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Karakassides, A.; Avgeropoulos, A.; Proestos, C. Performance of Thyme Oil@Na-Montmorillonite and Thyme Oil@Organo-Modified Montmorillonite Nanostructures on the Development of Melt-Extruded Poly-L-Lactic Acid Antioxidant Active Packaging Films. Molecules 2022, 27, 1231. [Google Scholar] [CrossRef] [PubMed]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Baikousi, M.; Kollia, E.; Tsigkou, V.; Karakassides, A.; Leontiou, A.; Kehayias, G.; Avgeropoulos, A.; et al. Nanocomposite Film Development Based on Chitosan/Polyvinyl Alcohol Using ZnO@Montmorillonite and ZnO@Halloysite Hybrid Nanostructures for Active Food Packaging Applications. Nanomaterials 2022, 12, 1843. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of Antiradical Properties of Antioxidants Using DPPH Assay: A Critical Review and Results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Barmpaki, A.A.; Zavvou, E.E.; Drivas, C.; Papapetros, K.; Sygellou, L.; Andrikopoulos, K.S.; Kennou, S.; Andritsos, N.D.; Giannakas, A.; Salmas, C.E.; et al. Atomic Layer Deposition of ZnO on PLA/TiO2 Bionanocomposites: Evaluation of Surface Chemistry and Physical Properties toward Food Packaging Applications. J. Appl. Polym. Sci. 2023, 140, e54465. [Google Scholar] [CrossRef]
- Tarladgis, B.G.; Watts, B.M.; Younathan, M.T.; Dugan, L. A Distillation Method for the Quantitative Determination of Malonaldehyde in Rancid Foods. J. Am. Oil Chem. Soc. 1960, 37, 44–48. [Google Scholar] [CrossRef]
- Clark, E.M.; Mahoney, A.W.; Carpenter, C.E. Heme and Total Iron in Ready-to-Eat Chicken. J. Agric. Food Chem. 1997, 45, 124–126. [Google Scholar] [CrossRef]
- Assanti, E.; Karabagias, V.K.; Karabagias, I.K.; Badeka, A.; Kontominas, M.G. Shelf Life Evaluation of Fresh Chicken Burgers Based on the Combination of Chitosan Dip and Vacuum Packaging under Refrigerated Storage. J. Food Sci. Technol. 2021, 58, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Hematizad, I.; Khanjari, A.; Basti, A.A.; Karabagias, I.K.; Noori, N.; Ghadami, F.; Gholami, F.; Teimourifard, R. In Vitro Antibacterial Activity of Gelatin-Nanochitosan Films Incorporated with Zataria Multiflora Boiss Essential Oil and Its Influence on Microbial, Chemical, and Sensorial Properties of Chicken Breast Meat during Refrigerated Storage. Food Packag. Shelf Life 2021, 30, 100751. [Google Scholar] [CrossRef]
- Takashima, Y.; Harada, A. Self-Healing Polymers. In Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–6. ISBN 978-3-642-36199-9. [Google Scholar]
- Triethyl Citrate(77-93-0) IR2 Spectrum. Available online: https://www.chemicalbook.com/SpectrumEN_77-93-0_IR2.htm (accessed on 26 October 2023).
