Chitosan-Based Films Blended with Tannic Acid and Moringa Oleifera for Application in Food Packaging: The Preservation of Strawberries (Fragaria ananassa)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Moringa Oleifera Seed Powder (MOSP)
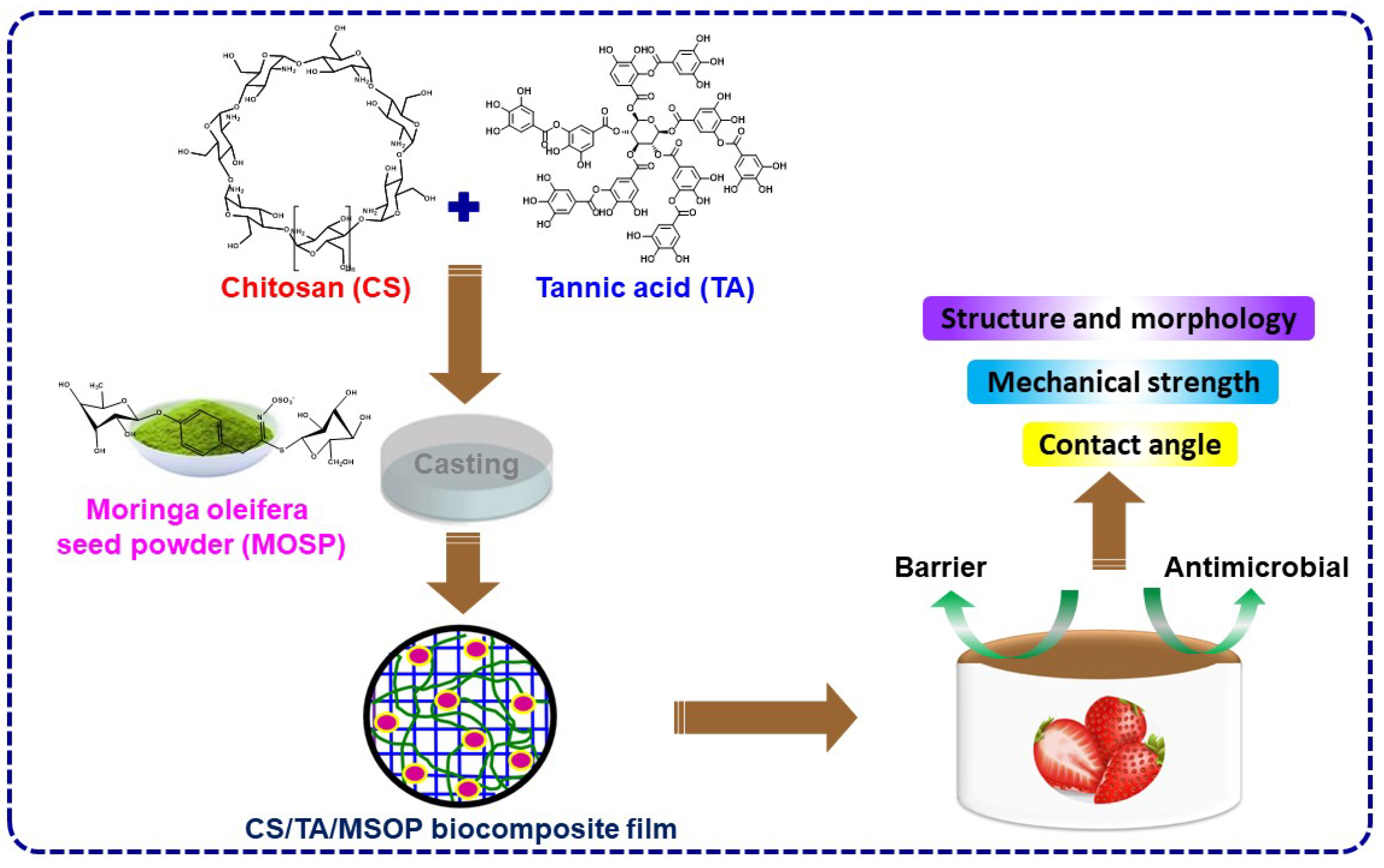
2.3. The Fabrication of the Chitosan-Based Composite Film
