Biopolymeric Nanocomposites for CO2 Capture
Abstract
1. Introduction
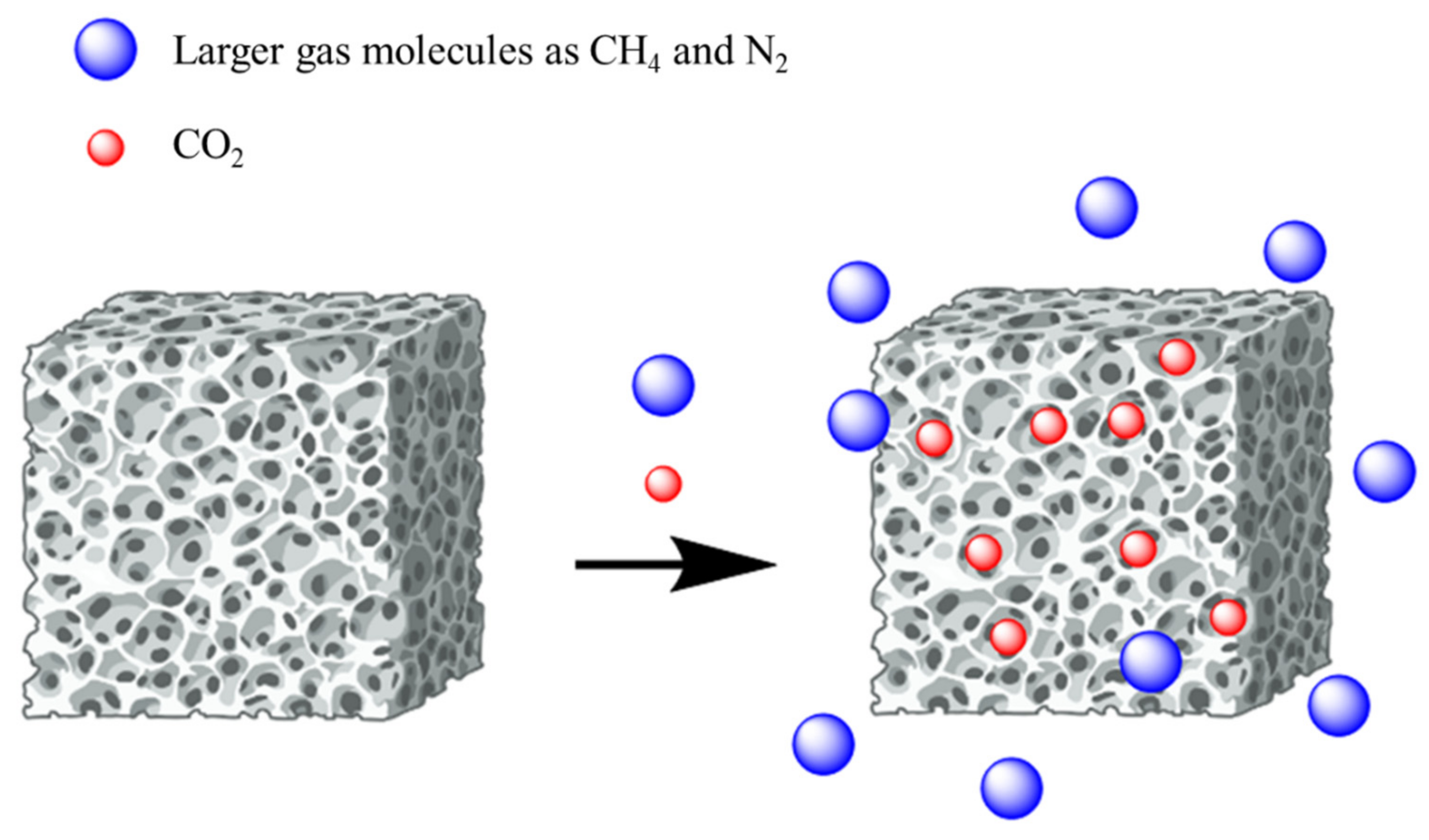
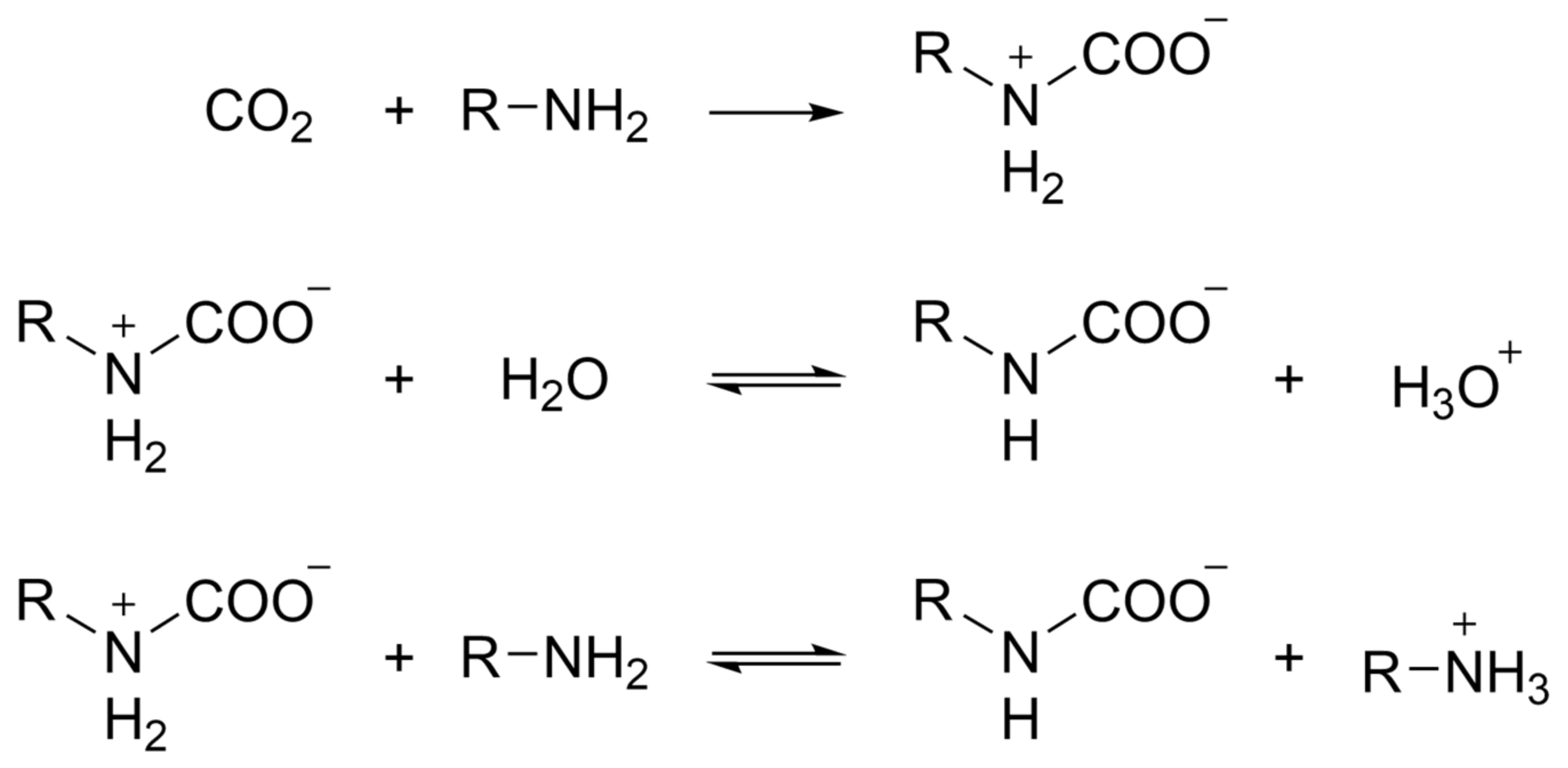
2. CO2 Capture Mechanism
- Post-combustion capture, where CO2 is captured after fuel combustion.
- Pre-combustion capture, where CO2 is captured before it is released into the atmosphere.
- Oxy-fuel combustion, which involves burning fossil fuels using oxygen (O2) and recycled flue gas as a substitute for air.