- Basha, R.K.; Konno, K.; Kani, H.; Kimura, T. Water Vapor Transmission Rate of Biomass Based Film Materials. Eng. Agric. Environ. Food 2011, 4, 37–42. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Salmas, C.E.; Moschovas, D.; Karabagias, V.K.; Karabagias, I.K.; Baikousi, M.; Georgopoulos, S.; Leontiou, A.; Katerinopoulou, K.; Zafeiropoulos, N.E.; et al. Development, Characterization, and Evaluation as Food Active Packaging of Low-Density-Polyethylene-Based Films Incorporated with Rich in Thymol Halloysite Nanohybrid for Fresh “Scaloppini” Type Pork Meat Fillets Preservation. Polymers 2023, 15, 282. [Google Scholar] [CrossRef]
- Stewart, G.S.A.B. Micro-Organisms in Food—2. Sampling for Microbiological Analysis: Principles and Specific Applications: ICMSF, Blackwell Scientific Publications, Oxford, 1986. 310 Pp. Price: £19·50 (Cloth). Meat Sci. 1987, 19, 315. [Google Scholar] [CrossRef]

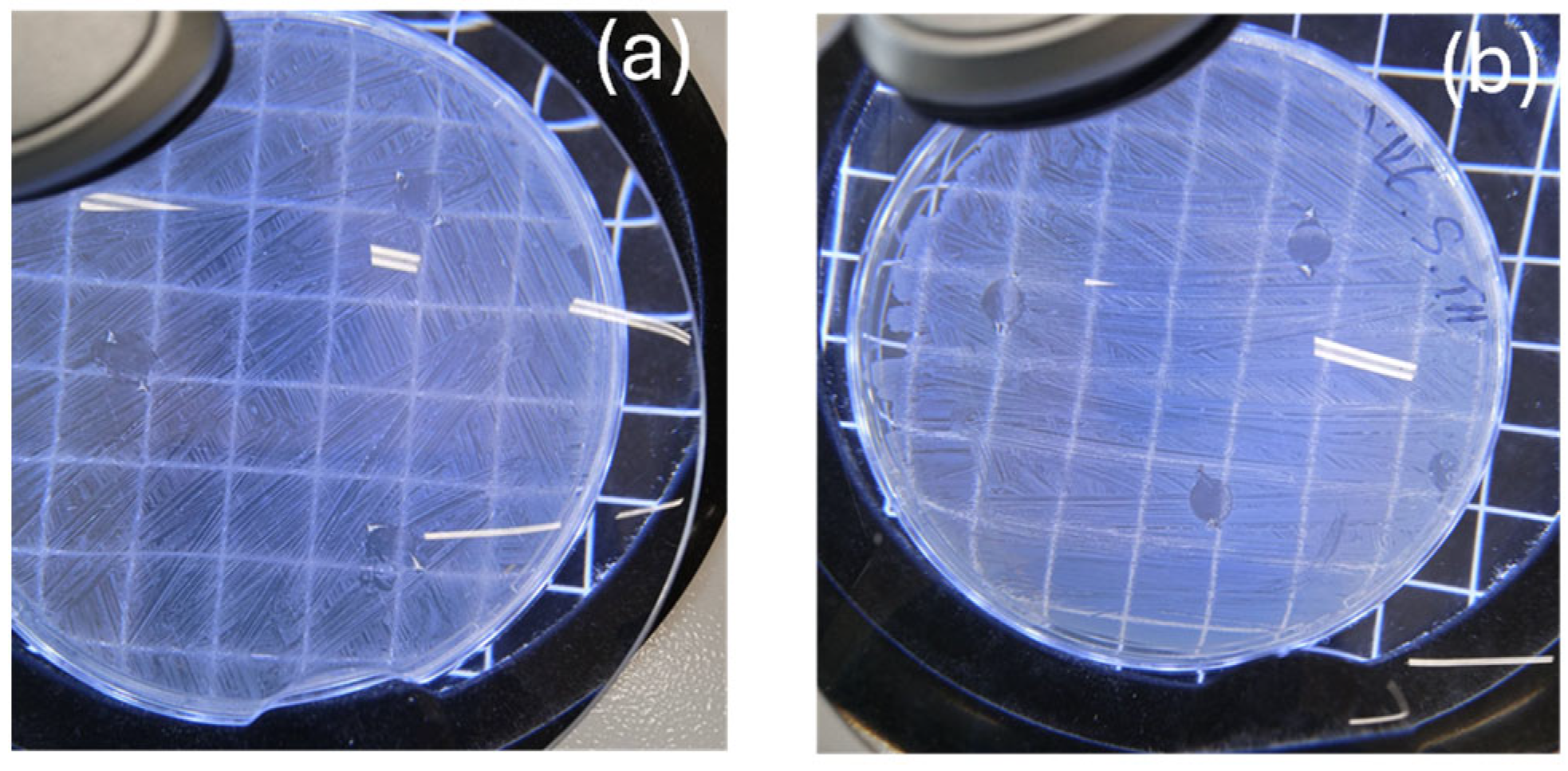
| Sample Code | PLA (g) | TEC mL (v/w) | Twin Extruder Processing Conditions | ||
|---|---|---|---|---|---|
| Temperature (°C) | Speed (rpm) | Time (min) | |||
| PLA | 4 | - | 180 | 120 | 5 |
| PLA/TEC0.2 | 4 | 0.2 (5 mL/100 g) | 180 | 120 | 5 |
| PLA/TEC0.4 | 4 | 0.4 (10 mL/100 g) | 180 | 120 | 5 |
| PLA/TEC0.6 | 4 | 0.6 (15 mL/100 g) | 180 | 120 | 5 |
| PLA/TEC0.8 | 4 | 0.8 (20 mL/100 g) | 180 | 120 | 5 |
| PLA/TEC1.0 | 4 | 1.0 (25 mL/100 g) | 180 | 120 | 5 |
| PLA/TEC1.2 | 4 | 1.2 (30 mL/100 g) | 180 | 120 | 5 |
| Sample Code | Elastic Modulus, E (MPa) | Ultim. Strength σuts (MPa) | % Elongation at Break (ε%) | Tg (°C) |
|---|---|---|---|---|
| PLA | 3102.0 ± 1221.9 a | 90.9 ± 24.81 a | 5.0 ± 1.0 a | 78 |
| PLA/TEC0.2 | 2832.6 ± 565.2 a | 51.1 ± 13.7 b | 3.4 ± 0.4 b | 64 |
| PLA/TEC0.4 | 2675.8 ± 484.28 a | 50.5 ± 23.1 b | 4.0 ± 2.2 a,b | 55 |
