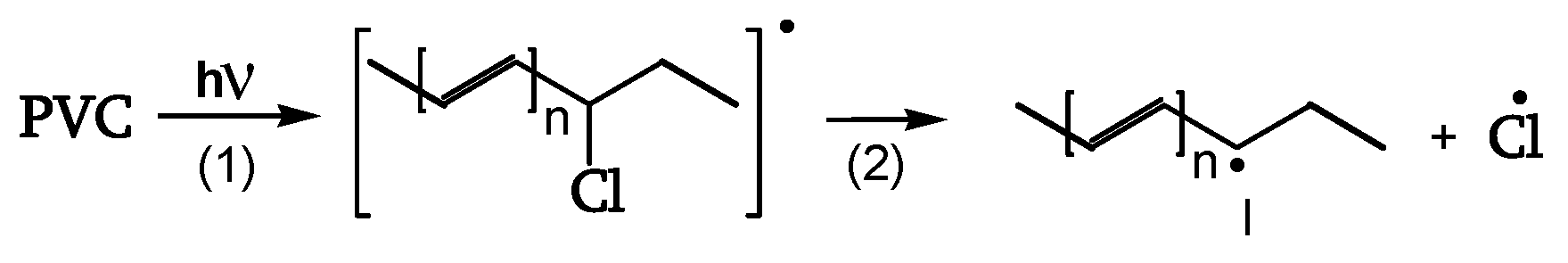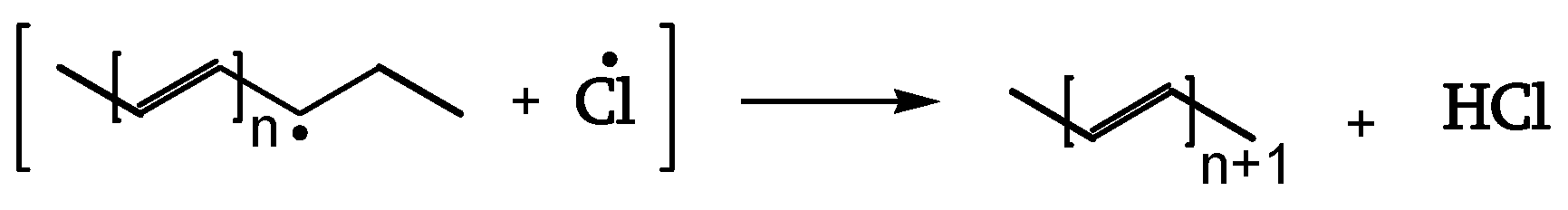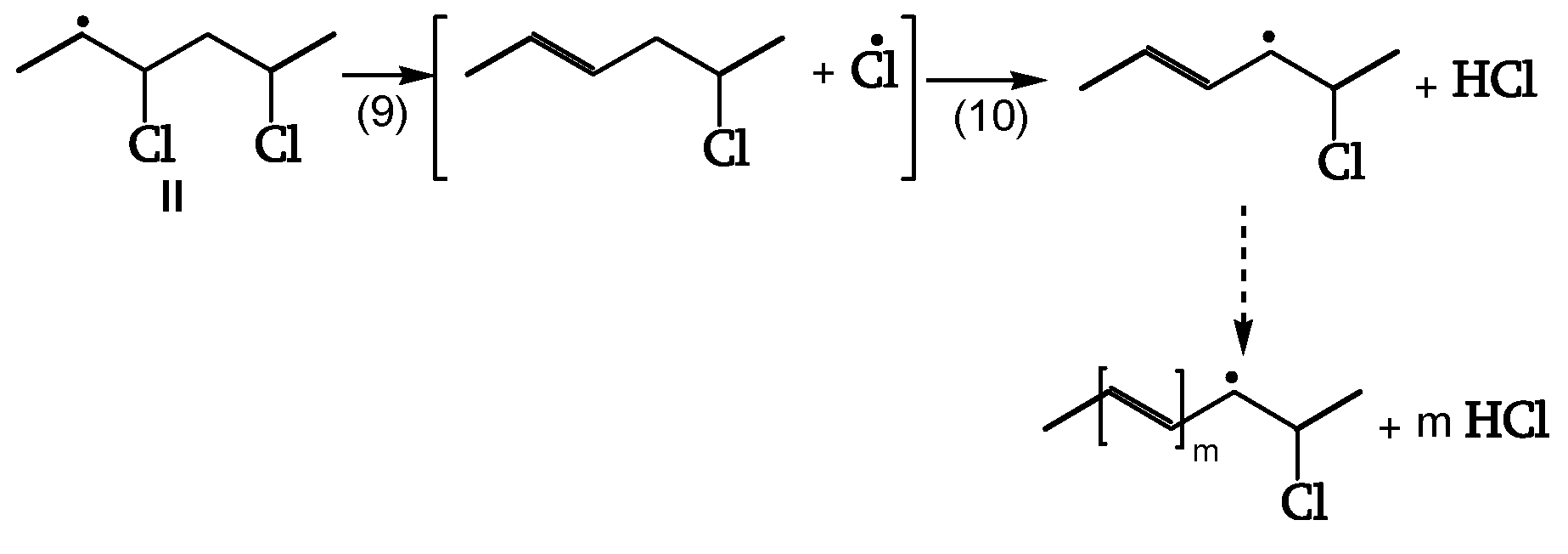1. Introduction
1.1. Scope
The durability of PVC items has gained increasing magnitude in order to save resources in the context of the eco-design of the articles [
1,
2]. PVC articles have a primary role in this scenario as they are made up of a sustainable material contributing significantly to energy saving [
3,
4]. However, despite their noteworthy durability, there is still the need to improve their stability, particularly outdoors, where degradation phenomena such as photodegradation, photooxidation, photo-catalyzed oxidation, photobleaching, side chemical reactions, and thermal degradation occur. Dark-colored articles are particularly susceptible to color change [
5,
6,
7] and it was also considered that the colors of plastics may play a role in plastic photoaging [
8]. In this context, a key role is played by the PVC stabilizers necessary to preserve the resin against degradation in processing and during items’ service life. Therefore, it is crucial to understand the chemical mechanism underlying the degradation phenomena to design more effective stabilizers. Many scientific papers have been published on the thermal degradation of PVC, and they are discussed in Refs. [
9,
10,
11,
12,
13,
14,
15,
16]. Although many studies have been published, a complete review of outdoor-weathering mechanisms leading to chalking is still missing.
This study reviews and investigates the complex mechanisms of weathering that lead to PVC outdoor degradation, particularly affecting articles like window frames and roller shutters. The study is particularly focused on the role of acid scavengers in preventing weathering drawbacks contributing to the eco-design and to enhance the sustainability of PVC items. As evident in the
Section 2, the novel conclusion of this article is that, contrary to what is expected, and contrary to what normally happens in thermal stability, not all acid scavengers act in the same way for the purpose of enhancing photostability.
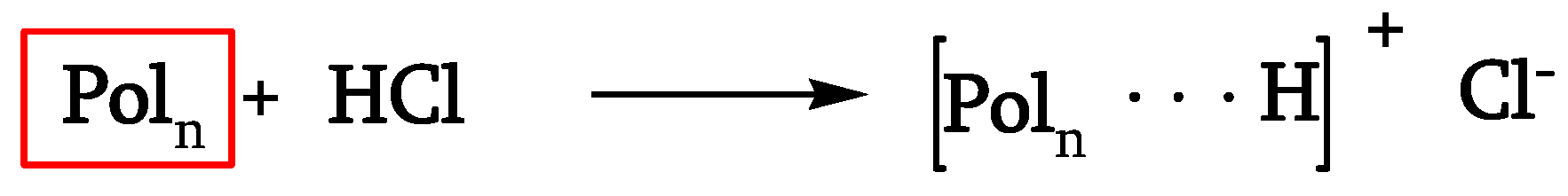
Thermal, photochemical, and chemical phenomena act simultaneously during the natural outdoor weathering of PVC. It is well known that PVC degrades thermally through a zip-like elimination of hydrogen chloride (HCl) with the consequent formation of conjugated-polyene sequences. Studies [
11,
13] have confirmed that it acts through an ion-pair mechanism where the free radicals generated by the interaction between HCl and polyene sequences play a crucial role [
17].
The theory section of this paper describes the weathering mechanisms in detail and step by step. The
Section 2 has the aim of studying the impact of some acid scavengers on the extension of the durability of dark-colored PVC articles upon weathering and, specifically, their impact on chalking. To this latter aim, natural- and accelerated-weathering tests are used to confirm the reliability of fast methods to screen the appropriate stabilizer recipes.
1.2. Theory
1.2.1. Introduction
As PVC is only transparent at wavelengths of more than 250 nm, the initiation is likely due to chromophores found in the PVC resin. However, polyenes rapidly become the main absorbing species among chromophores due to their high extinction coefficient [
18]. Only polyene sequences with more than four double bonds can interact with sunlight at the ground, as the atmosphere screens wavelengths shorter than about 290 nm (
Table 1, [
19]).
When photons hit the PVC item, two kinds of reactions take place. The first generates conjugated polyene sequences and HCl [
18]. The chromophores absorb the photons, and one of the non-radiative pathways releases Cl radicals, starting the zip-elimination quickly before oxygen can become involved [
18]. This yields conjugate-polyene sequences and their crosslinking. The UV screen from polyene sequences protects the matrix from further degradation.
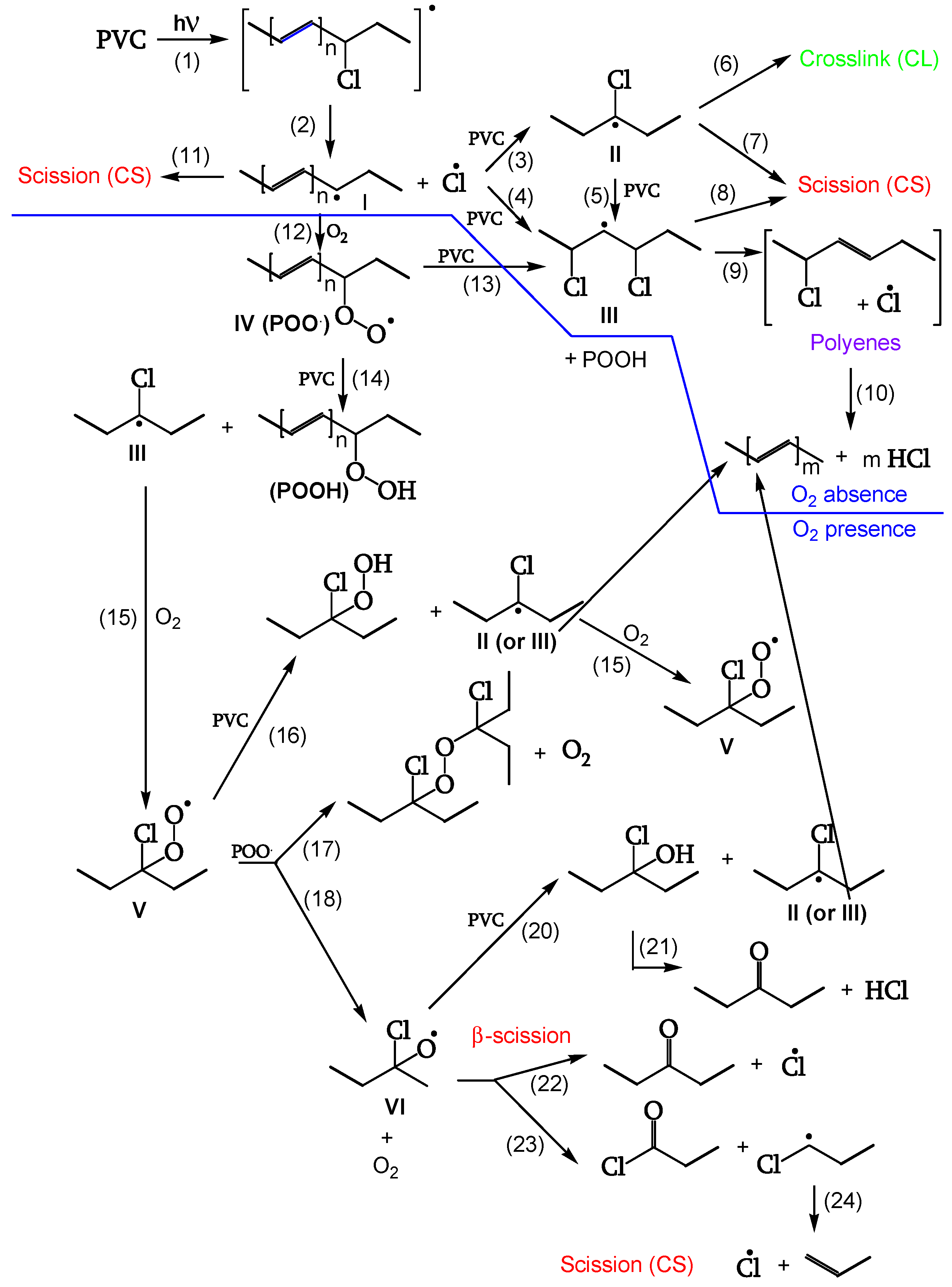
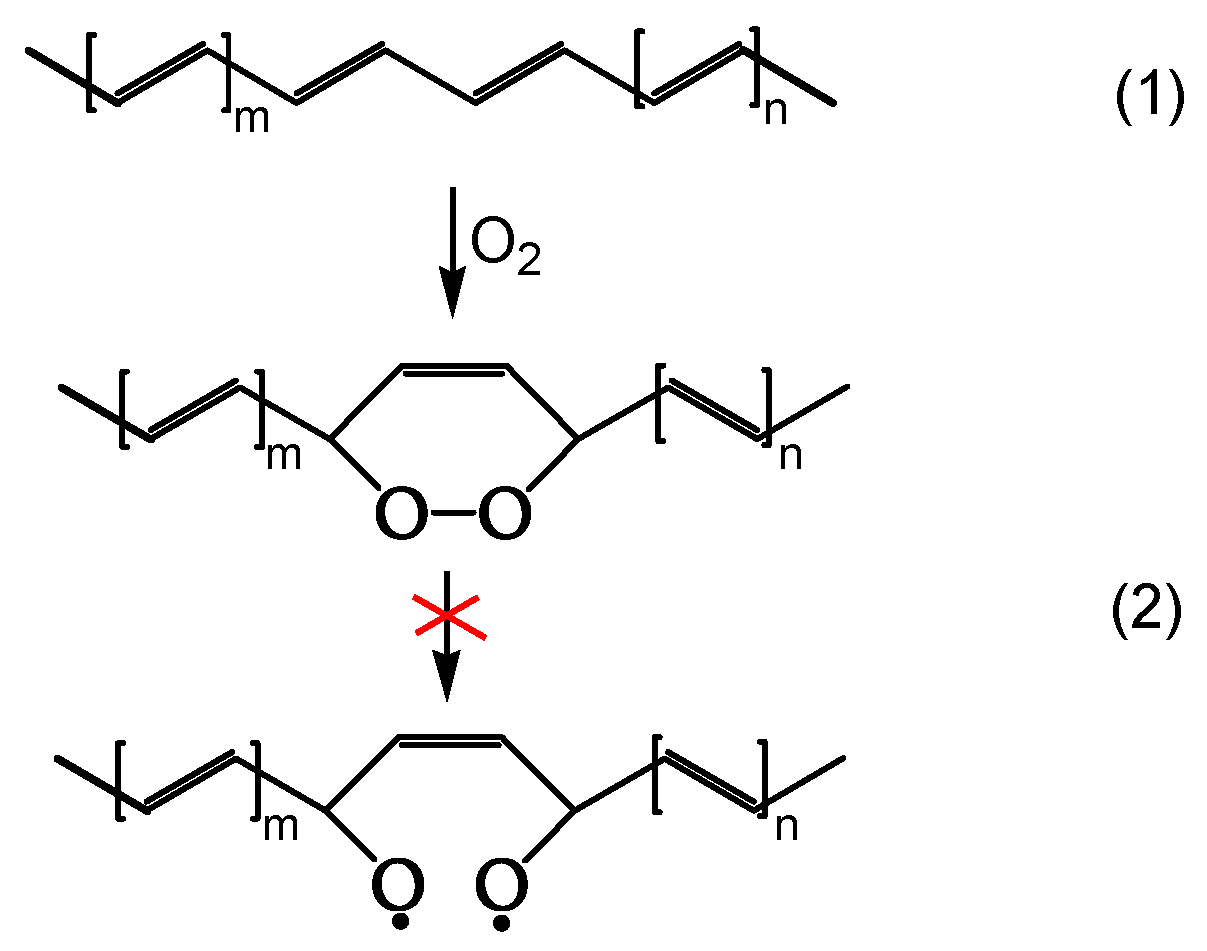
A second series of reactions involves oxygen, and it causes a severe photo-oxidation, bringing about chain scission and the formation of carbonyls (
Figure 1 and
Figure 2). Zip elimination will dominate where oxygen is lacking. Therefore, it will take place underskin and is called photodegradation. The second series of reactions will take place in the proximity of the surface, where oxygen concentration is high; it is called photo-oxidation (
Figure 1 and
Figure 2). Titanium dioxide can promote a strong photo-oxidation, and this is called photocatalyzed oxidation. Fillers, commonly found in the PVC compound, can react with HCl, impacting the weatherability of the article. When the photo or photocatalyzed oxidation destroys the surface, it exposes the photodegraded underskin to light and oxygen, and the polyene sequences are oxidized through photobleaching [
20].
