Composites of Poly(3-hydroxybutyrate) and Mesoporous SBA-15 Silica: Crystalline Characteristics, Confinement and Final Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. (Nano)composite and Film Preparation
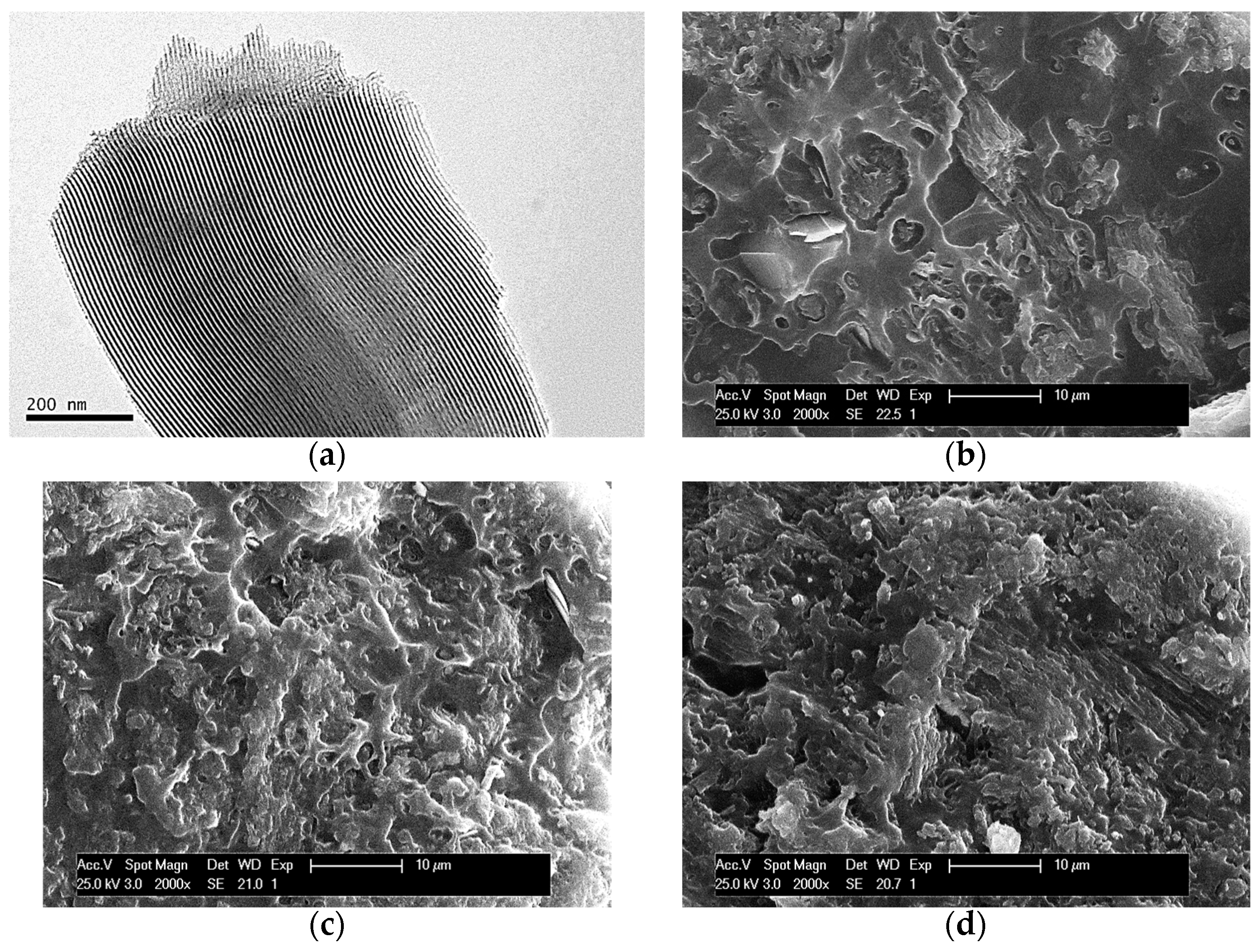
2.3. Transmission Electron Microscopy
2.4. Scanning Electron Microscopy
2.5. Thermogravimetric Analysis
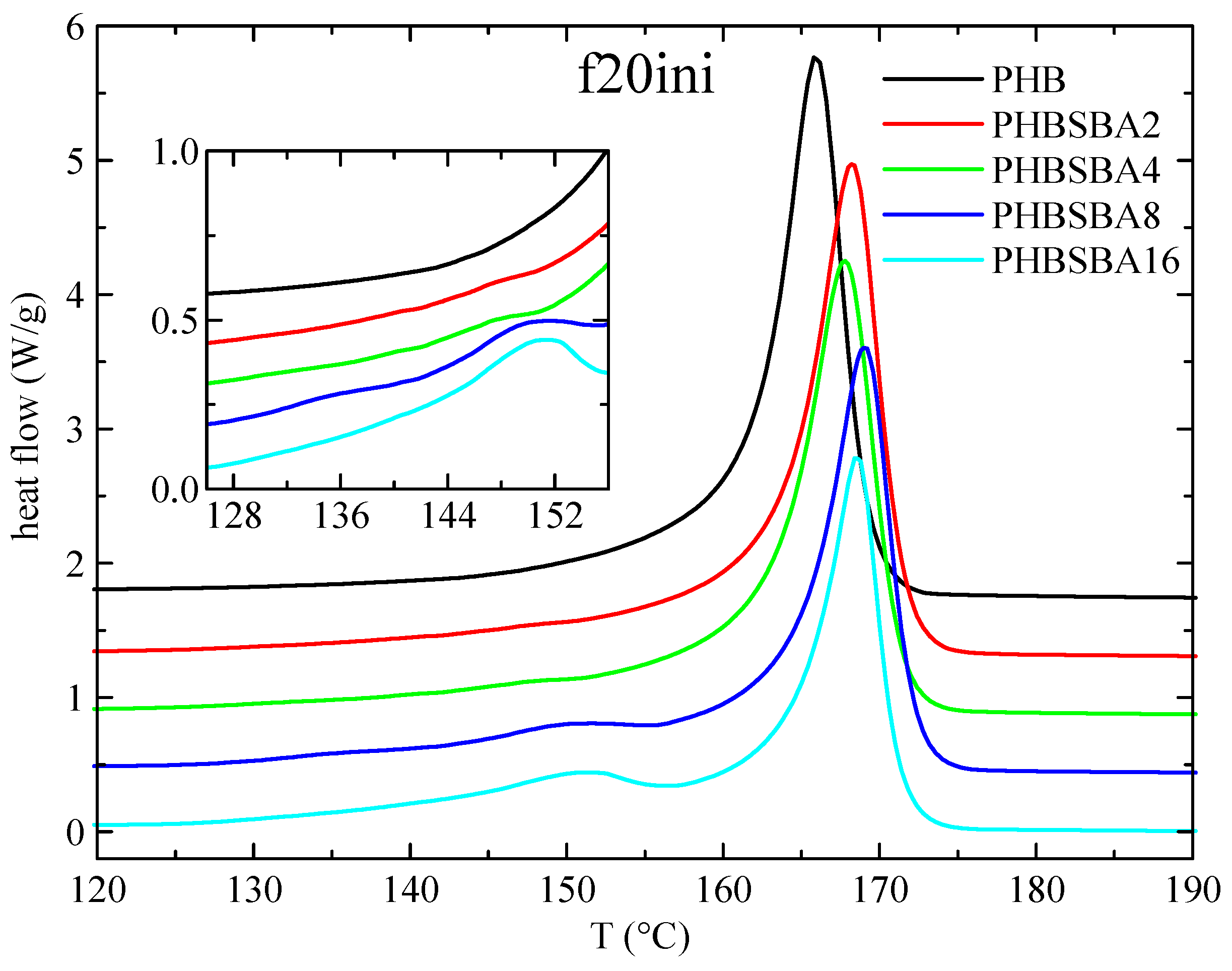
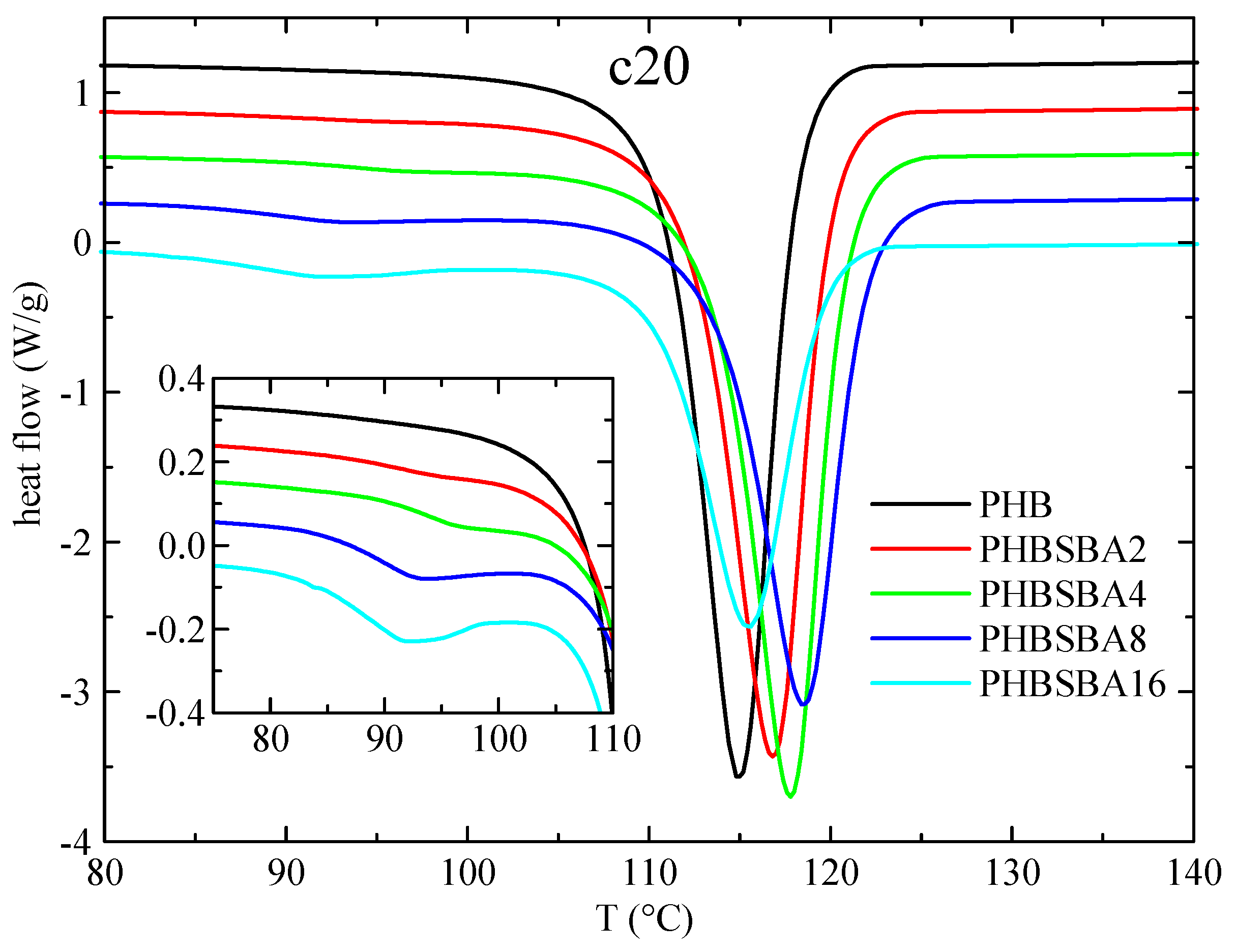
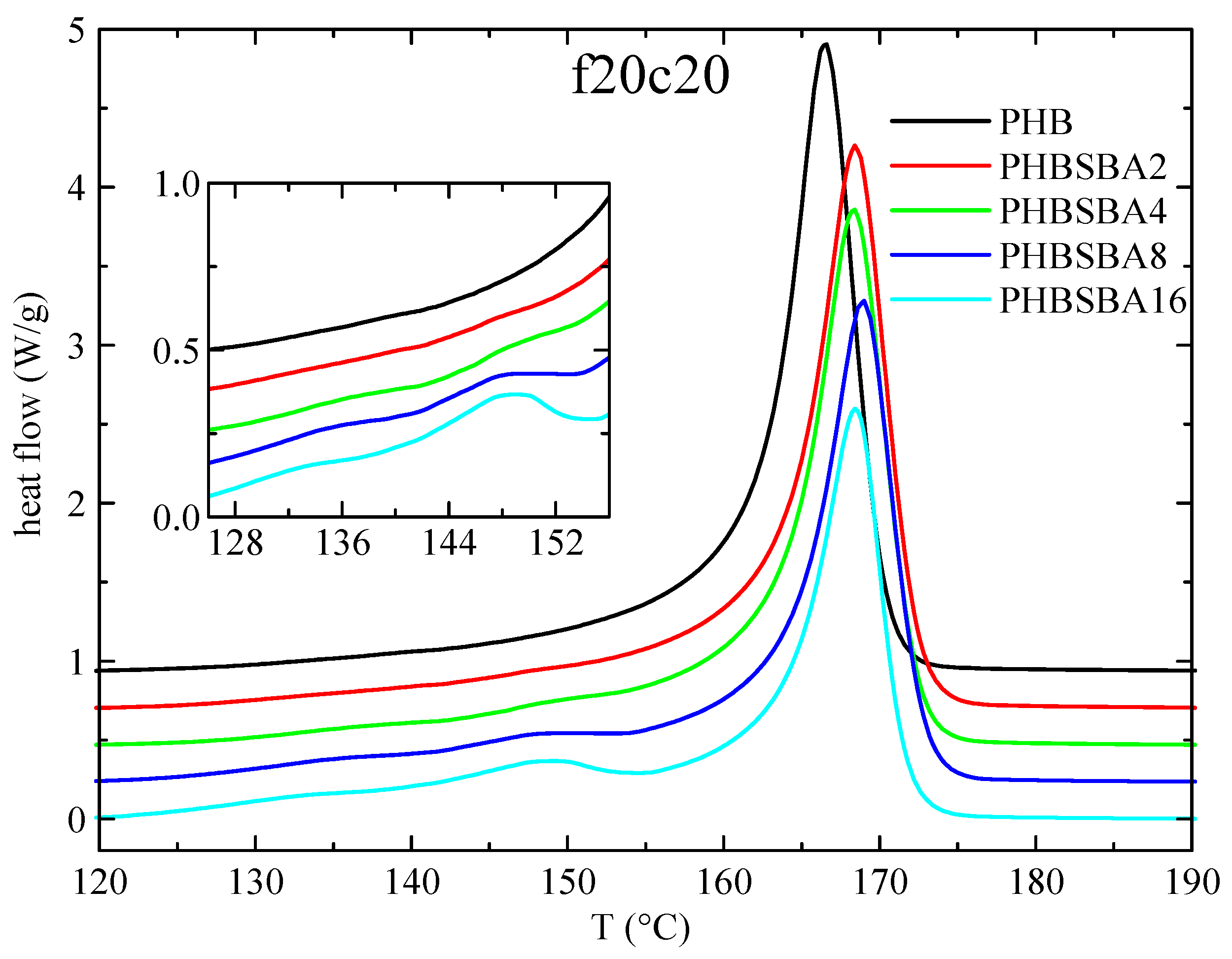
2.6. Differential Scanning Calorimetry
2.7. X-ray Experiments with Synchrotron Radiation
2.8. Dynamic Mechanical Thermal Analysis (DMTA)
3. Results and Discussion
3.1. Morphological Characteristics
3.2. Thermal Stability
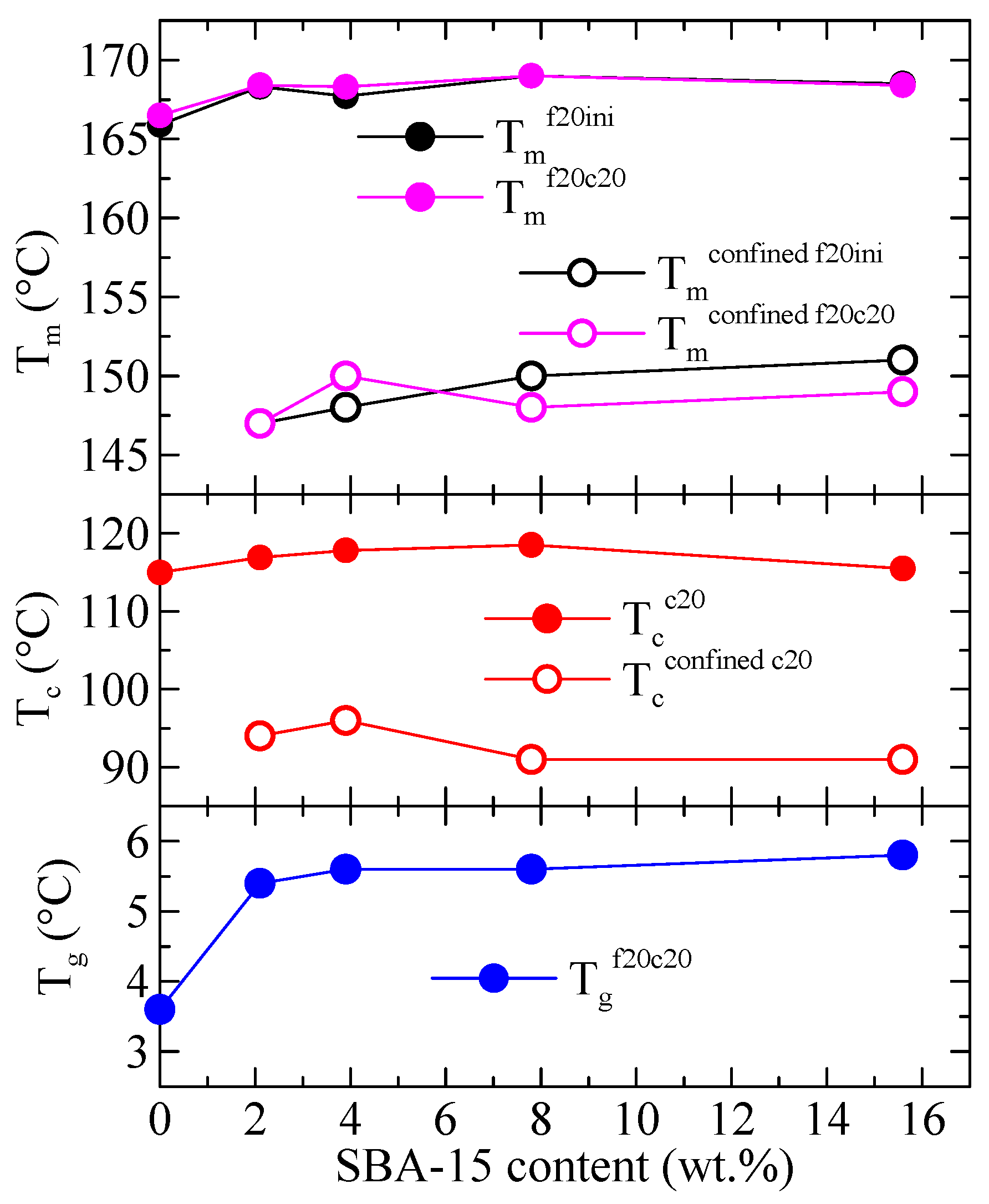
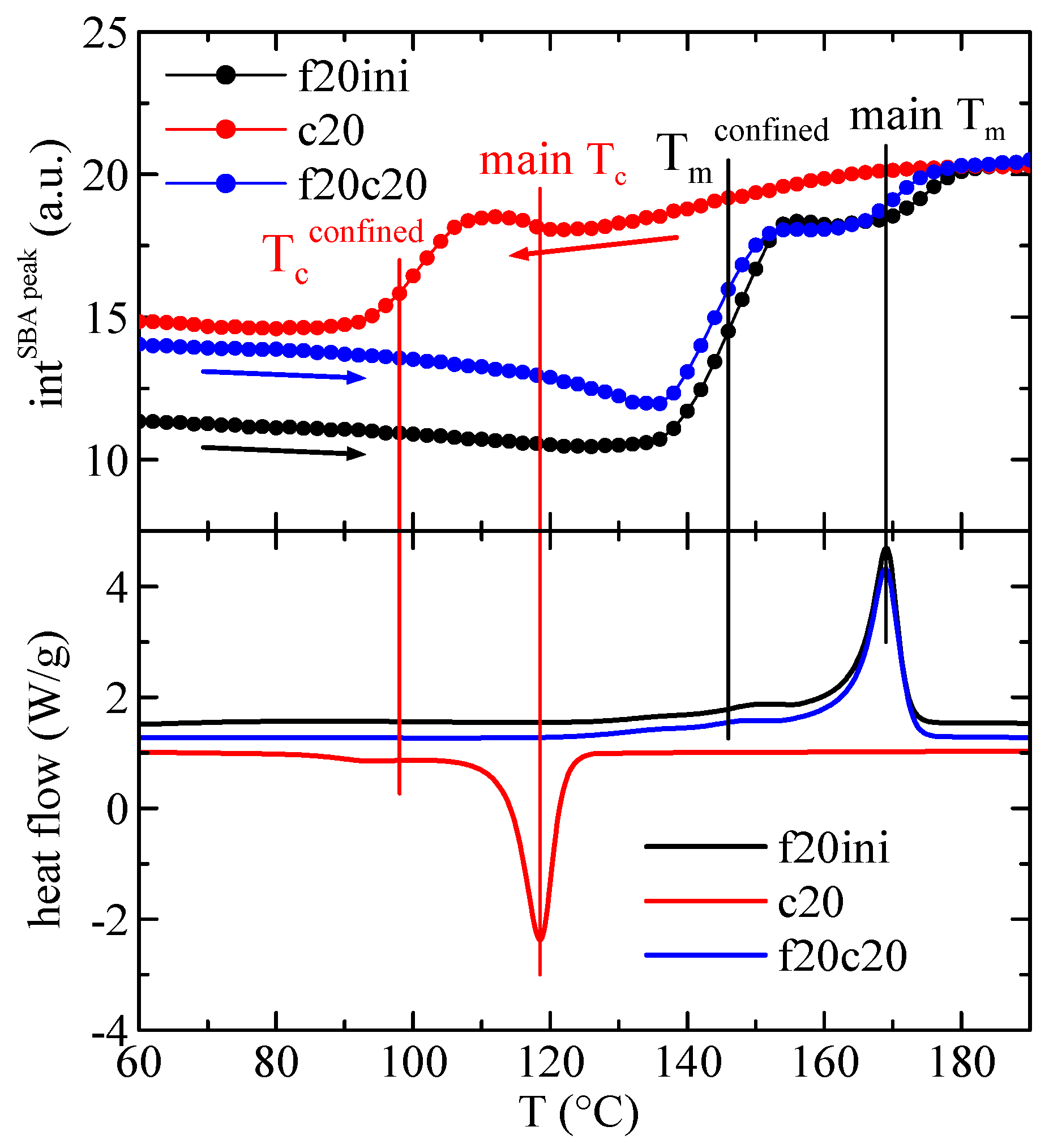
3.3. DSC Studies: Phase Transitions and Confinement of PHB Chains
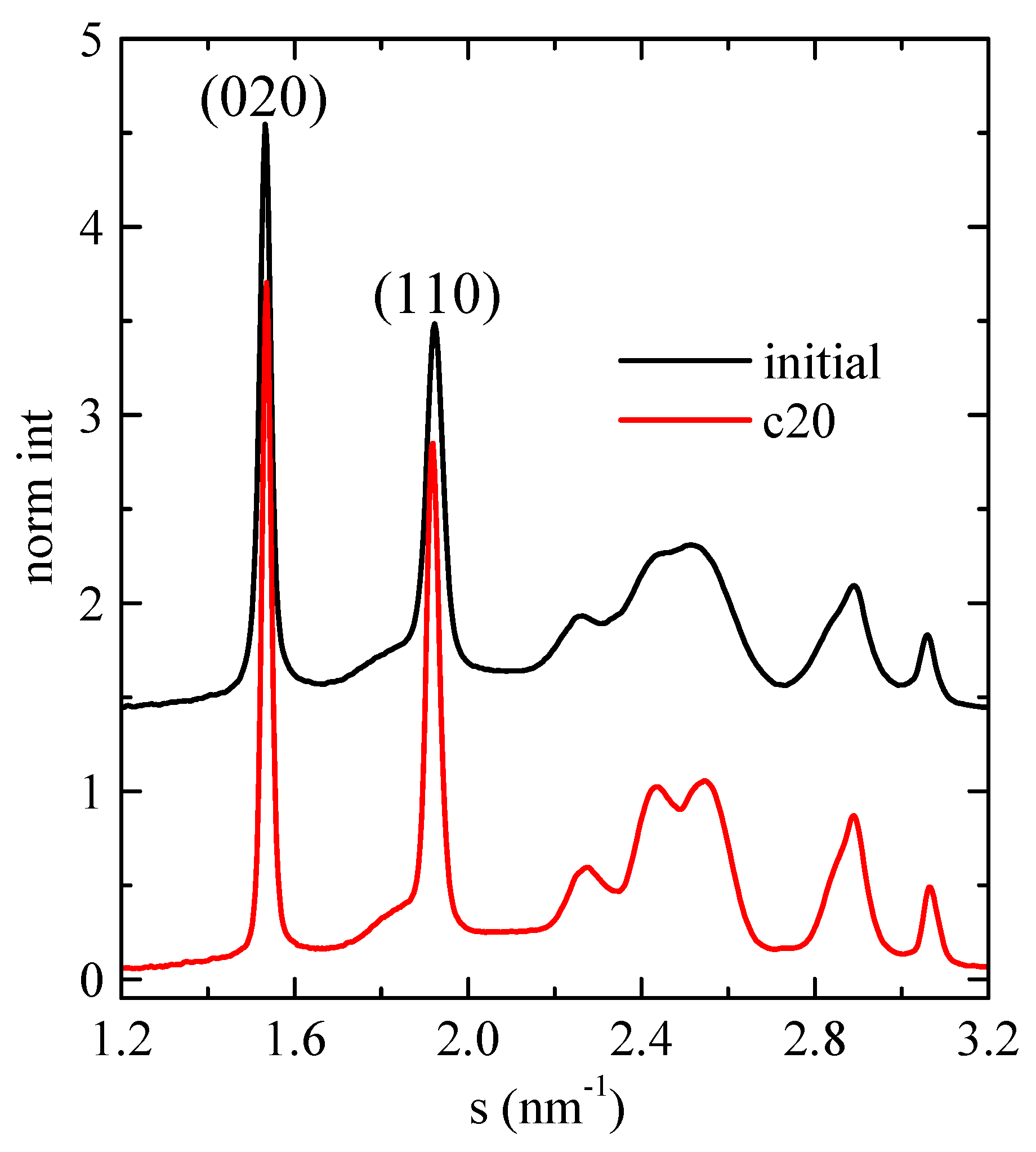
3.4. X-ray Experiments with Synchrotron Radiation
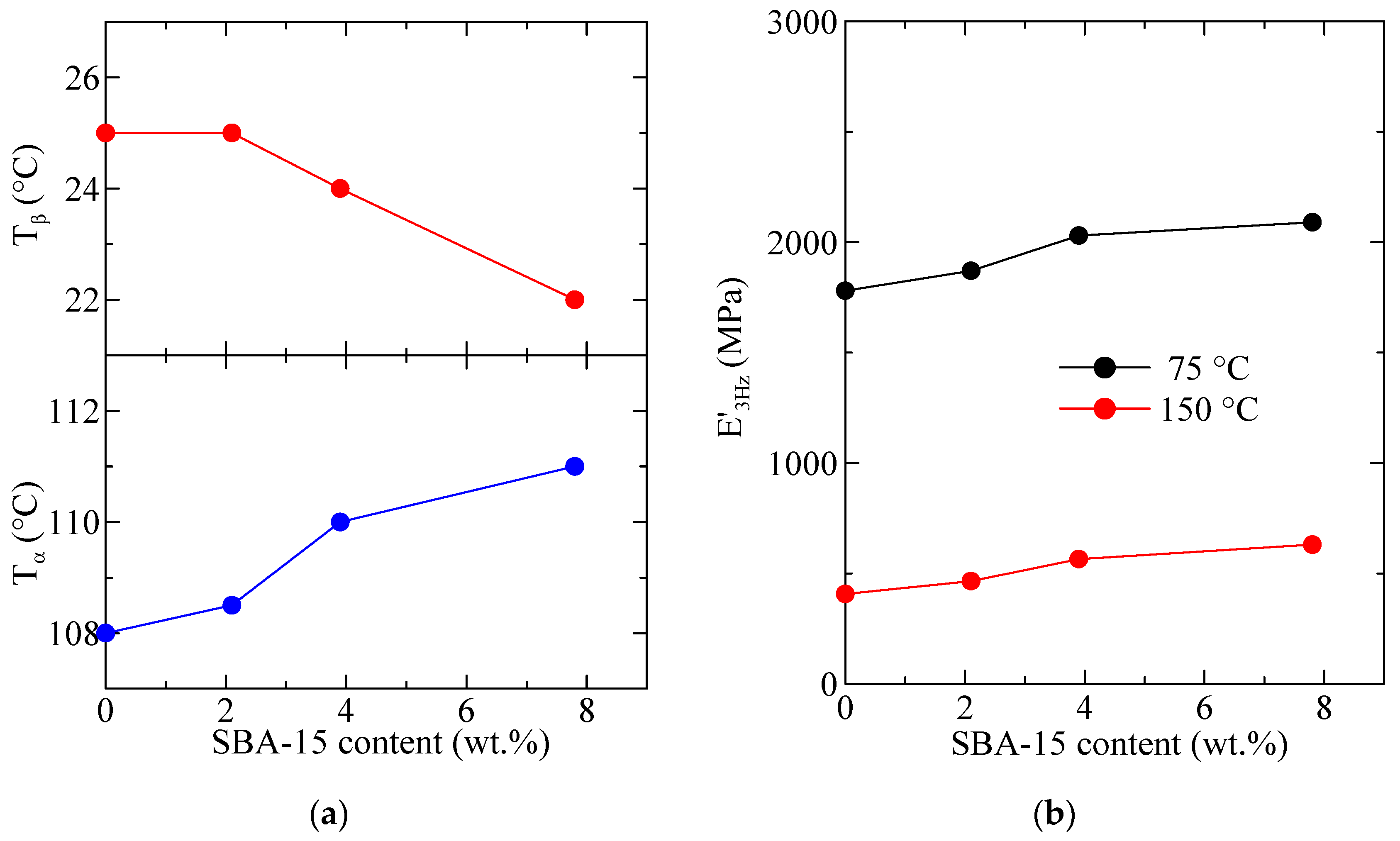
3.5. Dynamic Mechanical Thermal Analysis (DMTA) Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corre, Y.-M.; Bruzaud, S.; Audic, J.