2.4. Characterization
2.4.1. Evaluation of Mechanical Characteristics
2.4.2. Swelling Property Test
2.4.3. Water Solubility Test
2.4.4. Antimicrobial and Antifungal Activity
2.4.5. Food Quality Test
2.4.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Moringa Oleifera Seed Powder (MOSP)
3.2. Characterization of CS/TA/MOSP Biocomposite Film
3.2.1. FTIR Analysis
3.2.2. XRD Analysis
3.2.3. SEM Analysis
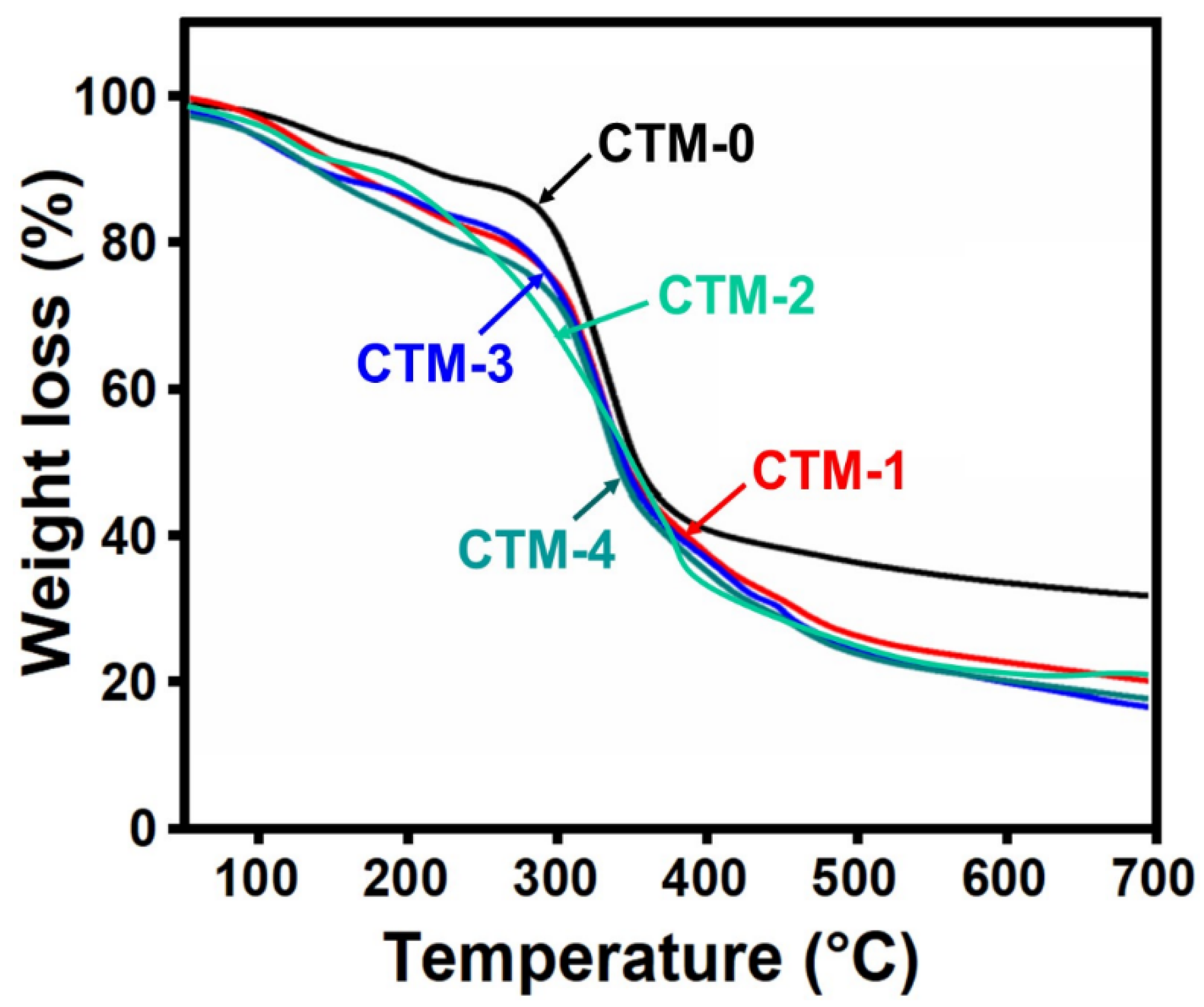
3.2.4. Thermogravimetric Analysis (TGA)
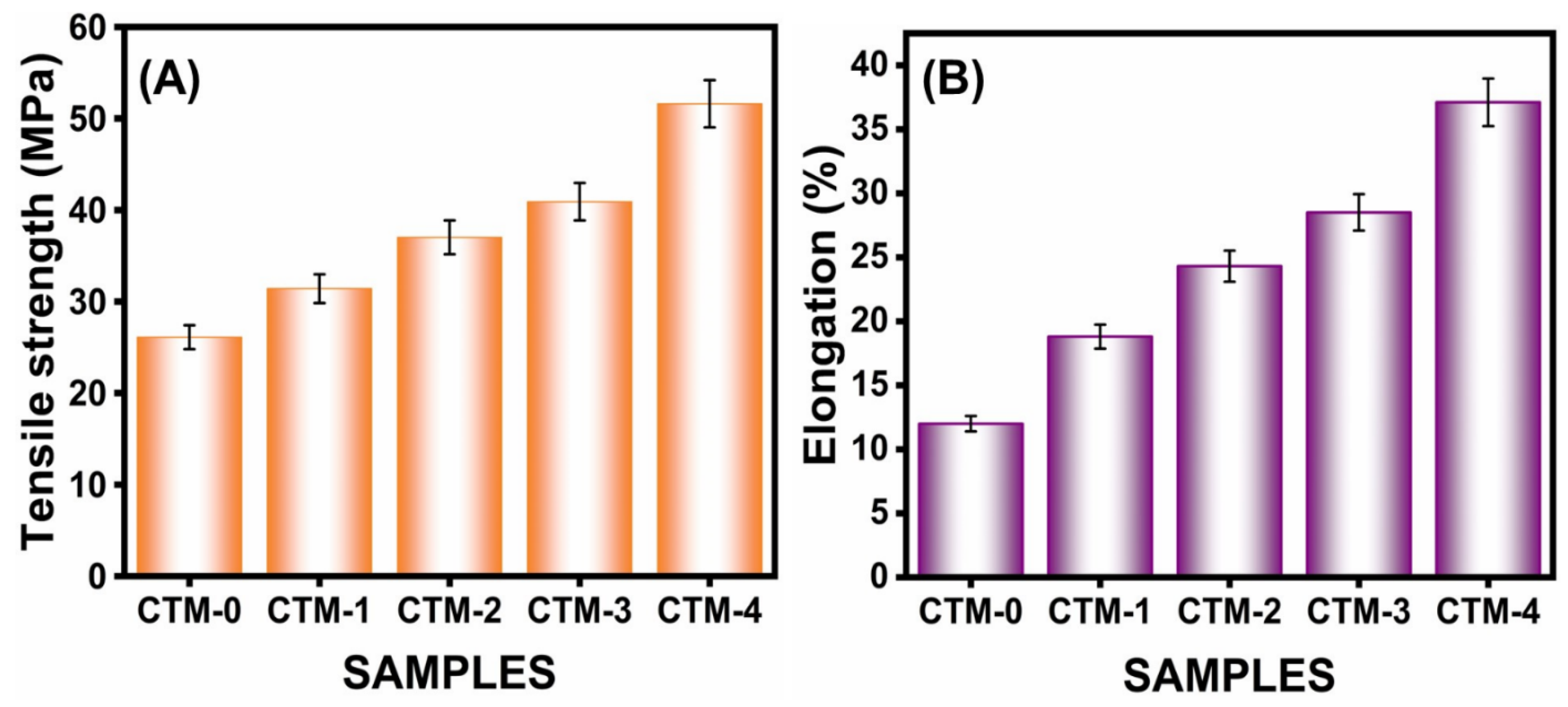
3.2.5. Mechanical Properties
3.2.6. Swelling Property
3.2.7. Water Solubility Test
3.2.8. Oxygen Transmission Rate (OTR)
- i.
