Optical and UV Shielding Properties of Inorganic Nanoparticles Embedded in Polymethyl Methacrylate Nanocomposite Freestanding Films
Abstract
1. Introduction
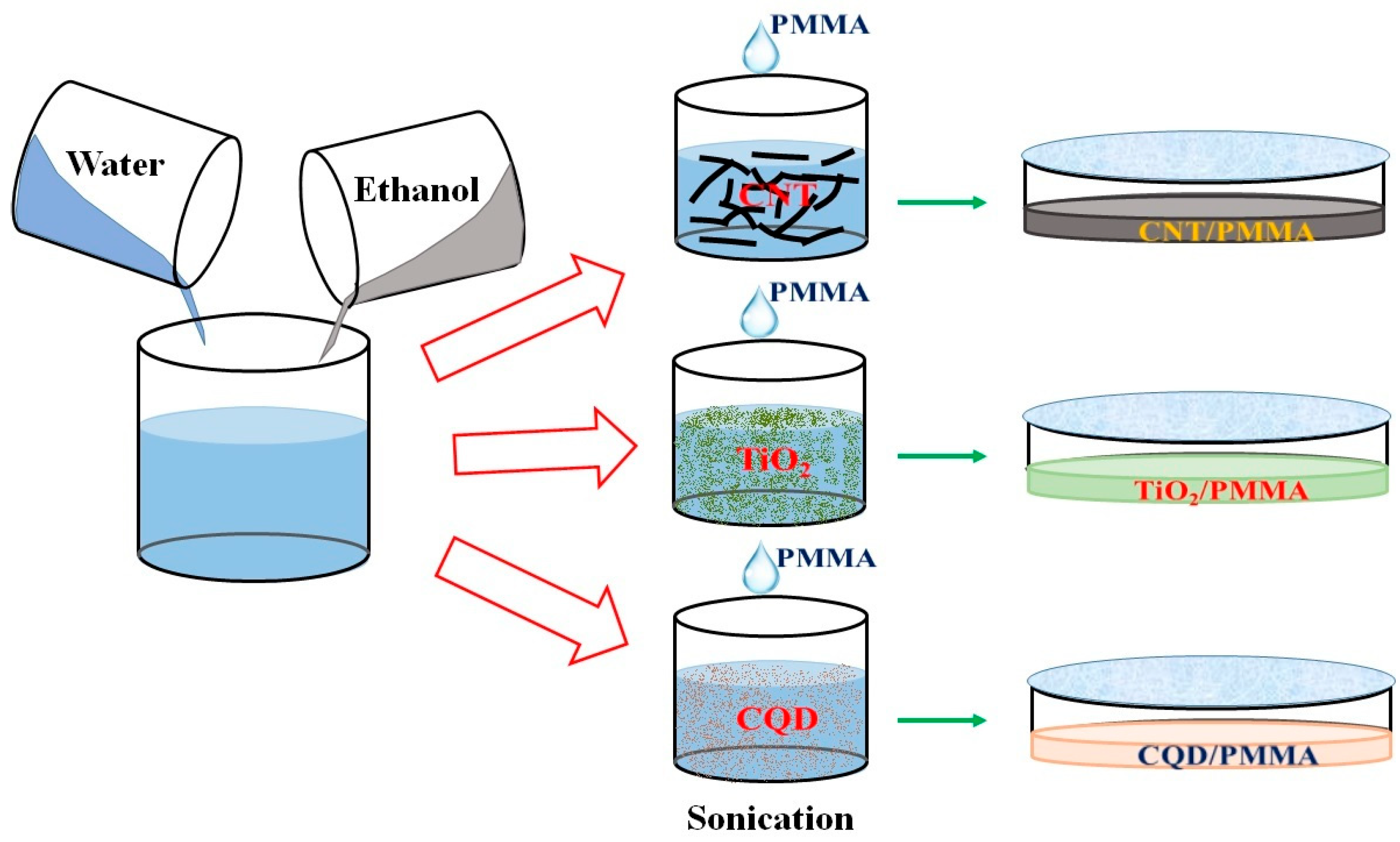
2. Experimental Details
3. Results and Analysis
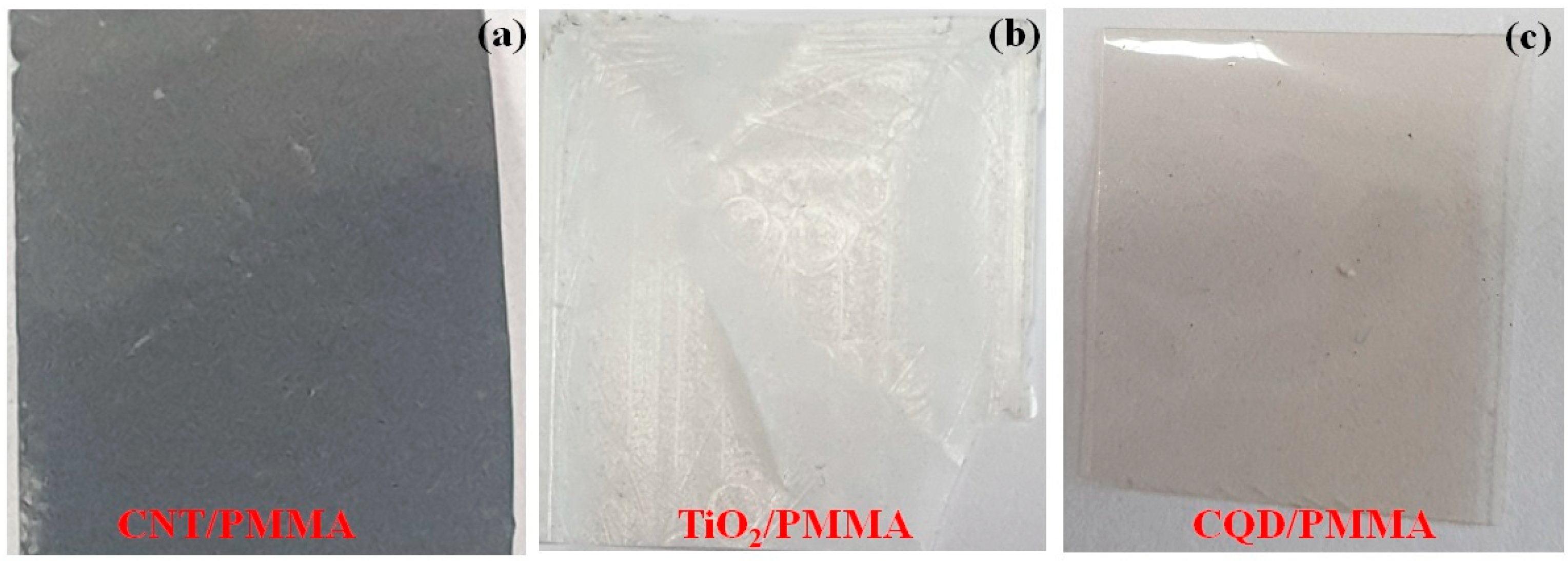
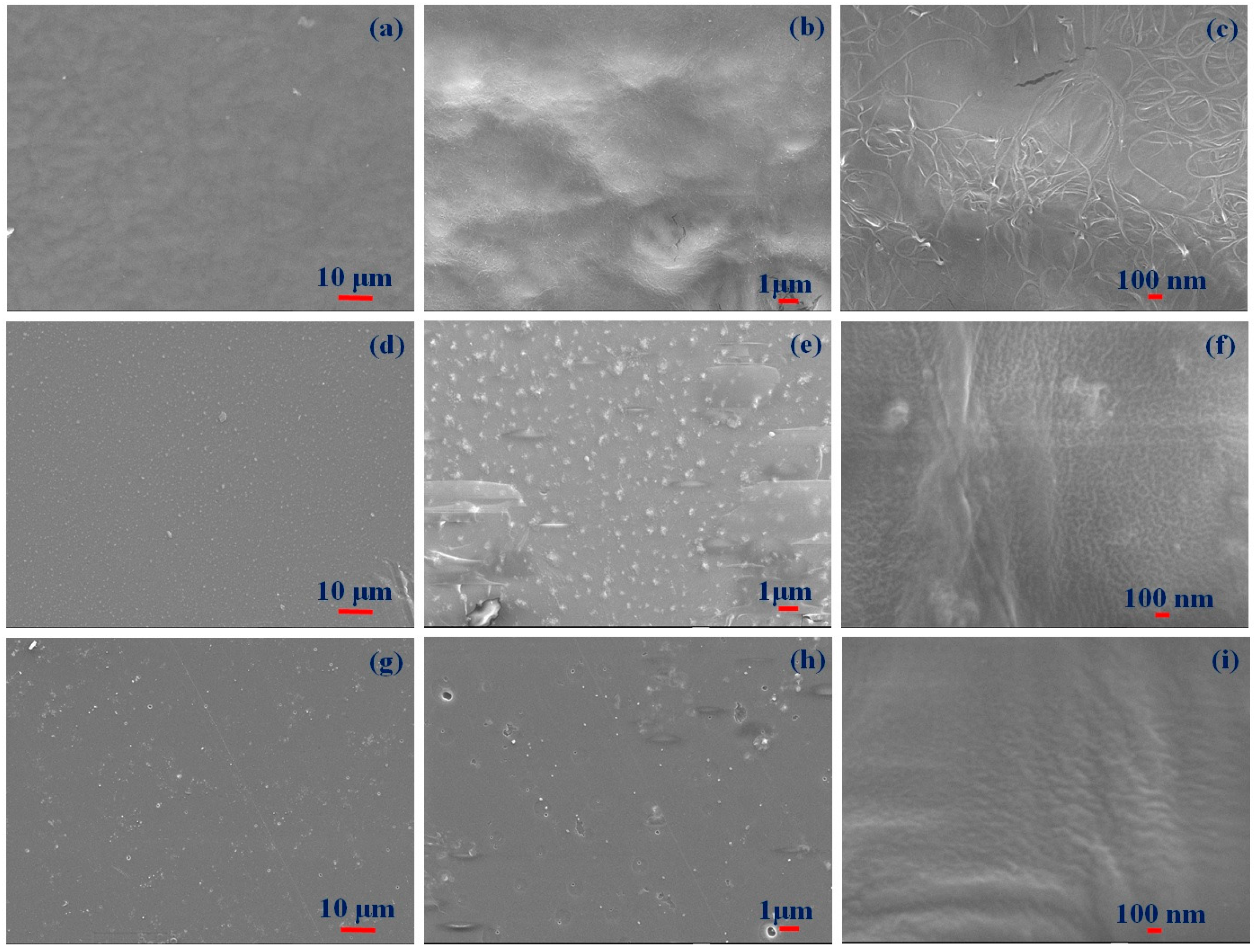
3.1. Morphological and Structural Studies
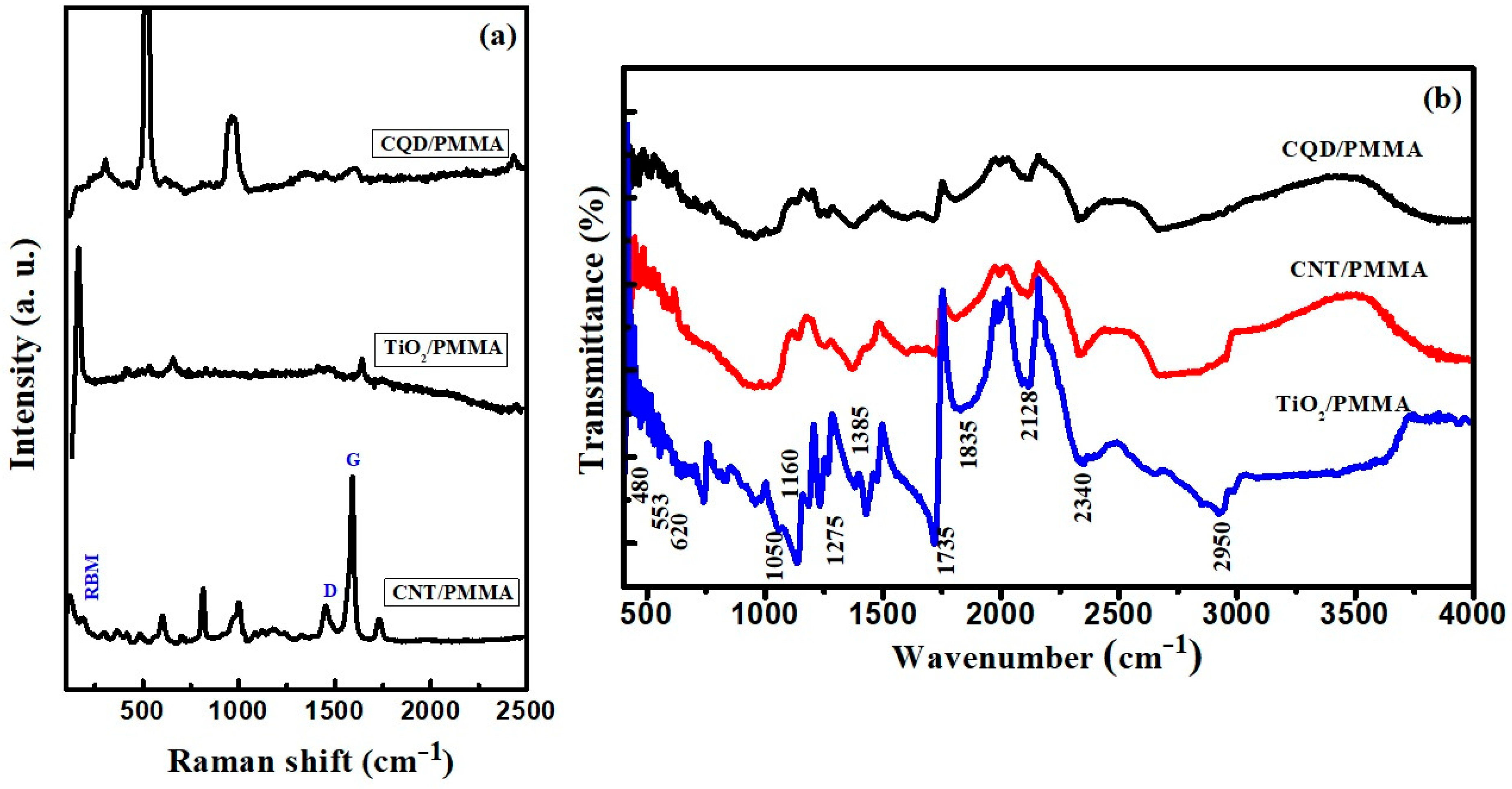
3.2. Raman and FTIR Spectroscopic Studies
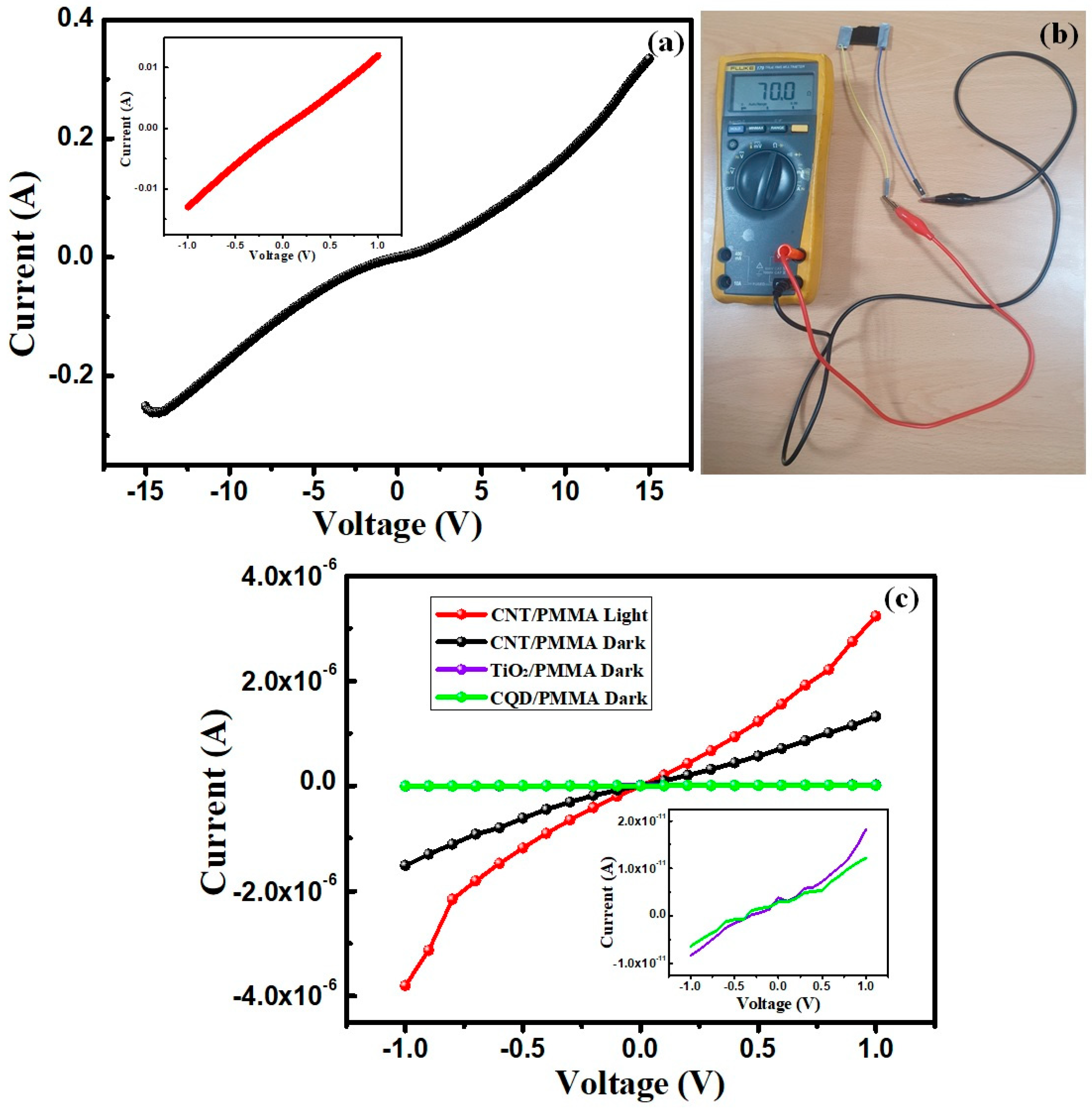
3.3. Electrical Properties
3.4. Optical Properties
3.5. Electrochemical Impedance Analysis
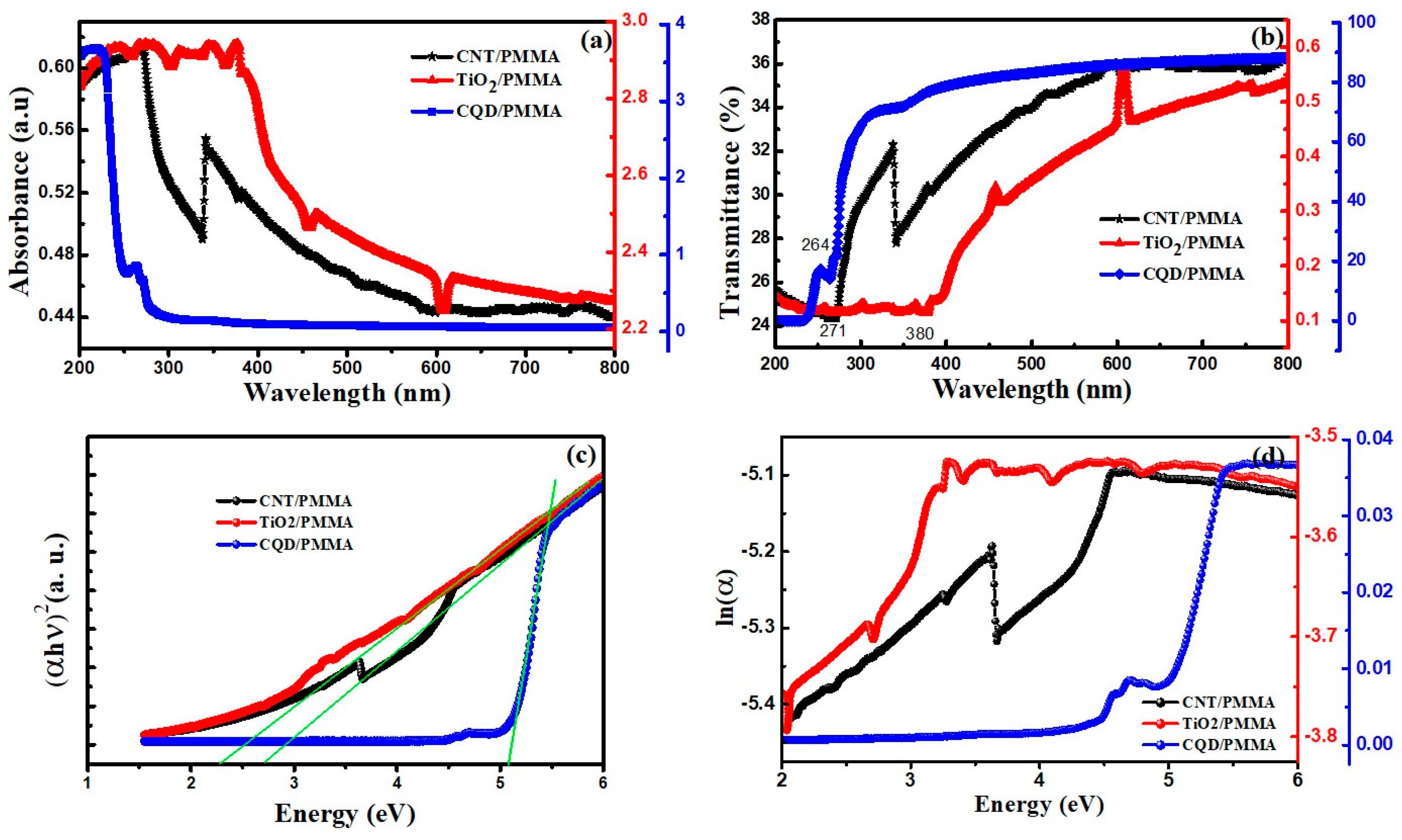
3.5.1. UV–Vis Absorbance and Transmittance
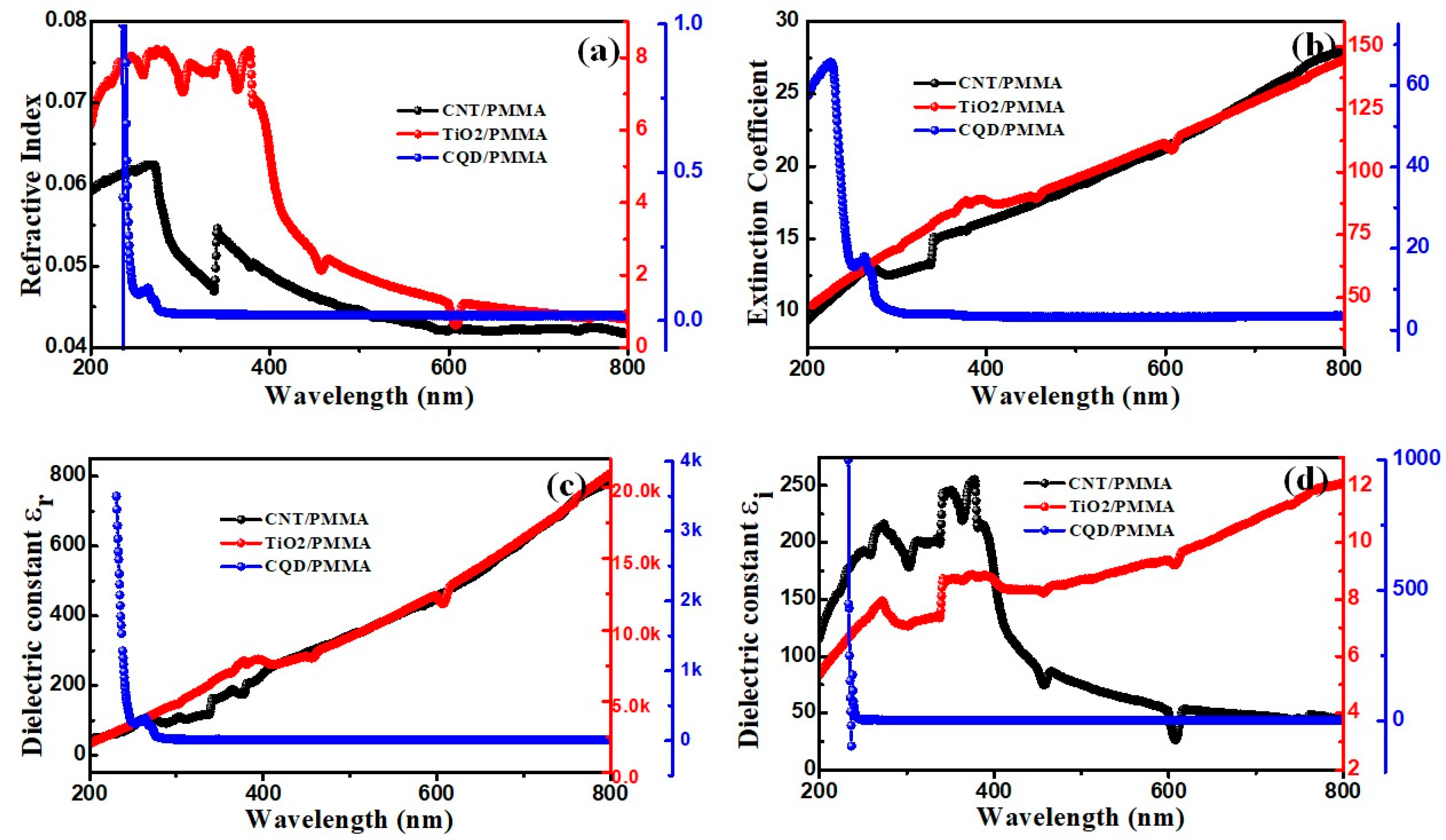
3.5.2. Refractive Index
3.5.3. Dielectric Constant
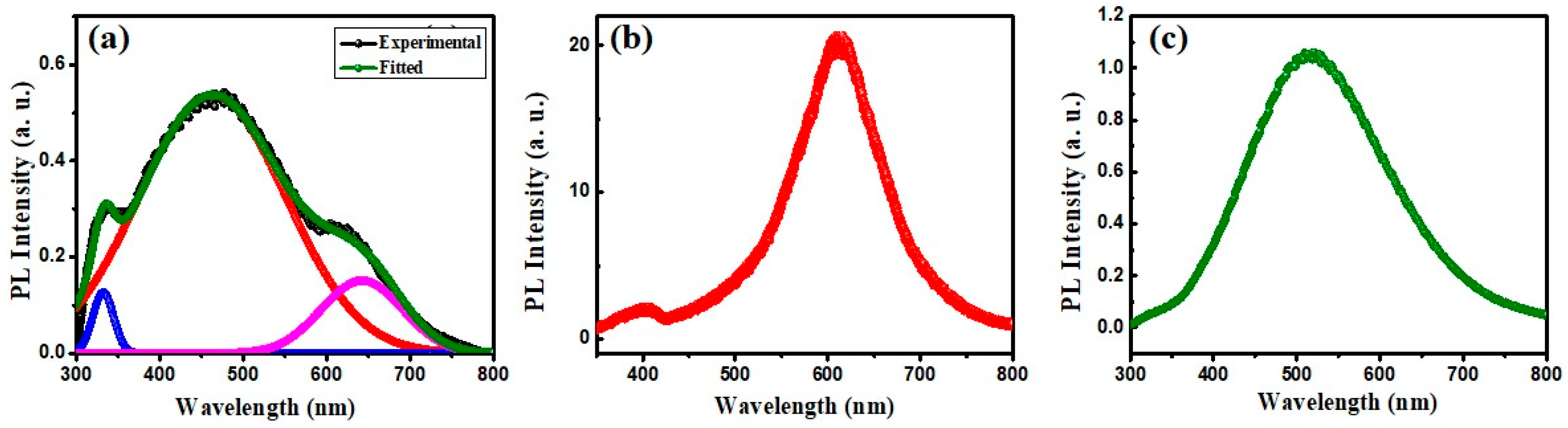
3.5.4. Fluorescence Spectroscopy
3.5.5. UV Shielding
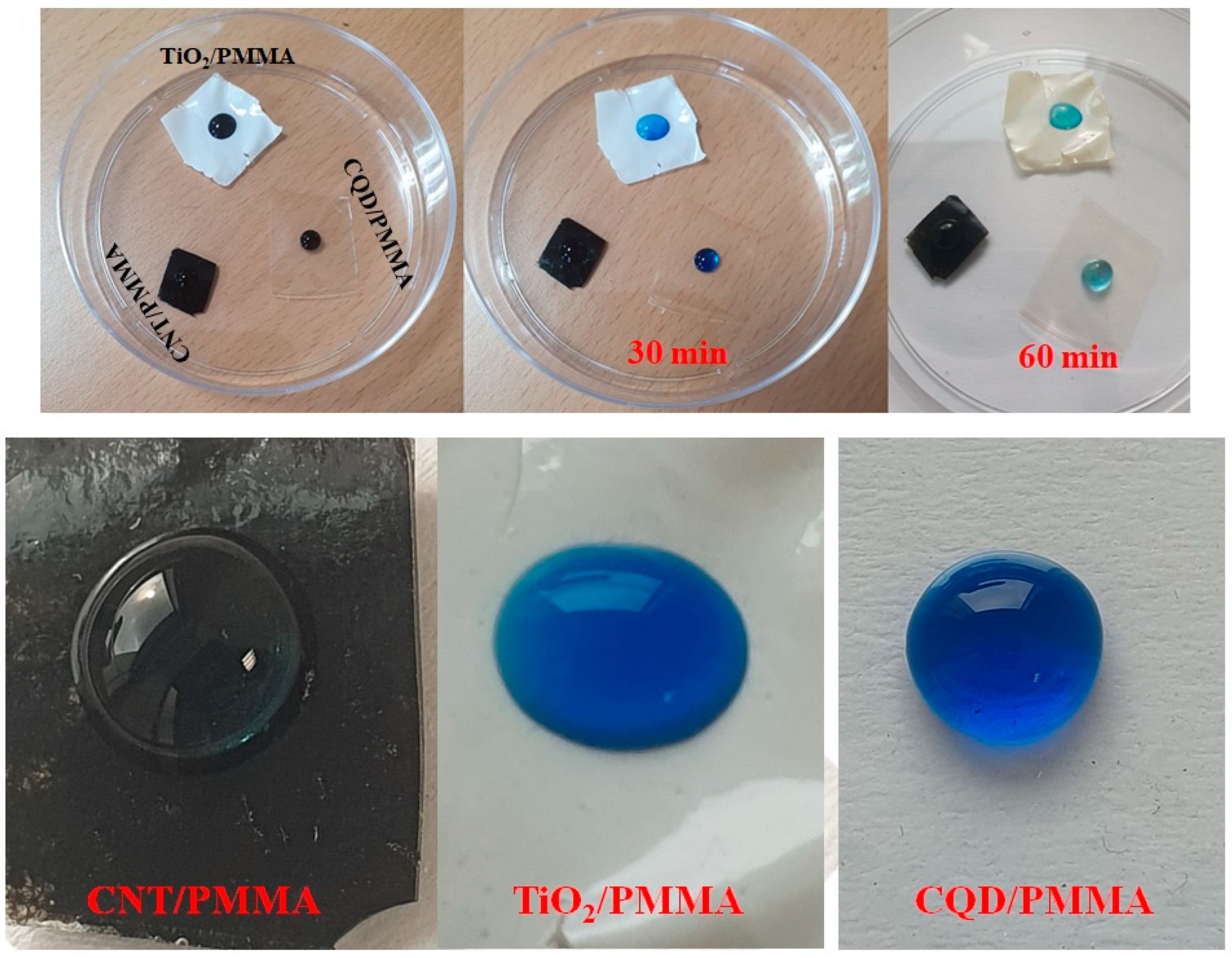
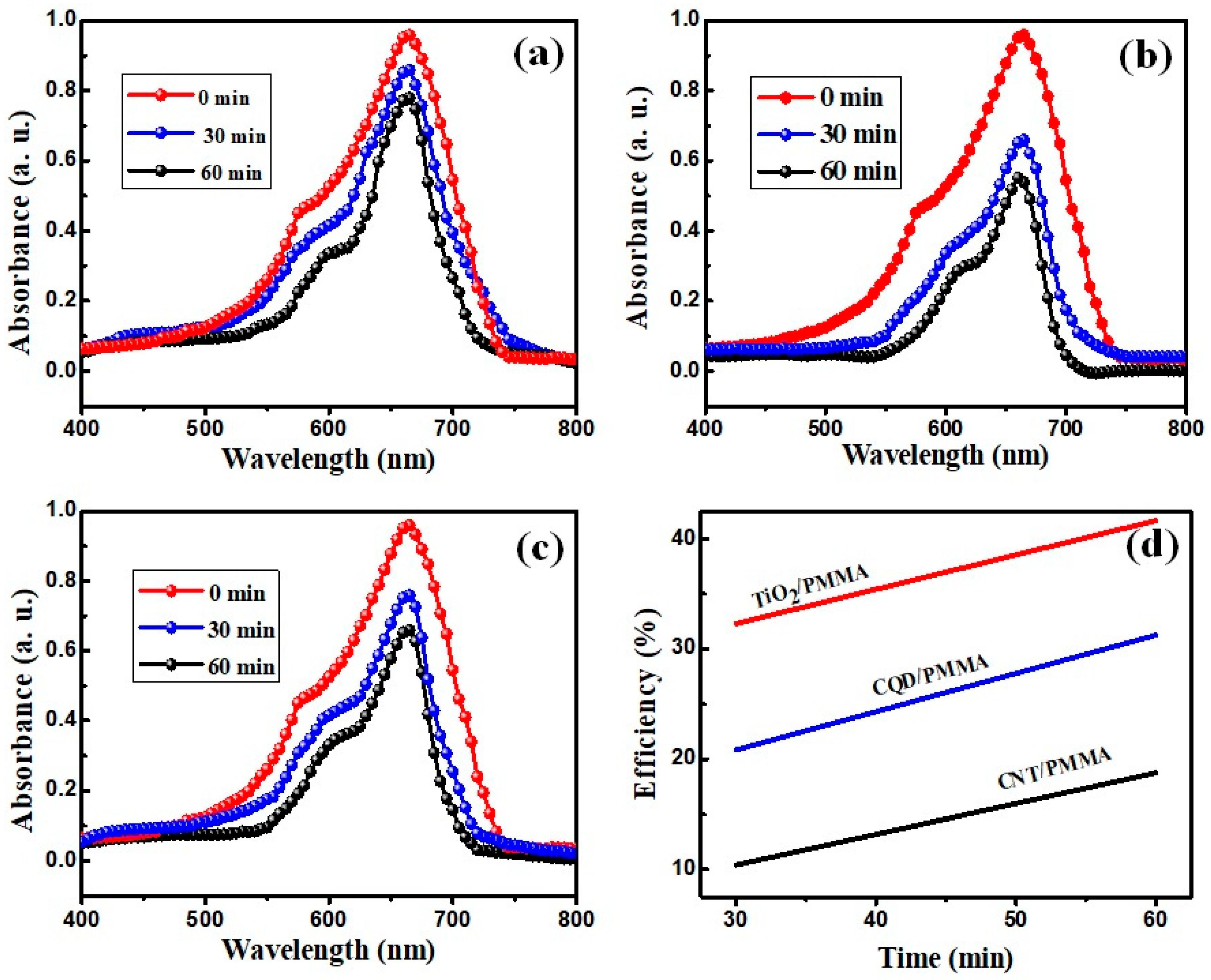
3.6. Photocatalytic Effects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Bataineh, Q.; Ahmad, A.; Alsaad, A.; Telfah, A. Optical characterizations of PMMA/metal oxide nanoparticles thin films: Bandgap engineering using a novel derived model. Heliyon 2021, 7, e05952. [Google Scholar] [CrossRef] [PubMed]
- Kaliramna, S.; Dhayal, S.S.; Kumar, N. Fabrication of PMMA thin film and its optical and photocatalytic activity. Mater. Today Proc. 2022, 69, 42–46. [Google Scholar] [CrossRef]
- Tuniz, A.; Lwin, R.; Argyros, A.; Fleming, S.C.; Kuhlmey, B.T. Fabricating metamaterials using the fiber drawing method. JoVE (J. Vis. Exp.) 2012, 68, e4299. [Google Scholar]
- Guo, X.; Ni, X.; Li, J.; Zhang, H.; Zhang, F.; Yu, H.; Wu, J.; Bai, Y.; Lei, H.; Huang, Y. Designing mechanical metamaterials with kirigami-inspired, hierarchical constructions for giant positive and negative thermal expansion. Adv. Mater. 2021, 33, 2004919. [Google Scholar] [CrossRef] [PubMed]