-L.; Grohens, Y. Morphology and functional properties of commercial polyhydroxyalkanoates: A comprehensive and comparative study. Polym. Test. 2012, 31, 226–235. [Google Scholar] [CrossRef]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Esposito, A.; Delpouve, N.; Causin, V.; Dhotel, A.; Delbreilh, L.; Dargent, E. From a Three-Phase Model to a Continuous Description of Molecular Mobility in Semicrystalline Poly(hydroxybutyrate-cohydroxyvalerate). Macromolecules 2016, 49, 4850–4861. [Google Scholar] [CrossRef]
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Inoue, Y. Polymorphism and isomorphism in biodegradable polyesters. Prog. Polym. Sci. 2009, 34, 605–640. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(lactic acid) crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.-W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Zhao, D.Y.; Feng, J.L.; Huo, Q.S.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Bento, A.; Pérez, E.; Lorenzo, V.; Lourenço, J.P.; Ribeiro, M.R. Hybrid Materials Based on Polyethylene and MCM-41 Particles Functionalized with Silanes: Catalytic Aspects of In Situ Polymerization, Crystalline Features and Mechanical Properties. Microporous Mesoporous Mater. 2016, 232, 86–96. [Google Scholar] [CrossRef]
- Barranco-García, R.; Ferreira, A.E.; Ribeiro, M.R.; Lorenzo, V.; García-Peñas, A.; Gómez-Elvira, J.M.; Pérez, E.; Cerrada, M.L. Hybrid materials obtained by in situ polymerization based on polypropylene and mesoporous SBA-15 silica particles: Catalytic aspects, crystalline details and mechanical behavior. Polymer 2018, 151, 218–230. [Google Scholar] [CrossRef]