Figure 1 outlines the overall first stage of the PVC photodegradation and photo-oxidation mechanism leading to chain scission, polyene sequence formation, and crosslinking, depending on the exposure conditions [
18].
As is evident from the calculated quantum yields (
Table 2), the presence of oxygen also enhances chain scission and triggers the generation of peroxides and ketones [
18].
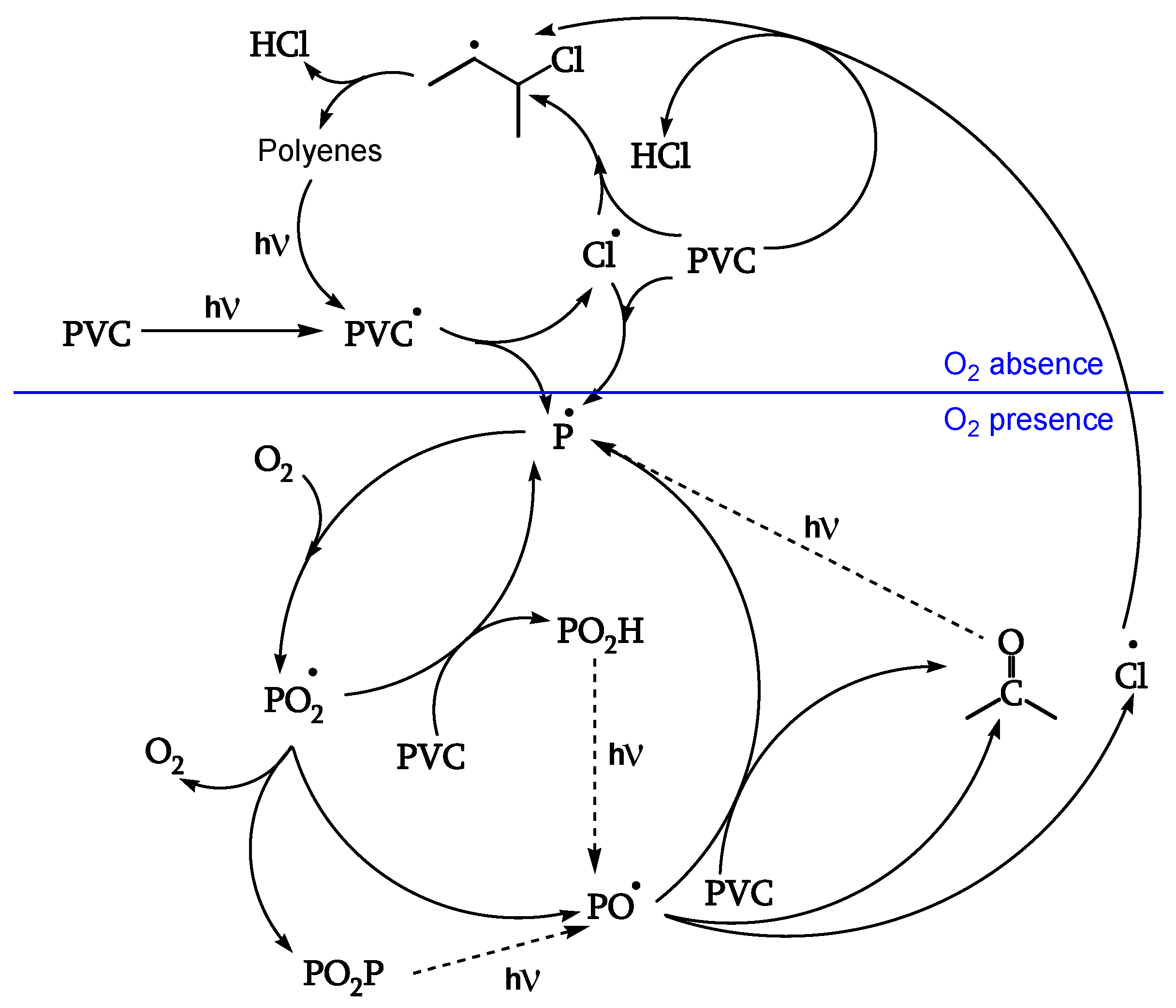
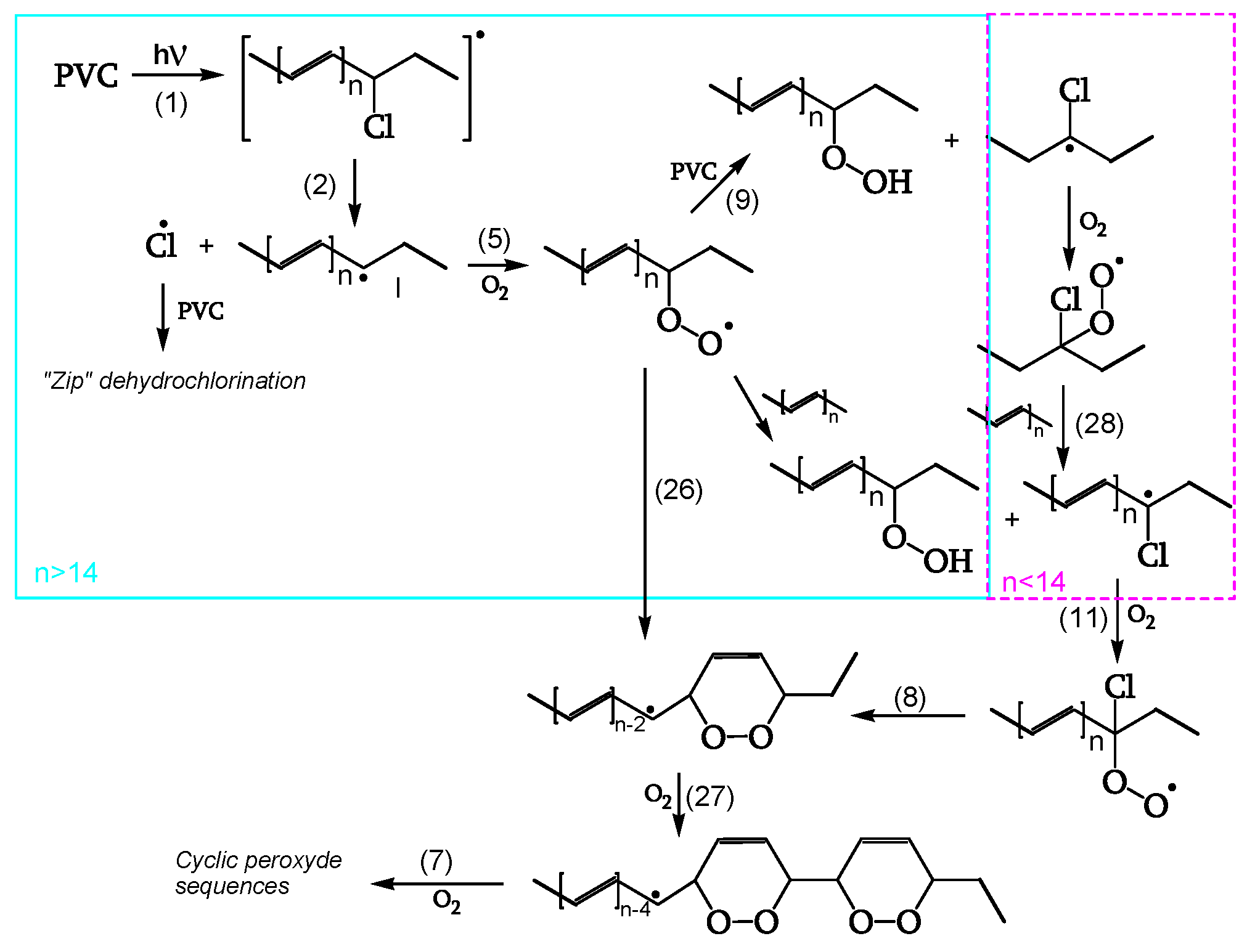
The reactions of
Figure 1 are also described in
Figure 2 in a simplified form with complete lines. This latter Figure, however, also outlines two subsequent degradation stages.
In fact, the reaction goes further in a second stage (dashed lines in
Figure 2), leading to photocleavage of peroxides and ketones and generating further initiating radicals [
18].
The following paragraphs will detail the chemistry of photodegradation and photo-oxidation.
1.2.2. Photodegradation
Initiation
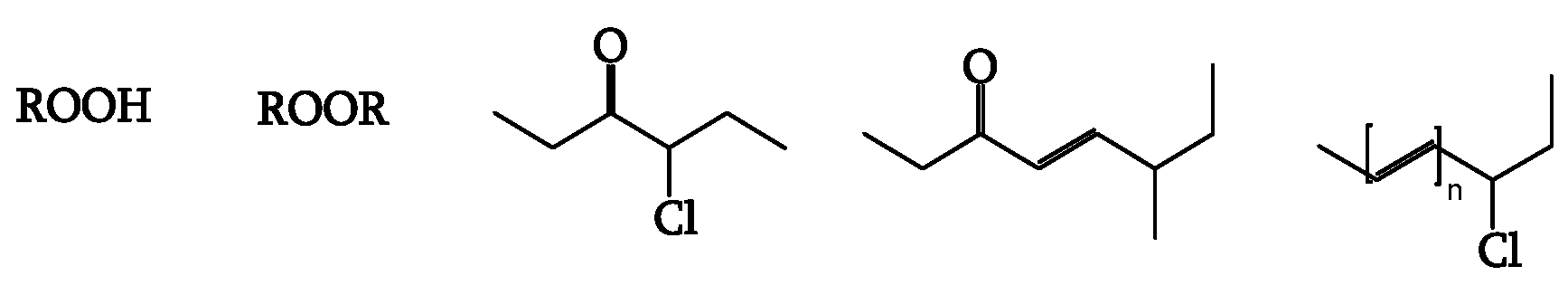
Impurities and chromophores trigger photodegradation. Polyenes, hydroperoxides, and ketones are possible chromophores (
Figure 3).
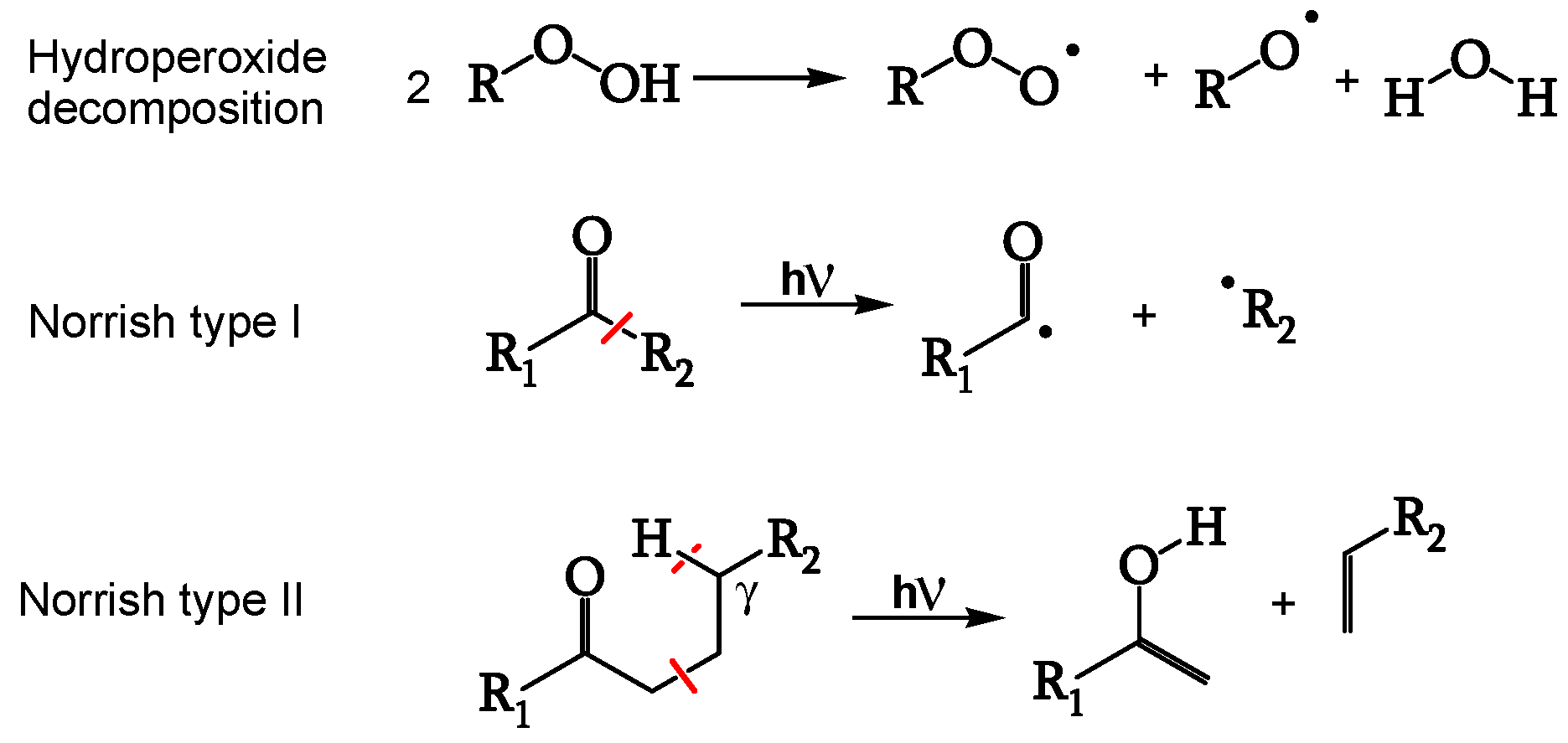
Hydroperoxides generate radicals upon light irradiation (
Figure 4, [
21]) as efficiently as ketones do through the Norrish type I reaction (
Figure 4, [
21]). Polyene sequences, having a high extinction coefficient, are the most absorbent chromophores [
18].
Table 1 [
19] gives the UV-visible absorptions of polyenes of different lengths.
Moreover, photodegradation studies in the presence and absence of oxygen showed the independence of the quantum yield of HCl on the irradiation time and the initial amount of unsaturation. That leads to the hypothesis of alkene-photo-sensitized degradation processes [
18,
22].
