The Effect of Silver Nanoparticles/Titanium Dioxide in Poly(acrylic acid-co-acrylamide)-Modified, Deproteinized, Natural Rubber Composites on Dye Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
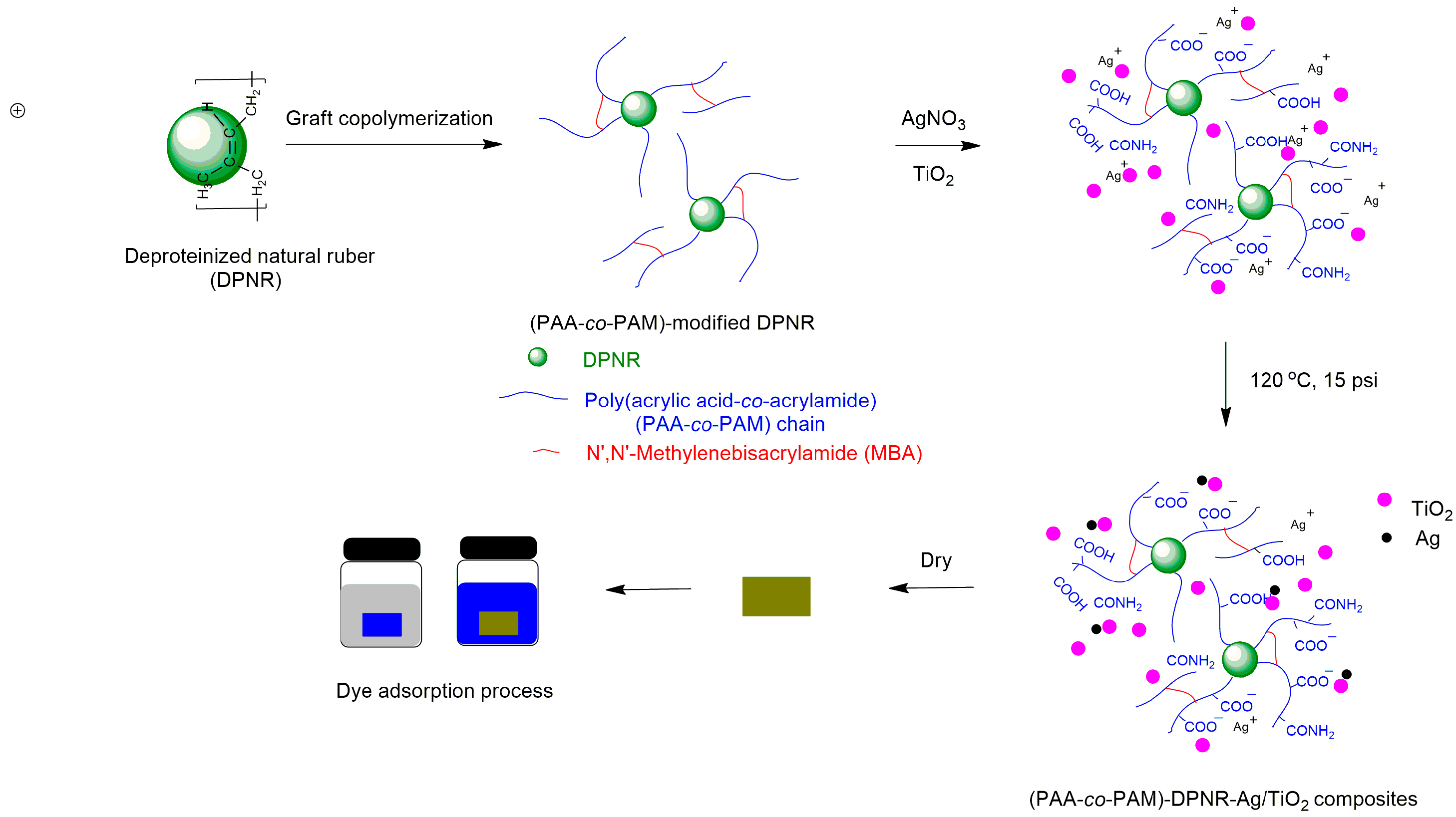
2.2. Preparation of Poly(acrylic acid-co-acrylamide)-Modified, Deproteinized, Natural Rubber
2.3. Characterization of (PAA-co-PAM)-Modified, Deproteinized, Natural Rubber
2.3.1. Determination of Monomer Conversion, Grafting Efficiency, and Grafting Percentage
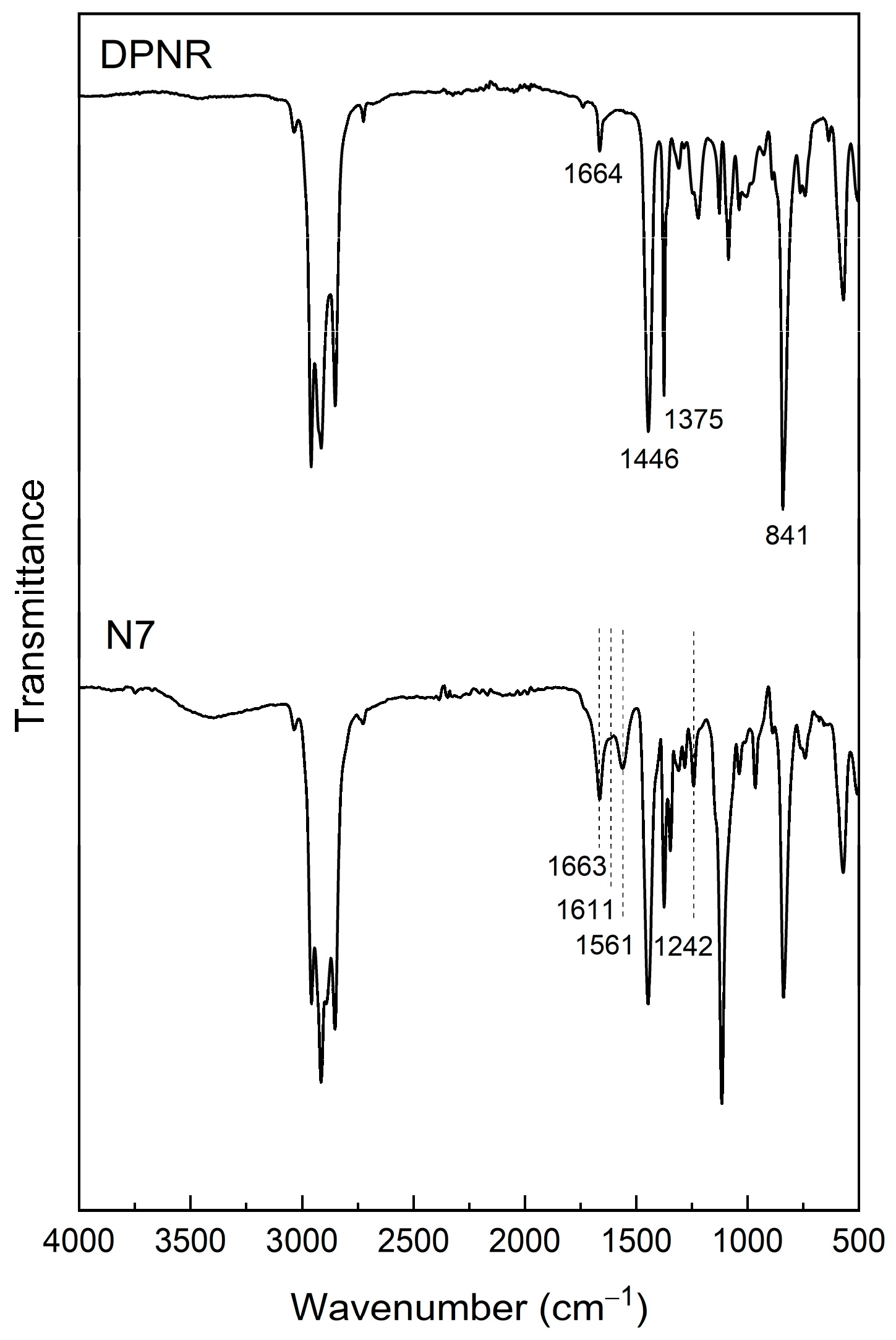
2.3.2. FTIR Analysis
2.4. Preparation of (PAA-co-PAM)-DPNR/Ag-TiO2 Composites
2.5. Characterization of (PAA-co-PAM)-DPNR/Ag-TiO2 Composites
2.5.1. UV-Vis Spectroscopy
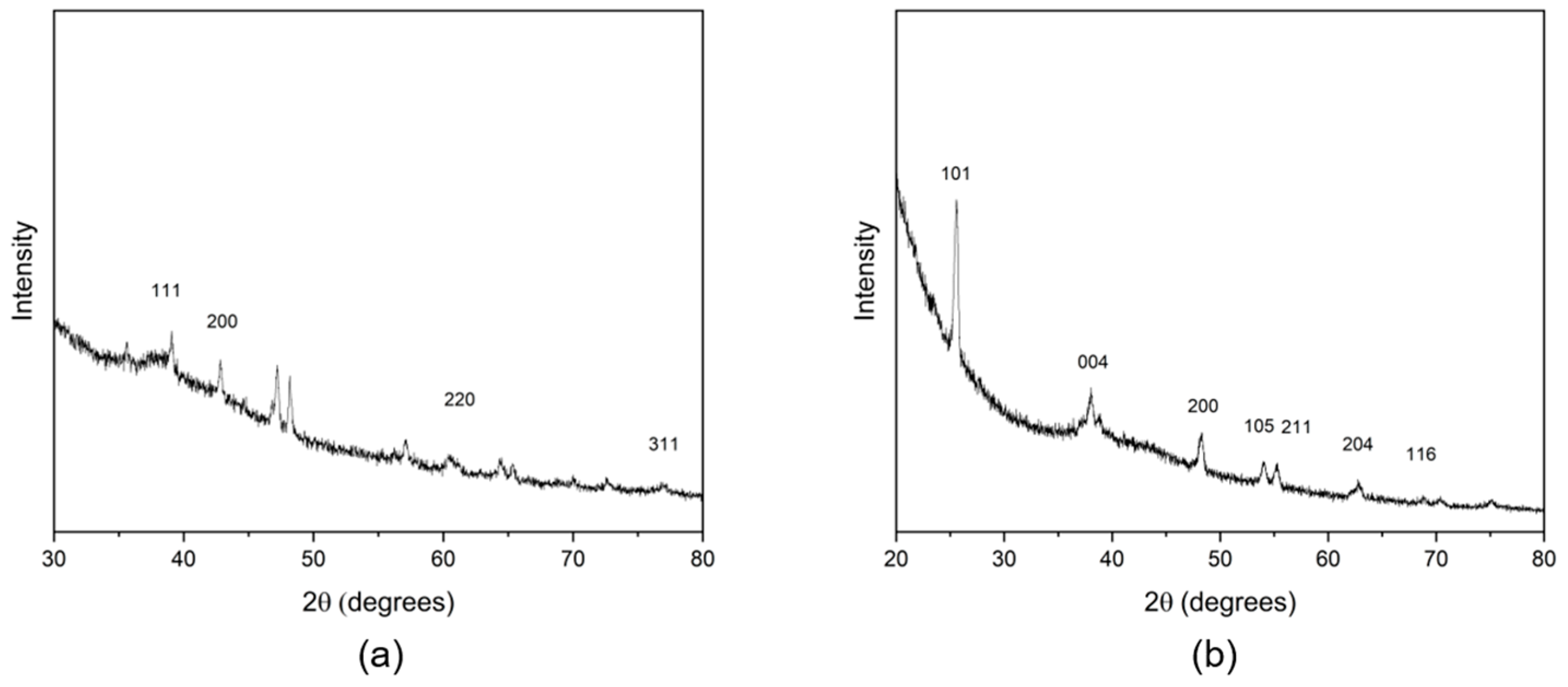
2.5.2. X-ray Diffraction
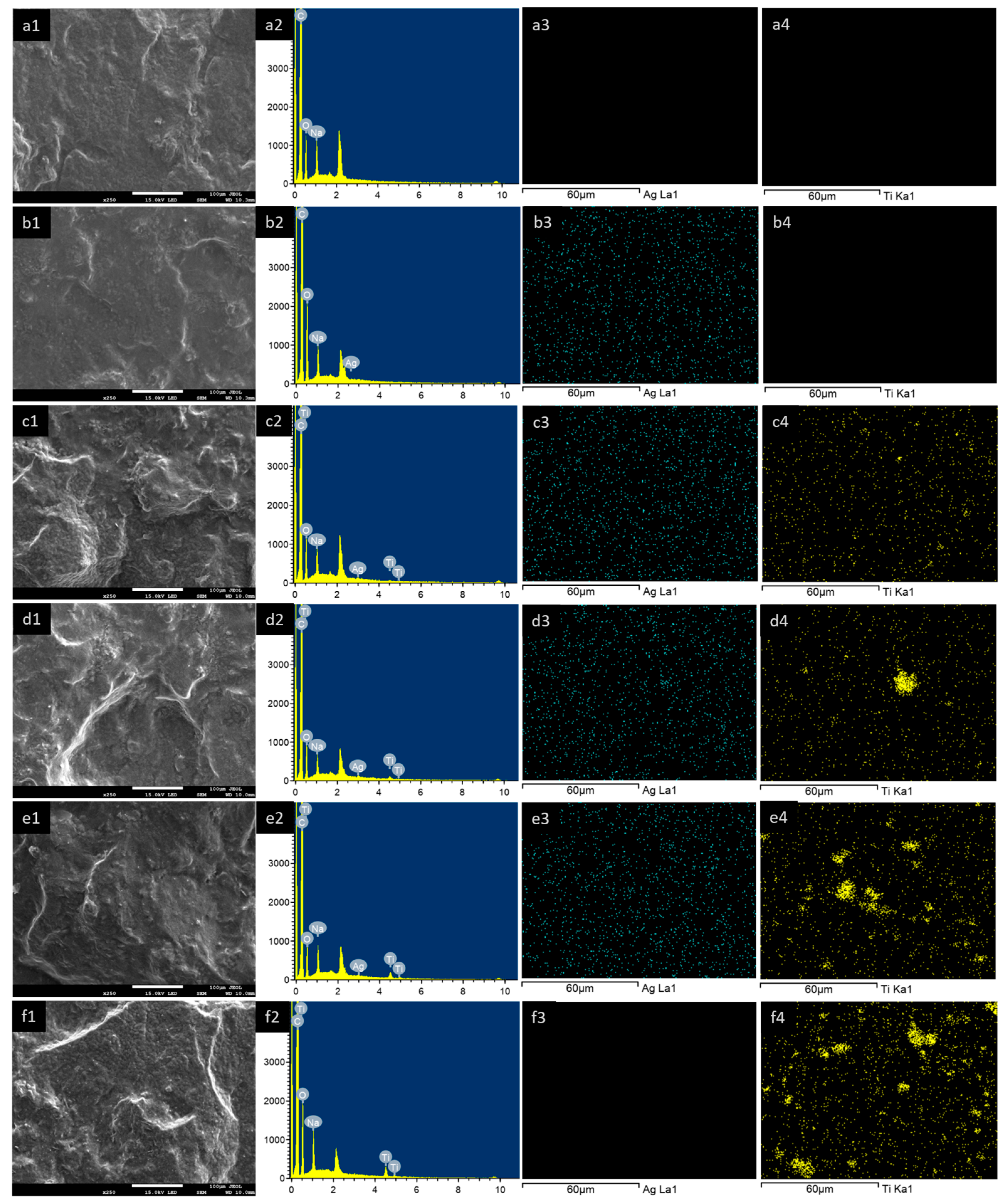
2.5.3. Morphology
2.5.4. Swelling Degree
2.5.5. Compressive Properties
2.6. Dye Adsorption Ability
2.6.1. Effect of Type of Adsorbent
2.6.2. Adsorption Kinetic
2.6.3. Adsorption Isotherm
2.6.4. Reusability
3. Results and Discussion
3.1. Characterization of (PAA-co-PAM)-Modified, Deproteinized, Natural Rubber
3.1.1. Conversion, Grafting Efficiency and Grafting Percentage
3.1.2. FTIR Analysis
3.2. Characterization of (PAA-co-PAM)-DPNR/Ag-TiO2 Composites
3.2.1. Formation of Silver Nanoparticles
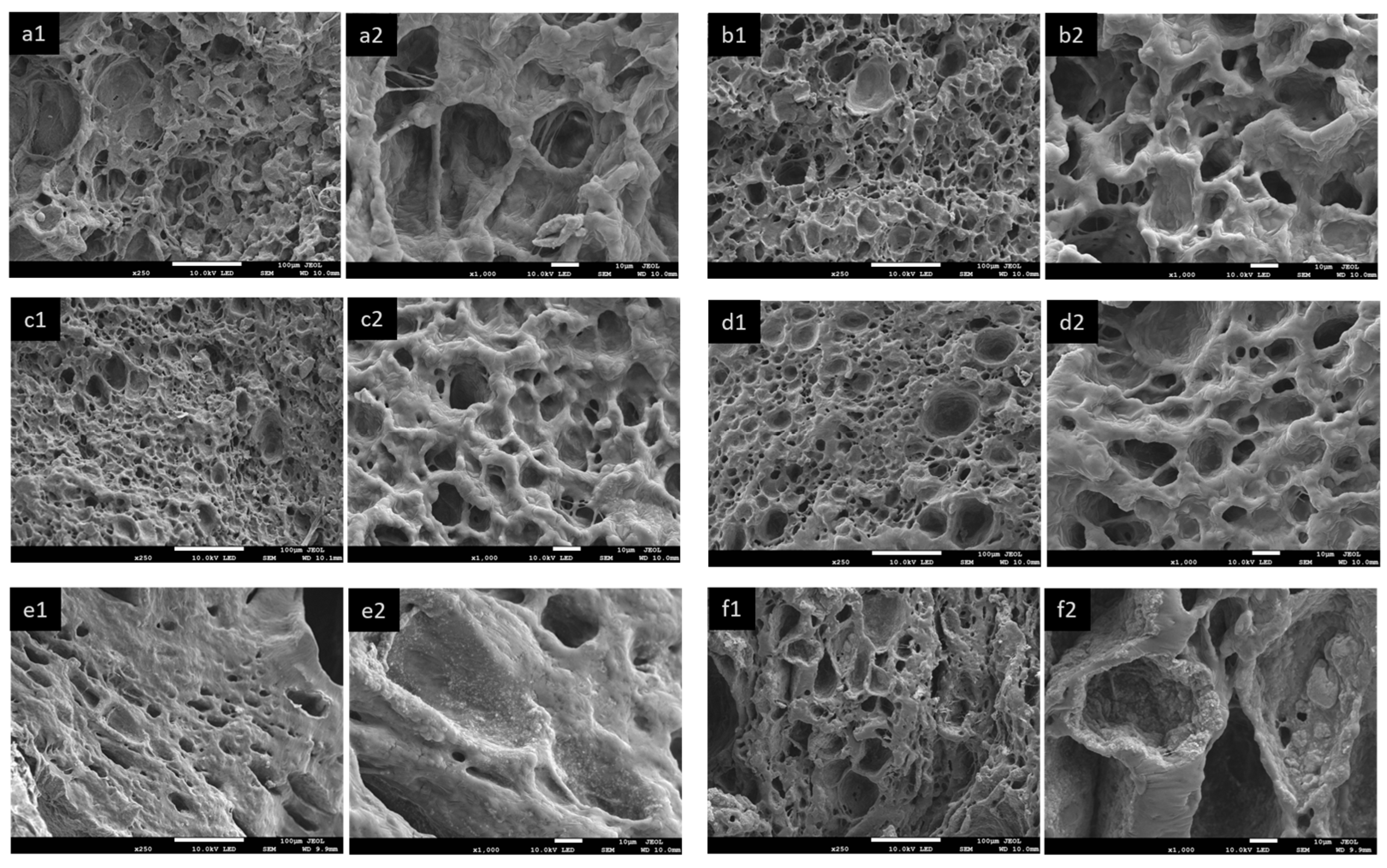
3.2.2. Morphology
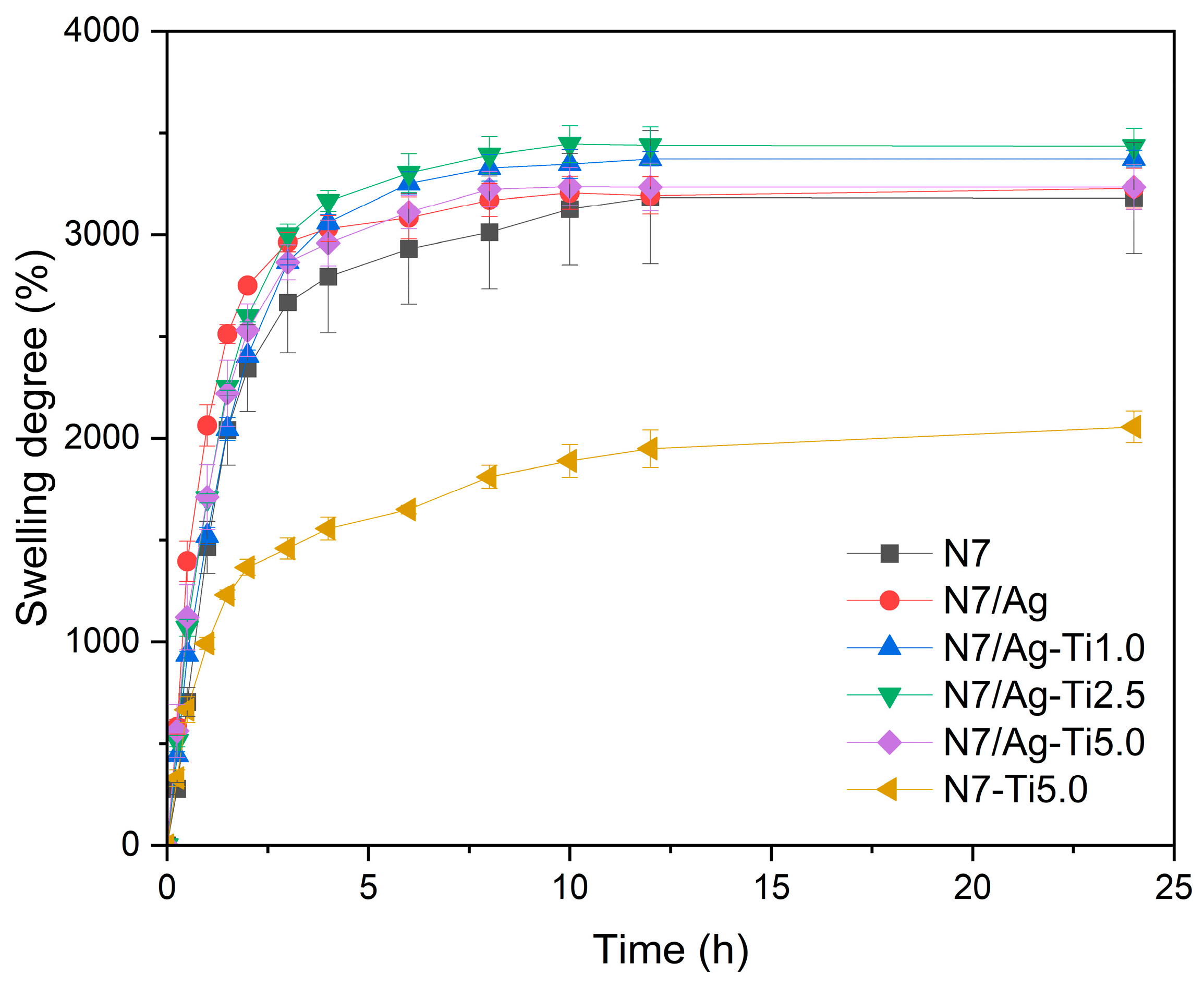
3.2.3. Swelling Degree
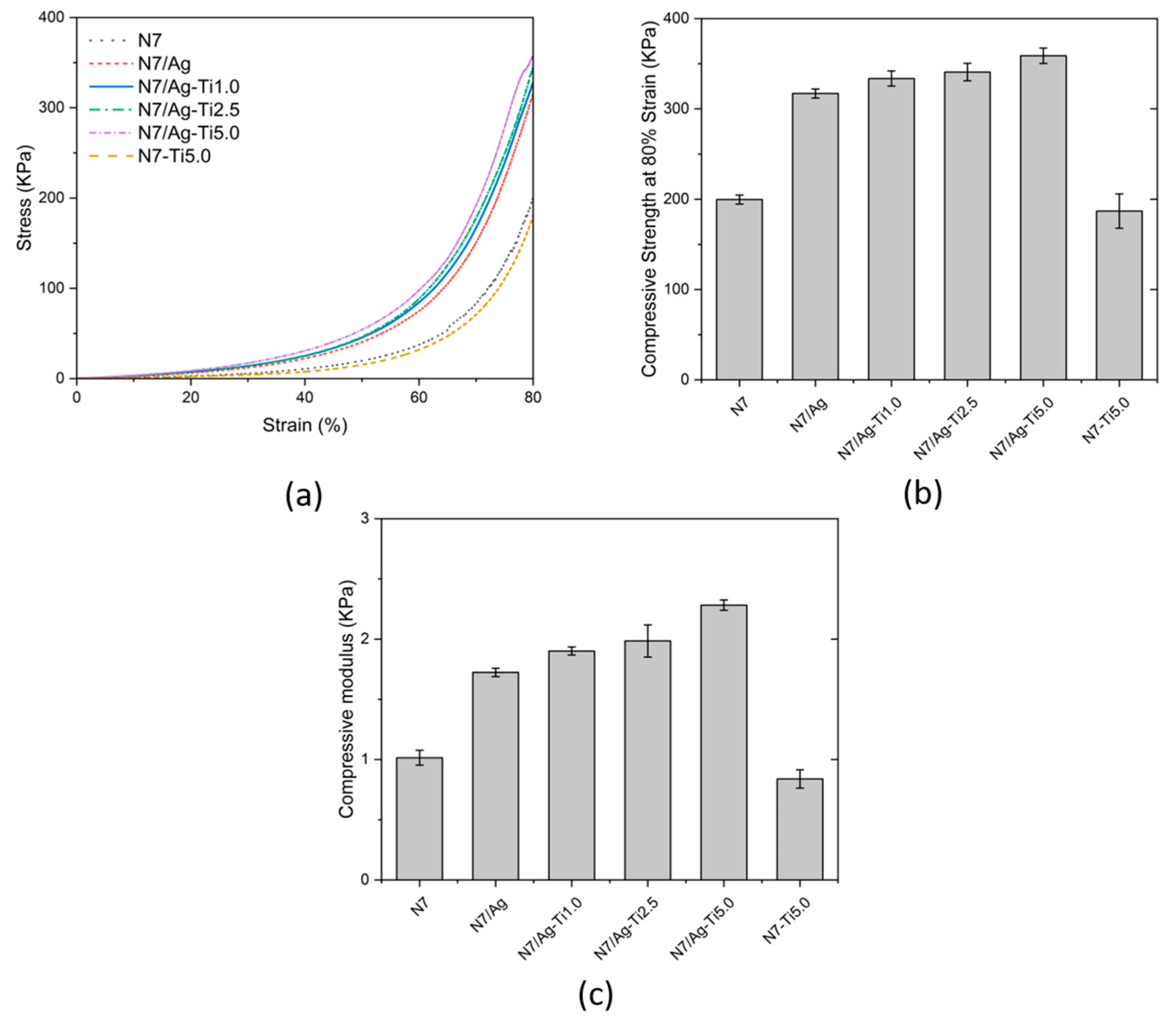
3.2.4. Compressive Properties
3.3. Dye Adsorption Studies
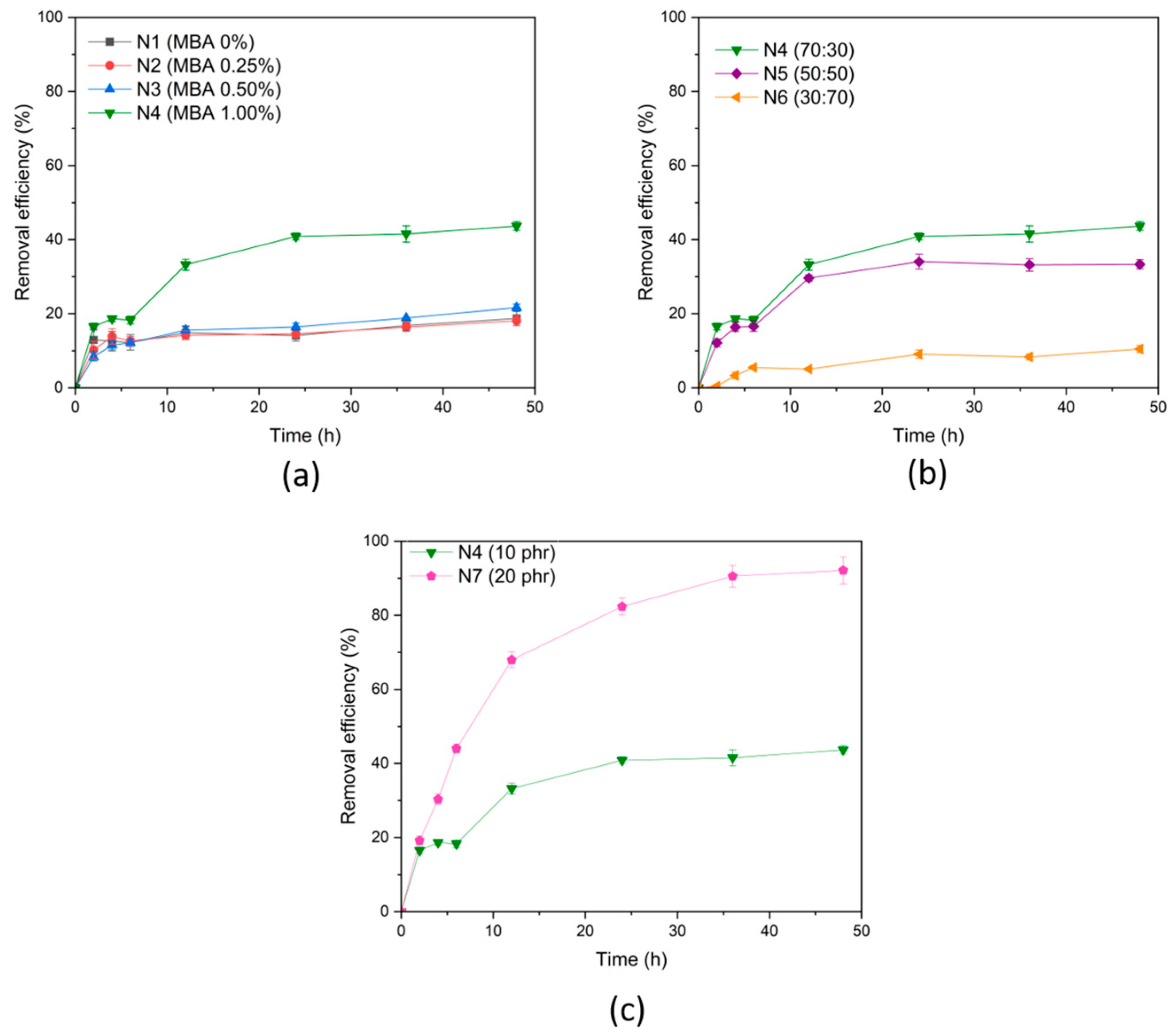
3.3.1. Effect of Type of (PAA-co-PAM)-DPNR
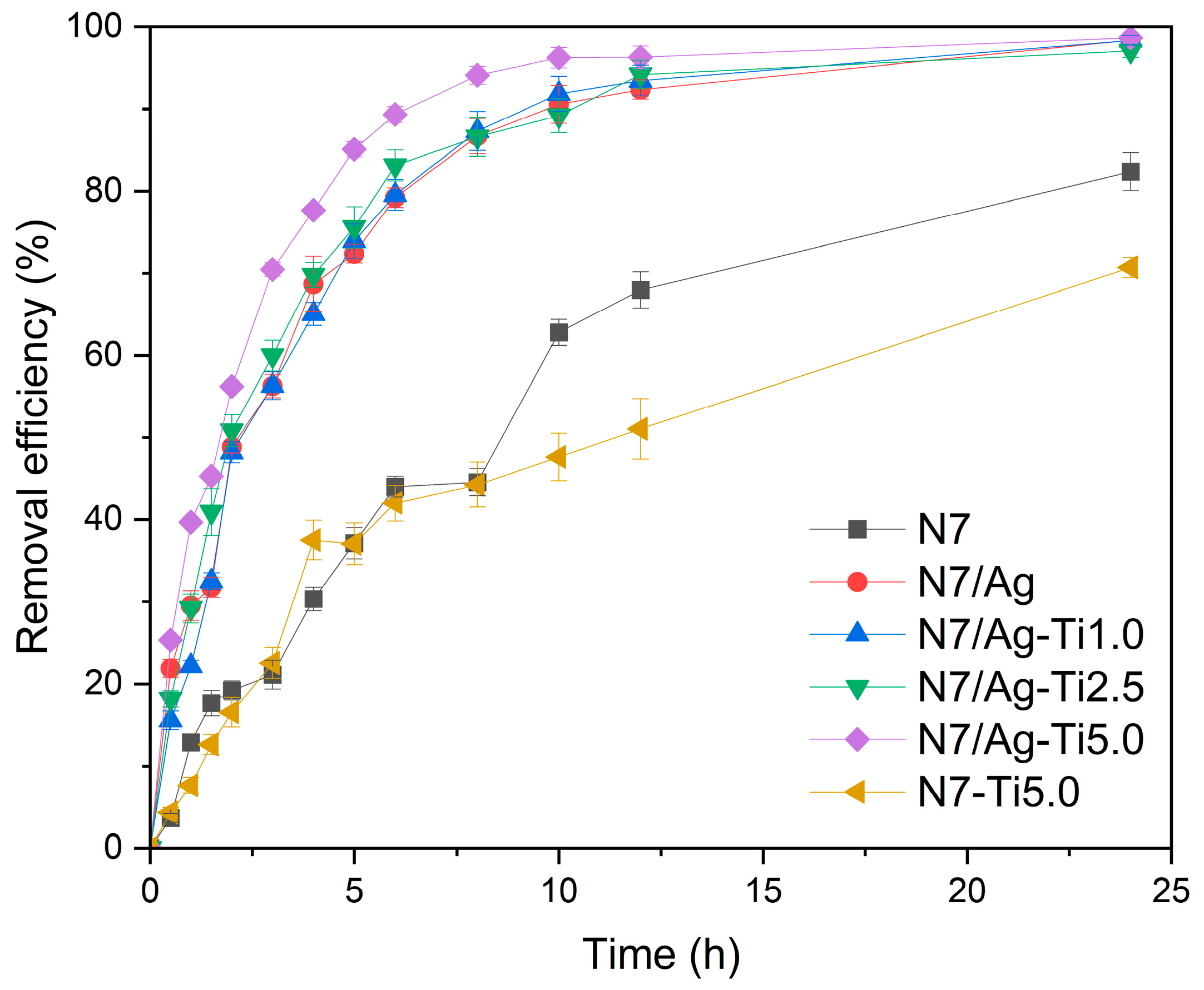
3.3.2. Effect of Type of (PAA-co-PAM)-DPNR/Ag-TiO2 Composites
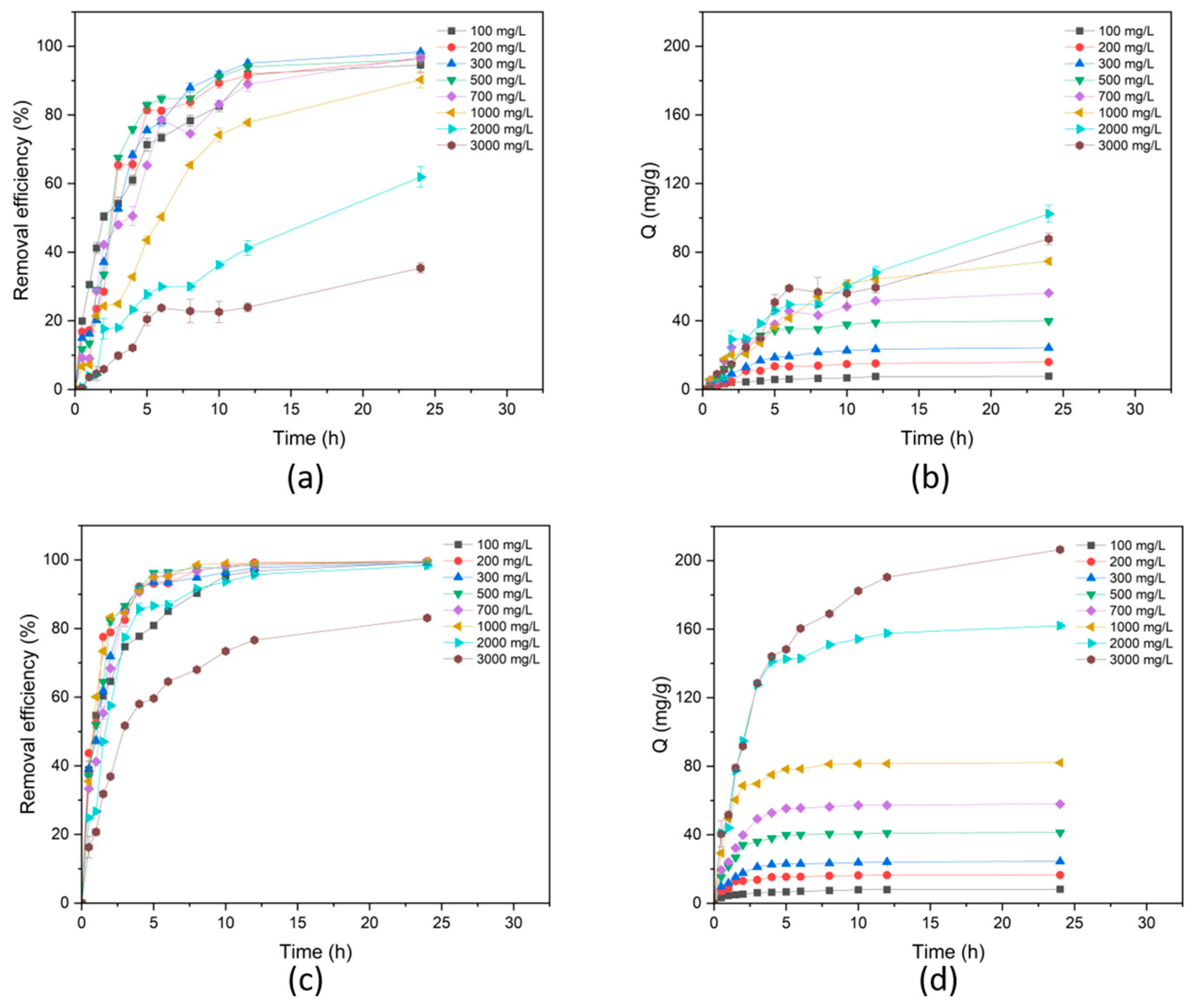
3.3.3. Adsorption Kinetic
3.3.4. Adsorption Isotherm
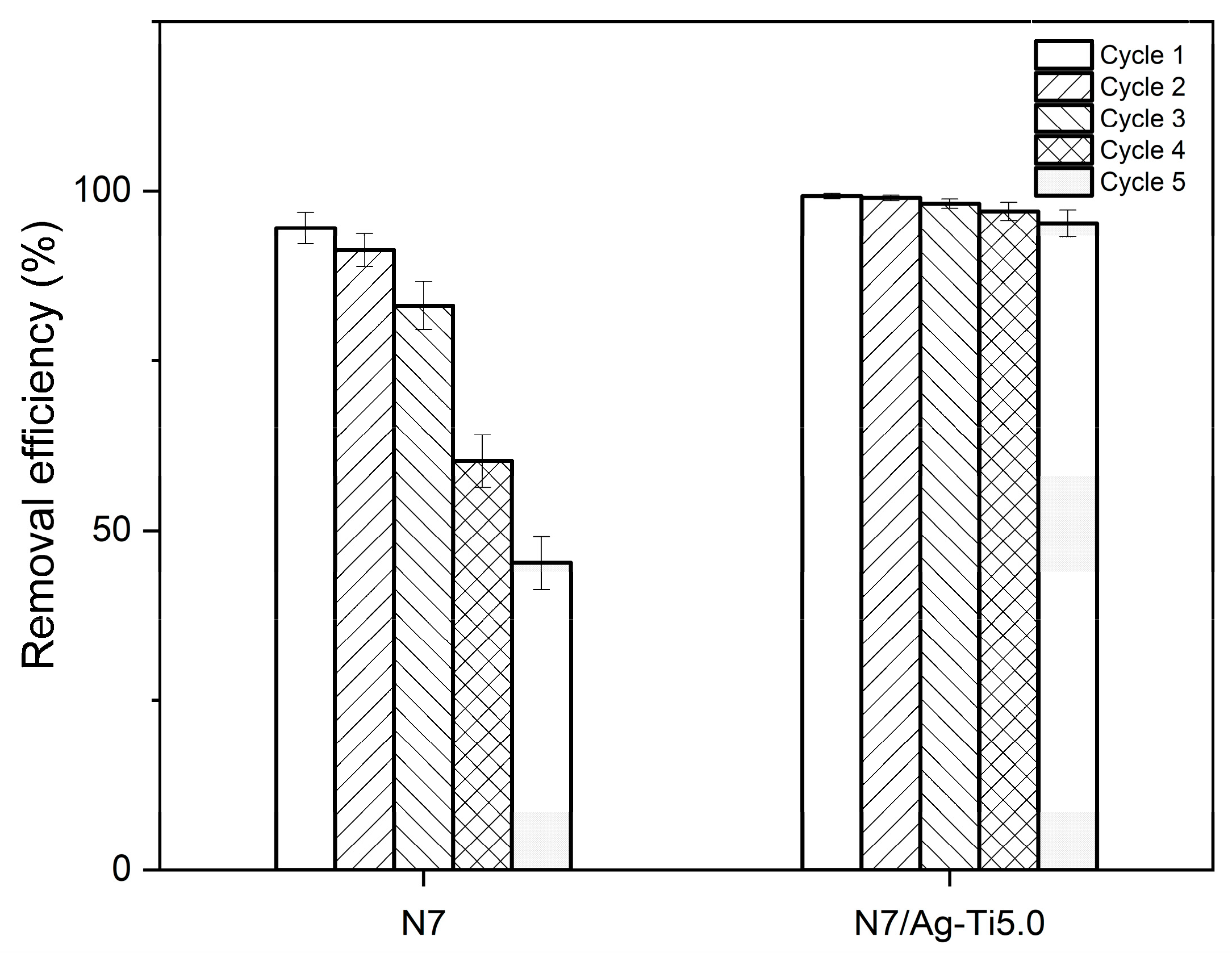
3.3.5. Reusability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cook, A.B.; Perrier, S. Branched and Dendritic Polymer Architectures: Functional Nanomaterials for Therapeutic Delivery. Adv. Funct. Mater. 2020, 30, 1901001. [Google Scholar] [CrossRef]
- Kavand, A.; Anton, N.; Vandamme, T.; Serra, C.A.; Chan-Seng, D. Synthesis and functionalization of hyperbranched polymers for targeted drug delivery. J. Control. Release 2020, 321, 285–311. [Google Scholar] [CrossRef]