- Díez-Rodríguez, T.M.; Blázquez-Blázquez, E.; Antunes, N.L.C.; Ribeiro, M.R.; Pérez, E.; Cerrada, M.L. Confinement in Extruded Nanocomposites based on PCL and Mesoporous Silicas: Effect of Pore Sizes and their Influence in Ultimate Mechanical Response. J. Compos. Sci. 2021, 5, 321. [Google Scholar] [CrossRef]
- Díez-Rodríguez, T.M.; Blázquez-Blázquez, E.; Pérez, E.; Cerrada, M.L. Composites Based on Poly(Lactic Acid) (PLA) and SBA-15: Effect of Mesoporous Silica on Thermal Stability and on Isothermal Crystallization from Either Glass or Molten State. Polymers 2020, 12, 2743. [Google Scholar] [CrossRef] [PubMed]
- Barranco-García, R.; Gómez-Elvira, J.M.; Ressia, J.A.; Quinzani, L.; Vallés, E.M.; Pérez, E.; Cerrada, M.L. Effect of iPP molecular weight on its confinement within mesoporous SBA-15 silica in extruded iPP-SBA-15 nanocomposites. Microporous Mesoporous Mater. 2020, 294, 109945. [Google Scholar] [CrossRef]
- Barranco-García, R.; Gómez-Elvira, J.M.; Ressia, J.A.; Quinzani, L.; Vallés, E.M.; Pérez, E.; Cerrada, M.L. Variation of Ultimate Properties in Extruded iPP-Mesoporous Silica Nanocomposites by Effect of