- Kathalingam, A.; Vikraman, D.; Marimuthu, K.P.; Karuppasamy, K.; Lee, H.; Maiyalagan, T.; Kim, H.-S. Characterization and application of flexible, highly conductive freestanding films of SWCNT and PMMA nanocomposite prepared by facile solution method. Surf. Interfaces 2023, 40, 103161. [Google Scholar] [CrossRef]
- Kim, K.-I.; Kim, D.-A.; Patel, K.D.; Shin, U.S.; Kim, H.-W.; Lee, J.-H.; Lee, H.-H. Carbon nanotube incorporation in PMMA to prevent microbial adhesion. Sci. Rep. 2019, 9, 4921. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liang, R.; Qin, W. Anti-fouling TiO2-Coated Polymeric Membrane Ion-Selective Electrodes with Photocatalytic Self-Cleaning Properties. Anal. Chem. 2023, 95, 6577–6585. [Google Scholar] [CrossRef] [PubMed]
- Hasanvandian, F.; Zehtab Salmasi, M.; Moradi, M.; Farshineh Saei, S.; Kakavandi, B.; Rahman Setayesh, S. Enhanced spatially coupling heterojunction assembled from CuCo2S4 yolk-shell hollow sphere capsulated by Bi-modified TiO2 for highly efficient CO2 photoreduction. Chem. Eng. J. 2022, 444, 136493. [Google Scholar] [CrossRef]
- Mehregan, S.; Hayati, F.; Mehregan, M.; Isari, A.A.; Jonidi Jafari, A.; Giannakis, S.; Kakavandi, B. Exploring the visible light-assisted conversion of CO2 into methane and methanol, using direct Z-scheme TiO2@g-C3N4 nanosheets: Synthesis and photocatalytic performance. Environ. Sci. Pollut. Res. Int. 2022, 29, 74951–74966. [Google Scholar] [CrossRef]
- Tang, B.; Chen, H.; Peng, H.; Wang, Z.; Huang, W. Graphene modified TiO2 composite photocatalysts: Mechanism, progress and perspective. Nanomaterials 2018, 8, 105. [Google Scholar] [CrossRef]
- Kanth, N.; Xu, W.; Prasad, U.; Ravichandran, D.; Kannan, A.M.; Song, K. PMMA-TiO2 fibers for the photocatalytic degradation of water pollutants. Nanomaterials 2020, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, W.A.; Abd El-Gawad, H.; Mekkey, S.; Galal, H.; Handal, H.; Mousa, H.; Labib, A. Quantum dots synthetization and future prospect applications. Nanotechnol. Rev. 2021, 10, 1926–1940. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Li, L. Tunable memristic characteristics based on graphene oxide charge-trap memory. Micromachines 2019, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Rameshkumar, C.; Sarojini, S.; Naresh, K.; Subalakshmi, R. Preparation and characterization of pristine PMMA and PVDF thin film using solution casting process for optoelectronic devices. J. Surf. Sci. Technol. 2017, 33, 12–18. [Google Scholar] [CrossRef]
- Ramoraswi, N.O.; Ndungu, P.G. Photo-catalytic properties of TiO2 supported on MWCNTs, SBA-15 and silica-coated MWCNTs nanocomposites. Nanoscale Res. Lett. 2015, 10, 427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Y.; Boyd, I.W.; O’sullivan, B.; Hurley, P.; Kelly, P.; Senateur, J.-P. Nanocrystalline TiO2 films studied by optical, XRD and FTIR spectroscopy. J. Non-Cryst. Solids 2002, 303, 134–138. [Google Scholar] [CrossRef]
- Toro, R.G.; Diab, M.; de Caro, T.; Al-Shemy, M.; Adel, A.; Caschera, D. Study of the effect of titanium dioxide hydrosol on the photocatalytic and mechanical properties of paper sheets. Materials 2020, 13, 1326. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Xu, L.; Hong, W.; Yang, Y.; Chen, X. The Glass-Transition Temperature of Supported PMMA Thin Films with Hydrogen Bond/Plasmonic Interface. Polymers 2019, 11, 601. [Google Scholar] [CrossRef]
- Dalla Bernardina, S.; Paineau, E.; Brubach, J.-B.; Judeinstein, P.; Rouzière, S.; Launois, P.; Roy, P. Water in carbon nanotubes: The peculiar hydrogen bond network revealed by infrared spectroscopy. J. Am. Chem. Soc. 2016, 138, 10437–10443. [Google Scholar] [CrossRef] [PubMed]
- Yuwono, A.H.; Liu, B.; Xue, J.; Wang, J.; Elim, H.I.; Ji, W.; Li, Y.; White, T.J. Controlling the crystallinity and nonlinear optical properties of transparent TiO2–PMMA nanohybrids. J. Mater. Chem. 2004, 14, 2978–2987. [Google Scholar] [CrossRef]