- Xiao, F.; Cheng, J.; Cao, W.; Yang, C.; Chen, J.; Luo, Z. Removal of heavy metals from aqueous solution using chitosan-combined magnetic biochars. J. Colloid Interface Sci. 2019, 540, 579–584. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, J.; Jin, P.; Xu, D.; Yuan, S.; Zhao, R.; Depuydt, S.; Gao, Y.; Xu, Z.-L.; Van der Bruggen, B. A PEI/TMC membrane modified with an ionic liquid with enhanced permeability and antibacterial properties for the removal of heavy metal ions. J. Hazard. Mater. 2022, 435, 129010. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, J.; Wang, Y.; Liu, Q.; Zhong, Y.; Wang, Y.; Li, L.; Lincoln, S.F.; Guo, X. Preparation of a poly (acrylic acid) based hydrogel with fast adsorption rate and high adsorption capacity for the removal of cationic dyes. RSC Adv. 2019, 9, 21075–21085. [Google Scholar] [CrossRef]
- Huang, T.; Shao, Y.-W.; Zhang, Q.; Deng, Y.-F.; Liang, Z.-X.; Guo, F.-Z.; Li, P.-C.; Wang, Y. Chitosan-Cross-Linked Graphene Oxide/Carboxymethyl Cellulose Aerogel Globules with High Structure Stability in Liquid and Extremely High Adsorption Ability. ACS Sustain. Chem. Eng. 2019, 7, 8775–8788. [Google Scholar] [CrossRef]
- Singha, N.R.; Roy, C.; Mahapatra, M.; Dutta, A.; Deb Roy, J.S.; Mitra, M.; Chattopadhyay, P.K. Scalable Synthesis of Collagenic-Waste and Natural Rubber-Based Biocomposite for Removal of Hg(II) and Dyes: Approach for Cost-Friendly Waste Management. ACS Omega 2019, 4, 421–436. [Google Scholar] [CrossRef]
- Guerra, N.B.; Sant’Ana Pegorin, G.; Boratto, M.H.; de Barros, N.R.; de Oliveira Graeff, C.F.; Herculano, R.D. Biomedical applications of natural rubber latex from the rubber tree Hevea brasiliensis. Mater. Sci. Eng. C 2021, 126, 112126. [Google Scholar] [CrossRef]
- Mosaku, A.M.; Akinlabi, A.K.; Sojinu, O.S.; Arifalo, M.K.O.; Falomo, A.A.; Oladipo, G.; Oni, S.; Falope, F.Y.; Ilesanmi, N.Y.; Diayi, V.N. Adsorptive Remediation of Oil Spill Contaminated Water Using Chitosan Modified Natural Rubber as Adsorbent. Chem. Afr. 2021, 4, 535–543. [Google Scholar] [CrossRef]
- Jermjun, K.; Khumho, R.; Thongoiam, M.; Yousatit, S.; Yokoi, T.; Ngamcharussrivichai, C.; Nuntang, S. Natural Rubber/Hexagonal Mesoporous Silica Nanocomposites as Efficient Adsorbents for the Selective Adsorption of (-)-Epigallocatechin Gallate and Caffeine from Green Tea. Molecules 2023, 28, 6019. [Google Scholar] [CrossRef]
- Inphonlek, S.; Jarukumjorn, K.; Chumsamrong, P.; Ruksakulpiwat, C.; Ruksakulpiwat, Y. Preparation of Crosslinked Poly(acrylic acid-co-acrylamide)-Grafted Deproteinized Natural Rubber/Silica Composites as Coating Materials for Controlled Release of Fertilizer. Polymers 2023, 15, 1770. [Google Scholar] [CrossRef]
- Maijan, P.; Junlapong, K.; Arayaphan, J.; Khaokong, C.; Chantarak, S. Synthesis and characterization of highly elastic superabsorbent natural rubber/polyacrylamide hydrogel. Polym. Degrad. Stab. 2021, 186, 109499. [Google Scholar] [CrossRef]