- The area available for gas diffusion is reduced when MOSP is introduced.
- ii.
- The increase in the length of the path that gaseous particles must travel to move via the film.
3.2.9. Water Vapor Transmission Rate (WVTR)
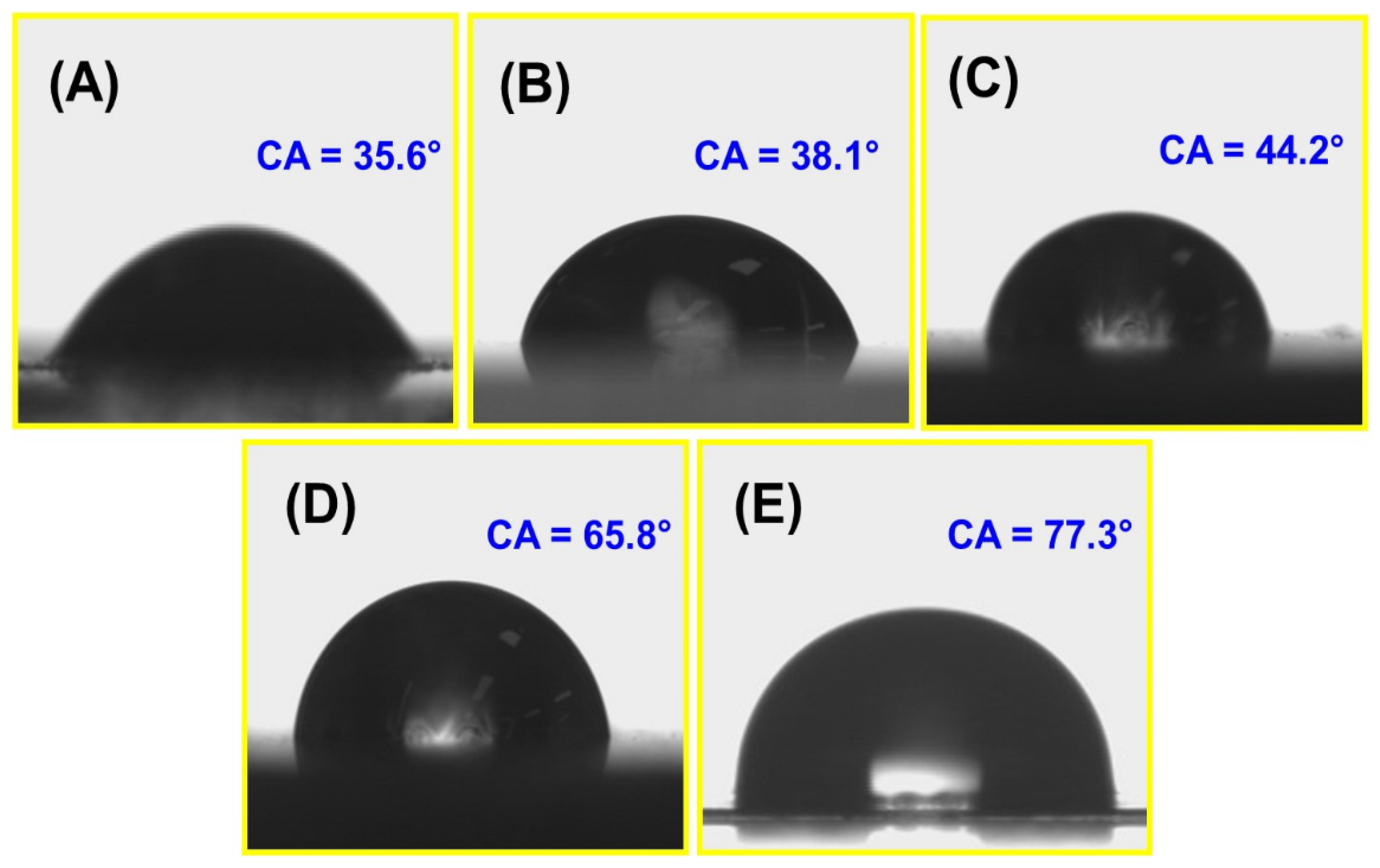
3.2.10. Water Contact Angle (WCA) Analysis
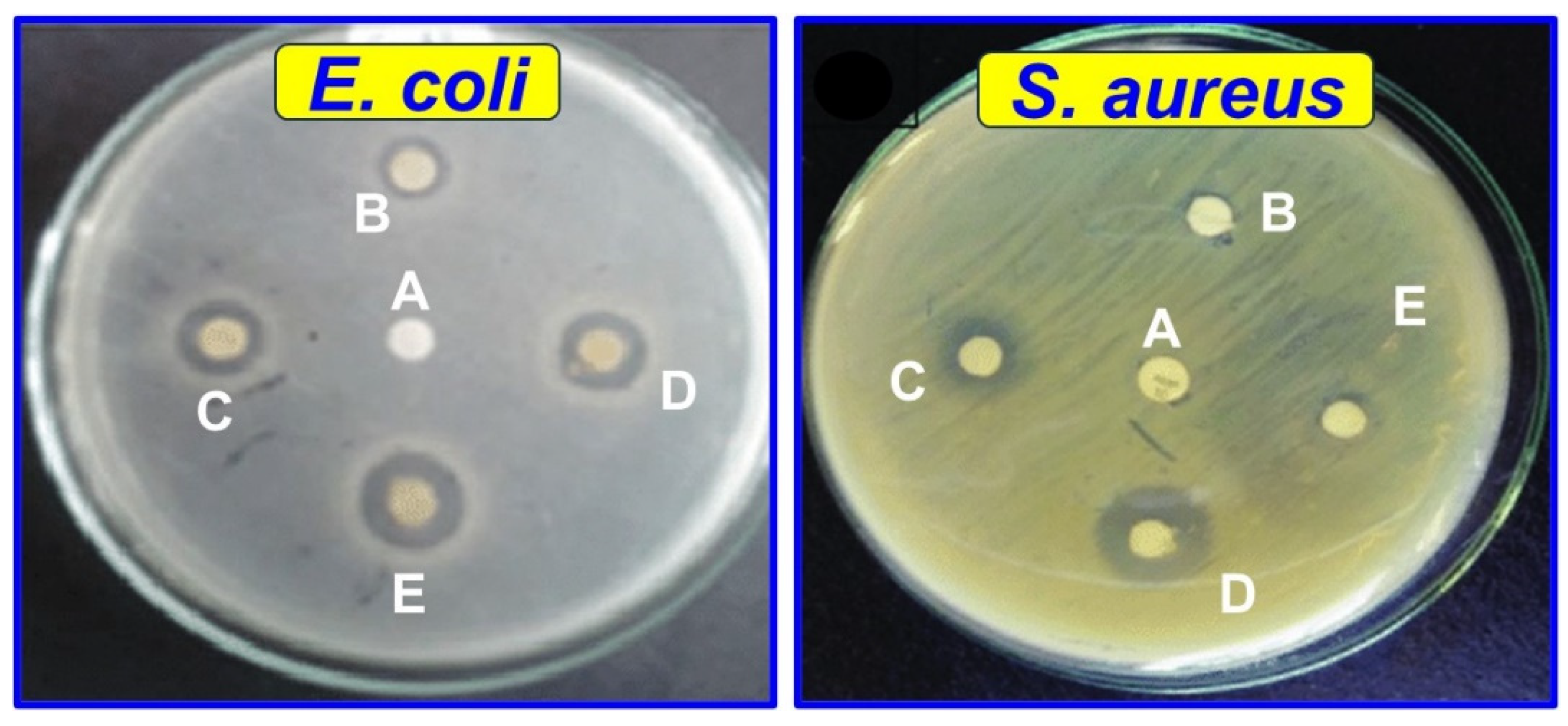
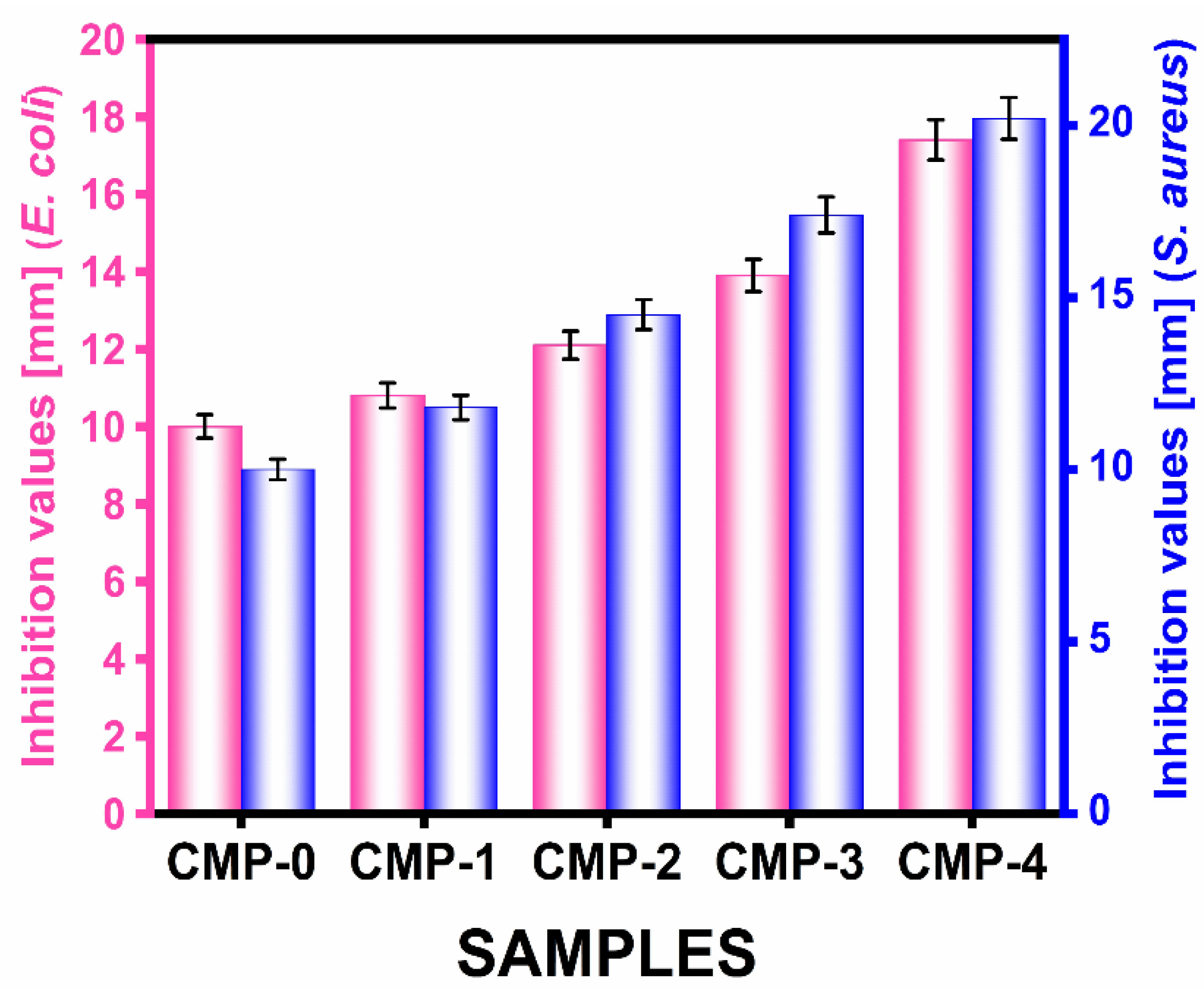
3.2.11. Antimicrobial and Antifungal Activity
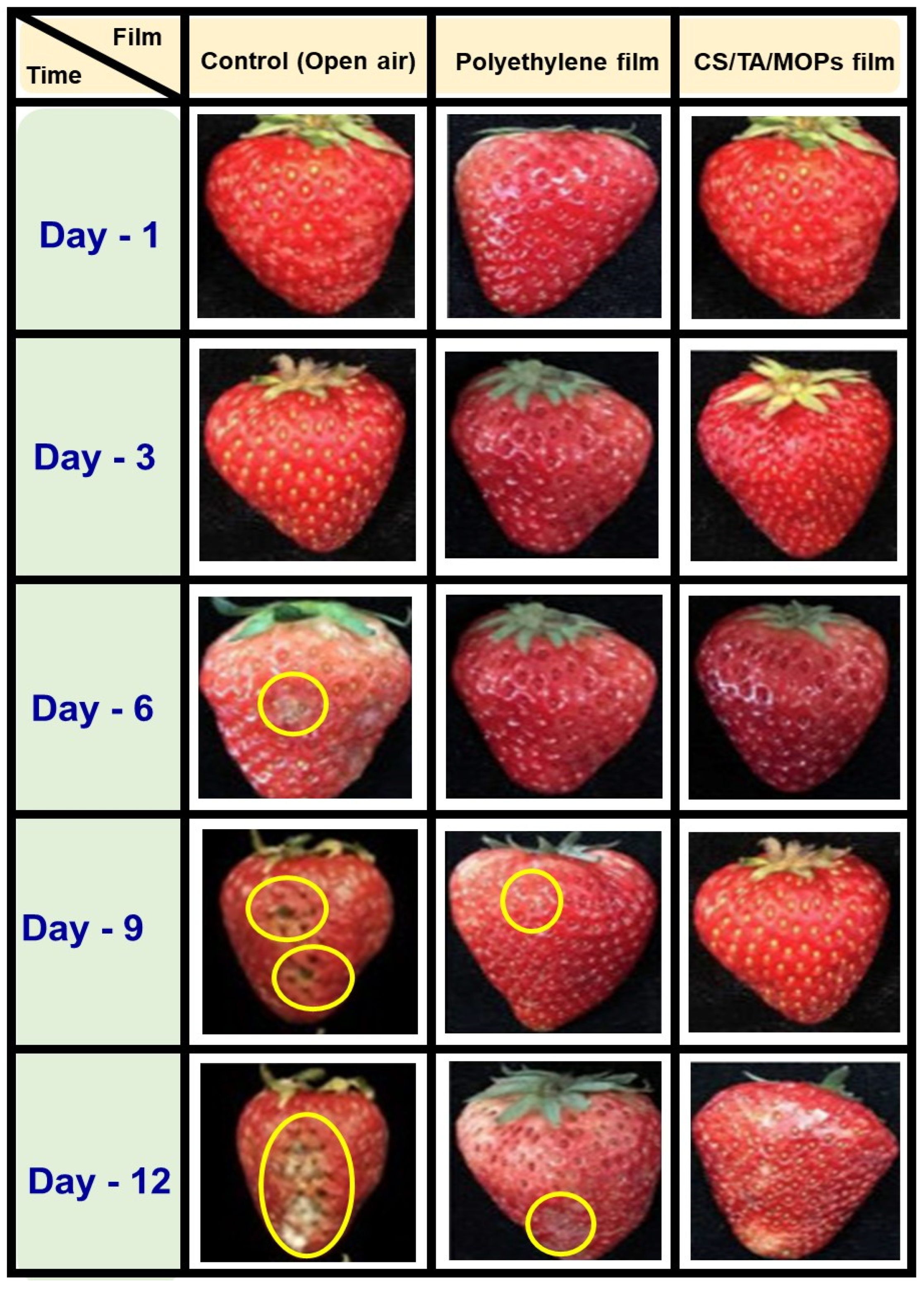
3.2.12. Shelf-Life Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Law, K.L.; Narayan, R. Reducing environmental plastic pollution by designing polymer materials for managed end-of-life. Nat. Rev. Mater. 2022, 7, 104–116. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Wagner, M. A sustainable future for plastics considering material safety and preserved value. Nat. Rev. Mater. 2022, 7, 71–73. [Google Scholar] [CrossRef]
- Shaikh, S.; Yaqoob, M.; Aggarwal, P. An overview of biodegradable packaging in food industry. Curr. Res. Food Sci. 2021, 4, 503–520. [Google Scholar] [CrossRef] [PubMed]