- Borandeh, S.; Abdolmaleki, A.; Zamani Nekuabadi, S.; Sadeghi, M. Methoxy poly (ethylene glycol) methacrylate-TiO2/poly (methyl methacrylate) nanocomposite: An efficient membrane for gas separation. Polym.-Plast. Technol. Mater. 2019, 58, 789–802. [Google Scholar] [CrossRef]
- Duarte, É.C.; Oréfice, R.L. Self-healing interfaces of poly (methyl methacrylate) reinforced with carbon fibers decorated with carbon quantum dots. J. Appl. Polym. Sci. 2021, 138, 49644. [Google Scholar] [CrossRef]
- Heiba, Z.K.; Mohamed, M.B.; Imam, N.G. Photophysical Parameters of Functional Transparent Polymethyl-Methacrylate/Double-Walled Carbon Nanotubes Nanocomposite Sheet Under UV-Irradiation. J. Inorg. Organomet. Polym. Mater. 2016, 26, 780–787. [Google Scholar] [CrossRef]
- Omri, K.; Alharbi, F. Synthesis and effect of temperature on morphological and photoluminescence properties of TiO2 nanoparticles. Appl. Phys. A 2019, 125, 696. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Brza, M.; Azawy, A.K.; Tahir, D.A. Effect of carbon nano-dots (CNDs) on structural and optical properties of PMMA polymer composite. Results Phys. 2019, 15, 102776. [Google Scholar] [CrossRef]
- Sellappan, R.; Sun, J.; Galeckas, A.; Lindvall, N.; Yurgens, A.; Kuznetsov, A.Y.; Chakarov, D. Influence of graphene synthesizing techniques on the photocatalytic performance of graphene–TiO2 nanocomposites. Phys. Chem. Chem. Phys. 2013, 15, 15528–15537. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, B.; McMeekin, S.G.; De La Rue, R.M.; Johnson, N.P. Enhanced Fano resonance of organic material films deposited on arrays of asymmetric splitring resonators (A-SRRs). Opt. Express 2013, 21, 9343–9352. [Google Scholar] [CrossRef]
- Rico, V.J.; Turk, H.; Yubero, F.; Gonzalez-Elipe, A.R. Titania Enhanced Photocatalysis and Dye Giant Absorption in Nanoporous 1D Bragg Microcavities. ACS Appl. Nano Mater. 2022, 5, 5487–5497. [Google Scholar] [CrossRef]
- Rooj, B.; Mandal, U. A review on characterization of carbon quantum dots. Vietnam J. Chem. 2023, 61, 693–718. [Google Scholar] [CrossRef]