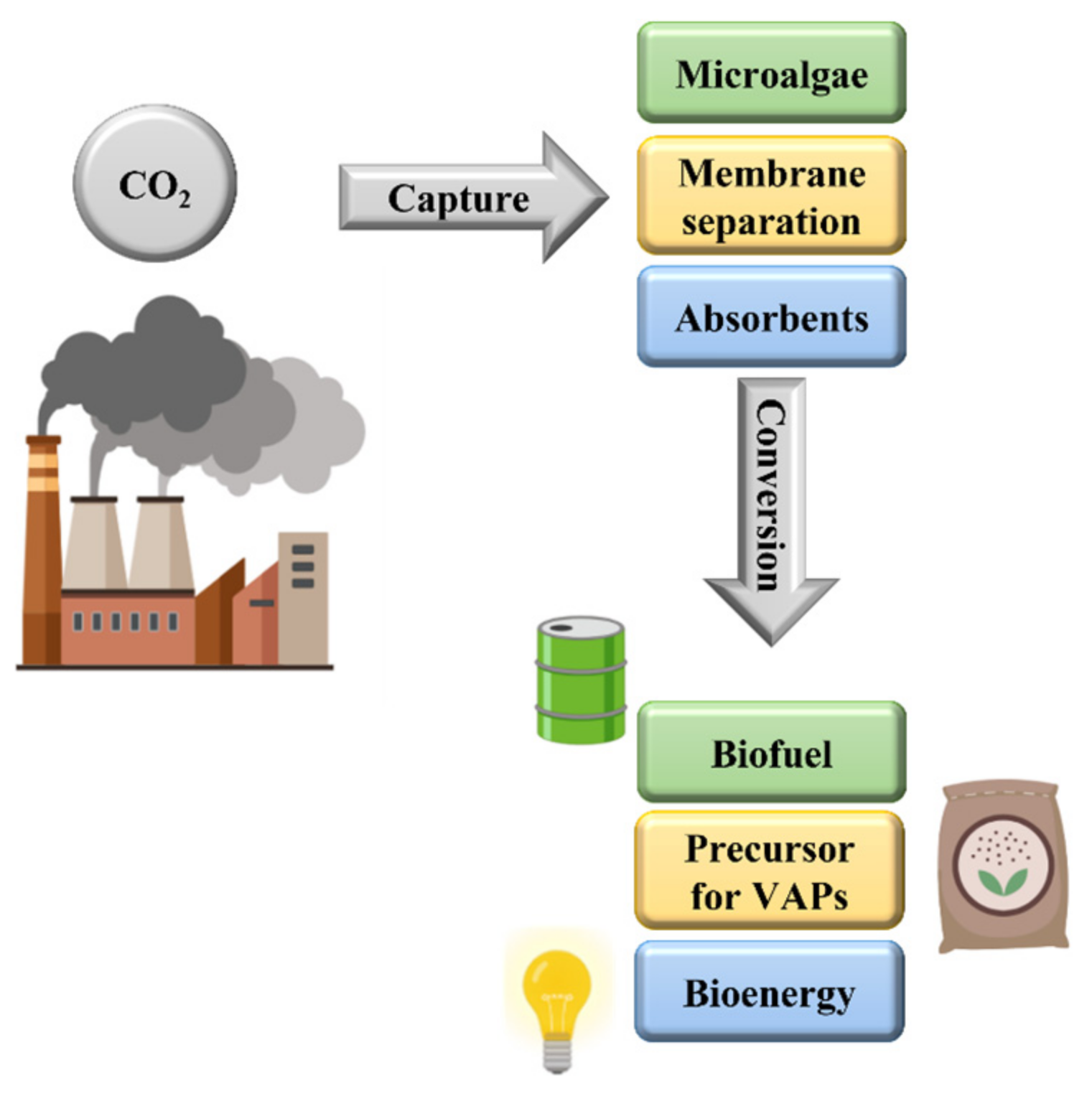
Enhancing CO2 Capture Capacity
3. Biopolymers for CO2 Capture
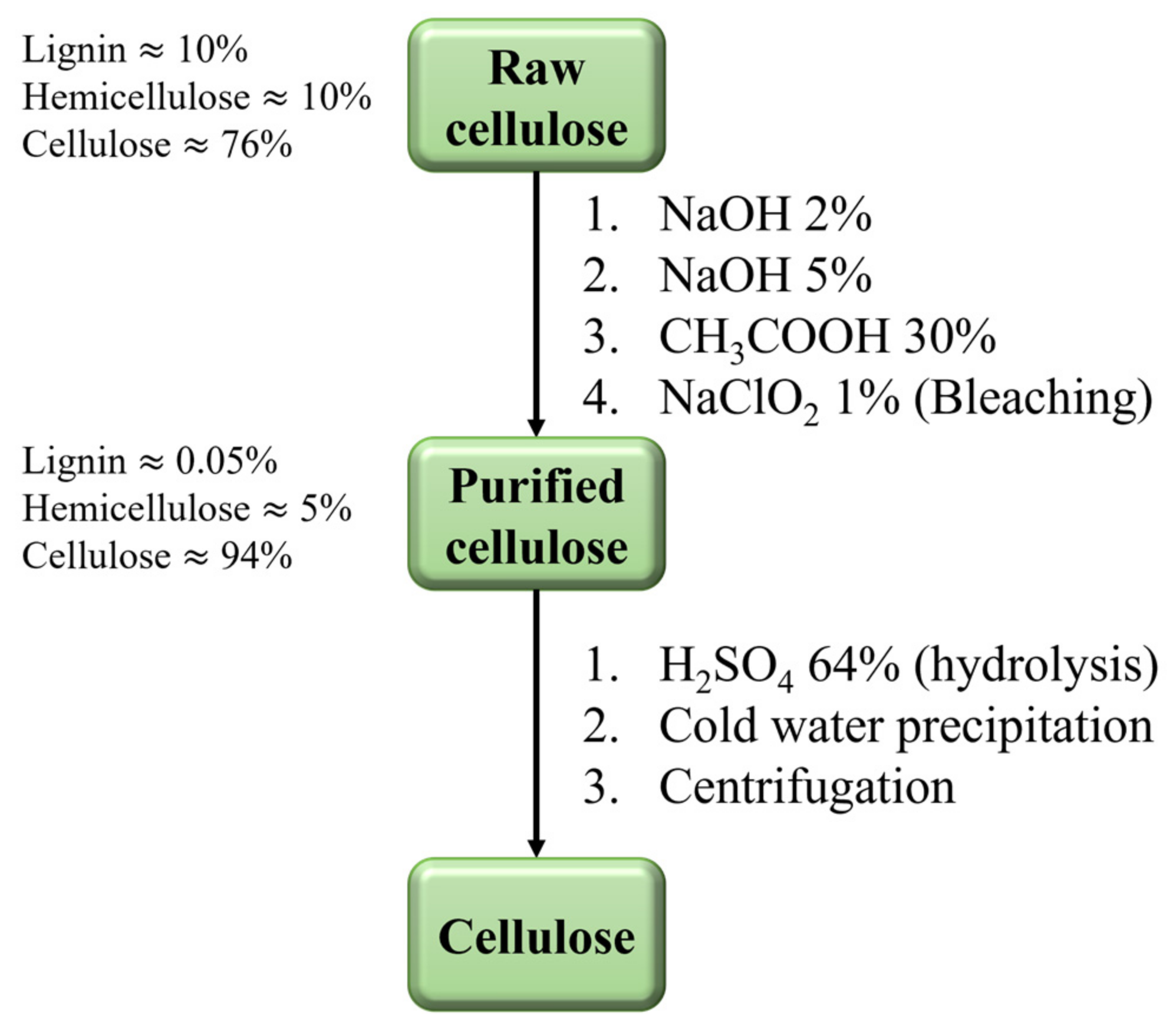
3.1. Cellulose
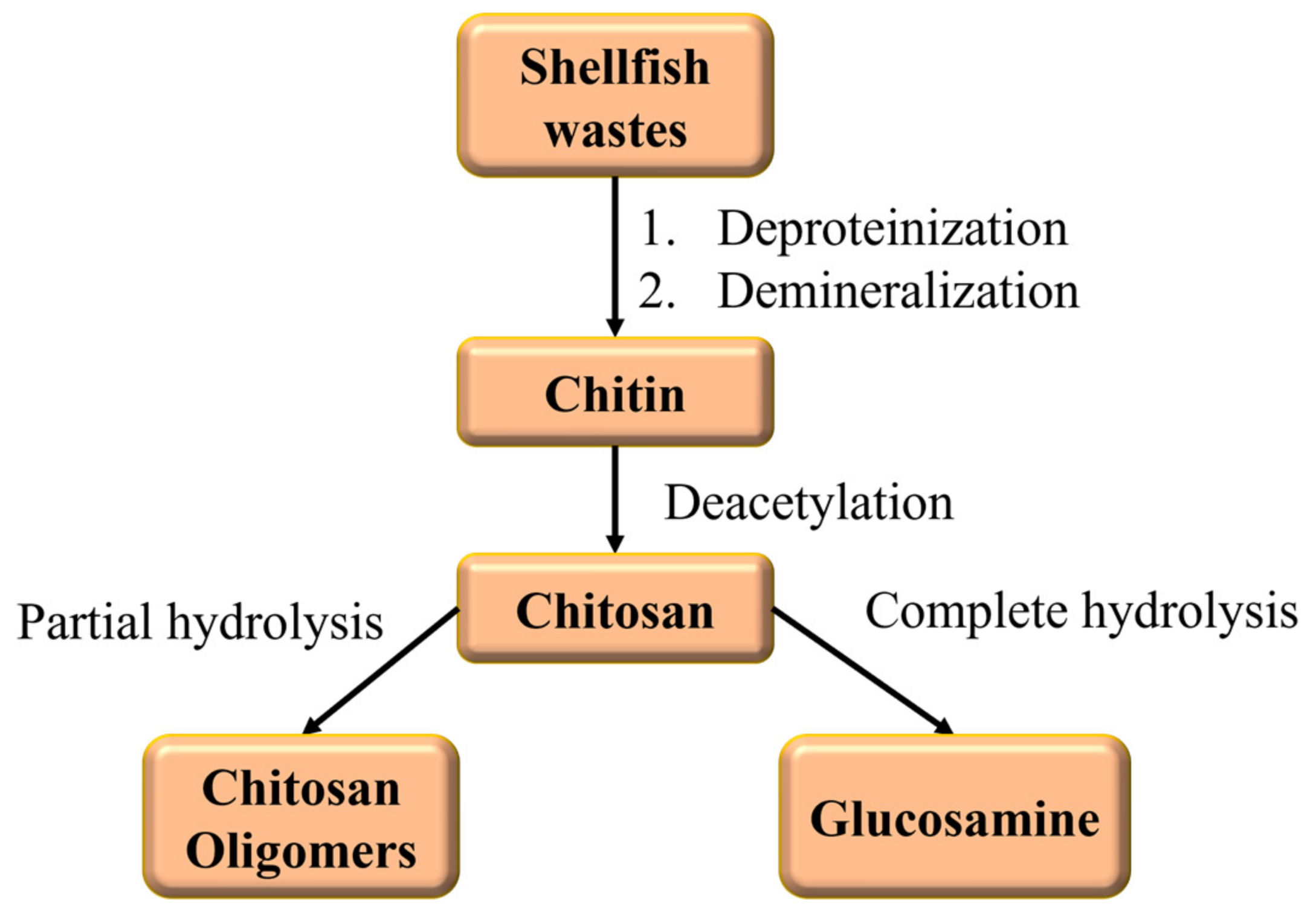
3.2. Alginate and Chitosan
3.3. Carrageenan
4. Industrial Scale-Up
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Raza, S.; Orooji, Y.; Ghasali, E.; Hayat, A.; Karimi-Maleh, H.; Lin, H. Engineering Approaches for CO2 Converting to Biomass Coupled with Nanobiomaterials as Biomediated towards Circular Bioeconomy. J. CO2 Util. 2023, 67, 102295. [Google Scholar] [CrossRef]
- Ghanbari, T.; Abnisa, F.; Wan Daud, W.M.A. A Review on Production of Metal Organic Frameworks (MOF) for CO2 Adsorption. Sci. Total Environ. 2020, 707, 135090. [Google Scholar] [CrossRef]
- Cheung, O.; Hedin, N. Zeolites and Related Sorbents with Narrow Pores for CO2 Separation from Flue Gas. RSC Adv. 2014, 4, 14480–14494. [Google Scholar] [CrossRef]
- Villari, V.; Mazzaglia, A.; Trapani, M.; Castriciano, M.A.; de Luca, G.; Romeo, A.; Scolaro, L.M.; Micali, N. Optical Enhancement and Structural Properties of a Hybrid Organic−Inorganic Ternary Nanocomposite. J. Phys. Chem. C 2011, 115, 5435–5439. [Google Scholar] [CrossRef]
- Li, Y.; Jia, P.; Xu, J.; Wu, Y.; Jiang, H.; Li, Z. The Aminosilane Functionalization of Cellulose Nanofibrils and the Mechanical and CO2 Adsorption Characteristics of Their Aerogel. Ind. Eng. Chem. Res. 2020, 59, 2874–2882. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nyström, G. Biopolymer Aerogels and Foams: Chemistry, Properties, and Applications. Angew. Chem. Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef] [PubMed]
- Bose, I.; Nousheen; Roy, S.; Yaduvanshi, P.; Sharma, S.; Chandel, V.; Biswas, D. Unveiling the Potential of Marine Biopolymers: Sources, Classification, and Diverse Food Applications. Materials 2023, 16, 4840. [Google Scholar] [CrossRef] [PubMed]
- Raghav, N.; Vashisth, C.; Mor, N.; Arya, P.; Sharma, M.R.; Kaur, R.; Bhatti, S.P.; Kennedy, J.F. Recent Advances in Cellulose, Pectin, Carrageenan and Alginate-Based Oral Drug Delivery Systems. Int. J. Biol. Macromol. 2023, 244, 125357. [Google Scholar] [CrossRef]
- Erans, M.; Sanz-Pérez, E.S.; Hanak, D.P.; Clulow, Z.; Reiner, D.M.; Mutch, G.A. Direct Air Capture: Process Technology, Techno-Economic and Socio-Political Challenges. Energy Environ. Sci. 2022, 15, 1360–1405. [Google Scholar] [CrossRef]
- Fasihi, M.; Efimova, O.; Breyer, C. Techno-Economic Assessment of CO2 Direct Air Capture Plants. J. Clean. Prod. 2019, 224, 957–980. [Google Scholar] [CrossRef]
- Daneshvar, E.; Wicker, R.J.; Show, P.-L.; Bhatnagar, A. Biologically-Mediated Carbon Capture and Utilization by Microalgae towards Sustainable CO2 Biofixation and Biomass Valorization—A Review. Chem. Eng. J. 2022, 427, 130884. [Google Scholar] [CrossRef]
- Alami, A.H.; Tawalbeh, M.; Alasad, S.; Ali, M.; Alshamsi, M.; Aljaghoub, H. Cultivation of Nannochloropsis Algae for Simultaneous Biomass Applications and Carbon Dioxide Capture. Energy Sources Part A Recovery Util. Environ. Eff. 2021, 1–12. [Google Scholar] [CrossRef]
- Yadav, G.; Dubey, B.K.; Sen, R. A Comparative Life Cycle Assessment of Microalgae Production by CO2 Sequestration from Flue Gas in Outdoor Raceway Ponds under Batch and Semi-Continuous Regime. J. Clean. Prod. 2020, 258, 120703. [Google Scholar] [CrossRef]
- Goli, A.; Shamiri, A.; Talaiekhozani, A.; Eshtiaghi, N.; Aghamohammadi, N.; Aroua, M.K. An Overview of Biological Processes and Their Potential for CO2 Capture. J. Environ. Manag. 2016, 183, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Onyeaka, H.; Ekwebelem, O.C. A Review of Recent Advances in Engineering Bacteria for Enhanced CO2 Capture and Utilization. Int. J. Environ. Sci. Technol. 2023, 20, 4635–4648. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Li, Y.; Ye, C.; Liu, L.; Chen, X. Engineering Microorganisms for Enhanced CO2 Sequestration. Trends Biotechnol. 2019, 37, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Soudan, B. Community-Scale Baseload Generation from Marine Energy. Energy 2019, 189, 116134. [Google Scholar] [CrossRef]
- Mohan, S.V.; Modestra, J.A.; Amulya, K.; Butti, S.K.; Velvizhi, G. A Circular Bioeconomy with Biobased Products from CO2 Sequestration. Trends Biotechnol. 2016, 34, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Luis, P.; Van der Bruggen, B. The Role of Membranes in Post-Combustion CO2 Capture. Greenh. Gases Sci. Technol. 2013, 3, 318–337. [Google Scholar] [CrossRef]
- Jana, A.; Modi, A. Recent Progress on Functional Polymeric Membranes for CO2 Separation from Flue Gases: A Review. Carbon Capture Sci. Technol. 2024, 11, 100204. [Google Scholar] [CrossRef]
- Han, Y.; Yang, Y.; Ho, W.S.W. Recent Progress in the Engineering of Polymeric Membranes for CO2 Capture from Flue Gas. Membranes 2020, 10, 365. [Google Scholar] [CrossRef]
- Jang, E.; Hong, S.; Kim, E.; Choi, N.; Cho, S.J.; Choi, J. Organic Template-Free Synthesis of High-Quality CHA Type Zeolite Membranes for Carbon Dioxide Separation. J. Membr. Sci. 2018, 549, 46–59. [Google Scholar] [CrossRef]