- Babaremu, K.; Oladijo, O.P.; Akinlabi, E. Biopolymers: A suitable replacement for plastics in product packaging. Adv. Ind. Eng. Polym. Res. 2023, 6, 333–340. [Google Scholar] [CrossRef]
- Tardy, B.L.; Richardson, J.J.; Greca, L.G.; Guo, J.; Bras, J.; Rojas, O.J. Advancing bio-based materials for sustainable solutions to food packaging. Nat. Sustain. 2023, 6, 360–367. [Google Scholar] [CrossRef]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A review on the modification of cellulose and its applications. Polymers 2023, 14, 3206. [Google Scholar] [CrossRef] [PubMed]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Islam, N.; Hoque, M.; Taharat, S.F. Recent advances in extraction of chitin and chitosan. World J. Microbiol. Biotechnol. 2023, 39, 28. [Google Scholar] [CrossRef]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jóźwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and chitosan as polymers of the future—Obtaining, modification, life cycle assessment and main directions of application. Polymers 2023, 15, 793. [Google Scholar] [CrossRef]
- Issahaku, I.; Tetteh, I.K.; Tetteh, A.Y. Chitosan and chitosan derivatives: Recent advancements in production and applications in environmental remediation. Environ. Adv. 2023, 11, 100351. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Hahn, T.; Zibek, S.; Gagliardini, A.; Panariello, L.; Coltelli, M.B.; et al. Characterization of chitin and chitosan derived from Hermetia illucens, a further step in a circular economy process. Sci. Rep. 2022, 12, 6613. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gong, C.; Qin, Y.; Hu, Y.; Jiao, A.; Jin, Z.; Qiu, C.; Wang, J. Bioactive and functional biodegradable packaging films reinforced with nanoparticles. J. Food Eng. 2022, 312, 110752. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Techn. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Techno. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Kabir, E.; Kaur, R.; Lee, J.; Kim, K.-H.; Kwon, E.E. Prospects of biopolymer technology as an alternative option for non-degradable plastics and sustainable management of plastic wastes. J. Clean. Prod. 2020, 258, 120536. [Google Scholar] [CrossRef]