- Li, Q.; Lu, J.; Gupta, P.; Qiu, M. Engineering Optical Absorption in Graphene and Other 2D Materials: Advances and Applications. Adv. Opt. Mater. 2019, 7, 1900595. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Wang, A.; Wu, L.; Wang, Q. Facile synthesis and photocatalytic activity of a novel titanium dioxide nanocomposite coupled with zinc porphyrin. Nanomater. Nanotechnol. 2016, 6, 1847980416669487. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Oxygen defect dependent variation of band gap, Urbach energy and luminescence property of anatase, anatase–rutile mixed phase and of rutile phases of TiO2 nanoparticles. Phys. E Low-Dimens. Syst. Nanostruct. 2014, 56, 364–371. [Google Scholar] [CrossRef]
- Akshay, V.; Arun, B.; Mandal, G.; Vasundhara, M. Visible range optical absorption, Urbach energy estimation and paramagnetic response in Cr-doped TiO2 nanocrystals derived by a sol–gel method. Phys. Chem. Chem. Phys. 2019, 21, 12991–13004. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, H.N.; Balakit, A.A.; Wahab, G.A.; Kodeary, A.K. Study of the optical properties of poly (methyl methaacrylate)(pmma) doped with a new diarylethen compound. Acad. Res. Int. 2014, 5, 48–56. [Google Scholar]
- Oboudi, S.F.; Yousif, E.; Abdul Nabi, M.; Yusop, R.M.; Derawi, D. Fabrication and characterization of nickel chloride doped PMMA films. Adv. Mater. Sci. Eng. 2015, 2015, 913260. [Google Scholar]
- Bhooshan, K.V.; Kumar, S.A.; SM, M.A.; Xiangping, L.; Aharon, G. Refractive-Index Tuning of Highly Fluorescent Carbon Dots. ACS Appl. Mater. Interfaces 2017, 9, 28930–28938. [Google Scholar]
- Mulkerns, N.M.; Hoffmann, W.H.; Ramos-Soriano, J.; de la Cruz, N.; Garcia-Millan, T.; Harniman, R.L.; Lindsay, I.D.; Seddon, A.M.; Galan, M.C.; Gersen, H. Measuring the refractive index and sub-nanometre surface functionalisation of nanoparticles in suspension. Nanoscale 2022, 14, 8145–8152. [Google Scholar] [CrossRef]