iPP Confinement within the Mesostructures. Polymers 2020, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Hammond, W.; Prouzet, E.; Mahanti, S.D.; Pinnavaia, T.J. Structure factor for the periodic walls of mesoporous MCM-41 molecular sieves. Microporous Mesoporous Mater. 1999, 27, 19–25. [Google Scholar] [CrossRef]
- Sauer, J.; Marlow, F.; Schüth, F. Simulation of powder diffraction patterns of modified ordered mesoporous materials. Phys. Chem. Chem. Phys. 2001, 3, 5579–5584. [Google Scholar] [CrossRef]
- Haller, W. Chromatography on Glass of Controlled Pore Size. Nature 1965, 206, 693–696. [Google Scholar] [CrossRef]
- Jackson, C.L.; McKenna, G.B. The melting behavior of organic materials confined in porous solids. J. Chem. Phys. 1990, 93, 9002–9011. [Google Scholar] [CrossRef]
- Gibbs, J.W. On the Equilibrium of Heterogeneous Substances. In Collected Works, Volume I: Thermodynamics; Chapter III; Longmans: Green, NY, USA, 1928; pp. 55–353. [Google Scholar]
- Defay, R.; Prigogine, I.; Bellemans, A.; Everett, D.H. Surface Tension and Adsorption; Wiley: New York, NY, USA, 1966. [Google Scholar]
- Thomson, W. On the equilibrium of vapor at a curved surface of liquid. Philos. Mag. 1871, 42, 448–452. [Google Scholar] [CrossRef]