| PLA/TEC0.6 | 1438.8 ± 321.55 b | 29.4 ± 5.7 c | 52.6 ± 48.9 c,d | 48 |
| PLA/TEC0.8 | 687.2 ± 225.7 c | 17.5 ± 3.1 d | 58.7 ± 6.8 c | 47 |
| PLA/TEC1.0 | 375.8 ± 129.2 c,d | 18.9 ± 4.5 b,c,d | 68.3 ± 53.2 c | 34 |
| PLA/TEC1.2 | 239.0 ± 53.03 d | 20.4 ± 3.7 d | 122.3 ± 22.0 d | 33 |
| Sample | Film Thickness (mm) | WVTR ×10−7 (g/cm2.s) | Diffusion Coefficient ×10−4 (cm2/s) |
|---|---|---|---|
| PLA | 0.098 ± 0.021 | 6.522 ± 1.092 | 1.40 ± 0.09 a |
| PLA/TEC0.2 | 0.089 ± 0.030 | 4.648 ± 1.146 | 0.91 ± 0.21 b |
| PLA/TEC0.4 | 0.088 ± 0.026 | 3.958 ± 0.996 | 0.76 ± 0.09 b |
| PLA/TEC0.6 | 0.102 ± 0.020 | 5.078 ± 1.763 | 1.15 ± 0.35 a,b |
| PLA/TEC0.8 | 0.081 ± 0.0171 | 4.091 ± 0.554 | 0.76 ± 0.21 b |
| PLA/TEC1.0 | 0.078 ± 0.024 | 6.093 ± 1.340 | 1.04 ± 0.28 a,b |
| PLA/TEC1.2 | 0.099 ± 0.010 | 4.500 ± 1.705 | 1.04 ± 0.35 a,b |
| Sample | Average Film Thickness (mm) | OTR [mL/(m2 × Day)] | PO2 ×10−9 (cm2/s) |
|---|---|---|---|
| PLA | 0.110 ± 0.002 | 189.5 ± 17.7 | 2.64 ± 0.28 a |
| PLA/TEC0.2 | 0.111 ± 0.016 | 203.0 ± 59.4 | 2.66 ± 1.14 a,b |
| PLA/TEC0.4 | 0.060 ± 0.004 | 339.0 ± 70.7 | 2.38 ± 0.65 a |
| PLA/TEC0.6 | 0.063 ± 0.012 | 298.0 ± 94.8 | 2.24 ± 1.11 a,b |
| PLA/TEC0.8 | 0.056 ± 0.004 | 503.0 ± 56.6 | 3.29 ± 0.61 a,b |
| PLA/TEC1.0 | 0.087 ± 0.002 | 317.0 ± 5.7 | 3.27 ± 0.02 b |
| PLA/TEC1.2 | 0.087 ± 0.002 | 489.0 ± 2.8 | 4.62 ± 0.03 c |
| Sample Code | % Weight Loss | EC50 |
|---|---|---|
| PLA | 0.06 ± 0.05 a | 571.4 ± 65.5 a |
| PLA/TEC0.2 | 0.10 ± 0.10 a | 532.0 ± 106.9 a |
| PLA/TEC0.4 | 0.66 ± 0.19 b | 330.8 ± 46.7 b |
| PLA/TEC0.6 | 1.99 ± 0.66 c | 212.6 ± 12.1 c |
| PLA/TEC0.8 | 2.60 ± 0.86 c | 220.5 ± 51.2 c,d |
| PLA/TEC1.0 | 2.00 ± 0.47 c | 178.0 ± 10.3 d |
| PLA/TEC1.2 | 2.29 ± 0.56 c | 101.8 ± 4.5 e |