In these processes, excited singlet polyenes deactivate by radiative and non-radiative processes. Only 1% of absorbed photons generate HCl due to the restricted mobility that favors radical recombination (reaction 2 of
Figure 5) [
18].
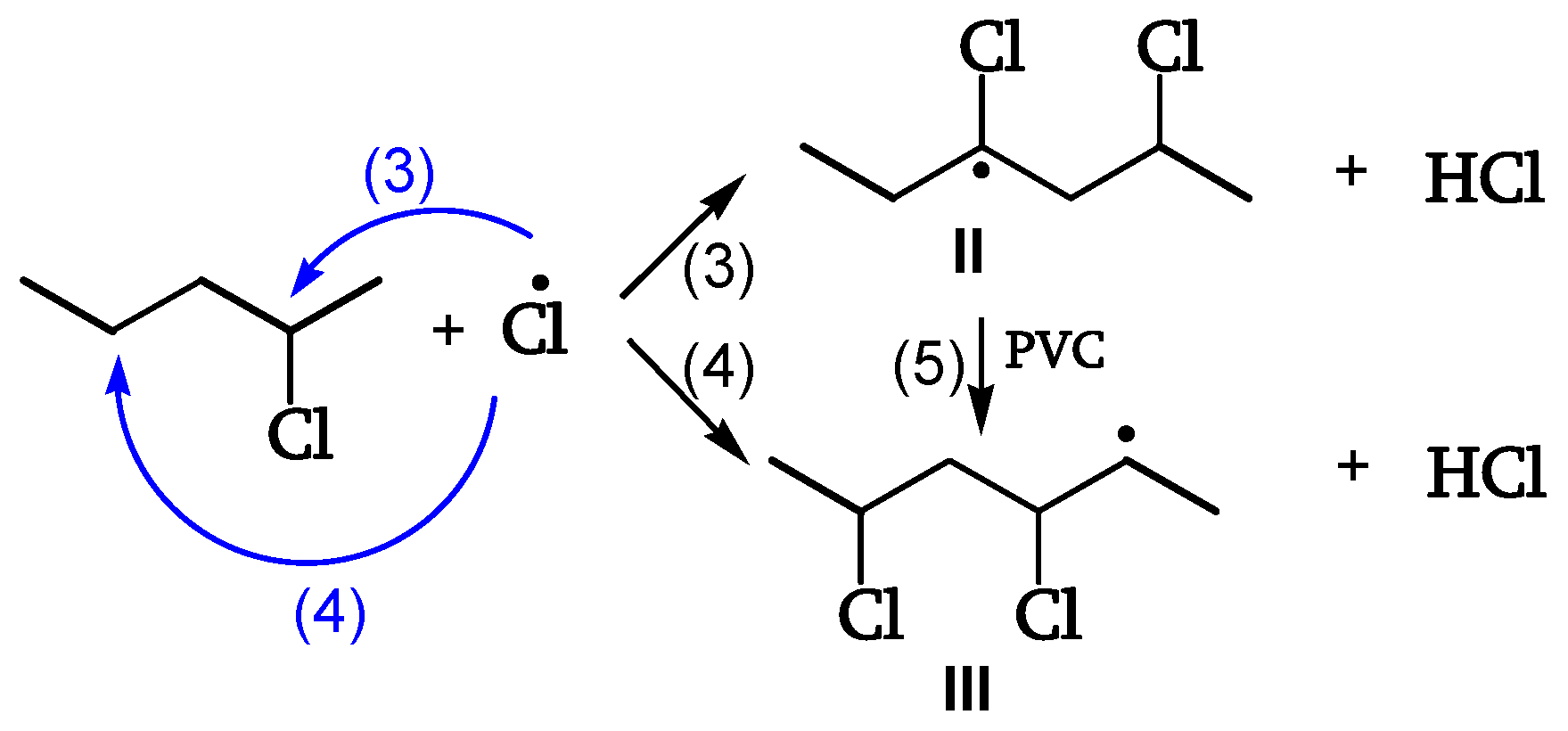
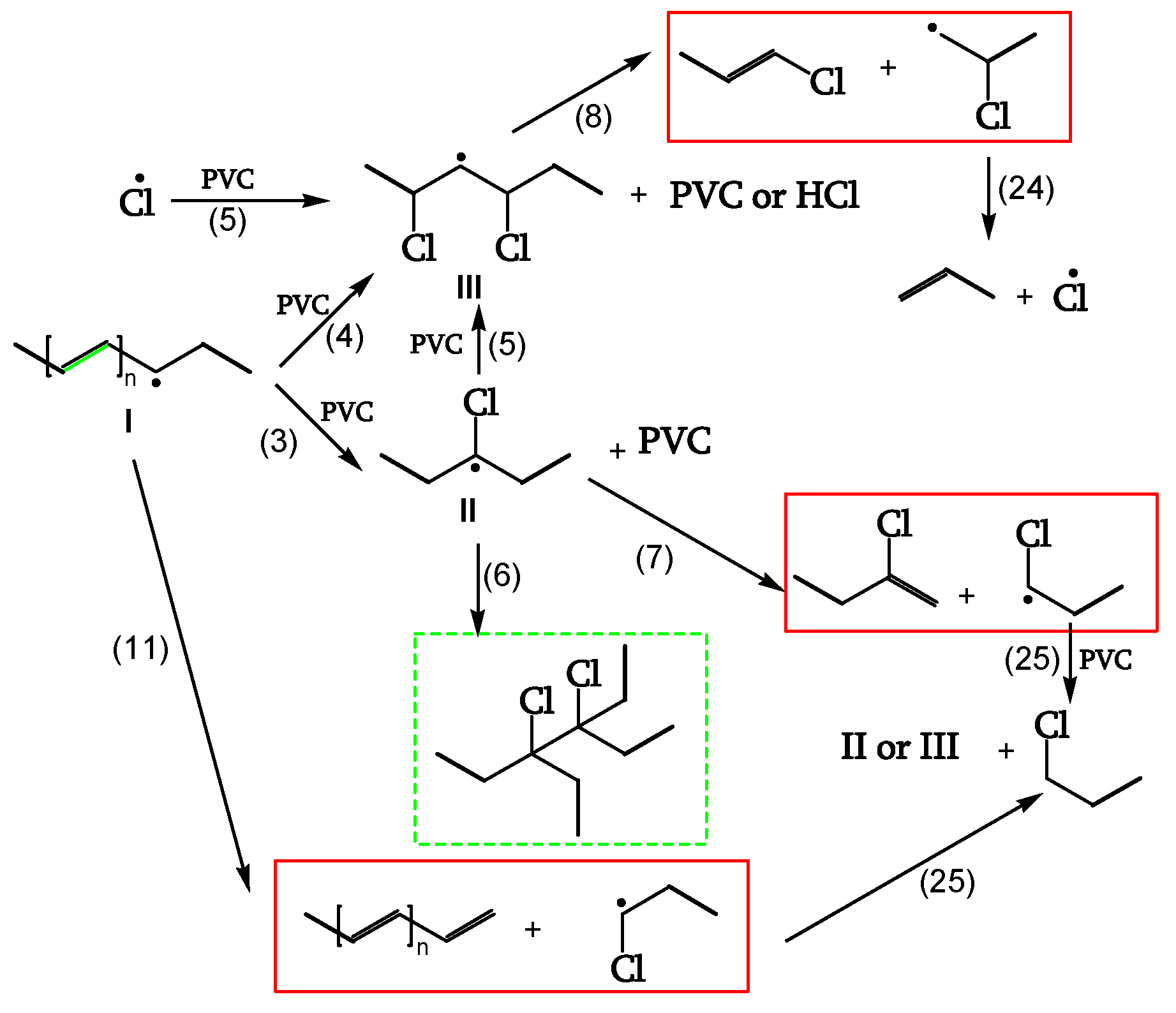
Chain Scission and Crosslinking
Radicals can be stabilized by β-scission (chain scission) of C-C bonds (reactions 7, 8, 11). The released radical fragments (•CHCl-CH
2- or •CH
2-CHCl-) propagate either by reacting with PVC to regenerate II and III (reaction 25) or by splitting off a Cl• (reaction 24). Radical III is sufficiently stable and provides crosslinking (reaction 6) (
Figure 9, [
18]).
1.2.3. Photooxidation
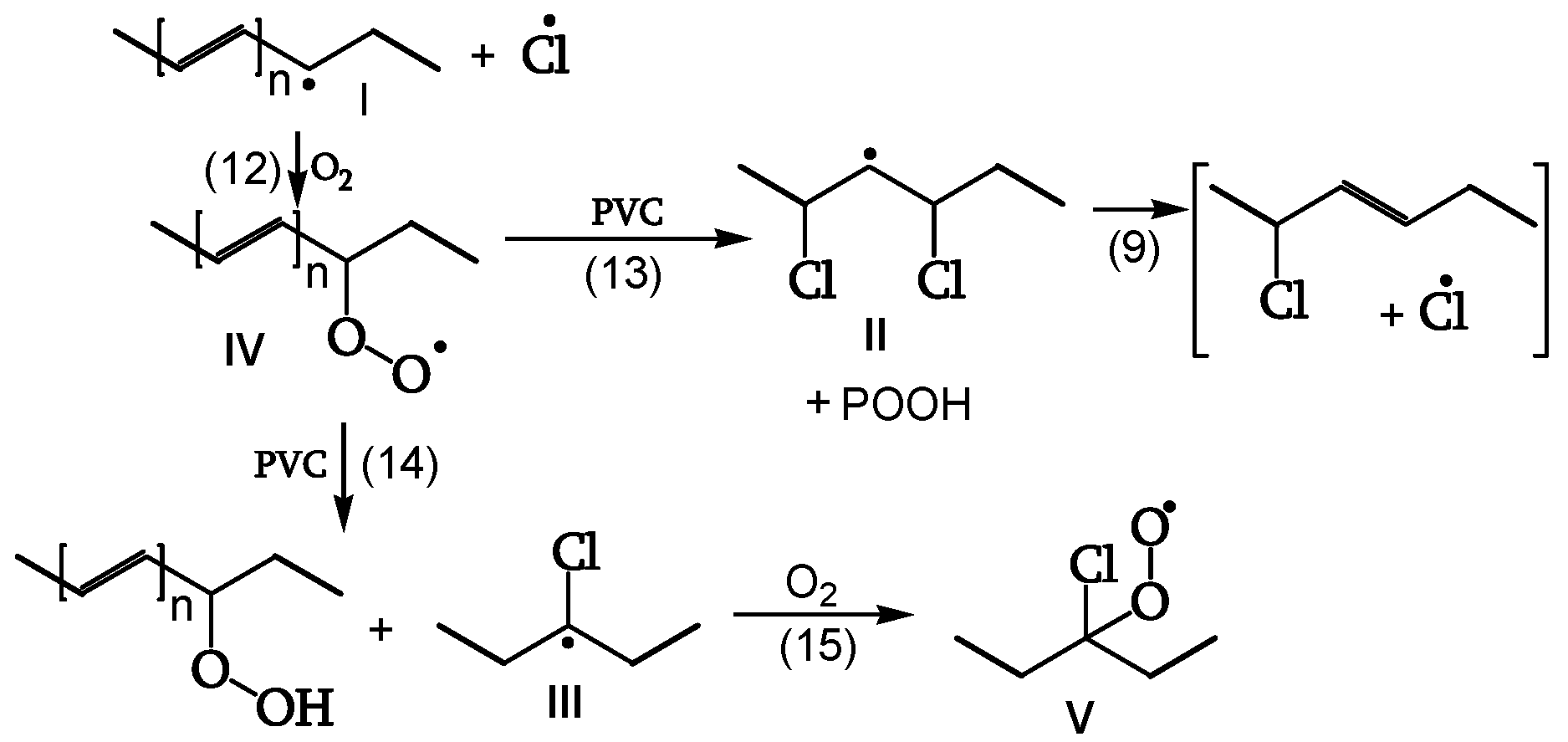
Initiation
The same initiation occurs in the presence or absence of oxygen, as the excited singlet polyenes (with very short lifetimes, about 10
−9 s) cannot be quenched by it (
Figure 5, [
18]).
Peroxidation
The polyenyl radical I (contrary to the less stable radical II) reacts with oxygen (reaction 12) to give peroxy radical IV. The attack on CH
2 of the PVC chain yields radical II (reaction 13). The attack on the CHCl of the PVC chain yields radical III (reaction 14). Radical III does not have labile β-Cl and reacts with oxygen to give γ-chloroalkylperoxy radical V (reaction 15) (
Figure 10, [
18]).
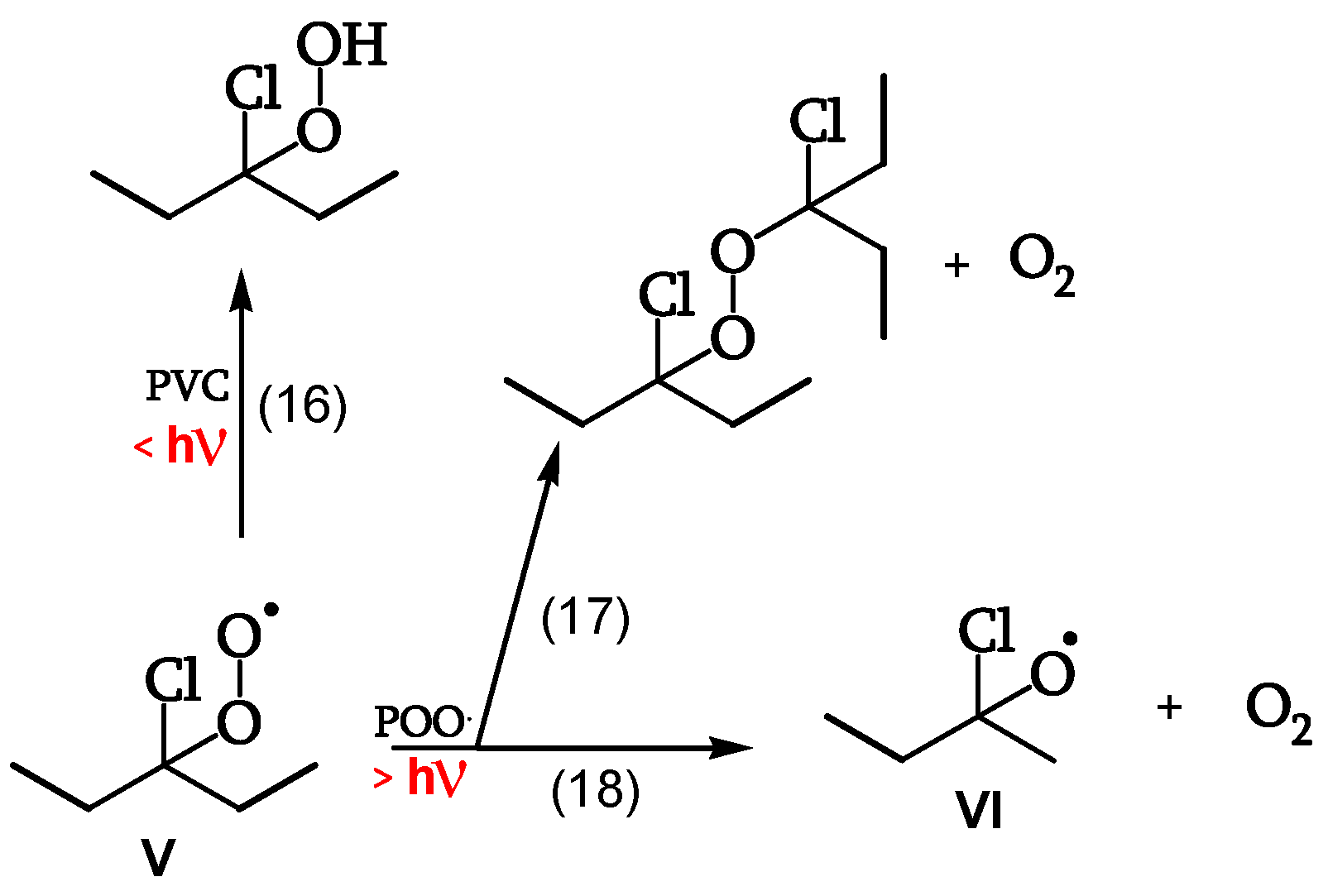
Reaction of the Peroxy Radical
At low irradiations, radical V abstracts H from PVC (reaction 16). The same radical V, at higher irradiations, either reacts with another peroxy radical to give “termination” (reaction 17) or reacts with another peroxy radical to give alkoxy radicals VI (reaction 18) (
Figure 11, [
18]). Peroxide obtained by reaction 17 is a photoreactive re-initiating site.