- Al-Muntaser, A.; Abdelghany, A.; Abdelrazek, E.; Elshahawy, A. Enhancement of optical and electrical properties of PVC/PMMA blend films doped with Li4Ti5O12 nanoparticles. J. Mater. Res. Technol. 2020, 9, 789–797. [Google Scholar] [CrossRef]
- Nassier, L.F.; Shinen, M.H. Study of the optical properties of poly (methyl methacrylate) (PMMA) by using spin coating method. Mater. Today: Proc. 2022, 60, 1660–1664. [Google Scholar] [CrossRef]
- Requena, S.; Lacoul, S.; Strzhemechny, Y.M. Luminescent properties of surface functionalized BaTiO3 embedded in poly (methyl methacrylate). Materials 2014, 7, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.C.; Cacioppo, M.; Amato, F.; Cabral, T.D.; Carreño, M.N.; Pereyra, I.; Ramos, C.A.; Cid, M.; Goveia, G.S.; Chubaci, J.F. Fabrication of fluorescent PMMA-carbon nanodots optical films and their feasibility in improving solar cells efficiency using low-cost sustainable materials. Braz. J. Chem. Eng. 2023, 1–13. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Samaddar, S.; Dasgupta, A.K. Reusable glucose sensing using carbon nanotube-based self-assembly. Nanoscale 2013, 5, 9231–9237. [Google Scholar] [CrossRef] [PubMed]
- Amato, F.; Cacioppo, M.; Arcudi, F.; Prato, M.; Mituo, M.; Fernandes, E.G.; Carreño, M.N.; Pereyra, I.; Bartoli, J.R. Nitrogen-doped carbon nanodots/pmma nanocomposites for solar cells applications. Chem. Eng. 2019, 74, 1105–1110. [Google Scholar]
- Yousefi, F.; Mousavi, S.B.; Heris, S.Z.; Naghash-Hamed, S. UV-shielding properties of a cost-effective hybrid PMMA-based thin film coatings using TiO2 and ZnO nanoparticles: A comprehensive evaluation. Sci. Rep. 2023, 13, 7116. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.-W.; Chen, X.-Y.; Tsai, W.-P. Poly (methyl methacrylate)/titanium dioxide (PMMA/TiO2) nanocomposite with shark-skin structure for preventing biofilm formation. Mater. Lett. 2021, 285, 129098. [Google Scholar] [CrossRef]