- Chernikova, V.; Shekhah, O.; Belmabkhout, Y.; Eddaoudi, M. Nanoporous Fluorinated Metal–Organic Framework-Based Membranes for CO2 Capture. ACS Appl. Nano Mater. 2020, 3, 6432–6439. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yang, H.-C.; Chung, D.-Y.; Yang, I.-H.; Choi, Y.J.; Moon, J. Functionalized Mesoporous Silica Membranes for CO2 Separation Applications. J. Chem. 2015, 2015, e202867. [Google Scholar] [CrossRef]
- Khdary, N.H.; Abdelsalam, M.E. Polymer-Silica Nanocomposite Membranes for CO2 Capturing. Arab. J. Chem. 2020, 13, 557–567. [Google Scholar] [CrossRef]
- Ghalei, B.; Sakurai, K.; Kinoshita, Y.; Wakimoto, K.; Isfahani, A.P.; Song, Q.; Doitomi, K.; Furukawa, S.; Hirao, H.; Kusuda, H.; et al. Enhanced Selectivity in Mixed Matrix Membranes for CO2 Capture through Efficient Dispersion of Amine-Functionalized MOF Nanoparticles. Nat. Energy 2017, 2, 17086. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the Right Stuff: The Trade-off between Membrane Permeability and Selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, N.; Tanaka, M.; Yamato, M.; Kawakami, H. Superhigh CO2-Permeable Mixed Matrix Membranes Composed of a Polymer of Intrinsic Microporosity (PIM-1) and Surface-Modified Silica Nanoparticles. ACS Appl. Polym. Mater. 2019, 1, 2516–2524. [Google Scholar] [CrossRef]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and Trends in CO2 Capture/Separation Technologies: A Review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Al-Hamed, K.H.M.; Dincer, I. A Comparative Review of Potential Ammonia-Based Carbon Capture Systems. J. Environ. Manag. 2021, 287, 112357. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; He, M.; Zhao, J.; Jin, W. Structural Manipulation of ZIF-8-Based Membranes for High-Efficiency Molecular Separation. Sep. Purif. Technol. 2021, 270, 118722. [Google Scholar] [CrossRef]
- Lei, L.; Bai, L.; Lindbråthen, A.; Pan, F.; Zhang, X.; He, X. Carbon Membranes for CO2 Removal: Status and Perspectives from Materials to Processes. Chem. Eng. J. 2020, 401, 126084. [Google Scholar] [CrossRef]
- Shewchuk, S.R.; Mukherjee, A.; Dalai, A.K. Selective Carbon-Based Adsorbents for Carbon Dioxide Capture from Mixed Gas Streams and Catalytic Hydrogenation of CO2 into Renewable Energy Source: A Review. Chem. Eng. Sci. 2021, 243, 116735. [Google Scholar] [CrossRef]
- Deline, A.R.; Frank, B.P.; Smith, C.L.; Sigmon, L.R.; Wallace, A.N.; Gallagher, M.J.; Goodwin, D.G., Jr.; Durkin, D.P.; Fairbrother, D.H. Influence of Oxygen-Containing Functional Groups on the Environmental Properties, Transformations, and Toxicity of Carbon Nanotubes. Chem. Rev. 2020, 120, 11651–11697. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; Wang, Y.-F.; You, S.-J.; Chao, H.-P. Insights into the Mechanism of Cationic Dye Adsorption on Activated Charcoal: The Importance of π–π Interactions. Process Saf. Environ. Prot. 2017, 107, 168–180. [Google Scholar] [CrossRef]
- Sher, F.; Iqbal, S.Z.; Albazzaz, S.; Ali, U.; Mortari, D.A.; Rashid, T. Development of Biomass Derived Highly Porous Fast Adsorbents for Post-Combustion CO2 Capture. Fuel 2020, 282, 118506. [Google Scholar] [CrossRef]
- Przepiórski, J.; Czyżewski, A.; Pietrzak, R.; Morawski, A.W. MgO/CaO-Loaded Activated Carbon for Carbon Dioxide Capture: Practical Aspects of Use. Ind. Eng. Chem. Res. 2013, 52, 6669–6677. [Google Scholar] [CrossRef]
- Przepiórski, J.; Czyżewski, A.; Pietrzak, R.; Tryba, B. MgO/CaO-Loaded Porous Carbons for Carbon Dioxide Capture. J. Therm. Anal. Calorim. 2013, 111, 357–364. [Google Scholar] [CrossRef]
- Wang, X.; Li, B. Chapter 1—Phase-Change Solvents for CO2 Capture. In Novel Materials for Carbon Dioxide Mitigation Technology; Shi, F., Morreale, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–22. ISBN 978-0-444-63259-3. [Google Scholar]
- Zhang, S.; Shen, Y.; Wang, L.; Chen, J.; Lu, Y. Phase Change Solvents for Post-Combustion CO2 Capture: Principle, Advances, and Challenges. Appl. Energy 2019, 239, 876–897. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A Systematic Review on CO2 Capture with Ionic Liquids: Current Status and Future Prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Chen, S.S.; Liao, W.; Wang, W.; Jang, M.-F.; Chen, W.-H.; Ahamad, T.; Alshehri, S.M.; Hou, C.-H.; Lin, K.-S.; et al. Assessment of Agricultural Waste-Derived Activated Carbon in Multiple Applications. Environ. Res. 2020, 191, 110176. [Google Scholar] [CrossRef]
- Rouzitalab, Z.; Maklavany, D.M.; Jafarinejad, S.; Rashidi, A. Lignocellulose-Based Adsorbents: A Spotlight Review of the Effective Parameters on Carbon Dioxide Capture Process. Chemosphere 2020, 246, 125756. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yu, G.; Yuan, J.; Strømme, M.; Hedin, N. Microporous Organic Polymers as CO2 Adsorbents: Advances and Challenges. Mater. Today Adv. 2020, 6, 100052. [Google Scholar] [CrossRef]
- Karatayeva, U.; Al Siyabi, S.A.; Brahma Narzary, B.; Baker, B.C.; Faul, C.F.J. Conjugated Microporous Polymers for Catalytic CO2 Conversion. Adv. Sci. 2024, 11, 2308228. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Tshikovhi, A.; Mishra, S.B.; Mishra, A.K. Nanocellulose-Based Composites for the Removal of Contaminants from Wastewater. Int. J. Biol. Macromol. 2020, 152, 616–632. [Google Scholar] [CrossRef]
- Benítez, A.J.; Walther, A. Cellulose Nanofibril Nanopapers and Bioinspired Nanocomposites: A Review to Understand the Mechanical Property Space. J. Mater. Chem. A 2017, 5, 16003–16024. [Google Scholar] [CrossRef]