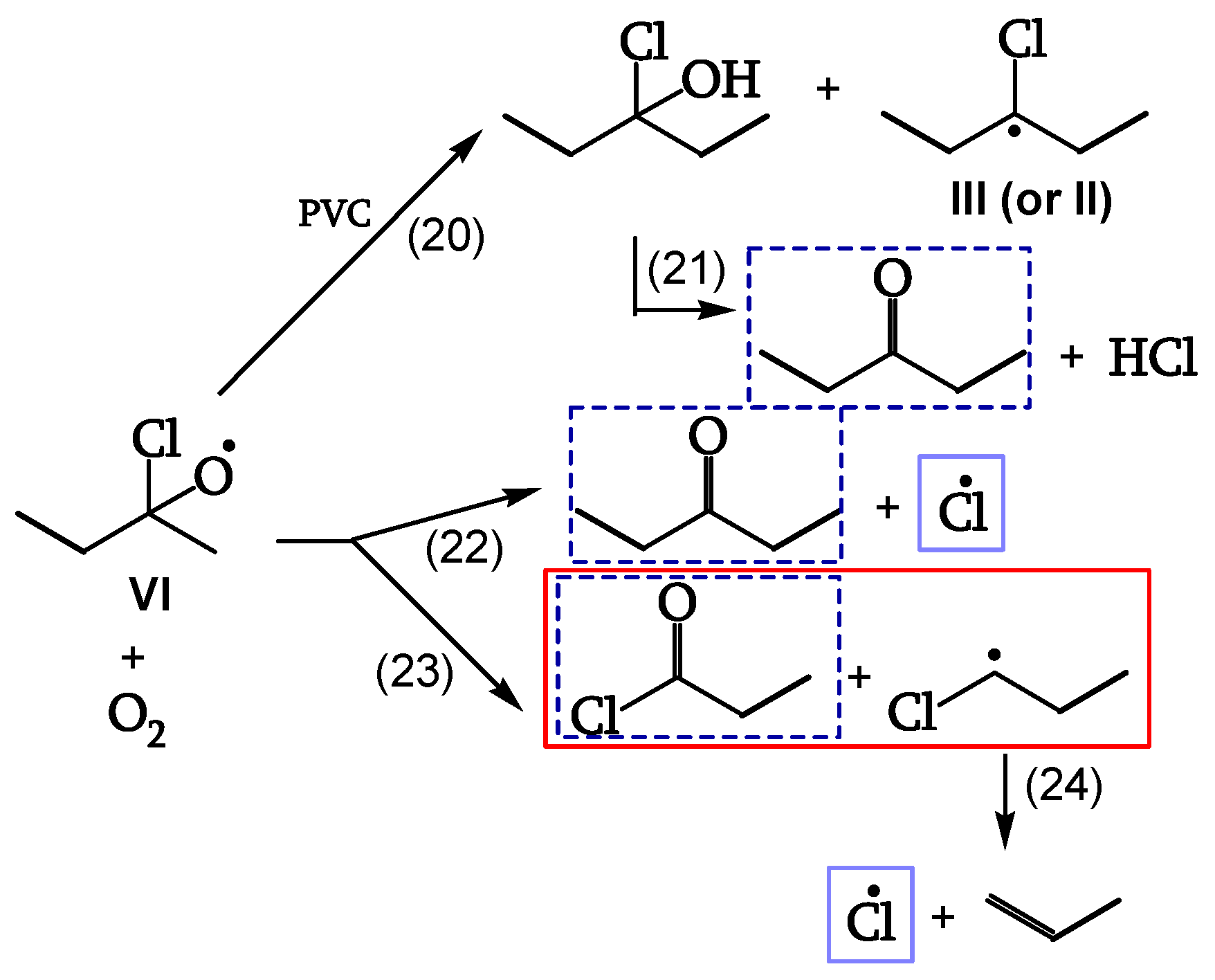
Reaction of the Alkoxy Radicals
Alkoxy radical VI either abstracts H from PVC (reaction 20), eventually decomposing to the corresponding ketone (reaction 21), or is stabilized by β-scission, giving ketones and either Cl• (reaction 22) or chain scission (reaction 23). These additional Cl• account for a 40% increase of θ
HCl in the presence of oxygen (
Figure 12, [
18]).
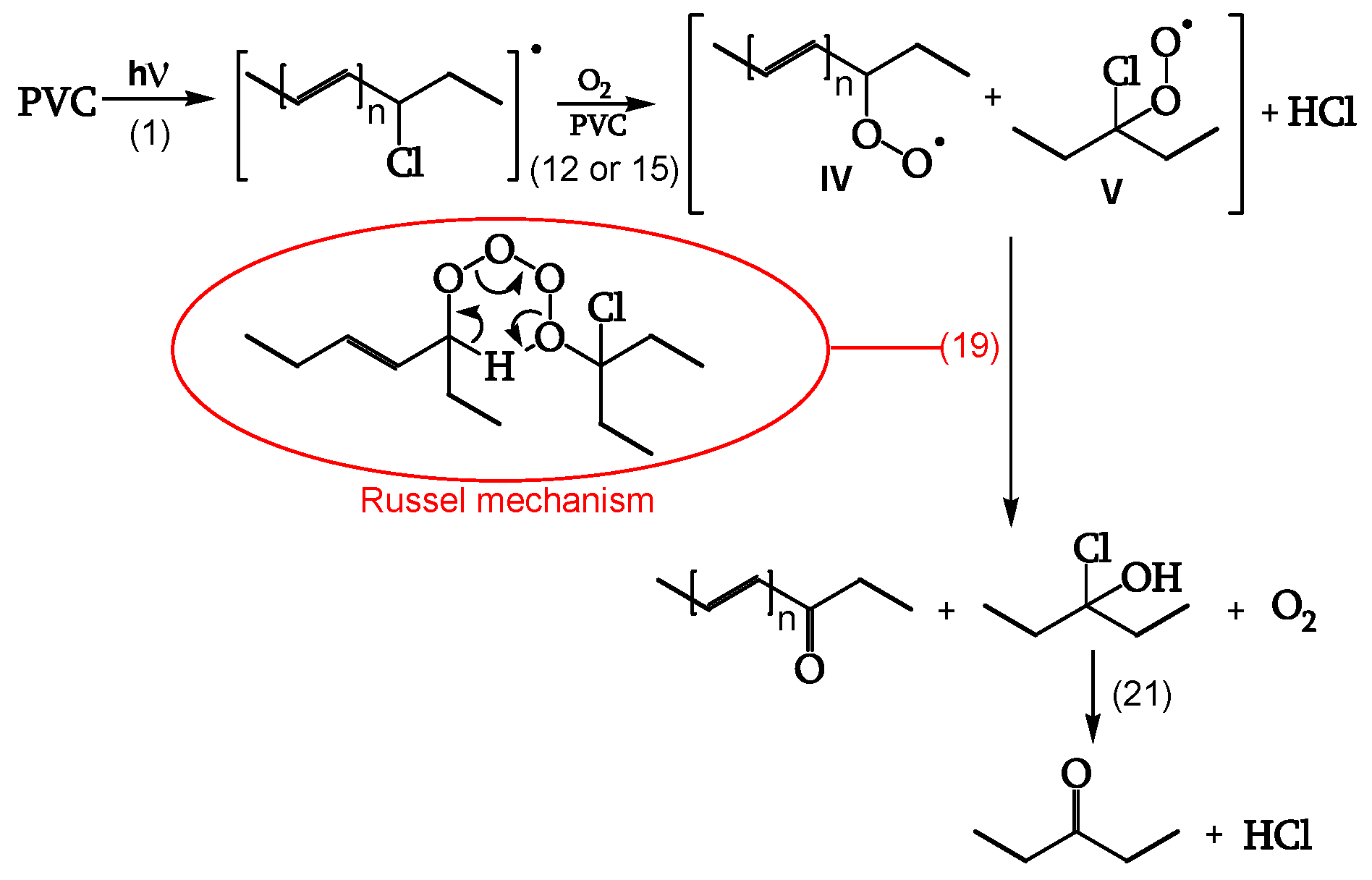
Termination
Cage reaction between peroxy radicals IV and V, via a Russel mechanism (reaction 19), leads to a ketone and an α-chloroalcohol. The α-chloroalcohol decomposes to give a ketone (reaction 21). That is why θ
C=O > θ
POOH, as evidenced in
Table 2 (
Figure 13, [
18]).
1.2.4. Photobleaching
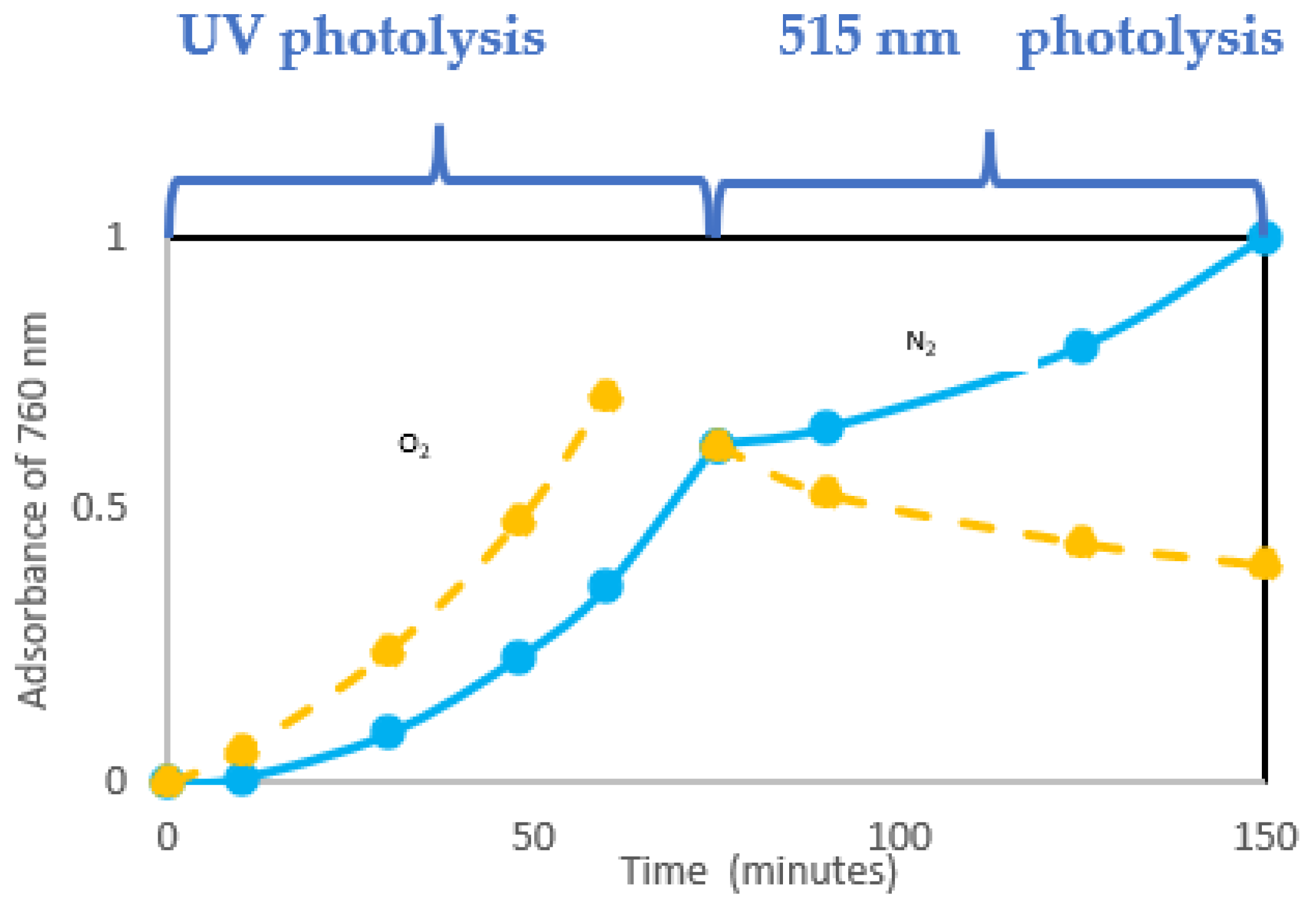
Experiments in Ref. [
20] were carried out, irradiating samples of PVC (with and without oxygen) with a UV lamp and then irradiating them with a lamp emitting in the solar spectrum region (i.e., 515 nm). In this second stage, a lowering of the absorbance was attributed to the photobleaching of conjugated double bonds by oxygen: in fact by far counterbalances double bond formation (
Figure 14).
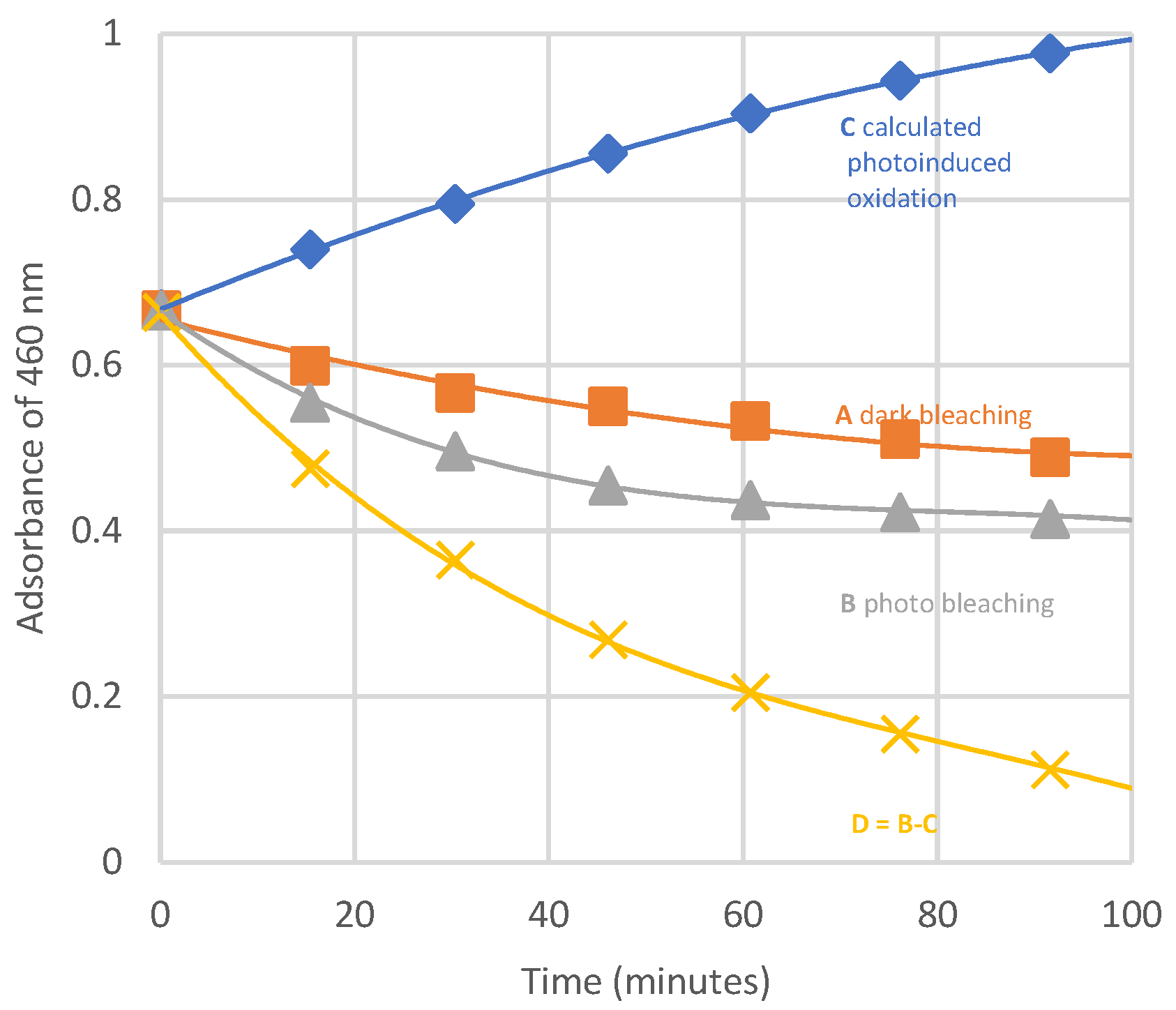
This kind of bleaching also occurs in the dark, and
Figure 15 shows that the neat contribution of photobleaching is reduced by the absorbance increase due to polyenes formation.