- Deryło-Marczewska, A.; Zienkiewicz-Strzałka, M.; Skrzypczyńska, K.; Światkowski, A.; Kuśmierek, K. Evaluation of the SBA-15 materials ability to accumulation of 4-chlorophenol on carbon paste electrode. Adsorption 2016, 22, 801–812. [Google Scholar] [CrossRef]
- Barham, P.J.; Keller, A.; Otun, E.L.; Holmes, P.A. Crystallization and morphology of a bacterial thermoplastic: Poly-3-hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]
- Marcilla, A.; Gomez, A.; Garcia, A.N.; Olaya, M.M. Kinetic study of the catalytic decomposition of different commercial polyethylenes over an MCM-41 catalyst. J. Anal. Appl. Pyrol. 2002, 64, 85–101. [Google Scholar] [CrossRef]
- Campos, J.; Lourenço, J.P.; Perez, E.; Cerrada, M.L.; Ribeiro, M.D.R. Self-Reinforced Hybrid Polyethylene/MCM-41 Nanocomposites: In-Situ Polymerisation and Effect of MCM-41 Content on Rigidity. J. Nanosci. Nanotechnol. 2009, 9, 3966–3974. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, B. Macromolecular Physics; Academic Press: New York, NY, USA, 1980; Volume 3. [Google Scholar]
- Darras, O.; Séguéla, R. Surface free energy of the chain-folding crystal faces of ethylene-butene random copolymers. Polymer 1993, 34, 2946–2950. [Google Scholar] [CrossRef]
- Lu, L.; Alamo, R.G.; Mandelkern, L. Lamellar thickness distribution in linear polyethylene and ethylene copolymers. Macromolecules 1994, 27, 6571–6576. [Google Scholar] [CrossRef]