| Sample Code | Replicates with Growth in the Contact Area of Sample/Total Replicates (6) | |
|---|---|---|
| S. aureus | S. Typhimurium | |
| PLA | 3/6 b | 0/6 a |
| PLA/TEC0.2 | 4/6 c | 0/6 a |
| PLA/TEC0.4 | 4/6 c | 0/6 a |
| PLA/TEC0.6 | 3/6 b | 0/6 a |
| PLA/TEC0.8 | 3/6 b | 0/6 a |
| PLA/TEC1.0 | 4/6 c | 0/6 a |
| PLA/TEC1.2 | 3/6 b | 0/6 a |
| Sample Code | Day 0 | Day 2 | Day 4 | Day 6 |
|---|---|---|---|---|
| TBARS (mg/kg) | ||||
| CONTROL | 0.46 ± 0.01 a | 0.59 ± 0.02 a | 0.75± 0.02 a | 0.81 ± 0.02 a |
| PLA | 0.46 ± 0.01 a | 0.58 ± 0.01 a | 0.71 ± 0.02 b | 0.80 ± 0.01 a |
| PLATEC0.6 | 0.46 ± 0.01 a | 0.55 ± 0.01 b | 0.66 ± 0.01 c | 0.76 ± 0.01 b |
| Heme iron (μg/g) | ||||
| CONTROL | 7.66 ± 0.12 a | 6.26 ± 0.36 a | 5.52 ± 0.18 a | 4.70 ± 0.33 a,b |
| PLA | 7.66 ± 0.12 a | 6.74 ± 0.21 a | 5.72 ± 0.12 a | 4.64 ± 0.19 a |
| PLATEC0.6 | 7.66 ± 0.12 a | 7.12 ± 0.12 b | 6.10 ± 0.21 b | 5.20 ± 0.27 b |
| Sample Code | logCFU/g | |||
|---|---|---|---|---|
| Day 0 | Day 2 | Day 4 | Day 6 | |
| CONTROL | 4.38 ± 0.03 a | 5.56 ± 0.13 a | 6.84 ± 0.06 a | 8.04 ± 0.01 a |
| PLA | 4.38 ± 0.03 a | 5.27 ± 0.16 a,b | 6.48 ± 0.06 b | 7.51 ± 0.04 b |
| PLATEC0.6 | 4.38 ± 0.03 a | 5.08 ± 0.04 b | 6.31 ± 0.07 c | 7.36 ± 0.05 c |
| Sample Name | Day 0 | Day 2 | Day 4 | Day 6 |
|---|---|---|---|---|
| Odor | ||||
| CONTROL | 5.00 ± 0.00 a | 4.32 ± 0.24 a | 3.94 ± 0.23 a | 3.50 ± 0.10 a |
| PLA | 5.00 ± 0.00 a | 4.55 ± 0.14 a,b | 4.22 ± 0.27 a,b | 3.60 ± 0.20 a |
| PLA/TEC0.6 | 5.00 ± 0.00 a | 4.71 ± 0.14 b | 4.50 ± 0.15 b | 4.05 ± 0.16 b |
| Color | ||||
| CONTROL | 5.00 ± 0.00 a | 4.30 ± 0.20 a | 3.98 ± 0.29 a | 2.85 ± 0.15 a |
| PLA | 5.00 ± 0.00 a | 4.50 ± 0.10 a,b | 4.12 ± 0.33 a | 3.50 ± 0.16 b |
| PLA/TEC0.6 | 5.00 ± 0.00 a | 4.60 ± 0.05 b | 4.35 ± 0.27 a | 4.02 ± 0.19 c |
| Cohesion | ||||
| Day 0 | Day 2 | Day 4 | Day 6 | |