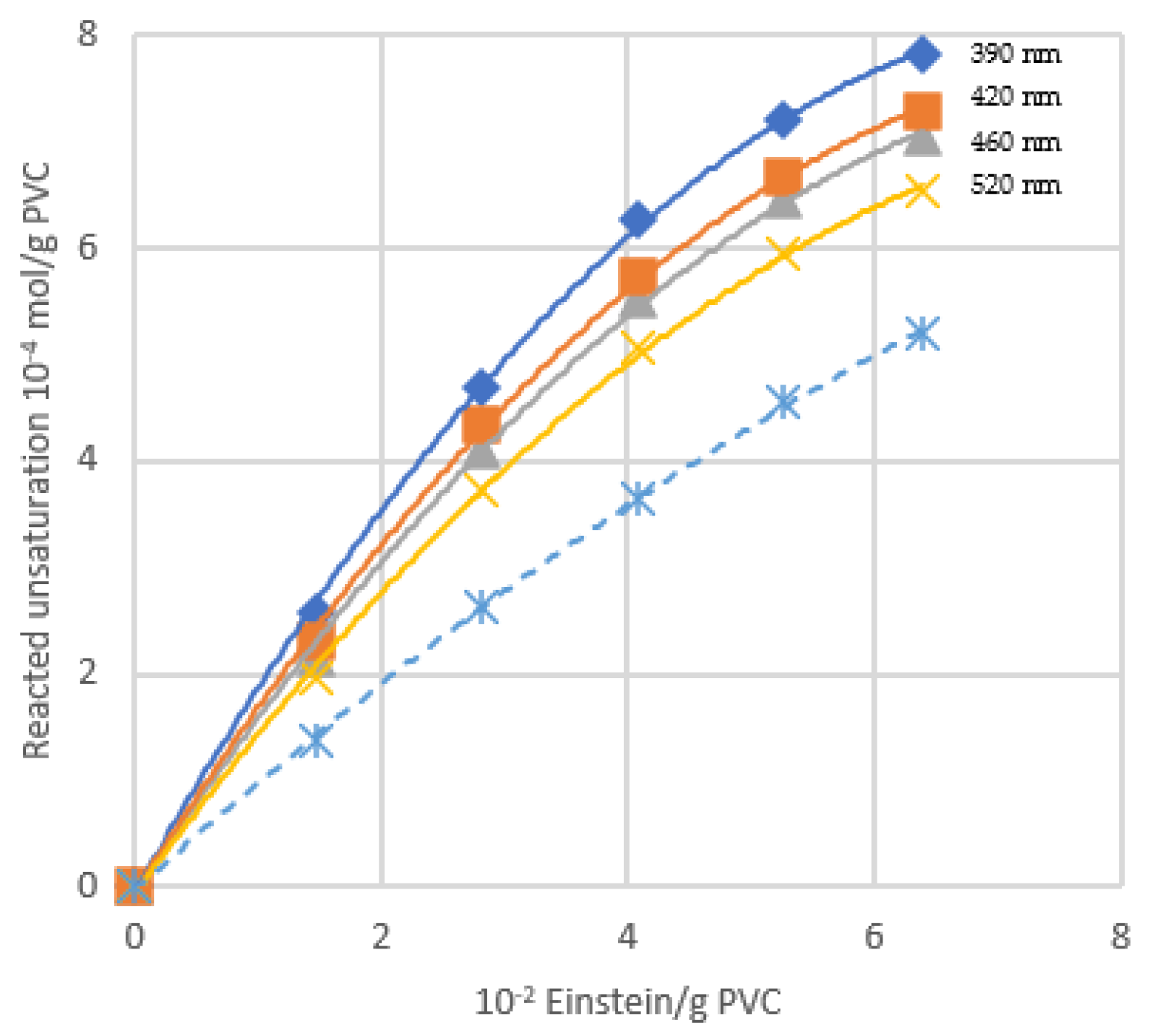
Figure 14 shows the extent of photobleaching (full lines) vs. dehydrochlorination (dashed line) under photolysis. The quantum yield of photobleaching is higher than dehydrochlorination one (θ
photobleaching = 1.5 × 10
−2 vs. θ
HCl = 0.9 × 10
−2). Θ
photobleaching is not very dependent on the wavelength. Thus, it is similar regardless of the length of the conjugated-polyene sequence (as will be schematically described in
Figure 16).
The mechanism leading to dark bleaching involves a step where ground-state oxygen reacts with polyenes (reaction 1 of
Figure 17), generating stable cyclic peroxides at room temperature. Their stability stems from very little HCl having evolved during oxygen bleaching in the dark.
Unlike dark-bleaching, in photobleaching (
Figure 18), polyenyl radical I is stable enough to react with oxygen (reaction 5). The resulting polyenyl peroxy radical may form cyclic peroxides (reaction 26). Then, a step-by-step polyene sequence-shortening follows, forming adjacent cyclic peroxides (reactions 7, 27). Reactions 26 and 27 also apply to shorter polyene peroxy radicals resulting from reactions 5, 11, and 28, thus accounting for the bleaching of polyenes with
n < 14 (not absorbing the 515 nm radiation).
1.2.5. Segmentation of Degradation Layers
Studies on the generation of polyenes and carbonyl species in the presence of stabilizers have been performed and led to the conclusion that the (photo)oxidation is diffusion-controlled [
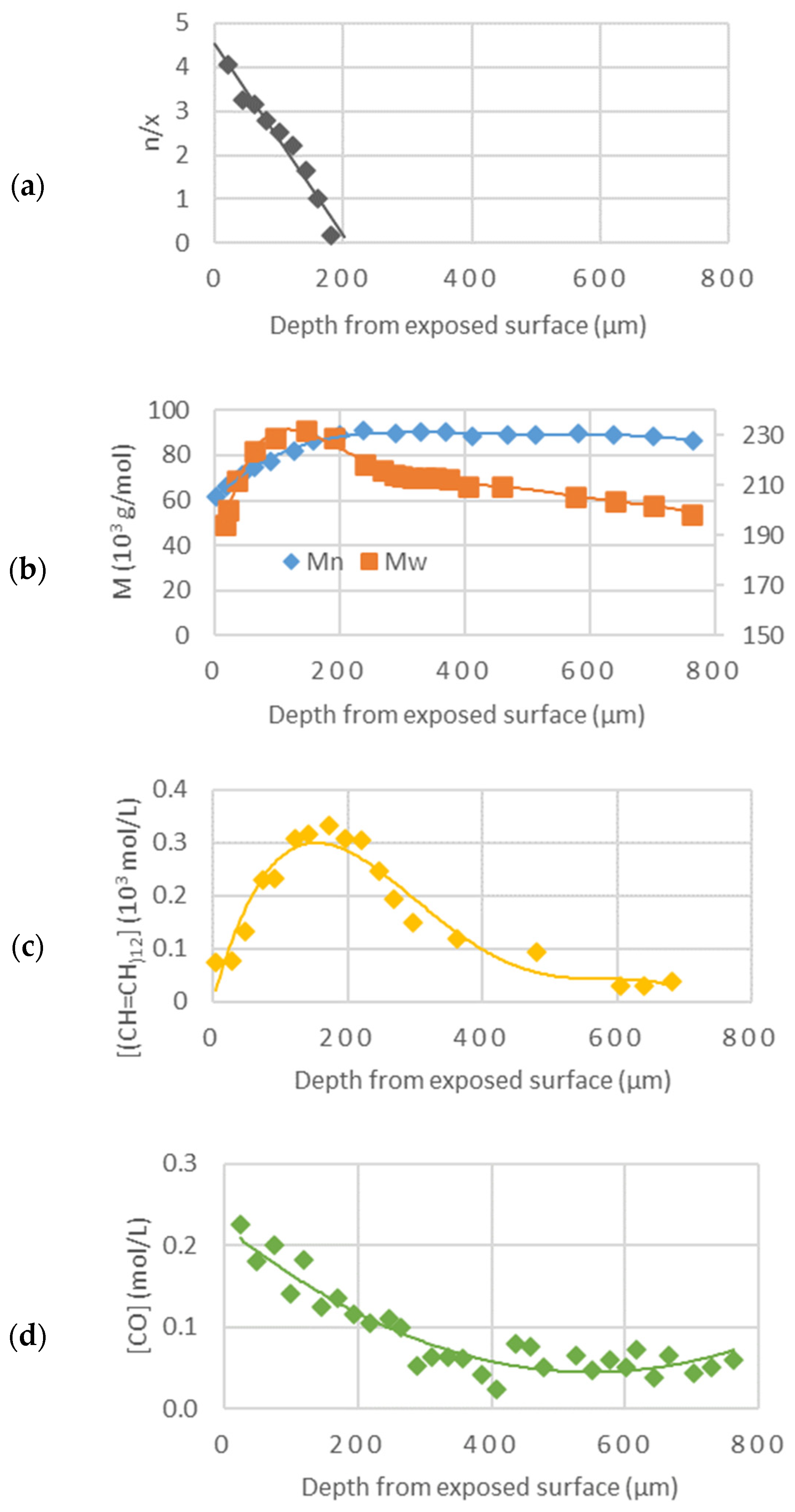
23]. This concept was completed by further studies on crosslink/chain-scission ratio, molecular weight, presence of polyenes, and carbonyl species along the thickness of an article exposed to sunlight (
Figure 19, [
24]).
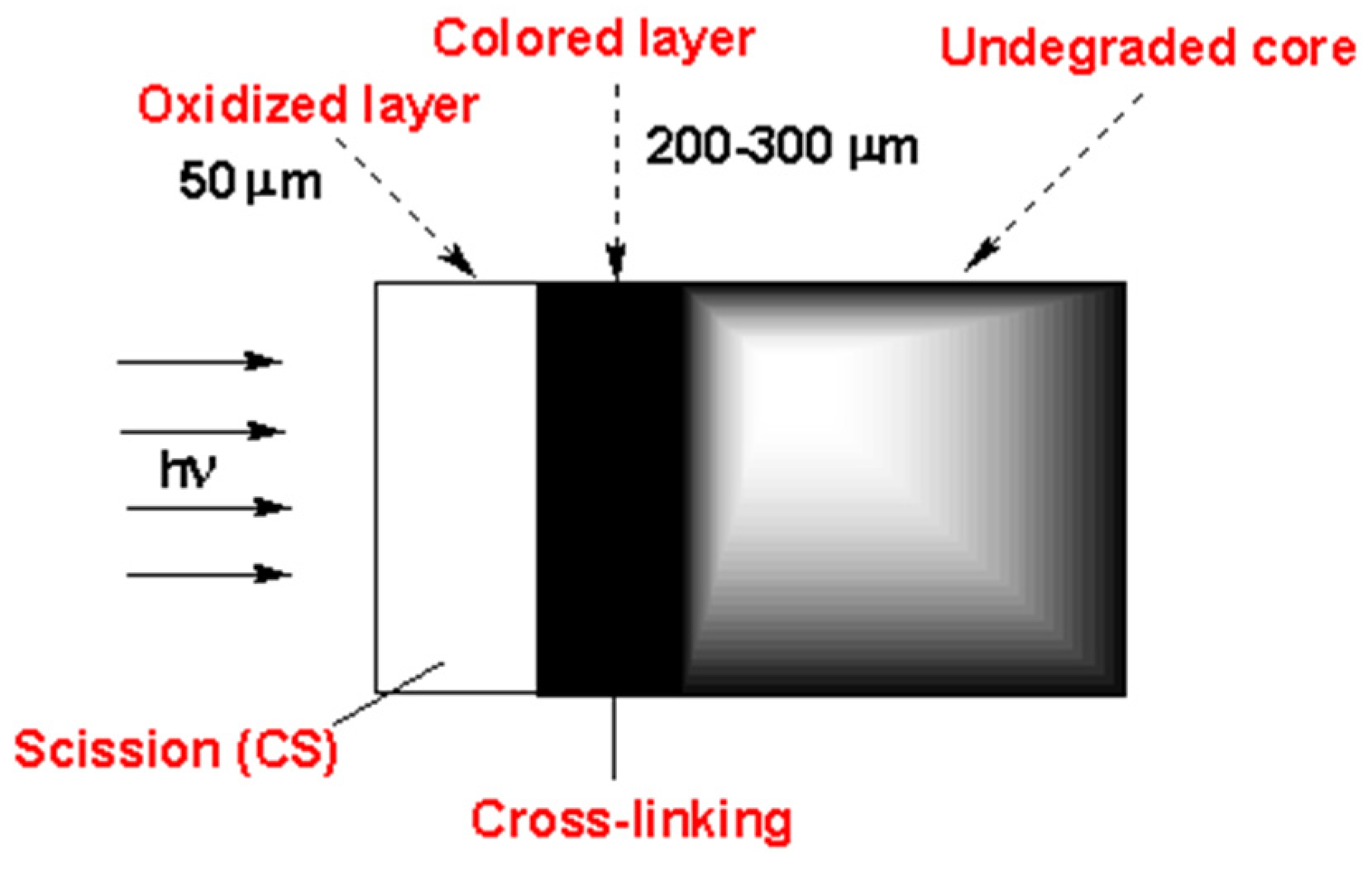
That experiment showed that there are three zones (roughly indicated by the different colors in
Figure 20) along the thickness of a PVC article exposed to sunlight:
A superficial zone (about 50 microns thick) with the predominance of oxidation products and chain scission: oxidation is oxygen diffusion-controlled in this region; chain scission results from oxidation reactions and is followed by chalking.
A lower underskin zone (between 50 and 300 microns) with a predominance of conjugated polyenes. This region is dominated by crosslinking (resulting from C• radicals) and polyene growth.
An undegraded core zone beyond 300 microns. In this region, photochemical reactions do not occur as the photons are screened by polyenes when they are increasingly generated after a specific time.
The formation of the two superficial degraded layers occurs because:
At a certain point in time, the superficial layer is embrittled by oxidative chain scission and cracks producing chalking that is, naturally, mechanically removed from the surface.
The polyenes, lacking their «protective layer», undergo photobleaching, shifting the boundary between degraded zones towards the core.