- Gedde, U.W. Polymer Physics; Chapman & Hall: London, UK, 1995. [Google Scholar]
- Shin, K.; Woo, E.; Jeong, Y.G.; Kim, C.; Huh, J.; Kim, K.-W. Crystalline structures, melting, and crystallization of linear polyethylene in cylindrical nanopores. Macromolecules 2007, 40, 6617–6623. [Google Scholar] [CrossRef]
- Wang, S.; Capoen, L.; D’hooge, D.R.; Cardon, L. Can the melt flow index be used to predict the success of fused deposition modelling of commercial poly(lactic acid) filaments into 3D printed materials? Plast. Rubber Compos. 2018, 47, 9–16. [Google Scholar] [CrossRef]
- Yokouchi, M.; Chatani, Y.; Tadokoro, H.; Teranish, K.; Tani, H. Structural studies of polyesters. 5. Molecular and crystal structures of optically-active and racemic poly(β-hydroxybutyrate). Polymer 1973, 14, 267–272. [Google Scholar] [CrossRef]
- Cornibert, J.; Marchessault, R.H. Physical properties of poly-β-hydroxybutyrate. IV. Conformational analysis and crystalline structure. J. Mol. Biol. 1972, 71, 735–756. [Google Scholar]
- Scandola, M.; Pizzofi, M.; Ceccorulli, G.; Cesàro, A.; Paoletti, S.; Navarini, L. Viscoelastic and thermal properties of bacterial poly(D-(-)-β-hydroxybutyrate). Int. J. Biol. Macromol. 1988, 10, 373–377. [Google Scholar] [CrossRef]