- Diop, C.I.K.; Lavoie, J.-M. Isolation of Nanocrystalline Cellulose: A Technological Route for Valorizing Recycled Tetra Pak Aseptic Multilayered Food Packaging Wastes. Waste Biomass Valor 2017, 8, 41–56. [Google Scholar] [CrossRef]
- Yang, X.; Biswas, S.K.; Han, J.; Tanpichai, S.; Li, M.-C.; Chen, C.; Zhu, S.; Das, A.K.; Yano, H. Surface and Interface Engineering for Nanocellulosic Advanced Materials. Adv. Mater. 2021, 33, 2002264. [Google Scholar] [CrossRef] [PubMed]
- Gebald, C.; Wurzbacher, J.A.; Borgschulte, A.; Zimmermann, T.; Steinfeld, A. Single-Component and Binary CO2 and H2O Adsorption of Amine-Functionalized Cellulose. Environ. Sci. Technol. 2014, 48, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
- Sehaqui, H.; Gálvez, M.E.; Becatinni, V.; cheng Ng, Y.; Steinfeld, A.; Zimmermann, T.; Tingaut, P. Fast and Reversible Direct CO2 Capture from Air onto All-Polymer Nanofibrillated Cellulose—Polyethylenimine Foams. Environ. Sci. Technol. 2015, 49, 3167–3174. [Google Scholar] [CrossRef]
- Dassanayake, R.S.; Gunathilake, C.; Dassanayake, A.C.; Abidi, N.; Jaroniec, M. Amidoxime-Functionalized Nanocrystalline Cellulose-Mesoporous Silica Composites for Carbon Dioxide Sorption at Ambient and Elevated Temperatures. J. Mater. Chem. A 2017, 5, 7462–7473. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Chen, N.; Dai, S.; Jiang, H.; Wang, S. Effects of Amine Loading on the Properties of Cellulose Nanofibrils Aerogel and Its CO2 Capturing Performance. Carbohydr. Polym. 2018, 194, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Y.; Jiang, H.; Wang, X.; Zhang, T.; Yao, Y. High CO2 Adsorption by Amino-Modified Bio-Spherical Cellulose Nanofibres Aerogels. Environ. Chem. Lett. 2018, 16, 605–614. [Google Scholar] [CrossRef]
- Jiang, F.; Hu, S.; Hsieh, Y. Aqueous Synthesis of Compressible and Thermally Stable Cellulose Nanofibril–Silica Aerogel for CO2 Adsorption. ACS Appl. Nano Mater. 2018, 1, 6701–6710. [Google Scholar] [CrossRef]
- Valdebenito, F.; García, R.; Cruces, K.; Ciudad, G.; Chinga-Carrasco, G.; Habibi, Y. CO2 Adsorption of Surface-Modified Cellulose Nanofibril Films Derived from Agricultural Wastes. ACS Sustain. Chem. Eng. 2018, 6, 12603–12612. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Jiang, H.; Wang, X. Aminosilane-Grafted Spherical Cellulose Nanocrystal Aerogel with High CO2 Adsorption Capacity. Environ. Sci. Pollut. Res. 2019, 26, 16716–16726. [Google Scholar] [CrossRef] [PubMed]
- Valencia, L.; Rosas, W.; Aguilar-Sanchez, A.; Mathew, A.P.; Palmqvist, A.E.C. Bio-Based Micro-/Meso-/Macroporous Hybrid Foams with Ultrahigh Zeolite Loadings for Selective Capture of Carbon Dioxide. ACS Appl. Mater. Interfaces 2019, 11, 40424–40431. [Google Scholar] [CrossRef] [PubMed]
- Valencia, L.; Abdelhamid, H.N. Nanocellulose Leaf-like Zeolitic Imidazolate Framework (ZIF-L) Foams for Selective Capture of Carbon Dioxide. Carbohydr. Polym. 2019, 213, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Wu, Y.; Wang, T.; Wang, X.; Gao, X. Preparation of Quaternized Bamboo Cellulose and Its Implication in Direct Air Capture of CO2. Energy Fuels 2019, 33, 1745–1752. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Zhang, Y.; Shen, M.; Zhang, J. Gas Phase Synthesis of Aminated Nanocellulose Aerogel for Carbon Dioxide Adsorption. Cellulose 2020, 27, 2953–2958. [Google Scholar] [CrossRef]
- Sepahvand, S.; Jonoobi, M.; Ashori, A.; Gauvin, F.; Brouwers, H.J.H.; Oksman, K.; Yu, Q. A Promising Process to Modify Cellulose Nanofibers for Carbon Dioxide (CO2) Adsorption. Carbohydr. Polym. 2020, 230, 115571. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Geng, S.; Hedlund, J.; Oksman, K. Lightweight, Flexible, and Multifunctional Anisotropic Nanocellulose-Based Aerogels for CO2 Adsorption. Cellulose 2020, 27, 2695–2707. [Google Scholar] [CrossRef]
- Tang, Y.; Tang, S.; Zhang, T. Homogeneous Preparation of Aerocellulose Grafted Acrylamide and Its CO2 Adsorption Properties. Cellulose 2020, 27, 3263–3275. [Google Scholar] [CrossRef]
- Miao, Y.; Luo, H.; Pudukudy, M.; Zhi, Y.; Zhao, W.; Shan, S.; Jia, Q.; Ni, Y. CO2 Capture Performance and Characterization of Cellulose Aerogels Synthesized from Old Corrugated Containers. Carbohydr. Polym. 2020, 227, 115380. [Google Scholar] [CrossRef]
- Ho, N.A.D.; Leo, C.P. A Review on the Emerging Applications of Cellulose, Cellulose Derivatives and Nanocellulose in Carbon Capture. Environ. Res. 2021, 197, 111100. [Google Scholar] [CrossRef]
- Pak, S.-H.; Jeon, Y.-W.; Shin, M.-S.; Koh, H.C. Preparation of Cellulose Acetate Hollow-Fiber Membranes for CO2/CH4 Separation. Environ. Eng. Sci. 2016, 33, 17–24. [Google Scholar] [CrossRef]
- Ansaloni, L.; Salas-Gay, J.; Ligi, S.; Baschetti, M.G. Nanocellulose-Based Membranes for CO2 Capture. J. Membr. Sci. 2017, 522, 216–225. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Hou, T.; Chen, J.; Feng, Y.; Li, B.; Gu, X.; He, M.; Yao, J. Facilitated Transport of CO2 Through the Transparent and Flexible Cellulose Membrane Promoted by Fixed-Site Carrier. ACS Appl. Mater. Interfaces 2018, 10, 24930–24936. [Google Scholar] [CrossRef] [PubMed]