The sequential generation of dark polyene sequences, their bleaching, and eventually the erosion of the cracked surface triggers the cyclic change of color typical in outdoor exposure [
26,
27].
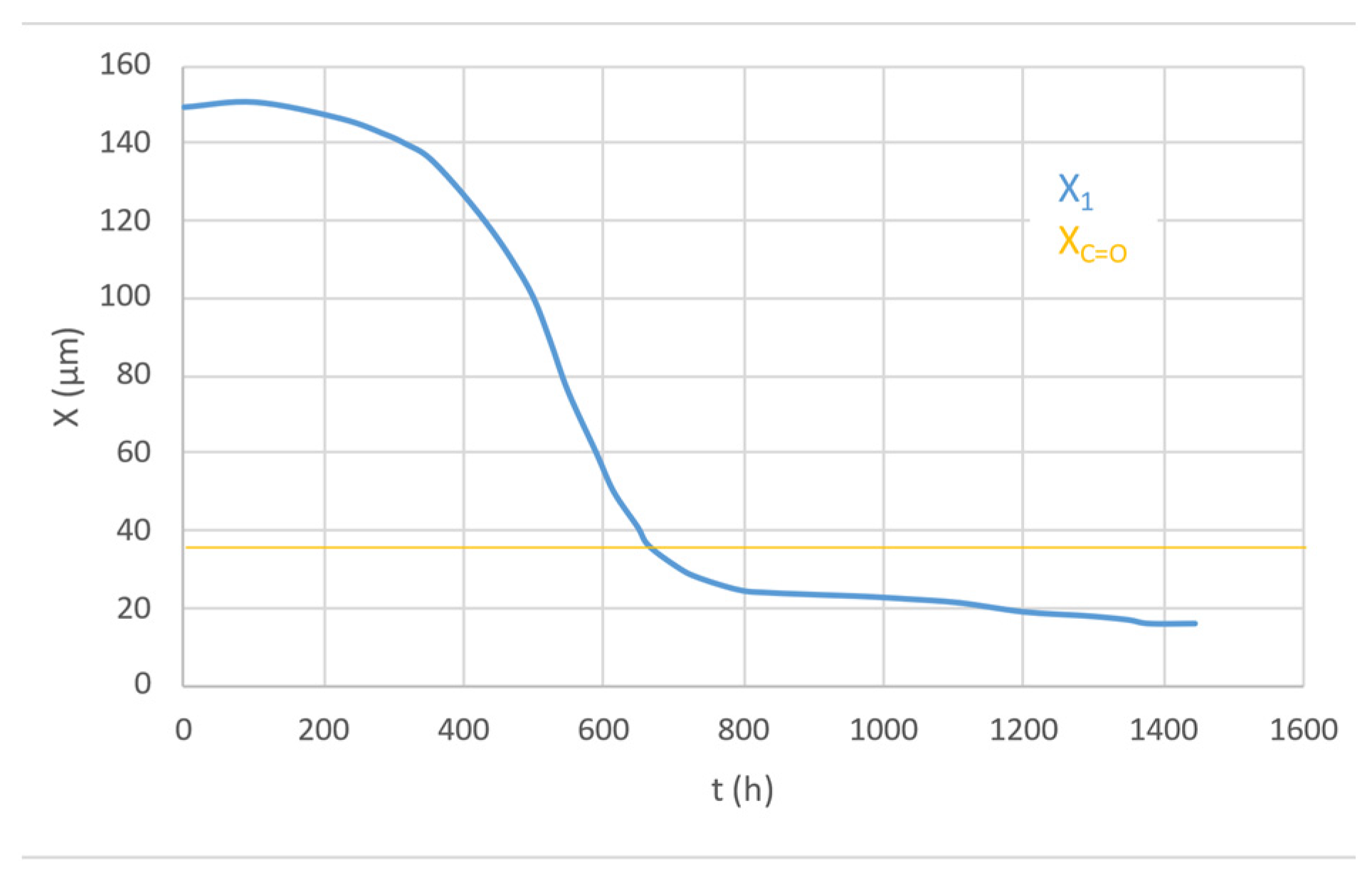
The thickness of the oxidation profile (Xco) increases with higher temperatures due to the higher diffusivity of oxygen, while it is independent of irradiation intensity. The depth of the max polyene concentration decreases with higher irradiation intensity and increases with higher temperature.
As the outer layer of PVC degrades, particles of pigment like titanium dioxide and fillers like CaCO
3 are released, producing a white powdery deposit; this phenomenon is known as “chalking.” It is known that different colors may affect plastic photoaging by influencing its solar absorbance [
8]. In addition, as chalking makes the article look whitish, this phenomenon is more apparent in dark-colored samples than in the white-pigmented ones. This particular weakness of dark-colored articles is well known and is a genuine challenge for manufacturers [
5,
6,
7].
1.2.6. Effects of Pigments and Fillers
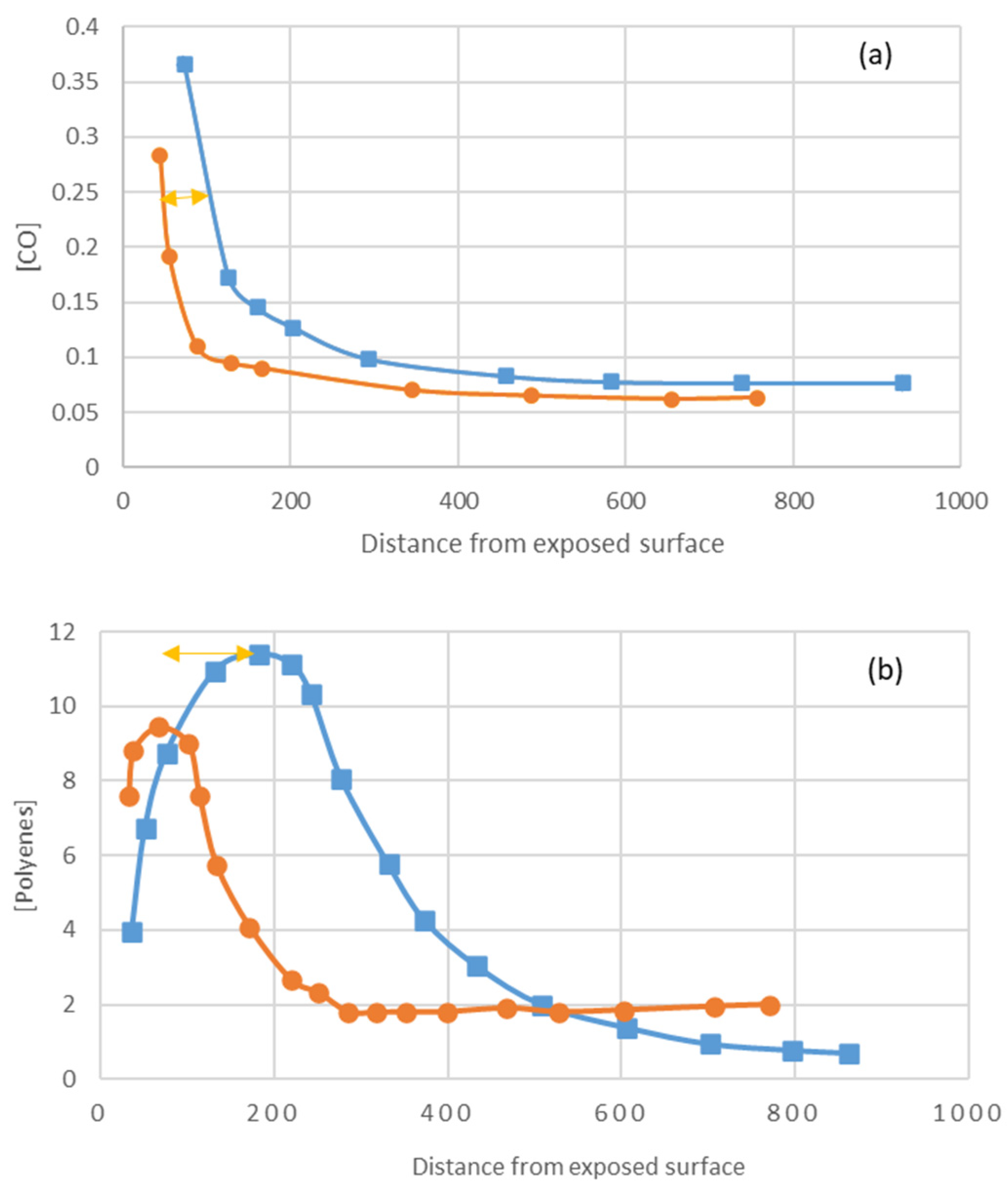
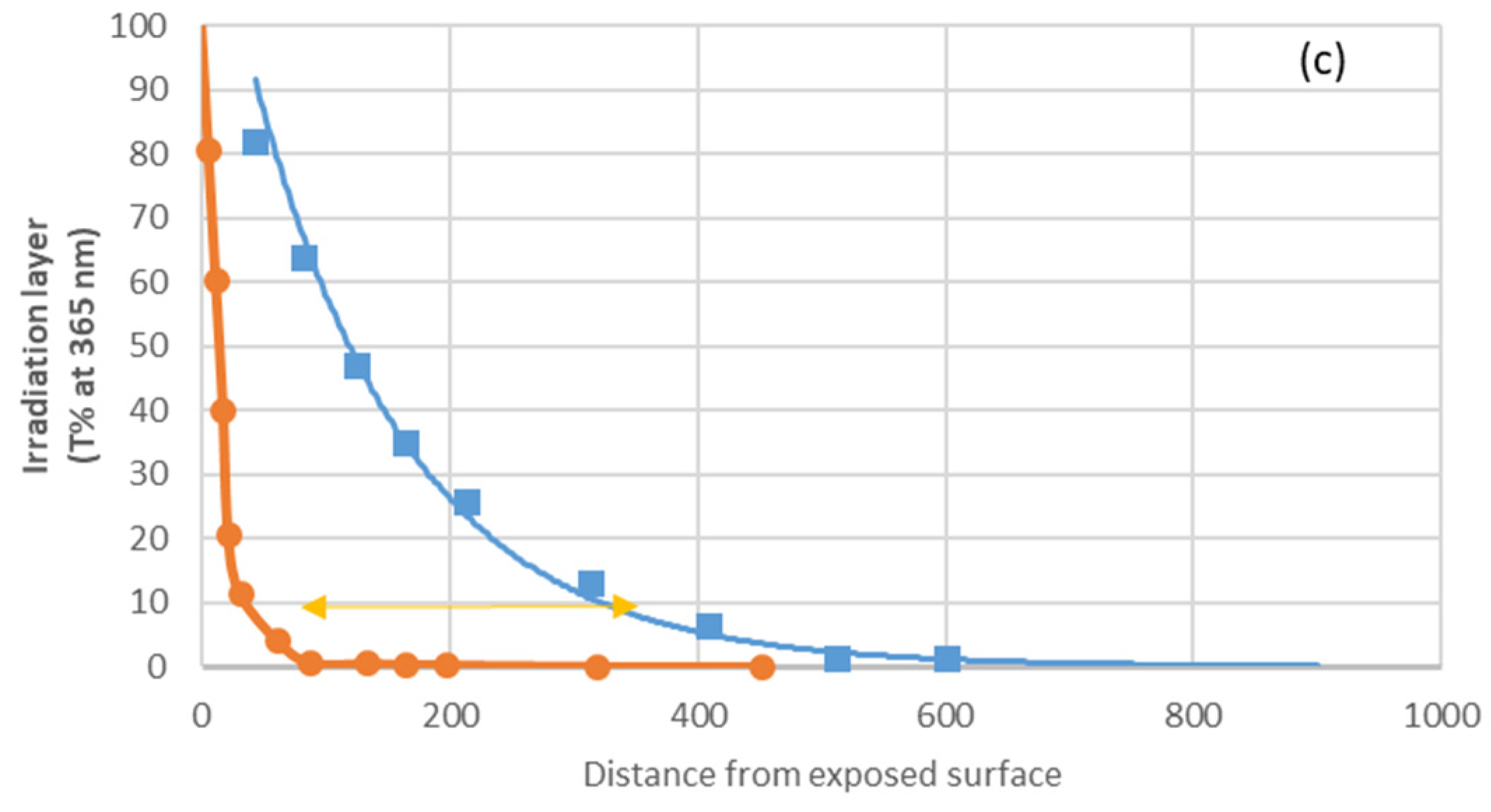
Effects of Titanium Dioxide Pigments
Figure 22 shows that titanium dioxide limits light penetration to 1-fifth. (
Figure 22c). The fact that the thickness of the degraded layer is 1-third (
Figure 22a for carbonyls, b for polyenes) [
28] is due to the following reasons:
UV light penetration decreases with time as polyenes build up.
Small radicals such as OH● and Cl● diffuse beyond the irradiation layer.
Photoreactions can be initiated by wavelength radiation close to the titanium dioxide cut-off (i.e., the limit between transmittance absorption and scattering, 365 nm).
In addition, it is also well known that, despite its screening effect, titanium dioxide (if not adequately coated or in the anatase form) promotes polymer degradation [
29]. Furthermore, due to the PVC surface layer’s degradation, titanium dioxide contributes to the chalking phenomenon described above, in the presence of water, as usually it happens in hot, humid climates such as Florida. On the other hand, chalking is not a common issue in hot, dry climates. This behavior is due to the typical mechanism through which TiO
2 brings about the oxidation of the organic substances in the presence of water [
18,
30].
Effects of Fillers
Fillers also influence the degradation and chalking of weathered PVC articles. CaCO
3 is an example of a filler capable of reacting with the HCl from PVC degradation to give the water-soluble calcium chloride. Its washout leaves holes, increasing the surface area subject to degradation and worsening the weathering. This phenomenon is increasingly evident if the filler amount rises [
31].
1.2.7. HCl Effect on Carbonyl Formation and Inhibition
Schemes of
Figure 1 and
Figure 2 do not involve HCl in the reactions in the presence of oxygen. However, it was observed that, in PVC photooxidation, there is a first autocatalytic phase in the formation and a subsequent inhibition phase for carbonyl species (
Figure 23, [
32]). As evident at short and long irradiation times, the initial auto-acceleration and the final auto-inhibition are favored when the thickness is high: the diffusion of a reactant from the sample to the atmosphere can thus control their kinetic behavior.
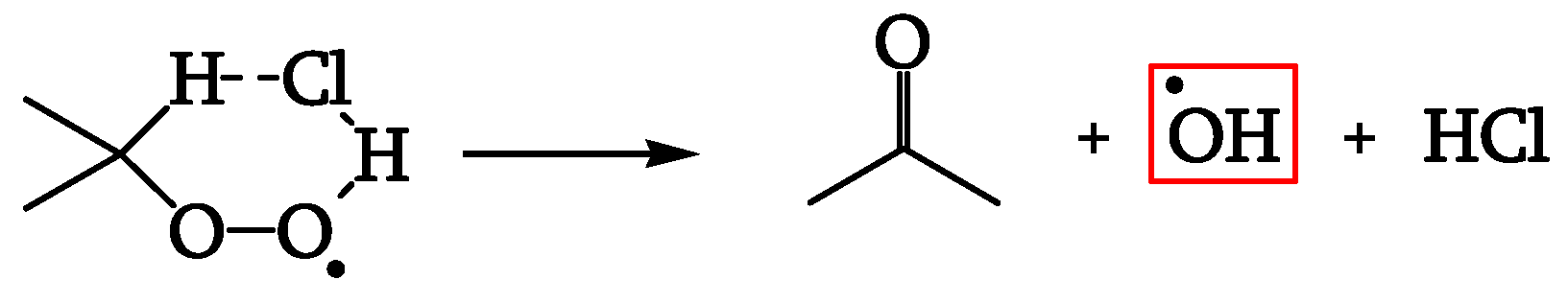
This reactant may, thus, be a gas such as HCl, which can explain the initial auto-acceleration with hydroxyl radical formation (
Figure 24).