- Pratt, G.J.; Smith, M.J.A. Dielectric relaxation spectroscopy of a poly-β-hydroxybutyrate homopolymer. Eur. Polym. J. 1997, 33, 857–861. [Google Scholar] [CrossRef]
- Šics, I.; Tupureina, V.; Kalninš, M.; Ezquerra, T.A.; Baltá-Calleja, F.J. Dielectric relaxation of poly-(β-hydroxybutyrate) relating to microstructure. J. Macromol. Sci.-Phys. 1998, 37, 851–862. [Google Scholar] [CrossRef]
- Ando, Y.; Fukada, E. Piezoelectric Properties and Molecular Motion of Poly(β-Hydroxybutyrate) Films. J. Polym. Sci. Polym. Phys. 1984, 22, 1821. [Google Scholar] [CrossRef]
- Fukada, E.; Ando, Y. Piezoelectric properties of poly-β-hydroxybutyrate and copolymers of β-hydroxybutyrate and β-hydroxyvalerate. Int. J. Biol. Macromol. 1986, 8, 361–366. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Pereña, J.M.; Benavente, R.; Pérez, E. Viscoelastic processes in vinyl alcohol-ethylene copolymers. Influence of composition and thermal treatment. Polymer 2000, 41, 6655–6661. [Google Scholar] [CrossRef]
- Ward, I.M.; Sweeney, J. Mechanical Properties of Solids Polymers, 3rd ed.; Wiley: Chichester, UK, 2012. [Google Scholar]





| Specimen | SBA-15 Content (wt.%) | TmaxDTGA (°C) | |
|---|---|---|---|
| Nominal | From TGA | ||
| PHB | 0 | 0 | 283.2 |