- Mohamad Idris, N.H.; Rajakumar, J.; Cheong, K.Y.; Kennedy, B.J.; Ohno, T.; Yamakata, A.; Lee, H.L. Titanium Dioxide/Polyvinyl Alcohol/Cork Nanocomposite: A Floating Photocatalyst for the Degradation of Methylene Blue under Irradiation of a Visible Light Source. ACS Omega 2021, 6, 14493–14503. [Google Scholar] [CrossRef]
- Al-Madanat, O.; AlSalka, Y.; Ramadan, W.; Bahnemann, D.W. TiO2 Photocatalysis for the Transformation of Aromatic Water Pollutants into Fuels. Catalysts 2021, 11, 317. [Google Scholar] [CrossRef]
- Suriyachai, N.; Chuangchote, S.; Laosiripojana, N.; Champreda, V.; Sagawa, T. Synergistic Effects of Co-Doping on Photocatalytic Activity of Titanium Dioxide on Glucose Conversion to Value-Added Chemicals. ACS Omega 2020, 5, 20373–20381. [Google Scholar] [CrossRef]
- Wongpreecha, J.; Polpanich, D.; Suteewong, T.; Kaewsaneha, C.; Tangboriboonrat, P. One-pot, large-scale green synthesis of silver nanoparticles-chitosan with enhanced antibacterial activity and low cytotoxicity. Carbohydr. Polym. 2018, 199, 641–648. [Google Scholar] [CrossRef]
- Ni, Z.; Wang, Z.; Sun, L.; Li, B.; Zhao, Y. Synthesis of poly acrylic acid modified silver nanoparticles and their antimicrobial activities. Mater. Sci. Eng. C 2014, 41, 249–254. [Google Scholar] [CrossRef]
- Ghorbanloo, M.; Nosrati Fallah, H. Silver nanoparticle embedded anionic crosslinked copolymer hydrogels: An efficient catalyst. J. Porous Mater. 2020, 27, 765–777. [Google Scholar] [CrossRef]
- Fahmy, A.; Agudo Jácome, L.; Schönhals, A. Effect of Silver Nanoparticles on the Dielectric Properties and the Homogeneity of Plasma Poly(acrylic acid) Thin Films. J. Phys. Chem. C 2020, 124, 22817–22826. [Google Scholar] [CrossRef]
- Kawahara, S.; Klinklai, W.; Kuroda, H.; Isono, Y. Removal of proteins from natural rubber with urea. Polym. Adv. Technol. 2004, 15, 181–184. [Google Scholar] [CrossRef]
- Pipertzis, A.; Kafetzi, M.; Giaouzi, D.; Pispas, S.; Floudas, G.A. Grafted Copolymer Electrolytes Based on the Poly(acrylic acid-co-oligo ethylene glycol acrylate) (P(AA-co-OEGA)) Ion-Conducting and Mechanically Robust Block. ACS Appl. Polym. Mater. 2022, 4, 7070–7080. [Google Scholar] [CrossRef]
- Rimdusit, N.; Jubsilp, C.; Mora, P.; Hemvichian, K.; Thuy, T.T.; Karagiannidis, P.; Rimdusit, S. Radiation Graft-Copolymerization of Ultrafine Fully Vulcanized Powdered Natural Rubber: Effects of Styrene and Acrylonitrile Contents on Thermal Stability. Polymers 2021, 13, 3447. [Google Scholar] [CrossRef]
- Shi, Z.; Jia, C.; Wang, D.; Deng, J.; Xu, G.; Wu, C.; Dong, M.; Guo, Z. Synthesis and characterization of porous tree gum grafted copolymer derived from Prunus cerasifera gum polysaccharide. Int. J. Biol. Macromol. 2019, 133, 964–970. [Google Scholar] [CrossRef]