- Mubashir, M.; Yeong, Y.F.; Lau, K.K.; Chew, T.L.; Norwahyu, J. Efficient CO2/N2 and CO2/CH4 Separation Using NH2-MIL-53(Al)/Cellulose Acetate (CA) Mixed Matrix Membranes. Sep. Purif. Technol. 2018, 199, 140–151. [Google Scholar] [CrossRef]
- Nikolaeva, D.; Azcune, I.; Tanczyk, M.; Warmuzinski, K.; Jaschik, M.; Sandru, M.; Dahl, P.I.; Genua, A.; Loïs, S.; Sheridan, E.; et al. The Performance of Affordable and Stable Cellulose-Based Poly-Ionic Membranes in CO2/N2 and CO2/CH4 Gas Separation. J. Membr. Sci. 2018, 564, 552–561. [Google Scholar] [CrossRef]
- Jahan, Z.; Niazi, M.B.K.; Hägg, M.-B.; Gregersen, Ø.W. Decoupling the Effect of Membrane Thickness and CNC Concentration in PVA Based Nanocomposite Membranes for CO2/CH4 Separation. Sep. Purif. Technol. 2018, 204, 220–225. [Google Scholar] [CrossRef]
- Venturi, D.; Grupkovic, D.; Sisti, L.; Baschetti, M.G. Effect of Humidity and Nanocellulose Content on Polyvinylamine-Nanocellulose Hybrid Membranes for CO2 Capture. J. Membr. Sci. 2018, 548, 263–274. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Feng, Y.; Wang, Z.; Jia, M.; Yao, J. Fabrication of Cellulose Nanofibrils/UiO-66-NH2 Composite Membrane for CO2/N2 Separation. J. Membr. Sci. 2018, 568, 10–16. [Google Scholar] [CrossRef]
- Yang, K.; Dai, Y.; Zheng, W.; Ruan, X.; Li, H.; He, G. ZIFs-Modified GO Plates for Enhanced CO2 Separation Performance of Ethyl Cellulose Based Mixed Matrix Membranesf. Sep. Purif. Technol. 2019, 214, 87–94. [Google Scholar] [CrossRef]
- Sundell, B.J.; Harrigan, D.J.; Hayden, S.C.; Vaughn, J.T.; Guzan, K.A.; Lawrence, J.A., III; Ostraat, M.L. Improved Gas Transport Properties of Cellulose Acetate via Sub-Tg Acid-Catalyzed Silanation. J. Membr. Sci. 2019, 573, 448–454. [Google Scholar] [CrossRef]
- Nguyen, H.; Wang, M.; Hsiao, M.-Y.; Nagai, K.; Ding, Y.; Lin, H. Suppression of Crystallization in Thin Films of Cellulose Diacetate and Its Effect on CO2/CH4 Separation Properties. J. Membr. Sci. 2019, 586, 7–14. [Google Scholar] [CrossRef]
- Jahan, Z.; Niazi, M.B.K.; Hagg, M.-B.; Gregersen, Ø.W.; Hussain, A. Phosphorylated Nanocellulose Fibrils/PVA Nanocomposite Membranes for Biogas Upgrading at Higher Pressure. Sep. Sci. Technol. 2020, 55, 1524–1534. [Google Scholar] [CrossRef]
- Torstensen, J.Ø.; Helberg, R.M.L.; Deng, L.; Gregersen, Ø.W.; Syverud, K. PVA/Nanocellulose Nanocomposite Membranes for CO2 Separation from Flue Gas. Int. J. Greenh. Gas Control. 2019, 81, 93–102. [Google Scholar] [CrossRef]
- Janakiram, S.; Yu, X.; Ansaloni, L.; Dai, Z.; Deng, L. Manipulation of Fibril Surfaces in Nanocellulose-Based Facilitated Transport Membranes for Enhanced CO2 Capture. ACS Appl. Mater. Interfaces 2019, 11, 33302–33313. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Deng, J.; Yu, Q.; Helberg, R.M.L.; Janakiram, S.; Ansaloni, L.; Deng, L. Fabrication and Evaluation of Bio-Based Nanocomposite TFC Hollow Fiber Membranes for Enhanced CO2 Capture. ACS Appl. Mater. Interfaces 2019, 11, 10874–10882. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Shu, L.; Guo, K.; Zhang, X.-F.; Zhou, S.; He, M.; Yao, J. Cellulose Membranes with Polyethylenimine-Modified Graphene Oxide and Zinc Ions for Promoted Gas Separation. Cellulose 2020, 27, 3277–3286. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Morisato, A.; Bhuwania, N.; Chinn, D.; Koros, W.J. Natural Gas Sweetening Using a Cellulose Triacetate Hollow Fiber Membrane Illustrating Controlled Plasticization Benefits. J. Membr. Sci. 2020, 601, 117910. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, X.-F.; Feng, Y.; Zhou, Y.; Yao, J. In-Situ Growing ZIF-8 on Cellulose Nanofibers to Form Gas Separation Membrane for CO2 Separation. J. Membr. Sci. 2020, 595, 117579. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, T.; Zhao, Q.; Liu, K.; Liang, D.; Si, C. Cellulose-Based Materials for Carbon Capture and Conversion. Carbon Capture Sci. Technol. 2024, 10, 100157. [Google Scholar] [CrossRef]
- Fujiki, J.; Yogo, K. The Increased CO2 Adsorption Performance of Chitosan-Derived Activated Carbons with Nitrogen-Doping. Chem. Commun. 2016, 52, 186–189. [Google Scholar] [CrossRef]
- Rasoulzadeh, H.; Motesaddi Zarandi, S.; Massoudinejad, M.; Amini, M.M. Modelling and Optimisation by Response Surface Technique for Adsorption of Carbon Dioxide by Aminated Biosilica/Alginate Composite: Experiments, Characterisation and Regeneration Studies. Int. J. Environ. Anal. Chem. 2021, 103, 3740–3761. [Google Scholar] [CrossRef]
- Molina-Fernández, C.; Luis, P. Immobilization of Carbonic Anhydrase for CO2 Capture and Its Industrial Implementation: A Review. J. CO2 Util. 2021, 47, 101475. [Google Scholar] [CrossRef]
- Dutta, S.; Bhaumik, A.; Wu, K.C.-W. Hierarchically Porous Carbon Derived from Polymers and Biomass: Effect of Interconnected Pores on Energy Applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G. Biochemistry; John Wiley & Sons: Hoboken, NJ, USA, 2010; ISBN 978-0-470-57095-1. [Google Scholar]
- Shen, J.; Yuan, Y.; Salmon, S. Carbonic Anhydrase Immobilized on Textile Structured Packing Using Chitosan Entrapment for CO2 Capture. ACS Sustain. Chem. Eng. 2022, 10, 7772–7785. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; No, H.K.; Rohani, S.M.R.; Oromiehie, A.; Ghasemi, S. Potential Inherent Properties of Chitosan and Its Applications in Preserving Muscle Food. J. Chitin Chitosan. 2010, 15, 35–45. Available online: http://www.chitosan.or.kr/JournalSearch/sub03_01.asp (accessed on 10 April 2024).