| CONTROL | 5.00 ± 0.00 a | 4.48 ± 0.29 a | 3.80 ± 0.20 a | 2.84 ± 0.11 a |
| PLA | 5.00 ± 0.00 a | 4.28 ± 0.40 a | 4.10 ± 0.25 a,b | 3.32 ± 0.28 b |
| PLA/TEC0.6 | 5.00 ± 0.00 a | 4.46 ± 0.27 a | 4.32 ± 0.11 b | 3.94 ± 0.18 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karabagias, V.K.; Giannakas, A.E.; Andritsos, N.D.; Moschovas, D.; Karydis-Messinis, A.; Leontiou, A.; Avgeropoulos, A.; Zafeiropoulos, N.E.; Proestos, C.; Salmas, C.E. Νovel Polylactic Acid/Tetraethyl Citrate Self-Healable Active Packaging Films Applied to Pork Fillets’ Shelf-Life Extension. Polymers 2024, 16, 1130. https://doi.org/10.3390/polym16081130
Karabagias VK, Giannakas AE, Andritsos ND, Moschovas D, Karydis-Messinis A, Leontiou A, Avgeropoulos A, Zafeiropoulos NE, Proestos C, Salmas CE. Νovel Polylactic Acid/Tetraethyl Citrate Self-Healable Active Packaging Films Applied to Pork Fillets’ Shelf-Life Extension. Polymers. 2024; 16(8):1130. https://doi.org/10.3390/polym16081130
Chicago/Turabian StyleKarabagias, Vassilios K., Aris E. Giannakas, Nikolaos D. Andritsos, Dimitrios Moschovas, Andreas Karydis-Messinis, Areti Leontiou, Apostolos Avgeropoulos, Nikolaos E. Zafeiropoulos, Charalampos Proestos, and Constantinos E. Salmas. 2024. "Νovel Polylactic Acid/Tetraethyl Citrate Self-Healable Active Packaging Films Applied to Pork Fillets’ Shelf-Life Extension" Polymers 16, no. 8: 1130. https://doi.org/10.3390/polym16081130
APA StyleKarabagias, V. K., Giannakas, A. E., Andritsos, N. D., Moschovas, D., Karydis-Messinis, A., Leontiou, A., Avgeropoulos, A., Zafeiropoulos, N. E., Proestos, C., & Salmas, C. E. (2024). Νovel Polylactic Acid/Tetraethyl Citrate Self-Healable Active Packaging Films Applied to Pork Fillets’ Shelf-Life Extension. Polymers, 16(8), 1130. https://doi.org/10.3390/polym16081130