- Abdel-Raouf, M.E.-S.; Farag, R.K.; Farag, A.A.; Keshawy, M.; Abdel-Aziz, A.; Hasan, A. Chitosan-Based Architectures as an Effective Approach for the Removal of Some Toxic Species from Aqueous Media. ACS Omega 2023, 8, 10086–10099. [Google Scholar] [CrossRef]
- Mokhtari, A.; Sabzi, M.; Azimi, H. 3D porous bioadsorbents based on chitosan/alginate/cellulose nanofibers as efficient and recyclable adsorbents of anionic dye. Carbohydr. Polym. 2021, 265, 118075. [Google Scholar] [CrossRef]
- Singh, N.; Riyajuddin, S.; Ghosh, K.; Mehta, S.K.; Dan, A. Chitosan-Graphene Oxide Hydrogels with Embedded Magnetic Iron Oxide Nanoparticles for Dye Removal. ACS Appl. Nano Mater. 2019, 2, 7379–7392. [Google Scholar] [CrossRef]
- Cao, W.; Ding, P.; Ding, Q.; Liu, C.; Yu, W.; Hu, L. Shear Responsive Gelation of Aqueous Polyacrylic Acid-co-polyacrylamide: Molecular Mechanism and Tribological Applications. ACS Appl. Polym. Mater. 2023, 5, 3247–3255. [Google Scholar] [CrossRef]
- Arunbabu, D.; Shahsavan, H.; Zhang, W.; Zhao, B. Poly(AAc-co-MBA) Hydrogel Films: Adhesive and Mechanical Properties in Aqueous Medium. J. Phys. Chem. B 2013, 117, 441–449. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.K.; Seyfoddin, A.; Fatihhi, S.J. Acrylic acid/acrylamide based hydrogels and its properties—A review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Konko, I.; Guriyanova, S.; Boyko, V.; Sun, L.; Liu, D.; Reck, B.; Men, Y. Role of the Hydrophilic Latex Particle Surface in Water Diffusion into Films from Waterborne Polymer Colloids. Langmuir 2019, 35, 6075–6088. [Google Scholar] [CrossRef]
- Jing, Z.; Xu, A.; Liang, Y.-Q.; Zhang, Z.; Yu, C.; Hong, P.; Li, Y. Biodegradable Poly(acrylic acid-co-acrylamide)/Poly(vinyl alcohol) Double Network Hydrogels with Tunable Mechanics and High Self-healing Performance. Polymers 2019, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Ishiaku, U.S.; Shaharum, A.; Ismail, H.; Ishak, Z.A.M. The effect of an epoxidized plasticizer on the thermo-oxidative ageing of poly(vinyl chloride)/epoxidized natural rubber thermoplastic elastomers. Polymer Int. 1998, 45, 83–91. [Google Scholar] [CrossRef]
- Chalid, M.; Husnil, Y.A.; Puspitasari, S.; Cifriadi, A. Experimental and Modelling Study of the Effect of Adding Starch-Modified Natural Rubber Hybrid to the Vulcanization of Sorghum Fibers-Filled Natural Rubber. Polymers 2020, 12, 3017. [Google Scholar] [CrossRef]
- Xu, C.; Chen, Y.; Zheng, Z.; Liu, Y.; Cao, S.; Xu, Y. Mussel-Inspired Biocompatible PAADOPA/PAAm Hydrogel Adhesive for Amoxicillin Delivery. Ind. Eng. Chem. Res. 2020, 59, 13556–13563. [Google Scholar] [CrossRef]
- Yan, K.-Y.; Chen, J.-Y.; Li, X.-Y.; Wang, Q.; Kuang, G.-C. Carboxylic Acid Enriched Porous Organic Polymer as a Platform for Highly Efficient Removal of Methylene Blue from Aqueous Solution. Macromol. Chem. Phys. 2020, 221, 1900553. [Google Scholar] [CrossRef]
- Liufu, S.; Xiao, H.; Li, Y. Adsorption of Poly(Acrylic Acid) onto the Surface of Titanium Dioxide and the Colloidal Stability of Aqueous Suspension. J. Colloid Interface Sci. 2005, 281, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Mofidfar, M.; Kim, E.S.; Larkin, E.L.; Long, L.; Jennings, W.D.; Ahadian, S.; Ghannoum, M.A.; Wnek, G.E. Antimicrobial Activity of Silver Containing Crosslinked Poly(Acrylic Acid) Fibers. Micromachines 2019, 10, 829. [Google Scholar] [CrossRef]
- Varghese Alex, K.; Tamil Pavai, P.; Rugmini, R.; Shiva Prasad, M.; Kamakshi, K.; Sekhar, K.C. Green Synthesized Ag Nanoparticles for Bio-Sensing and Photocatalytic Applications. ACS Omega 2020, 5, 13123–13129. [Google Scholar] [CrossRef]
- Singh, J.; Dhaliwal, A. Water retention and controlled release of KCl by using microwave-assisted green synthesis of xanthan gum-cl-poly (acrylic acid)/AgNPs hydrogel nanocomposite. Polym. Bull. 2020, 77, 4867–4893. [Google Scholar] [CrossRef]
- Alim-Al-Razy, M.; Asik Bayazid, G.M.; Rahman, R.U.; Bosu, R.; Shamma, S.S. Silver nanoparticle synthesis, UV-Vis spectroscopy to find particle size and measure resistance of colloidal solution. J. Phys. Conf. Ser. 2020, 1706, 012020. [Google Scholar] [CrossRef]
- Lu, Y.; Mei, Y.; Schrinner, M.; Ballauff, M.; Möller, M.W.; Breu, J. In Situ Formation of Ag Nanoparticles in Spherical Polyacrylic Acid Brushes by UV Irradiation. J. Phys. Chem. C 2007, 111, 7676–7681. [Google Scholar] [CrossRef]
- Abutalib, M.M.; Rajeh, A. Enhanced structural, electrical, mechanical properties and antibacterial activity of Cs/PEO doped mixed nanoparticles (Ag/TiO2) for food packaging applications. Polym. Test. 2021, 93, 107013. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Jiang, S.; Zhang, X.; Bi, F.; Yang, Y.; Wang, Y.; Wang, Z. Enhanced photocatalytic degradation of gaseous toluene and liquidus tetracycline by anatase/rutile titanium dioxide with heterophase junction derived from materials of Institut Lavoisier-125(Ti): Degradation pathway and mechanism studies. J. Colloid Interface Sci. 2021, 588, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Puskás, I.; Sohajda, T.; Varga, E.; Vass, P.; Nagy, Z.K.; Farkas, A.; Várnai, B.; Béni, S.; Hazai, E. Sulfobutylether-beta-cyclodextrin-enabled antiviral remdesivir: Characterization of electrospun- and lyophilized formulations. Carbohydr. Polym. 2021, 264, 118011. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Fang, G.; Jiang, Y.; Song, H.; Zhang, Y.; Zhao, Q. Emulsion Lyophilization as a Facile Pathway to Fabricate Stretchable Polymer Foams Enabling Multishape Memory Effect and Clip Application. ACS Appl. Mater. Interfaces 2019, 11, 32423–32430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, P.; Lan, G.; Liu, Y.; Cai, Q.; Xi, J. High crosslinked sodium carboxyl methylstarch-g-poly (acrylic acid-co-acrylamide) resin for heavy metal adsorption: Its characteristics and mechanisms. Environ. Sci. Pollut. Res. 2020, 27, 38617–38630. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Ma, K.; Jiao, T.; Xing, R.; Li, K.; Zhou, J.; Zhang, L. Preparation and dye removal capacities of porous silver nanoparticle-containing composite hydrogels via poly (acrylic acid) and silver ions. RSC Adv. 2016, 6, 110799–110807. [Google Scholar] [CrossRef]
- Toader, G.; Ginghina, R.E.; Diacon, A.; Rusen, E.; Bratu, A.E.; Podaru, A.; Rotariu, T. Design and Application of Photocrosslinkable Hydrogel Films for Fast and Efficient Decontamination of Chemical Warfare Agents. ACS Appl. Polym. Mater. 2023, 5, 877–891. [Google Scholar] [CrossRef]
- Fan, M.; Wu, L.; Hu, Y.; Qu, M.; Yang, S.; Tang, P.; Pan, L.; Wang, H.; Bin, Y. A highly stretchable natural rubber/buckypaper/natural rubber (NR/N-BP/NR) sandwich strain sensor with ultrahigh sensitivity. Adv. Compos. Hybrid Mater. 2021, 4, 1039–1047. [Google Scholar] [CrossRef]
- Hosseini, H.; Zirakjou, A.; McClements, D.J.; Goodarzi, V.; Chen, W.-H. Removal of methylene blue from wastewater using ternary nanocomposite aerogel systems: Carboxymethyl cellulose grafted by polyacrylic acid and decorated with graphene oxide. J. Hazard. Mater. 2022, 421, 126752. [Google Scholar] [CrossRef]
- Lv, Q.; Shen, Y.; Qiu, Y.; Wu, M.; Wang, L. Poly(acrylic acid)/poly(acrylamide) hydrogel adsorbent for removing methylene blue. J. Appl. Polym. Sci. 2020, 137, 49322. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Q.; Zhang, F. Preparation of Fe3O4@SiO2@ P(AANa-co-AM) Composites and Their Adsorption for Pb(II). ACS Omega 2020, 5, 8816–8824. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Rangaiah, G.P.; Zhao, X.S. Photocatalytic Degradation of Methylene Blue by Titanium Dioxide: Experimental and Modeling Study. Ind. Eng. Chem. Res. 2014, 53, 14641–14649. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Visible-Light Active Titanium Dioxide Nanomaterials with Bactericidal Properties. Nanomaterials 2020, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Skiba, M.; Vorobyova, V.; Pasenko, O. Surface modification of titanium dioxide with silver nanoparticles for application in photocatalysis. Appl. Nanosci. 2022, 12, 1175–1182. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A.E.K.; Bouhfid, R. Recent progress on Ag/TiO2 photocatalysts: Photocatalytic and bactericidal behaviors. Environ. Sci. Pollut. Res. 2021, 28, 44638–44666. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Q.; Li, Y.; Zhang, Q.; Huang, J.; Wu, Q.; Wang, S. Fabrication of Cellulose Nanocrystal-g-Poly(Acrylic Acid-Co-Acrylamide) Aerogels for Efficient Pb(II) Removal. Polymers 2020, 12, 333. [Google Scholar] [CrossRef]
- Ghanei, M.; Rashidi, A.; Tayebi, H.-A.; Yazdanshenas, M.E. Removal of Acid Blue 25 from Aqueous Media by Magnetic-SBA-15/CPAA Super Adsorbent: Adsorption Isotherm, Kinetic, and Thermodynamic Studies. J. Chem. Eng. Data 2018, 63, 3592–3605. [Google Scholar] [CrossRef]
- Yang, J.; Wang, K.; Lv, Z.; Li, W.; Luo, K.; Cao, Z. Facile Preparation and Dye Adsorption Performance of Poly(N-isopropylacrylamide-co-acrylic acid)/Molybdenum Disulfide Composite Hydrogels. ACS Omega 2021, 6, 28285–28296. [Google Scholar] [CrossRef]

| Samples | Monomer Contents (phr) * | AA:AM | MBA Contents ** (%) | Conversion (%) | Grafting Efficiency (%) | Grafting Percentage (%) |
|---|---|---|---|---|---|---|
| N1 | 10 | 70:30 | 0 | 81.51 ± 2.04 | 12.44 ± 1.36 | 1.24 ± 0.14 |
| N2 | 10 | 70:30 | 0.25 | 80.69 ± 0.14 | 15.60 ± 2.65 | 1.56 ± 0.27 |
| N3 | 10 | 70:30 | 0.50 | 79.19 ± 4.40 | 27.60 ± 0.69 | 2.76 ± 0.07 |
| N4 | 10 | 70:30 | 1.00 | 82.66 ± 2.38 | 48.48 ± 4.32 | 4.85 ± 0.43 |
| N5 | 10 | 50:50 | 1.00 | 81.91 ± 3.23 | 19.30 ± 3.32 | 1.93 ± 0.33 |
| N6 | 10 | 30:70 | 1.00 | 80.62 ± 2.49 | 10.20 ± 2.33 | 1.02 ± 0.23 |
| N7 | 20 | 70:30 | 1.00 | 83.34 ± 3.01 | 54.26 ± 1.55 | 10.63 ± 0.31 |
| Samples | N7 | N7/Ag | N7/Ag-Ti1.0 | N7/Ag-Ti2.5 | N7/Ag-Ti5.0 | N7-Ti5.0 |
|---|---|---|---|---|---|---|
| (PAA-co-PAM)-DPNR (phr) | 100 | 100 | 100 | 100 | 100 | 100 |
| AgNO3 (phr) | 0 | 0.5 | 0.5 | 0.5 | 0.5 | 0 |
| TiO2 (phr) | 0 | 0 | 1.0 | 2.5 | 5.0 | 5.0 |
| Characteristics |  |  |  |  |  |  |
| Samples | Weight (%) | Atomic (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | O | Na | Ag | Ti | C | O | Na | Ag | Ti | |
| N7 | 83.13 | 14.79 | 2.08 | 0.00 | 0.00 | 87.21 | 11.65 | 1.14 | 0.00 | 0.00 |
| N7/Ag | 75.98 | 21.95 | 1.74 | 0.32 | 0.00 | 81.34 | 17.64 | 0.97 | 0.04 | 0.00 |
| N7/Ag-Ti1.0 | 83.73 | 14.18 | 1.53 | 0.43 | 0.13 | 87.90 | 11.17 | 0.84 | 0.05 | 0.04 |
| N7/Ag-Ti2.5 | 84.81 | 12.72 | 1.67 | 0.47 | 0.33 | 88.93 | 10.01 | 0.91 | 0.06 | 0.09 |
| N7/Ag-Ti5.0 | 87.66 | 9.31 | 1.70 | 0.55 | 0.79 | 91.51 | 7.30 | 0.93 | 0.06 | 0.21 |
| N7-Ti5.0 | 78.90 | 18.24 | 1.93 | 0.00 | 0.94 | 84.08 | 14.60 | 1.07 | 0.00 | 0.25 |
| Initial Dye Concentration (mg/L) | Qe(exp) (mg/g) | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|---|---|
| k1 (L/h) | Qe(theo) (mg/g) | R2 | k2 (g/mg·h) | Qe(theo) (mg/g) | R2 | ||
| N7 | |||||||
| 100 | 7.78 | 0.0991 | 8.19 | 0.9250 | 0.0482 | 8.60 | 0.9974 |
| 200 | 15.97 | 0.0816 | 15.90 | 0.6289 | 0.0133 | 19.51 | 0.9519 |
| 300 | 24.28 | 0.1194 | 24.07 | 0.7490 | 0.0076 | 30.30 | 0.9495 |
| 500 | 39.95 | 0.1190 | 36.00 | 0.8154 | 0.0053 | 49.26 | 0.9357 |
| 700 | 56.24 | 0.0840 | 54.98 | 0.4931 | 0.0022 | 74.07 | 0.8769 |
| 1000 | 74.68 | 0.0675 | 77.87 | 0.9721 | 0.0007 | 117.65 | 0.8394 |
| 2000 | 102.35 | 0.0193 | 197.60 | 0.6736 | 0.0006 | 140.85 | 0.9060 |
| 3000 | 87.77 | 0.0685 | 74.54 | 0.8757 | 0.0005 | 140.85 | 0.8311 |
| N7/Ag-Ti5.0 | |||||||
| 100 | 8.20 | 0.2676 | 7.24 | 0.9765 | 0.1034 | 8.59 | 0.9991 |
| 200 | 16.54 | 0.1500 | 16.00 | 0.9324 | 0.1013 | 17.06 | 0.9994 |
| 300 | 24.43 | 0.1579 | 22.74 | 0.9021 | 0.0519 | 25.38 | 0.9990 |
| 500 | 41.25 | 0.2702 | 40.33 | 0.9704 | 0.0375 | 42.74 | 0.9989 |
| 700 | 57.89 | 0.0779 | 57.64 | 0.9511 | 0.0181 | 60.98 | 0.9973 |
| 1000 | 82.04 | 0.0791 | 72.00 | 0.9308 | 0.0208 | 84.75 | 0.9993 |
| 2000 | 162.02 | 0.1152 | 162.34 | 0.9680 | 0.0036 | 175.44 | 0.9933 |
| 3000 | 206.42 | 0.0980 | 198.00 | 0.9855 | 0.0015 | 232.56 | 0.9976 |
| Samples | Langmuir Adsorption Isotherm | Freundlich Adsorption Isotherm | |||||
|---|---|---|---|---|---|---|---|
| Qm (mg/g) | KL (L/mg) | RL | R2 | KF (mg/g)(L/mg)1/n | n | R2 | |
| N7 | |||||||
| 90.09 | 0.0982 | 0.0053 | 0.9963 | 0.0131 | 0.4604 | 0.6933 | |
| N7/Ag-Ti5.0 | |||||||
| 208.33 | 0.1387 | 0.0142 | 0.9998 | 0.0010 | 0.5673 | 0.7406 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inphonlek, S.; Ruksakulpiwat, C.; Ruksakulpiwat, Y. The Effect of Silver Nanoparticles/Titanium Dioxide in Poly(acrylic acid-co-acrylamide)-Modified, Deproteinized, Natural Rubber Composites on Dye Removal. Polymers 2024, 16, 92. https://doi.org/10.3390/polym16010092
Inphonlek S, Ruksakulpiwat C, Ruksakulpiwat Y. The Effect of Silver Nanoparticles/Titanium Dioxide in Poly(acrylic acid-co-acrylamide)-Modified, Deproteinized, Natural Rubber Composites on Dye Removal. Polymers. 2024; 16(1):92. https://doi.org/10.3390/polym16010092
Chicago/Turabian StyleInphonlek, Supharat, Chaiwat Ruksakulpiwat, and Yupaporn Ruksakulpiwat. 2024. "The Effect of Silver Nanoparticles/Titanium Dioxide in Poly(acrylic acid-co-acrylamide)-Modified, Deproteinized, Natural Rubber Composites on Dye Removal" Polymers 16, no. 1: 92. https://doi.org/10.3390/polym16010092
APA StyleInphonlek, S., Ruksakulpiwat, C., & Ruksakulpiwat, Y. (2024). The Effect of Silver Nanoparticles/Titanium Dioxide in Poly(acrylic acid-co-acrylamide)-Modified, Deproteinized, Natural Rubber Composites on Dye Removal. Polymers, 16(1), 92. https://doi.org/10.3390/polym16010092