- Kim, H.-L.; Jung, G.-Y.; Yoon, J.-H.; Han, J.-S.; Park, Y.-J.; Kim, D.-G.; Zhang, M.; Kim, D.-J. Preparation and Characterization of Nano-Sized Hydroxyapatite/Alginate/Chitosan Composite Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2015, 54, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Dai, Y.-N.; Zhang, J.-P.; Wang, A.-Q.; Wei, Q. Chitosan-Alginate Nanoparticles as a Novel Drug Delivery System for Nifedipine. Int. J. Biomed. Sci. 2008, 4, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Chen, X.; Tang, K.; Meng, Q.; Shen, C.; Zhang, G. Zeolite Imidazolate Framework Membranes on Polymeric Substrates Modified with Poly(Vinyl Alcohol) and Alginate Composite Hydrogels. ACS Appl. Mater. Interfaces 2019, 11, 12605–12612. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Eghbali Babadi, F.; Masoudi Soltani, S.; Aroua, M.K.; Babamohammadi, S.; Mousavi Moghadam, A. Carbon Dioxide Adsorption on Nitrogen-Enriched Gel Beads from Calcined Eggshell/Sodium Alginate Natural Composite. Process Saf. Environ. Prot. 2017, 109, 387–399. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Sun, G.; Tang, Q.; Bian, H. Enzymatic Properties of Immobilized Carbonic Anhydrase and the Biocatalyst for Promoting CO2 Capture in Vertical Reactor. Int. J. Greenh. Gas Control. 2016, 49, 290–296. [Google Scholar] [CrossRef]
- Sneddon, G.; Ganin, A.Y.; Yiu, H.H.P. Sustainable CO2 Adsorbents Prepared by Coating Chitosan onto Mesoporous Silicas for Large-Scale Carbon Capture Technology. Energy Technol. 2015, 3, 249–258. [Google Scholar] [CrossRef]
- Song, J.; Liu, J.; Zhao, W.; Chen, Y.; Xiao, H.; Shi, X.; Liu, Y.; Chen, X. Quaternized Chitosan/PVA Aerogels for Reversible CO2 Capture from Ambient Air. Ind. Eng. Chem. Res. 2018, 57, 4941–4948. [Google Scholar] [CrossRef]
- Hsan, N.; Dutta, P.K.; Kumar, S.; Bera, R.; Das, N. Chitosan Grafted Graphene Oxide Aerogel: Synthesis, Characterization and Carbon Dioxide Capture Study. Int. J. Biol. Macromol. 2019, 125, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Primo, A.; Forneli, A.; Corma, A.; García, H. From Biomass Wastes to Highly Efficient CO2 Adsorbents: Graphitisation of Chitosan and Alginate Biopolymers. ChemSusChem 2012, 5, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.E.; Olivieri, G.; Marzocchella, A.; Salatino, P.; Caramuscio, P.; Cavaleiro, C. Post-Combustion Carbon Capture Mediated by Carbonic Anhydrase. Sep. Purif. Technol. 2013, 107, 331–339. [Google Scholar] [CrossRef]
- Simsek-Ege, F.A.; Bond, G.M.; Stringer, J. Polyelectrolyte Cages For A Novel Biomimetic CO2 Sequestration System. In Environmental Challenges and Greenhouse Gas Control for Fossil Fuel Utilization in the 21st Century; Maroto-Valer, M.M., Song, C., Soong, Y., Eds.; Springer: Boston, MA, USA, 2002; pp. 133–145. ISBN 978-1-4613-5232-7. [Google Scholar]
- Oviya, M.; Sukumaran, V.; Giri, S.S. Immobilization and Characterization of Carbonic Anhydrase Purified from E. coli MO1 and Its Influence on CO2 Sequestration. World J. Microbiol. Biotechnol. 2013, 29, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Z.; Zhang, C.; Wang, J.; Wang, S. A Novel Membrane Prepared from Sodium Alginate Cross-Linked with Sodium Tartrate for CO2 Capture. Chin. J. Chem. Eng. 2013, 21, 1098–1105. [Google Scholar] [CrossRef]
- Barroso-Martín, I.; Cecilia, J.A.; Vilarrasa-García, E.; Ballesteros-Plata, D.; Jiménez-Gómez, C.P.; Vílchez-Cózar, Á.; Infantes-Molina, A.; Rodríguez-Castellón, E. Modification of the Textural Properties of Chitosan to Obtain Biochars for CO2-Capture Processes. Polymers 2022, 14, 5240. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, Y.; Zhang, T.C.; Ouyang, L.; Yuan, S. Phytic Acid-Induced Self-Assembled Chitosan Gel-Derived N, P–Co-Doped Porous Carbon for High-Performance CO2 Capture and Supercapacitor. J. Power Sources 2022, 517, 230727. [Google Scholar] [CrossRef]
- Marin, L.; Dragoi, B.; Olaru, N.; Perju, E.; Coroaba, A.; Doroftei, F.; Scavia, G.; Destri, S.; Zappia, S.; Porzio, W. Nanoporous Furfuryl-Imine-Chitosan Fibers as a New Pathway towards Eco-Materials for CO2 Adsorption. Eur. Polym. J. 2019, 120, 109214. [Google Scholar] [CrossRef]
- De Ruiter, G.A.; Rudolph, B. Carrageenan Biotechnology. Trends Food Sci. Technol. 1997, 8, 389–395. [Google Scholar] [CrossRef]
- Dębowski, M.; Krzemieniewski, M.; Zieliński, M.; Kazimierowicz, J. Immobilized Microalgae-Based Photobioreactor for CO2 Capture (IMC-CO2PBR): Efficiency Estimation, Technological Parameters, and Prototype Concept. Atmosphere 2021, 12, 1031. [Google Scholar] [CrossRef]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent Advances in Hydroxyapatite-Based Biocomposites for Bone Tissue Regeneration in Orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.K.S.; Menyhárd, A.; Klébert, S.; Mohai, M.; Nagy, B.; László, K. Effect of Carbon Nanoparticles on the Porous Texture of ι-Carrageenan-Based N-Doped Nanostructured Porous Carbons and Implications for Gas Phase Applications. C 2023, 9, 68. [Google Scholar] [CrossRef]
- De Luca, G.; Romeo, A.; Scolaro, L.M.; Pasternack, R.F. Conformations of a Model Protein Revealed by an Aggregating CuII Porphyrin: Sensing the Difference. Chem. Commun. 2010, 46, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Castriciano, M.A.; Carbone, A.; Saccà, A.; Donato, M.G.; Micali, N.; Romeo, A.; De Luca, G.; Scolaro, L.M. Optical and Sensing Features of TPPS4 J-Aggregates Embedded in Nafion® Membranes: Influence of Casting Solvents. J. Mater. Chem. 2010, 20, 2882–2886. [Google Scholar] [CrossRef]
- Epuran, C.; Fratilescu, I.; Macsim, A.-M.; Lascu, A.; Ianasi, C.; Birdeanu, M.; Fagadar-Cosma, E. Excellent Cooperation between Carboxyl-Substituted Porphyrins, k-Carrageenan and AuNPs for Extended Application in CO2 Capture and Manganese Ion Detection. Chemosensors 2022, 10, 133. [Google Scholar] [CrossRef]
- Rosace, G.; Cardiano, P.; Urzì, C.; De Leo, F.; Galletta, M.; Ielo, I.; Plutino, M.R. Potential Roles of Fluorine-Containing Sol-Gel Coatings against Adhesion to Control Microbial Biofilm. IOP Conf. Ser. Mater. Sci. Eng. 2018, 459, 012021. [Google Scholar] [CrossRef]
- Cammarano, A.; De Luca, G.; Amendola, E. Surface Modification and Adhesion Improvement of Polyester Films. Cent. Eur. J. Chem. 2012, 11, 35–45. [Google Scholar] [CrossRef]
- In-na, P.; Lee, J.; Caldwell, G. Living Textile Biocomposites Deliver Enhanced Carbon Dioxide Capture. J. Ind. Text. 2022, 51, 5683S–5707S. [Google Scholar] [CrossRef]
- Sadrul Islam, A.K.M.; Ahiduzzaman, M. Biomass Energy: Sustainable Solution for Greenhouse Gas Emission. AIP Conf. Proc. 2012, 1440, 23–32. [Google Scholar] [CrossRef]
- Ketabchi, M.R.; Babamohammadi, S.; Davies, W.G.; Gorbounov, M.; Masoudi Soltani, S. Latest Advances and Challenges in Carbon Capture Using Bio-Based Sorbents: A State-of-the-Art Review. Carbon Capture Sci. Technol. 2023, 6, 100087. [Google Scholar] [CrossRef]
- Sam, D.K.; Sam, E.K.; Durairaj, A.; Lv, X.; Zhou, Z.; Liu, J. Synthesis of Biomass-Based Carbon Aerogels in Energy and Sustainability. Carbohydr. Res. 2020, 491, 107986. [Google Scholar] [CrossRef] [PubMed]
- Zanco, S.E.; Pérez-Calvo, J.-F.; Gasós, A.; Cordiano, B.; Becattini, V.; Mazzotti, M. Postcombustion CO2 Capture: A Comparative Techno-Economic Assessment of Three Technologies Using a Solvent, an Adsorbent, and a Membrane. ACS Eng. Au 2021, 1, 50–72. [Google Scholar] [CrossRef]
- Wang, C.; Okubayashi, S. Polyethyleneimine-Crosslinked Cellulose Aerogel for Combustion CO2 Capture. Carbohydr. Polym. 2019, 225, 115248. [Google Scholar] [CrossRef] [PubMed]
- Razman, K.K.; Hanafiah, M.M.; Mohammad, A.W.; Lun, A.W. Life Cycle Assessment of an Integrated Membrane Treatment System of Anaerobic-Treated Palm Oil Mill Effluent (POME). Membranes 2022, 12, 246. [Google Scholar] [CrossRef]




| Cellulose Derivative | Chemical Modification | CO2 Adsorption Capacity (mmol/g) | Notes | Refs. |
|---|---|---|---|---|
| CNFs aerogel | 3-Aminopropylmethyl-diethoxysilane | 2.26 | Absorption capacity increases linearly with humidity | [51] |
| CNFs foam | PEI a | 2.22 | Reduced surface area after modification | [52] |
| CNC composite | Silica, triethoxysilylpropyl-3-pentanyldinitrile carbamate | 5.54 | [53] | |
| CNFs aerogel | N-(2-aminoethyl)-3-aminopropylmethyldimethoxysilane and acetic acid | 1.91 | Reduced surface area after modification | [54] |
| CNFs aerogel | N-(2-aminoethyl)-3-aminopropylmethyldimethoxysilane | 1.78 | Reduced surface area after modification | [55] |
| CNFs aerogel | Silica, Na2SiO3, APTES b | 2.2 | Improved surface area with silica incorporation; reduced surface area after silanization. | [56] |
| CNFs thin film | (3-trimethoxysilylpropyl) diethylenetriamine | 2.11 | [57] | |
| CNC aerogel | N-(2-aminoethyl)-3-aminopropylmethyldimethoxysilane | 1.68 | Reduced surface area after modification | [58] |
| CNFs foam | Silicalite-1 zeolite | 1.2 | [59] | |
| CNFs foam | ZIM c | 0.62 | [60] | |
| Cellulose | (3-Chloro-2 hydroxypropyl) trimethylammonium chloride | 0.14 | [61] | |
| CNFs aerogel | N-(2-aminoethyl)-3-aminopropylmethyldimethoxysilane | 1.01 | Reduced surface area after modification | [5] |
| CNFs aerogel | N-(2-aminoethyl)-3-aminopropylmethyldimethoxysilane | 1.59 | Reduced surface area after modification | [62] |
| CNFs aerogel | phthalimide (1,3-dihydro-1,3-dioxoisoindole) | 5.3 | [63] | |
| CNFs aerogel | Sodium acetate | 1.14 | [64] | |
| Cellulose aerogel | Acrylamide | 1.07 | [65] | |
| Cellulose aerogel | Silica | 1.96–11.87 | Gas selectivity increases with silica gel content. | [66] |
| Cellulose Membrane | Chemical Modification | CO2 Permeability/Permanence | CO2 Selectivity | Refs. |
|---|---|---|---|---|
| Polysulfone | CNF/polyvinyl amine coating | 25 Barrer | CO2/N2: 500 CO2/CH4: 350 | [69] |
| Regenerated cellulose | - | 155.0 Barrer | CO2/N2: 27.2 | [70] |
| Cellulose acetate | Amine functionalized MIL-53(Al) a | 52.6 Barrer | CO2/N2: 23.4 | [71] |
| Cellulose acetate | Poly(ionic liquid) | 8.9 Barrer | CO2/N2: 26.8 | [72] |
| Polysulfone | PVA/CNC b coating | 0.27 m3(STP)/(m2⋅bar⋅h) | CO2/CH4: 39 | [73] |
| Polyvinylamine | CNF | 187 Barrer | CO2/N2: 100 | [74] |
| CNF | UiO-66 c | 139 Barrer | CO2/N2: 43.6 | [75] |
| Ethylcellulose | ZIF-8 d ZIF-8/graphene oxide | 203.3 Barrer | CO2/N2: 33.4 | [76] |
| Cellulose acetate | Vinyltrimethoxysilane with acetic acid | 24.5 Barrer | CO2/CH4: 28.8 | [77] |
| Cellulose diacetate | – | 9 Barrer | CO2/CH4: 30–35 | [78] |
| Polysulfone | PVA/phosphoryl-CNC coating | 0.21 m3(STP)/(m2⋅bar⋅h) | CO2/CH4: 46 | [79] |
| Polysulfone | PVA/CNC Phosphorylated CNF Oxidized CNF coating | 27.8 ± 5.5 GPU e; 100 ± 3.7 GPU; 90.7 ± 3.7 GPU | CO2/N2: 39 ± 0.4; 42 ± 1.8; 90.7 ± 3.7; 42 ± 0.7 | [80] |
| PVDF f | PVA/polyallylamine/functionalized CNF coating | 652 GPU | CO2/N2: 41.3 | [81] |
| PPO g | PVA/CNC coating | 672 GPU | CO2/N2: 43.6 | [82] |
| Regenerated cellulose | PEI-modified graphene oxide | 268.9 Barrer | CO2/N2: 48.9 CO2/CH4: 57.4 | [83] |
| Cellulose triacetate | – | 110 GPU | CO2/CH4: 22 | [84] |
| CNF | ZIF-8 | 550 Barrer | CO2/N2: 45.5 CO2/CH4: 36.2 | [85] |
| Advantages | Disadvantages | Refs. |
|---|---|---|
| Abundant and renewable resource, primarily derived from plant sources | Poor solubility in most common solvents, requiring specialized processing methods | [63] |
| Biodegradable and environmentally friendly | Limited thermal stability | [74] |
| High strength and stiffness, making it suitable for reinforcing composite materials | Susceptible to degradation by microbial activity under certain conditions | [81,83] |
| Good compatibility with other materials due to its hydrophilic nature | Processing can be energy-intensive and require expensive processes | [61] |
| Can be easily processed into 0D to 2D nanostructured materials (nanoparticles, fibers, films) | [78,80,81] |
| Biopolymer | Chemical Modification | Mechanism of CO2 Capture | CO2 Captured (mmol g−1) | Refs. |
|---|---|---|---|---|
| Alginate | PVA a, ZIF b | Membrane gas separation | - | [96] |
| NH2-functionalized | Adsorption | 0.2380 c | [97] | |
| NH2-SiO2 | Adsorption | 7.865 d | [88] | |
| CA e | Absorption | 0.025 f | [98] | |
| Chitosan | SiO2 | Adsorption | 0.98 | [99] |
| PVA | Adsorption | 0.18 | [100] | |
| GO g or MWCN h | Adsorption | 0.257 | [101] | |
| Alginate and Chitosan | Pyrolyzed | Adsorption | 5 | [102] |
| CA Source | CA Immobilization Technique | Activity a | Thermal Stability b/Storage c | CA Reusability d | Refs. |
|---|---|---|---|---|---|
| Bovine | Entrapment | 30.8 | ~35.7/7.1 (3 h, 343 K) | - | [104] |
| Purified bacterial | 94.5 | 43.3 (2 h, 343 K)/81.2 (28 d, 277 K) | 53% (8 c) | [105] | |
| Mammals/extremophile bacteria | 60 | - | - | [103] |
| Biopolymer | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| Sodium alginate | Derived from seaweed and algae, thus a sustainable and abundant resource | Limited mechanical strength compared to synthetic polymers | [102] |
| Biocompatible and non-toxic | Susceptible to enzymatic degradation in the presence of alginate lyases | [102] | |
| Forms hydrogels with divalent cations, offering versatility in material properties | Solubility and gelation properties can be affected by pH and temperature | [96] | |
| Good film-forming ability, enabling the production of thin films for various applications | Relatively high cost compared to some other natural polymers | [106] | |
| Can be easily cross-linked to improve mechanical properties and stability | [106] | ||
| Chitosan | Derived from chitin, a biopolymer found in fungi and arthropods | Limited solubility in water at neutral pH, requiring acidic conditions for dissolution | [102] |
| Biocompatible, biodegradable, and non-toxic | Mechanical properties possibly affected by moisture absorption | [99] | |
| Antimicrobial properties | Challenging solution processing due to high viscosity | [92] | |
| Forms films, gels, and fibers with excellent mechanical properties | Sensitive to enzymatic degradation by chitinases | [107,108,109] | |
| Can be chemically tailored to introduce specific functionalities | [107,109] |
| κ-Carrageenan Biocomposite | CO2 mmol g−1 Adsorbent | Refs. |
|---|---|---|
| Chlorella vulgaris on cotton sheet | 41.29 ± 2.17 | [119] |
| Chlorella vulgaris on polyester sheet | 11.09 ± 0.85 | [119] |
| Carboxyl-substituted porphyrin | 6.97 | [116] |
| Advantages | Disadvantages | Refs. |
|---|---|---|
| Extracted from red seaweed, making it a renewable and sustainable resource | Limited mechanical strength compared to synthetic polymers | [110] |
| Forms strong and flexible gels in the presence of potassium ions | Susceptible to degradation by microbial enzymes and acidic conditions | [116] |
| Excellent stabilizing and thickening properties in aqueous solutions | Gelation properties can be affected by the presence of certain ions and pH | [119] |
| Biocompatible and non-toxic | [110] | |
| Can be modified to tailor its properties for specific applications | [113,116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cigala, R.M.; De Luca, G.; Ielo, I.; Crea, F. Biopolymeric Nanocomposites for CO2 Capture. Polymers 2024, 16, 1063. https://doi.org/10.3390/polym16081063
Cigala RM, De Luca G, Ielo I, Crea F. Biopolymeric Nanocomposites for CO2 Capture. Polymers. 2024; 16(8):1063. https://doi.org/10.3390/polym16081063
Chicago/Turabian StyleCigala, Rosalia Maria, Giovanna De Luca, Ileana Ielo, and Francesco Crea. 2024. "Biopolymeric Nanocomposites for CO2 Capture" Polymers 16, no. 8: 1063. https://doi.org/10.3390/polym16081063
APA StyleCigala, R. M., De Luca, G., Ielo, I., & Crea, F. (2024). Biopolymeric Nanocomposites for CO2 Capture. Polymers, 16(8), 1063. https://doi.org/10.3390/polym16081063