HCl can also explain the final auto-inhibition. A charge transfer complex of HCl with polyenes (not photochemically active, different from polyenes themselves) is described in the literature (
Figure 25).
2. Experimental
As degradation generates cracking and the final effect is the chalking of the surface, this mainly affects the color variation of dark-colored articles. For this reason, several dark-green samples were prepared, as this color is particularly affected by weathering.
In the presence of pigments with high light fastness, the color change is due mainly to the chalking from the degraded surface rather than the degradation of the pigment itself. As shown above, carbonyl formation is limited to the surface as well (
Section 1.2.5). Therefore, as HCl seems to play a role in the carbonyl formation (
Section 1.2.7), samples with an increasingly higher loading of acid scavengers were prepared to study their effect on surface degradation of the dark-green-colored samples. Several acid scavengers are available and among them are hydrotalcites (also exchanged with different metals such as zinc, lithium, tin, titanium, and zirconium), hydrocalumite, ettringites, dawsonites, garnets, calcium and magnesium hydroxides, zeolites, metal carboxylates, organic phosphites, and epoxy compounds. Their general mechanism of action is well known and reviewed in the literature [
33].
2.1. Materials and Methods
Samples are manufactured using a base formulation made up of 100 parts w/w of PVC K65 from Vynova (Tessenderlo, Belgium) (about Mw of 81 × 103 g/mol and Mn of 41 × 103 g/mol), 15 parts w/w of calcium Carbonate Valtochim from Umbriafiller (Nocera Scalo, Perugia, Italy), 0.25 parts w/w of acrylic processing aid Reamod P220 from Reagens (San Giorgio di Piano, Bologna, Italy), lubricants REALUBE RL/105 CP from Reagens (San Giorgio di Piano, Bologna, Italy) (0.3 parts w/w), REALUBE SS 16-18 from Reagens (San Giorgio di Piano, Bologna, Italy) (0.2 parts w/w), REALUBE PO from Reagens (San Giorgio di Piano, Bologna, Italy) (0.15 parts w/w), Calcium-Zinc Corepack (1.8 parts w/w) from Reagens (San Giorgio di Piano, Bologna, Italy). V5000179 precolor PVC Dark green Cool Type from MASTER TEC GmbH (Oberhaid, Germany) is the selected pigment, has a high light fastness and contains titanium dioxide.
2.2. Sample Preparation
The formulations in
Table 3 were prepared by adding the indicated acid scavengers to the base formulation, prepared as described above, into a dry blend obtained using a PlasMec (Lonate Pozzolo, Varese, Italy) laboratory high-speed heater and cooler mixer combination. A sample without any acid scavenger is not presented as, in its absence, PVC samples would not have been obtained, as it is a key component of thermal stabilizers too.
The following protocol was used for the blending:
Ambient temperature: Start low-speed, add all components, and switch to high speed
110 °C: Discharge the heater mixer into the cooler mixer
40 °C: Discharge cooler mixer
All specimens (cut into pieces of 2 bv 10 cm) have been produced by a Bausano (Rivarolo Canavese, Torino, Italy) MD30 fully instrumented parallel twin-screw extruder with the following parameters:
Temperature profile: 145, 150, 160, 170, 165, 190, 195 °C
Screw speed: 22 rpm
Torque: about 21–22 Nm
Mass Temperature: about 180 °C
2.3. Weathering
2.3.1. Natural Outdoor Exposure
Natural outdoor exposure was carried out at the Reagens plant in San Giorgio di Piano (BO, Italy), southward 45°. San Giorgio di Piano is characterized by:
A typical annual solar irradiance of around 5.40 GJ/m
2 (Registered by Dexter Arpae San Pietro CapodiFiume available at
https://simc.arpae.it/dext3r/, access date 13 November 2022).
The average temperature of the warmest month of the year being 25 °C (Registered by Dexter Arpae San Pietro CapodiFiume available at
https://simc.arpae.it/dext3r/, access date 13 November 2022).
Severe climate, according to DIN EN 12608-1:2020 [
34].
2.3.2. Accelerated Exposure
Accelerated exposure was carried out both with a xenon-arc tester Altas (Mount Prospect, Illinois, USA) Ci4000 Weather-Ometer according to DIN EN 513:2018 [
35], method 2 (hot climate—Irradiation 12 GJ/m
2, wavelength 280–800 nm, black panel temperature 65 °C, 114 min dry/6 min water spray, RH dry cycle 65%) and with a Q-Lab (Westlake, Ohio, USA) QUV Accelerated Weathering Tester according to ISO 4892-3:2016 [
36] Method A/Cycle 1 (Irradiation 0,76 Wm
2 at 340 nm, UVA 340 (Type 1 A), black panel temperature 50 °C).
2.4. Color Measurement
Color has been measured on three replicates with an X-Rite SP 62 colorimeter working at D65/10. Evaluations were based on Delta E, representing the overall color change. The definition of Delta E and L*, a*. and b* is in DIN EN 13245-1:2010 [
37].
3. Results and Discussion
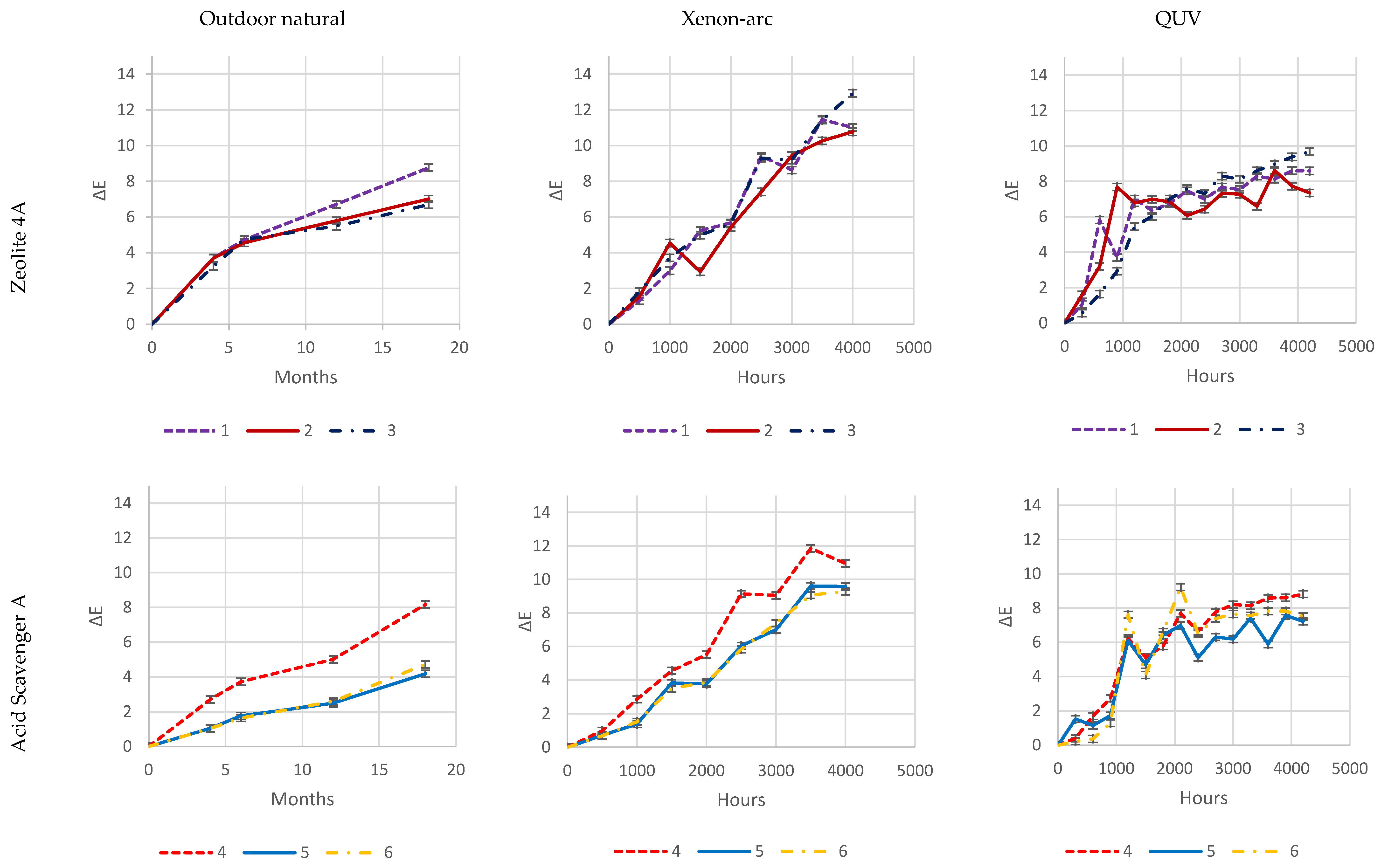
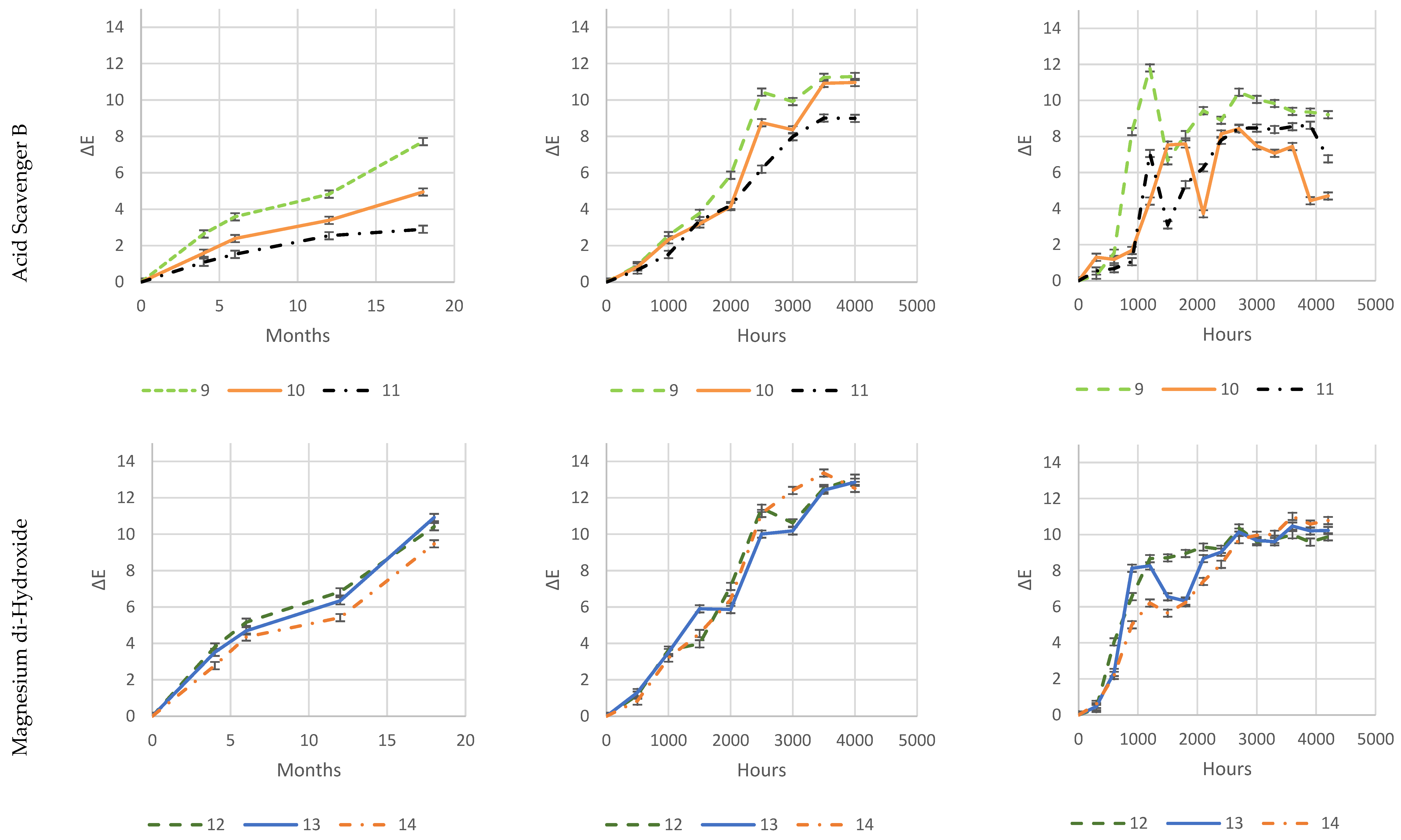
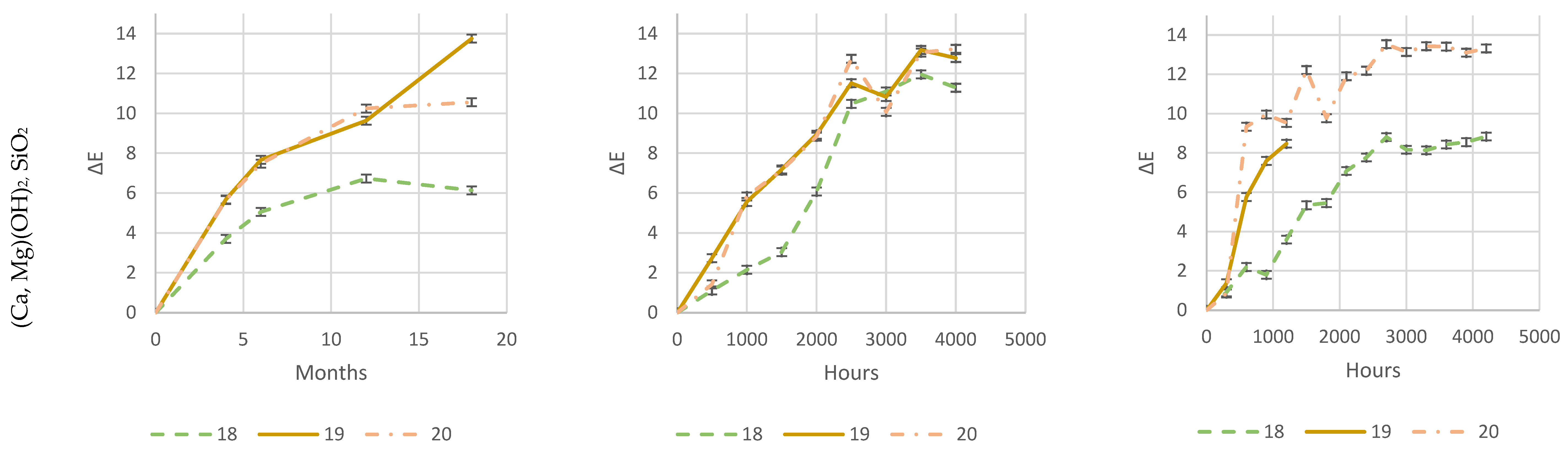
As it is evident from
Figure 26, both natural-outdoor and accelerated-weathering data are in accordance and show that higher loadings of some acid scavengers are more effective than others against photodegradation (e.g., Acid Scavenger One-pack A and Acid Scavenger One-pack B), while some of them are almost ineffective (e.g., simple Magnesium di-hydroxide, or simple Zeolite 4A), or even detrimental as a trivial (Ca, Mg)(OH)
2, SiO
2.
Increased loadings of Zeolite 4A and, in particular, Magnesium di-hydroxide, has no impact on color change during exposure, which is evident in all three exposure types.