- Nanda, S.; Patra, B.R.; Patel, R.; Bakos, J.; Dalai, A.K. Innovations in applications and prospects of bioplastics and biopolymers: A review. Environ. Chem. Lett. 2022, 20, 379–395. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Sheikh, M.F.; Zolfagharian, A.; Bodaghi, M. Biopolymeric sustainable materials and their emerging applications. J. Environ. Chem. Eng. 2022, 10, 108159. [Google Scholar] [CrossRef]
- Vedula, S.S.; Yadav, G.D. Chitosan-based membranes preparation and applications: Challenges and opportunities. J. Indian Chem. Soc. 2021, 98, 100017. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.; Caballero, A.H. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef]
- Cui, L.; Gao, S.; Song, X.; Huang, L.; Dong, H.; Liu, J.; Chen, F.; Yu, S. Preparation and characterization of chitosan membranes. RSC Adv. 2018, 8, 28433–28439. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.; Bugusu, B. Food packaging—Roles, materials, and environmental issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, E.B.; Sankhla, M.S.; Bhat, R.A.; Bhagat, D.S. Microplastics from food packaging: An overview of human consumption, health threats, and alternative solutions. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100608. [Google Scholar] [CrossRef]
- Leite, L.S.F.; Pham, C.; Bilatto, S.; Azeredo, H.M.C.; Cranston, E.D.; Moreira, F.K.; Mattoso, L.H.C.; Bras, J. Effect of tannic acid and cellulose nanocrystals on antioxidant and antimicrobial properties of gelatin films. ACS Sustain. Chem. Eng. 2021, 9, 8539–8549. [Google Scholar] [CrossRef]
- Chen, C.; Yanga, H.; Yang, X.; Ma, Q. Tannic acid: A crosslinker leading to versatile functional polymeric networks: A review. RSC Adv. 2022, 12, 7689–7711. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, M.; Yilmaz, A.S.; Demirci, S.; Sahiner, N. Physically and chemically crosslinked, tannic acid embedded linear PEI-based hydrogels and cryogels with natural antibacterial and antioxidant properties. Biomedicines 2023, 11, 706. [Google Scholar] [CrossRef]
- Venkatesan, R.; