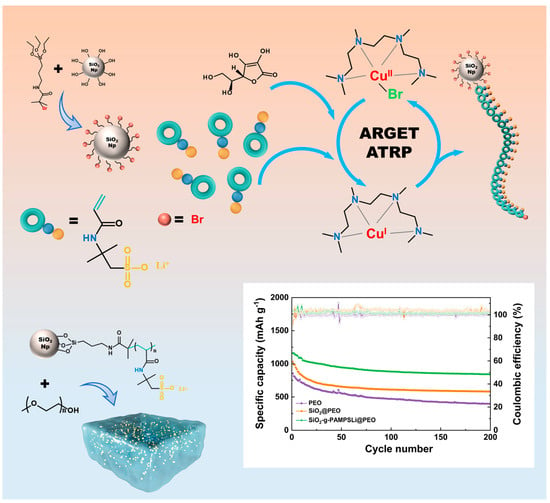
ARGET-ATRP-Mediated Grafting of Bifunctional Polymers onto Silica Nanoparticles Fillers for Boosting the Performance of High-Capacity All-Solid-State Lithium–Sulfur Batteries with Polymer Solid Electrolytes
Abstract
1. Introduction
2. Materials and Methods
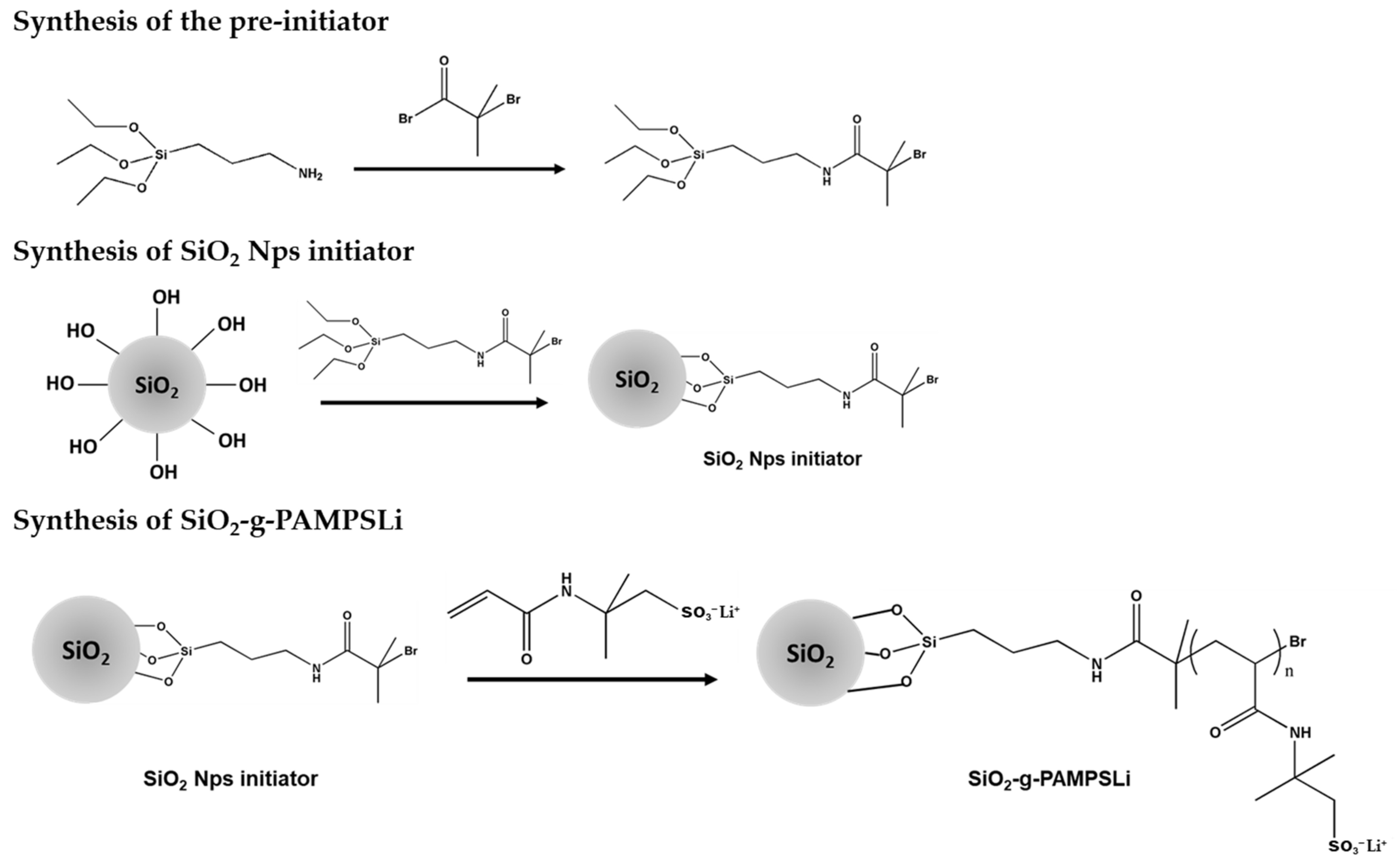
2.1. Preparation of SiO2-g-PAMPSLi
2.2. Preparation of SiO2-g-PAMPSLi@PEO-Based Polymer Composite Solid Electrolytes
2.3. Preparation of Carbon Sulfur Composite Cathode Materials
2.4. Preparation of Cathodes and Lithium–Sulfur Batteries
2.5. Electrochemical Measurements
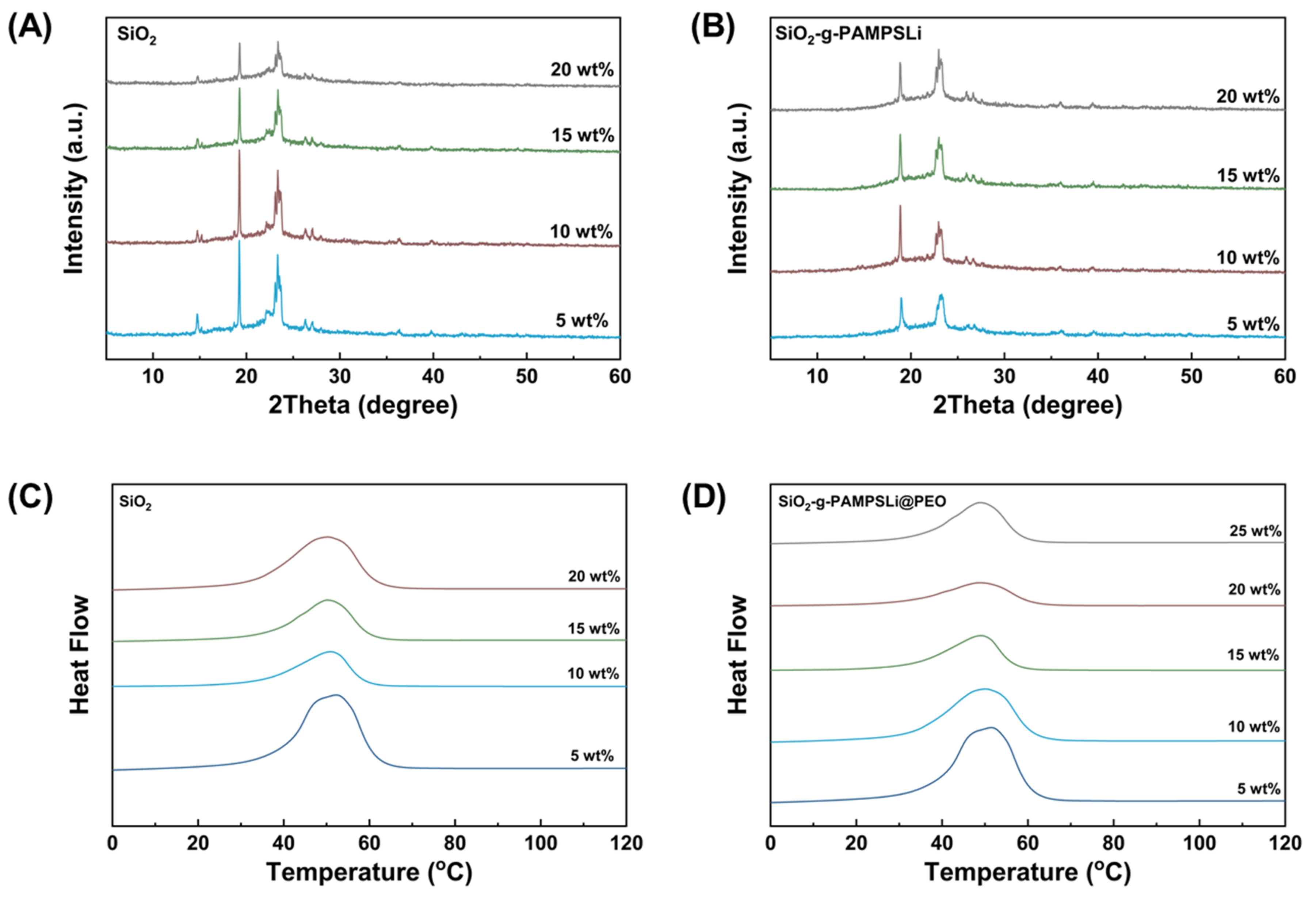
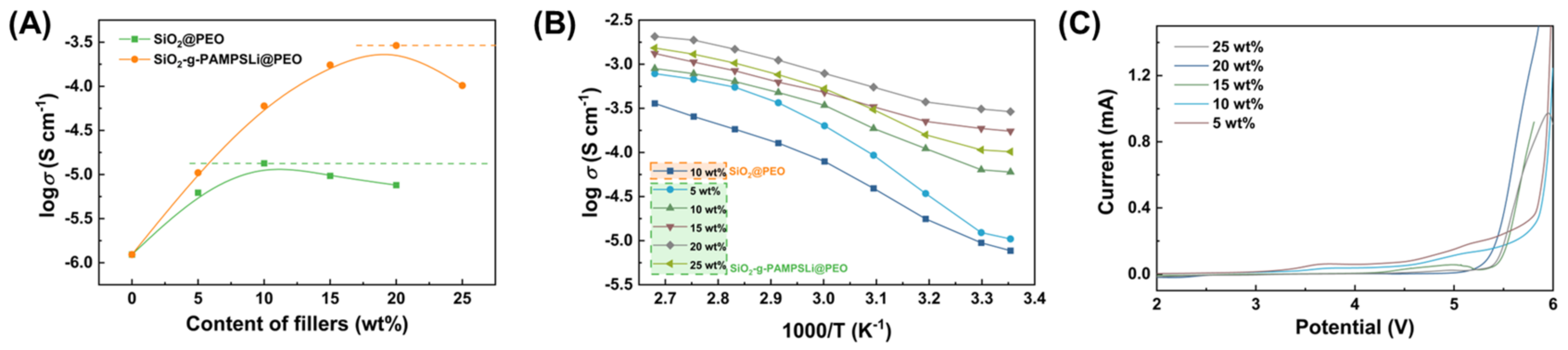
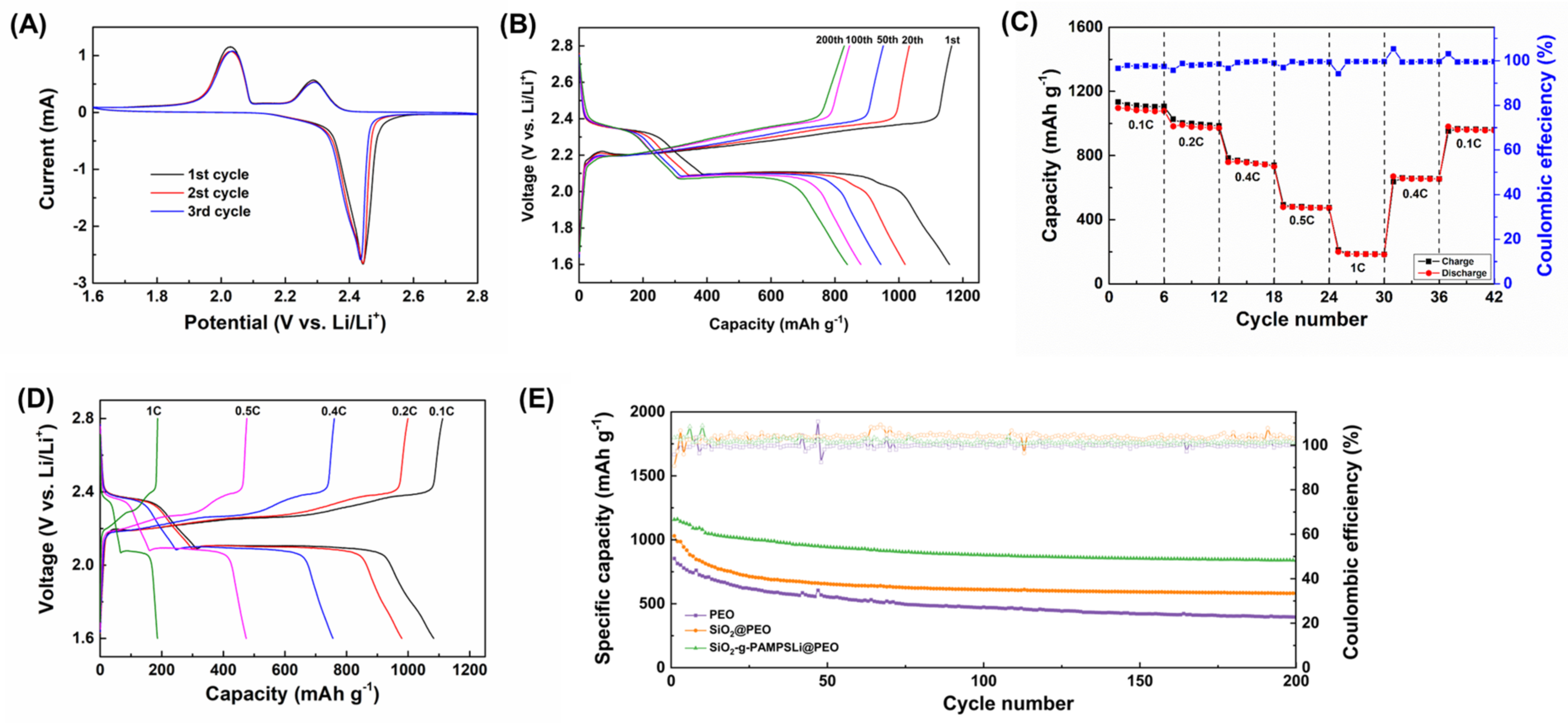
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Su, X.; Xu, X.-P.; Ji, Z.-Q.; Wu, J.; Ma, F.; Fan, L.-Z. Polyethylene Oxide-Based Composite Solid Electrolytes for Lithium Batteries: Current Progress, Low-Temperature and High-Voltage Limitations, and Prospects. Electrochem. Energy Rev. 2024, 7, 2–39. [Google Scholar] [CrossRef]
- Chen, K.; Barai, P.; Kahvecioglu, O.; Wu, L.; Pupek, K.Z.; Ge, M.; Ma, L.; Ehrlich, S.N.; Zhong, H.; Zhu, Y.; et al. Cobalt-free composite-structured cathodes with lithium-stoichiometry control for sustainable lithium-ion batteries. Nat. Commun. 2024, 15, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Rahn, C.D.; Archer, L.A.; You, F. Second life and recycling: Energy and environmental sustainability perspectives for high-performance lithium-ion batteries. Sci. Adv. 2021, 7, eabi7633–eabi7648. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, L.; Pan, F.; Gong, C.; Sun, L.; Gao, H.; Zhang, J.; Zhao, Y.; Wang, G.; Liu, H. Engineering Strategies for Suppressing the Shuttle Effect in Lithium–Sulfur Batteries. Nano-Micro Lett. 2023, 16, 12–46. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Hong, X.; Jiang, Y.; Shi, J.; Zhang, M.; Yan, W.; Lai, C. A Review on Engineering Design for Enhancing Interfacial Contact in Solid-State Lithium–Sulfur Batteries. Nano-Micro Lett. 2024, 16, 71–104. [Google Scholar] [CrossRef]
- Shaibani, M.; Mirshekarloo, M.S.; Singh, R.; Easton, C.D.; Cooray, M.C.D.; Eshraghi, N.; Abendroth, T.; Dörfler, S.; Althues, H.; Kaskel, S.; et al. Expansion-tolerant architectures for stable cycling of ultrahigh-loading sulfur cathodes in lithium-sulfur batteries. Sci. Adv. 2020, 6, eaay2757–eaay2766. [Google Scholar] [CrossRef]
- Pei, F.; Wu, L.; Zhang, Y.; Liao, Y.; Kang, Q.; Han, Y.; Zhang, H.; Shen, Y.; Xu, H.; Li, Z.; et al. Interfacial self-healing polymer electrolytes for long-cycle solid-state lithium-sulfur batteries. Nat. Commun. 2024, 15, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Liu, Y.; Li, Y.; Wang, X.; Fu, J.; Wang, X. Application Progress of Polyaniline, Polypyrrole and Polythiophene in Lithium-Sulfur Batteries. Polymers 2020, 12, 331. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.-X.; Xin, S.; Guo, Y.-G.; Wan, L.-J. Lithium–Sulfur Batteries: Electrochemistry, Materials, and Prospects. Angew. Chem. Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef]
- Wang, C.; Liu, R.; Liu, W.; Zhu, W.; Yang, X.; Wu, Q.; Xie, K.; Shen, L.; Wu, J.; Liu, Y.; et al. Porous Carbon Cloth@CoSe2 as Kinetics-Enhanced and High-Loading Integrated Sulfur Host for Lithium–Sulfur Batteries. Adv. Funct. Mater. 2024, 2316221–2316231. [Google Scholar] [CrossRef]
- Wang, Z.; Song, C.; Shen, H.; Ma, S.; Li, G.; Li, Y. RuOx Quantum Dots Loaded on Graphdiyne for High-Performance Lithium–Sulfur Batteries. Adv. Mater. 2023, 36, 2307786–2307798. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Su, H.; Zhong, Y.; Hu, X.; Wang, X.; Gu, C.; Tu, J. Localized S-Li2s Conversion with Accelerated Kinetics Mediated by Mixed Conductive Shell for High-Performance Solid-State Lithium-Sulfur Battery. Adv. Energy Mater. 2024, 14, 2302255. [Google Scholar] [CrossRef]
- Mikhaylik, Y.V.; Akridge, J.R. Polysulfide Shuttle Study in the Li/S Battery System. J. Electrochem. Soc. 2004, 151, A1969–A1976. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, W.; Wu, X.; Zha, Y.; Liu, S. Preparation of GO/Diatomite/Polyacrylonitrile Functional Separator and Its Application in Li–S Batteries. Materials 2024, 17, 789. [Google Scholar] [CrossRef] [PubMed]
- Talukder, N.; Wang, Y.; Nunna, B.B.; Lee, E.S. N-Doped Graphene (N-G)/MOF(ZIF-8)-Based/Derived Materials for Electrochemical Energy Applications: Synthesis, Characteristics, and Functionality. Batteries 2024, 10, 47. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, Y.; Ou, J.; Li, M.; Luo, D.; Dou, H.; Li, Z.; Amine, K.; Yu, A.; Chen, Z. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures. Chem. Soc. Rev. 2020, 49, 8790–8839. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liao, Y.; Wu, X.; Ye, L.; Wang, Z.; Wu, F.; Lin, Z. In Situ Construction of Elastic Solid-State Polymer Electrolyte with Fast Ionic Transport for Dendrite-Free Solid-State Lithium Metal Batteries. Nanomaterials 2024, 14, 433. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Raghupathy, R.; Vittadello, M. Sodium Polymer Electrolytes: A Review. Batteries 2024, 10, 73. [Google Scholar] [CrossRef]
- Patodia, T.; Gupta, M.K.; Singh, R.; Ichikawa, T.; Jain, A.; Tripathi, B. Electrochemical Performance of Graphene-Modulated Sulfur Composite Cathodes Using LiBH4 Electrolyte for All-Solid-State Li-S Battery. Energies 2021, 14, 7362. [Google Scholar] [CrossRef]
- Wang, L. Development of Novel High Li-Ion Conductivity Hybrid Electrolytes of Li10GeP2S12 (LGPS) and Li6.6La3Zr1.6Sb0.4O12 (LLZSO) for Advanced All-Solid-State Batteries. Oxygen 2021, 1, 16–21. [Google Scholar] [CrossRef]
- Li, S.; Zhang, W.; Zheng, J.; Lv, M.; Song, H.; Du, L. Inhibition of Polysulfide Shuttles in Li–S Batteries: Modified Separators and Solid-State Electrolytes. Adv. Energy Mater. 2021, 11, 2000779–2000802. [Google Scholar] [CrossRef]
- Zhang, J.; Chou, J.; Luo, X.-X.; Yang, Y.-M.; Yan, M.-Y.; Jia, D.; Zhang, C.-H.; Wang, Y.-H.; Wang, W.-P.; Tan, S.-J.; et al. A Fully Amorphous, Dynamic Cross-Linked Polymer Electrolyte for Lithium-Sulfur Batteries Operating at Subzero-Temperatures. Angew. Chem. Int. Ed. 2024, 63–71, e202316087. [Google Scholar] [CrossRef]
- Song, Z.; Jiang, W.; Li, B.; Qu, Y.; Mao, R.; Jian, X.; Hu, F. Advanced Polymers in Cathodes and Electrolytes for Lithium–Sulfur Batteries: Progress and Prospects. Small 2024, 2308550. [Google Scholar] [CrossRef]
- Wu, Z.-H.; Huang, A.-C.; Tang, Y.; Yang, Y.-P.; Liu, Y.-C.; Li, Z.-P.; Zhou, H.-L.; Huang, C.-F.; Xing, Z.-X.; Shu, C.-M.; et al. Thermal Effect and Mechanism Analysis of Flame-Retardant Modified Polymer Electrolyte for Lithium-Ion Battery. Polymers 2021, 13, 1675. [Google Scholar] [CrossRef]
- Meyer, W.H. Polymer Electrolytes for Lithium-Ion Batteries. Adv. Mater. 1998, 10, 439–448. [Google Scholar] [CrossRef]
- Song, Z.; Wang, L.; Jiang, W.; Pei, M.; Li, B.; Mao, R.; Liu, S.; Zhang, T.; Jian, X.; Hu, F. “Like Compatible Like” Strategy Designing Strong Cathode-Electrolyte Interface Quasi-Solid-State Lithium–Sulfur Batteries. Adv. Energy Mater. 2024, 14, 2302688–2302701. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Chen, Y.; Wang, P.; Zhang, H.; He, X. PEO based polymer-ceramic hybrid solid electrolytes: A review. Nano Converg. 2021, 8, 2–13. [Google Scholar] [CrossRef]
- Le Mong, A.; Shin, J.C.; Lee, M.; Kim, D. Accelerated Single Li-Ion Transport in Solid Electrolytes for Lithium–Sulfur Batteries: Poly(Arylene Ether Sulfone) Grafted with Pyrrolidinium-Terminated Poly(Ethylene Glycol). Small 2023, 2309162. [Google Scholar] [CrossRef]
- Chiu, L.-L.; Chung, S.-H. A Poly(ethylene oxide)/Lithium bis(trifluoromethanesulfonyl)imide-Coated Polypropylene Membrane for a High-Loading Lithium–Sulfur Battery. Polymers 2021, 13, 535. [Google Scholar] [CrossRef]
- Babkova, T.; Kiefer, R.; Le, Q.B. Hybrid Electrolyte Based on PEO and Ionic Liquid with In Situ Produced and Dispersed Silica for Sustainable Solid-State Battery. Sustainability 2024, 16, 1683. [Google Scholar] [CrossRef]
- Croce, F.; Appetecchi, G.B.; Persi, L.; Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 1998, 394, 456–458. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, Q.; Ma, W.; Yang, L. Polyethylene Oxide-Based Composites as Solid-State Polymer Electrolytes for Lithium Metal Batteries: A Mini Review. Front. Chem. 2020, 8, 4581–4601. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Saboungi, M.-L.; Price, D.L.; Armand, M.B.; Howells, W.S. Structure of Liquid PEO-LiTFSI Electrolyte. Phys. Rev. Lett. 2000, 84, 5536–5539. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Wang, Y.Y.; Wan, C.C. Review of gel-type polymer electrolytes for lithium-ion batteries. J. Power Sources 1999, 77, 183–197. [Google Scholar] [CrossRef]
- Song, Z.; Chen, F.; Martinez-Ibañez, M.; Feng, W.; Forsyth, M.; Zhou, Z.; Armand, M.; Zhang, H. A reflection on polymer electrolytes for solid-state lithium metal batteries. Nat. Commun. 2023, 14, 4884–4896. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; He, D.; Xie, X. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Marmorstein, D.; Yu, T.H.; Striebel, K.A.; McLarnon, F.R.; Hou, J.; Cairns, E.J. Electrochemical performance of lithium/sulfur cells with three different polymer electrolytes. J. Power Sources 2000, 89, 219–226. [Google Scholar] [CrossRef]
- Yang, Q.; Deng, N.; Zhao, Y.; Gao, L.; Cheng, B.; Kang, W. A review on 1D materials for all-solid-state lithium-ion batteries and all-solid-state lithium-sulfur batteries. Chem. Eng. J. 2023, 451, 138532–138549. [Google Scholar] [CrossRef]
- Tian, W.; Li, Z.; Miao, L.; Sun, Z.; Wang, Q.; Jiao, L. Composite Quasi-Solid-State Electrolytes with Organic–Inorganic Interface Engineering for Fast Ion Transport in Dendrite-Free Sodium Metal Batteries. Adv. Mater. 2023, 36, 2308586. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, J.; Zhang, W.; Lu, Y. Recent Advances of Composite Solid-State Electrolytes for Lithium-Based Batteries. Energy Fuels 2021, 35, 11118–11140. [Google Scholar] [CrossRef]
- Tambelli, C.C.; Bloise, A.C.; Rosário, A.V.; Pereira, E.C.; Magon, C.J.; Donoso, J.P. Characterisation of PEO–Al2O3 composite polymer electrolytes. Electrochim. Acta 2002, 47, 1677–1682. [Google Scholar] [CrossRef]
- Zhao, W.; Su, Y.; Wen, X.; Wang, D. Manipulating crystallization behavior of poly(ethylene oxide) by functionalized nanoparticle inclusion. Polymer 2019, 165, 28–38. [Google Scholar] [CrossRef]
- Jiang, S.; Yu, D.; Ji, X.; An, L.; Jiang, B. Confined crystallization behavior of PEO in silica networks. Polymer 2000, 41, 2041–2046. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Wang, L.; Zhu, S.; Zhong, Q.; Li, Y.; Zhou, Q. Li+ Conduction in a Polymer/Li1.5Al0.5Ge1.5(PO4)3 Solid Electrolyte and Li-Metal/Electrolyte Interface. Molecules 2023, 28, 8029. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, L.; Yang, Y.; Zhang, H.; Yao, R.; Yuan, C.; Cheng, S. In Situ Nano-SiO2 Electrospun Polyethylene-Oxide-Based Nano-Fiber Composite Solid Polymer Electrolyte for High-Performance Lithium-Ion Batteries. Nanomaterials 2023, 13, 1294. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhou, Z.; Jia, M.; Chen, X.; Shi, C.; Zhao, N.; Guo, X. Solid Polymer Electrolytes with Flexible Framework of SiO2 Nanofibers for Highly Safe Solid Lithium Batteries. Polymers 2020, 12, 1324. [Google Scholar] [CrossRef]
- Zhou, T.H.; Ruan, W.H.; Rong, M.Z.; Zhang, M.Q.; Mai, Y.L. Keys to Toughening of Non-layered Nanoparticles/Polymer Composites. Adv. Mater. 2007, 19, 2667–2671. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Gucci, F.; Grasso, M.; Russo, S.; Leighton, G.J.T.; Shaw, C.; Brighton, J. Electrical and Mechanical Characterisation of Poly(ethylene)oxide-Polysulfone Blend for Composite Structural Lithium Batteries. Polymers 2023, 15, 2581. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Judez, X.; Li, C.; Martinez-Ibanez, M.; Gracia, I.; Bondarchuk, O.; Carrasco, J.; Rodriguez-Martinez, L.M.; Zhang, H.; Armand, M. Ultrahigh Performance All Solid-State Lithium Sulfur Batteries: Salt Anion’s Chemistry-Induced Anomalous Synergistic Effect. J. Am. Chem. Soc. 2018, 140, 9921–9933. [Google Scholar] [CrossRef]
- Fang, R.; Xu, H.; Xu, B.; Li, X.; Li, Y.; Goodenough, J.B. Reaction Mechanism Optimization of Solid-State Li–S Batteries with a PEO-Based Electrolyte. Adv. Funct. Mater. 2020, 31, 2001812. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, K.; Liu, M.; Chen, S.; Liu, X.; Yang, B.; Wang, Z.; Huang, W.; Song, Z.; Xue, S.; et al. PIM-1 as a Multifunctional Framework to Enable High-Performance Solid-State Lithium–Sulfur Batteries. Adv. Funct. Mater. 2021, 31, 2104830. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, X.; Wang, J.; Zhang, Z.; Xiao, X.; Wan, J.; Ye, Y.; Chou, L.Y.; Lee, H.K.; Wang, J.; et al. Incorporating the Nanoscale Encapsulation Concept from Liquid Electrolytes into Solid-State Lithium-Sulfur Batteries. Nano Lett. 2020, 20, 5496–5503. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Wang, Y.; Kim, N.; Lee, S.B. Polymer-based electrolytes for all-solid-state lithium–sulfur batteries: From fundamental research to performance improvement. J. Mater. Sci. 2021, 56, 8358–8382. [Google Scholar] [CrossRef]
- Li, M.; Frerichs, J.E.; Kolek, M.; Sun, W.; Zhou, D.; Huang, C.J.; Hwang, B.J.; Hansen, M.R.; Winter, M.; Bieker, P. Solid-State Lithium–Sulfur Battery Enabled by Thio-LiSICON/Polymer Composite Electrolyte and Sulfurized Polyacrylonitrile Cathode. Adv. Funct. Mater. 2020, 30, 1910123. [Google Scholar] [CrossRef]
- Xu, X.; Hou, G.; Nie, X.; Ai, Q.; Liu, Y.; Feng, J.; Zhang, L.; Si, P.; Guo, S.; Ci, L. Li7P3S11/poly(ethylene oxide) hybrid solid electrolytes with excellent interfacial compatibility for all-solid-state batteries. J. Power Sources 2018, 400, 212–217. [Google Scholar] [CrossRef]