- Lee, S.Y.; San Lim, H.; Lee, N.E.; Cho, S.O. Biocompatible UV-absorbing polymer nanoparticles prepared by electron irradiation for application in sunscreen. RSC Adv. 2020, 10, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, J.; Strømme, M.; Welch, K. Enhanced UV protection and water adsorption properties of transparent poly (methyl methacrylate) films through incorporation of amorphous magnesium carbonate nanoparticles. J. Polym. Res. 2021, 28, 281. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Melinte, V.; Stroea, L.; Chibac-Scutaru, A.L. Polymer nanocomposites for photocatalytic applications. Catalysts 2019, 9, 986. [Google Scholar] [CrossRef]
- Jiang, D.; Otitoju, T.A.; Ouyang, Y.; Shoparwe, N.F.; Wang, S.; Zhang, A.; Li, S. A review on metal ions modified TiO2 for photocatalytic degradation of organic pollutants. Catalysts 2021, 11, 1039. [Google Scholar] [CrossRef]
- Vasiljevic, Z.; Dojcinovic, M.; Vujancevic, J.; Jankovic-Castvan, I.; Ognjanovic, M.; Tadic, N.; Stojadinovic, S.; Brankovic, G.; Nikolic, M. Photocatalytic degradation of methylene blue under natural sunlight using iron titanate nanoparticles prepared by a modified sol–gel method. R. Soc. Open Sci. 2020, 7, 200708. [Google Scholar] [CrossRef] [PubMed]
- Abd El Khalk, A.A.; Betiha, M.A.; Mansour, A.S.; Abd El Wahed, M.G.; Al-Sabagh, A.M. High degradation of methylene blue using a new nanocomposite based on zeolitic imidazolate framework-8. ACS Omega 2021, 6, 26210–26220. [Google Scholar] [CrossRef] [PubMed]
- Kalaycıoğlu, Z.; Uysal, B.; Pekcan, Ö.; Erim, F. Efficient photocatalytic degradation of methylene blue dye from aqueous solution with cerium oxide nanoparticles and graphene oxide-doped polyacrylamide. ACS Omega 2023, 8, 13004–13015. [Google Scholar] [CrossRef] [PubMed]
- Chahar, D.; Kumar, D.; Thakur, P.; Thakur, A. Visible light induced photocatalytic degradation of methylene blue dye by using Mg doped Co-Zn nanoferrites. Mater. Res. Bull. 2023, 162, 112205. [Google Scholar] [CrossRef]