| PHBSBA2 | 2 | 2.1 | 287.2 |
| PHBSBA4 | 4 | 3.9 | 292.8 |
| PHBSBA8 | 8 | 7.8 | 285.8 |
| PHBSBA16 | 16 | 15.6 | 284.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díez-Rodríguez, T.M.; Blázquez-Blázquez, E.; Pérez, E.; Cerrada, M.L. Composites of Poly(3-hydroxybutyrate) and Mesoporous SBA-15 Silica: Crystalline Characteristics, Confinement and Final Properties. Polymers 2024, 16, 1037. https://doi.org/10.3390/polym16081037
Díez-Rodríguez TM, Blázquez-Blázquez E, Pérez E, Cerrada ML. Composites of Poly(3-hydroxybutyrate) and Mesoporous SBA-15 Silica: Crystalline Characteristics, Confinement and Final Properties. Polymers. 2024; 16(8):1037. https://doi.org/10.3390/polym16081037
Chicago/Turabian StyleDíez-Rodríguez, Tamara M., Enrique Blázquez-Blázquez, Ernesto Pérez, and María L. Cerrada. 2024. "Composites of Poly(3-hydroxybutyrate) and Mesoporous SBA-15 Silica: Crystalline Characteristics, Confinement and Final Properties" Polymers 16, no. 8: 1037. https://doi.org/10.3390/polym16081037
APA StyleDíez-Rodríguez, T. M., Blázquez-Blázquez, E., Pérez, E., & Cerrada, M. L. (2024). Composites of Poly(3-hydroxybutyrate) and Mesoporous SBA-15 Silica: Crystalline Characteristics, Confinement and Final Properties. Polymers, 16(8), 1037. https://doi.org/10.3390/polym16081037