- Kou, W.; Wang, J.; Li, W.; Lv, R.; Peng, N.; Wu, W.; Wang, J. Asymmetry-structure electrolyte with rapid Li+ transfer pathway towards high-performance all-solid-state lithium–sulfur battery. J. Membr. Sci. 2021, 634, 119432. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Lin, Y.; Zhao, Y.; Yuan, H.; Su, Y.; Zhang, J.; Ren, S.; Fan, H.; Zhang, Y. Mechanistic Investigation of Polymer-Based All-Solid-State Lithium/Sulfur Battery. Adv. Funct. Mater. 2021, 31, 2104863. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Huang, J.; Shen, Y.; Ma, M.; Ruan, W.; Zhang, M. ARGET-ATRP-Mediated Grafting of Bifunctional Polymers onto Silica Nanoparticles Fillers for Boosting the Performance of High-Capacity All-Solid-State Lithium–Sulfur Batteries with Polymer Solid Electrolytes. Polymers 2024, 16, 1128. https://doi.org/10.3390/polym16081128
Wang L, Huang J, Shen Y, Ma M, Ruan W, Zhang M. ARGET-ATRP-Mediated Grafting of Bifunctional Polymers onto Silica Nanoparticles Fillers for Boosting the Performance of High-Capacity All-Solid-State Lithium–Sulfur Batteries with Polymer Solid Electrolytes. Polymers. 2024; 16(8):1128. https://doi.org/10.3390/polym16081128
Chicago/Turabian StyleWang, Liang, Junyue Huang, Yujian Shen, Mengqi Ma, Wenhong Ruan, and Mingqiu Zhang. 2024. "ARGET-ATRP-Mediated Grafting of Bifunctional Polymers onto Silica Nanoparticles Fillers for Boosting the Performance of High-Capacity All-Solid-State Lithium–Sulfur Batteries with Polymer Solid Electrolytes" Polymers 16, no. 8: 1128. https://doi.org/10.3390/polym16081128
APA StyleWang, L., Huang, J., Shen, Y., Ma, M., Ruan, W., & Zhang, M. (2024). ARGET-ATRP-Mediated Grafting of Bifunctional Polymers onto Silica Nanoparticles Fillers for Boosting the Performance of High-Capacity All-Solid-State Lithium–Sulfur Batteries with Polymer Solid Electrolytes. Polymers, 16(8), 1128. https://doi.org/10.3390/polym16081128