- Negash, A.; Mohammed, S.; Weldekirstos, H.D.; Ambaye, A.D.; Gashu, M. Enhanced photocatalytic degradation of methylene blue dye using eco-friendly synthesized rGO@ ZnO nanocomposites. Sci. Rep. 2023, 13, 22234. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, A.; Torres, C.R.; Marchetti, S.; Stewart, S. Degradation of methylene blue dye under dark and visible light conditions in presence of hybrid composites of nanostructured MgFe2O4 ferrites and oxygenated organic compounds. J. Environ. Chem. Eng. 2020, 8, 104274. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U. Sun-light driven rapid photocatalytic degradation of methylene blue by poly (methyl methacrylate)/metal oxide nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 136–147. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adaikalam, K.; Vikraman, D.; Lee, D.-H.; Cho, Y.-A.; Kim, H.-S. Optical and UV Shielding Properties of Inorganic Nanoparticles Embedded in Polymethyl Methacrylate Nanocomposite Freestanding Films. Polymers 2024, 16, 1048. https://doi.org/10.3390/polym16081048
Adaikalam K, Vikraman D, Lee D-H, Cho Y-A, Kim H-S. Optical and UV Shielding Properties of Inorganic Nanoparticles Embedded in Polymethyl Methacrylate Nanocomposite Freestanding Films. Polymers. 2024; 16(8):1048. https://doi.org/10.3390/polym16081048
Chicago/Turabian StyleAdaikalam, Kathalingam, Dhanasekaran Vikraman, Du-Hee Lee, Yoon-A Cho, and Hyun-Seok Kim. 2024. "Optical and UV Shielding Properties of Inorganic Nanoparticles Embedded in Polymethyl Methacrylate Nanocomposite Freestanding Films" Polymers 16, no. 8: 1048. https://doi.org/10.3390/polym16081048
APA StyleAdaikalam, K., Vikraman, D., Lee, D.-H., Cho, Y.-A., & Kim, H.-S. (2024). Optical and UV Shielding Properties of Inorganic Nanoparticles Embedded in Polymethyl Methacrylate Nanocomposite Freestanding Films. Polymers, 16(8), 1048. https://doi.org/10.3390/polym16081048