An increased loading of (Ca, Mg)(OH)
2, SiO
2, is even increasingly detrimental for the color change when it raises from 1 phr to 4 or 8 phr. This is probably due to the reaction of HCl with calcium, generating water-soluble CaCl
2. As in the case of CaCO
3, outlined in
Section 1.2.6, CaCl
2 brings water into the matrix and makes the surface more prone to photo-oxidation. Furthermore, it is washed out during natural outdoor rain times and in accelerated-weathering devices during their wet cycles, leaving holes, cavities, and an uneven surface. Thus, the increased surface area and a stronger photo-oxidation worsen the weathering (
Section 1.2.6). As in the case of CaCO
3, this phenomenon is increasingly evident as the amount rises. This adverse effect probably offsets the beneficial HCl scavenging mechanism.
Zeolite 4A provides slightly higher protection at 4 and 8 phr, while an increase in Magnesium di-Hydroxide loading does not.
On the contrary, well-balanced acid-scavenger packages like Acid Scavenger One-Pack A and Acid Scavenger One-Pack B provide increasingly stronger protection against weathering as their loading increases. In particular, Acid Scavenger One-Pack B provides the strongest protection effect, lowering Delta E when it is increased from 4 to 8 phr. On the contrary, Acid Scavenger One-Pack A effect is leveled off at a concentration above 4 phr.
It is noteworthy that milder (and closer to reality) weathering methods allow for better discrimination between the performance of formulations than accelerated ones do. For example, this is evident for Acid Scavengers One-Pack A where the difference in performance between its 2 and 4–8 phr is well evident in natural weathering. On the contrary, it is less noticeable in the more aggressive xenon-arc tester, and almost not evident in the far more aggressive QUV tester. Regarding Acid Scavenger One-Pack B, natural weathering well discriminates among the performance of its 1, 4, and 8 phr loading. This gap is narrower in xenon-arc tester where the difference between 1 and 4 phr is negligible. In the QUV test apparatus, the Acid Scavenger One-Pack B loading effect is not evident at all. The slightly improved protection of Zeolite 4A at higher loading is not evident at all, not only in the most accelerated QUV tester, but also in the intermediate accelerated xenon-arc tester.
This emphasizes the importance of extended testing in the real field for a better understanding of the weathering performance, while accelerated-weathering tests should be used only for screening purposes. From a practical point of view, an accelerated screening QUV test would have been helpful to discriminate between evidently detrimental additives (e.g., (Ca, Mg)(OH)2, SiO2) and beneficial ones (e.g., Acid Scavenger One-Pack B), but only natural outdoor exposure has been used to investigate the loading effect of the latter.
4. Conclusions
Acid scavengers are remarkable substances designed to prevent thermal degradation and scavenge HCl during the thermal decomposition and combustion of low-acidity PVC compounds for cables [
38,
39,
40]. For these reasons, they already are crucial ingredients in formulating PVC compounds.
This paper shows another, somehow new aspect of some acid scavengers in preventing the drawbacks of exposure of the items to the outdoors, where thermal degradation and photodegradation, photooxidation, photo-catalyzed oxidation, and chemical reactions bring about phenomena affecting not only the aesthetic but also the mechanical properties of the articles.
The comprehensive understanding of the photodegradation mechanism of PVC gained from the experiments carried out proves that acid scavengers not only are effective against the HCl-catalyzed zip-like elimination that proceeds in the absence of oxygen but also against carbonyl formation, chain scission, and surface chalking in the presence of oxygen.
This new experimental evidence confirms the mechanism outlined in
Section 1.2.7, where HCl plays a crucial role in photo-oxidation of the matrix; a role that, until now, was not considered at all in commonly accepted theories of weathering.
Therefore, acid scavengers are beneficial to prevent weathering degradation, acting in several ways:
First, protecting the polymer during processing limits the generation of conjugated double bonds acting as chromophores that initiate photodegradation and photo-oxidation (
Section 1.2.2 and
Section 1.2.3).
Providing a reservoir of stabilizers limits the evolution of colored conjugated double bonds underskin in the absence of oxygen (
Section 1.2.5)
Providing a reservoir of stabilizers limits the evolution of cracking, and eventually chalking, on the surface in the presence of oxygen (
Section 1.2.7).
It is worth noting that not all acid scavengers have the same positive effect, but that some of them are neutral or even detrimental to surface discoloration, probably due to the water uptake stimulating the photo-oxidation of the matrix.
The design of the appropriate stabilizer formulation, thus, still requires a complex choice of proper ingredients and needs several laboratory and, eventually, real field tests.
Supplementary Materials
The following supporting information can be downloaded at:
https://www.mdpi.com/article/10.3390/polym16081047/s1, Table S1: Outdoor weathering data; Table S2: Xenon-arc tester data; Table S3: QUV Accelerated Weathering Tester. The data presented in this study are available as Supplementary material.
Author Contributions
Conceptualization, S.G., G.S. (Gianfranco Sarti) and G.S. (Gianluca Sarti); methodology, S.G., L.G., G.S. (Gianfranco Sarti) and G.S. (Gianluca Sarti); Validation, G.S. (Gianfranco Sarti) and G.S. (Gianluca Sarti); Formal Analysis, S.G., L.G., G.S. (Gianfranco Sarti) and G.S. (Gianluca Sarti); Investigation, S.G., L.G., G.S. (Gianfranco Sarti) and G.S. (Gianluca Sarti); Resources, G.S. (Gianluca Sarti); Data Curation, S.G., L.G., G.S. (Gianfranco Sarti) and G.S. (Gianluca Sarti); writing—original draft preparation, S.G.; writing—review and editing, S.G., L.G., G.S. (Gianfranco Sarti) and G.S. (Gianluca Sarti); Visualization, L.G.; Supervision, G.S. (Gianluca Sarti); Project Administration, G.S. (Gianfranco Sarti) All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Conflicts of Interest
Stefano Gardi, Lorenzo Giannone, Gianfranco Sarti and Gianluca Sarti were employed by the Reagens S.p.A. The all authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Royo, M.; Chulvi, V.; Mulet, E.; Ruiz-Pastor, L. Analysis of parameters about useful life extension in 70 tools and methods related to eco-design and circular economy. J. Ind. Ecol. 2023, 27, 562–586. [Google Scholar] [CrossRef]
- Michael, S. Chapter 5. PVC and Sustainability. In “PVC Additives”; Hanser: Munich, Germany, 2015; pp. 369–410. [Google Scholar]
- Everard, M. Twenty Years of the Polyvinyl Chloride Sustainability Challenges. J. Vinyl Addit. Technol. 2020, 26, 390–402. [Google Scholar] [CrossRef]
- Jörg, F. Optimising Weathering for Dark-Coloured PVC. In Pipe & Profile Extrusion; AMI: Bristol, UK, July–Agust (2018) 13–17. Available online: https://content.yudu.com/web/1rl19/0A1utvq/PPEJulAug18/html/index.html?refUrl=https%253A%252F%252Fmagazines.amiplastics.com%252F (accessed on 1 November 2018).
- Girois, S.; Schipper, P.S. Enhanced weatherability of exterior PVC building products. J. Vinyl Addit. Technol. 2001, 7, 61–66. [Google Scholar] [CrossRef]
- Summers, J.W. Formulations for vinyl house siding: History, present, future. J. Vinyl Addit. Technol. 1983, 5, 43–46. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Leung, K.M.Y.; Wu, F. Color: An Important but Overlooked Factor for Plastic Photoaging and Microplastic Formation. Environ. Sci. Technol. 2022, 56, 9161–9163. [Google Scholar] [CrossRef]
- Starnes, W.H. How and to what extent are free radicals involved in the nonoxidative thermal dehydrochlorination of poly(vinyl chloride)? J. Vinyl Addit. Technol. 2012, 18, 71–75. [Google Scholar] [CrossRef]
- Starnes, W.H.; Ge, X. Mechanism of Autocatalysis in the Thermal Dehydrochlorination of Poly(vinyl chloride). Macromolecules 2004, 37, 352–359. [Google Scholar] [CrossRef]
- Starnes, W.H.; Wallach, J.A.; Yao, H. Six-Center Concerted Mechanism for Poly(vinyl chloride) Dehydrochlorination. Requiescat Pace Macromol. 1996, 29, 7631–7633. [Google Scholar] [CrossRef]
- Starnes, W. Structural and mechanistic aspects of the thermal degradation of poly(vinyl chloride). Prog. Polym. Sci. 2002, 27, 2133–2170. [Google Scholar] [CrossRef]
- Payne, L.B. The Dehydrochlorination Mechanism of the Internal Allylic Chloride Structure in Poly(Vinyl Chloride). College of William & Mary—Arts & Sciences: Williamsburg, Virginia, USA, 2000. [Google Scholar] [CrossRef]
- Bacaloglu, R.; Fisch, M. Degradation and stabilization of poly (vinyl chloride). V. Reaction mechanism of poly(vinyl chloride) degradation. Polym. Degrad. Stab. 1995, 47, 33–57. [Google Scholar] [CrossRef]
- Fisch, M.H.; Bacaloglu, R. Degradation and stabilization of poly(vinyl chloride). 6: Model studies on dehydrochlorination of 6(4)-chloro-4(5)-tetradecenes in the presence of alkyl phosphites and zinc di(dialkyl phosphites). J. Vinyl Addit. Technol. 1999, 5, 205–217. [Google Scholar] [CrossRef]
- Yanborisov, V.M.; Minsker, K.S.; Zaikov, G.E.; Zaikov, V.G. Some aspects of the thermal degradation of PVC. Crosslinking of macromolecules. J. Vinyl Addit. Technol. 2002, 8, 176–179. [Google Scholar] [CrossRef]
- Kelen, T. Secondary Processes of Thermal Degradation of PVC. J. Macromol. Sci. Part A Chem. 1978, 12, 349–360. [Google Scholar] [CrossRef]
- Decker, C. Degradation of poly(vinyl chloride) by u.v. radiation—II: Mechanism. Eur. Polym. J. 1984, 20, 149–155. [Google Scholar] [CrossRef]
- Michael, S. Chapter 4. Unchartered Territory in the Use of PVC Products: Photo Effects. In “PVC Additives”; Hanser: Munich, Germany, 2015; pp. 281–289, 344–363. [Google Scholar]
- Decker, C.; Balandier, M. Laser-induced degradation of polyvinyl chloride II: Oxygen bleaching of polyenes. J. Photochem. 1981, 15, 221–228. [Google Scholar] [CrossRef]
- Gugumus, F. Plastic Additives Hand Book; Hanser: Munich, Germany, 2015; pp. 155,157. [Google Scholar]
- Decker, C.; Balandier, M. Laser-induced degradation of polyvinyl chloride I: Quantum yield of dehydrochlorination. J. Photochem. 1981, 15, 213–219. [Google Scholar] [CrossRef]
- Anton-Prinet, C.; Mur, G.; Gay, M.; Audouin, L.; Verdu, J. Photoageing of rigid PVC—I. Films containing CaZn thermal stabiliser. Polym. Degrad. Stab. 1998, 60, 265–273. [Google Scholar] [CrossRef]
- Anton-Prinet, C.; Dubois, J.; Mur, G.; Gay, M.; Audouin, L.; Verdu, J. Photoageing of rigid PVC—II. Degradation thickness profiles. Polym. Degrad. Stab. 1998, 60, 275–281. [Google Scholar] [CrossRef]
- Anton-Prinet, C.; Mur, G.; Gay, M.; Audouin, L.; Verdu, J. Photoageing of rigid PVC—III. Influence of exposure conditions on the thickness distribution of photoproducts. Polym. Degrad. Stab. 1998, 60, 283–289. [Google Scholar] [CrossRef]
- Gardi, S.; Giannone, L.; Sarti, G.; Sarti, G.; Costa, M. Influence of initial season on PVC weathering. Polym. Test. 2023, 125, 108123. [Google Scholar] [CrossRef]
- Gardi, S.; Lorenzo, G.; Gianfranco, S.; Sarti, G.; Costa, M. Influence of initial season on PVC weathering. In Proceedings of the AMI PVC Formulation, Cologne, Germany, 12–14 September 2023. [Google Scholar] [CrossRef]
- Anton-Prinet, C.; Mur, G.; Gay, M.; Audouin, L.; Verdu, J. Photoageing of rigid PVC—IV. Effects of titanium dioxide. Polym. Degrad. Stab. 1998, 61, 211–216. [Google Scholar] [CrossRef]
- Michael, S. Chapter 2. Additional PVC Additives. In “PVC Additives”; Hanser: Munich, Germany, 2015; pp. 187–189. [Google Scholar]
- Mull, B.; Möhlmann, L.; Wilke, O. Photocatalytic Degradation of Toluene, Butyl Acetate and Limonene under UV and Visible Light with Titanium Dioxide-Graphene Oxide as Photocatalyst. Environments 2017, 4, 9. [Google Scholar] [CrossRef]
- Bolt j., D.; Feng A., A.; Johnson R., W. The Role of Titanium Dioxide Pigments in Durability of Exterior PVC. In Proceedings of the PVC Conference, Brighton, UK, 26–28 April 2005. [Google Scholar]
- Verdu, J. Photooxidation of Polyvinyl Chloride). I. Influence of Film Thickness and Temperature on the Kinetics of Formation of CO Groups. J. Macromol. Sci. Part A Chem. 1978, 12, 551–567. [Google Scholar] [CrossRef]
- Michael, S. Chapter 1. PVC Stabilizers. In “PVC Additives”; Hanser: Munich, Germany, 2015; pp. 8–46. [Google Scholar]
- DIN EN 12608-1:2020; Unplasticized Poly(vinyl chloride) (PVC-U) Profiles for the Fabrication of Windows and Doors—Classification, Requirements and Test Methods—Part 1: Non-Coated PVC-U Profiles with Light Coloured Surfaces. CENELEC: Brussels, Belgium, 2020. Available online: https://my.ceinorme.it/home.html (accessed on 1 June 2020).
- DIN EN 513:2018; Plastics—Poly(vinyl chloride) (PVC) Based Profiles—Determination of the Resistance to Artificial Weathering. CENELEC: Brussels, Belgium, 2019. Available online: https://my.ceinorme.it/home.html (accessed on 1 August 2019).
- ISO 4892-3:2016; Plastics—Methods of Exposure to Laboratory Light Sources—Part 3: Fluorescent UV Lamps. CENELEC: Brussels, Belgium, 2016. Available online: https://my.ceinorme.it/home.html (accessed on 1 May 2016).
- EN 13245-1; Plastics—Unplasticized Poly(vinyl chloride) (PVC-U) Profiles for Building Applications—Part 1: Designation of PVC-U Profiles. UNI: Milano, Italy, 2010; (accessed on 1 May 2010).
- Bassi, I.; Bandinelli, C.; Delchiaro, F.; Piana, M.; Sarti, G. A New Perspective on Hydrogen Chloride Scavenging at High Temperatures for Reducing the Smoke Acidity of PVC Cables in Fires V: Comparison between EN 60754-1 and EN 60754-2. Fire 2023, 6, 326. [Google Scholar] [CrossRef]
- Bassi, I.; Delchiaro, F.; Bandinelli, C.; Mazzocchetti, L.; Salatelli, E.; Sarti, G. A New Perspective on Hydrogen Chloride Scavenging at High Temperatures for Reducing the Smoke Acidity of PVC Cables in Fires, IV: The Impact of Acid Scavengers at High Temperatures on Flame Retardance and Smoke Emission. Fire 2023, 6, 259. [Google Scholar] [CrossRef]
- Sarti, G. A New Perspective on Hydrogen Chloride Scavenging at High Temperatures for Reducing the Smoke Acidity of PVC Cables in Fires. I: An Overview of the Theory, Test Methods, and the European Union Regulatory Status. Fire 2022, 5, 127. [Google Scholar] [CrossRef]
- Sarti, G. A New Perspective on Hydrogen Chloride Scavenging at High Temperatures for Reducing the Smoke Acidity of PVC Cables in Fires. II: Some Examples of Acid Scavengers at High Temperatures in the Condensed Phase. Fire 2022, 5, 142. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).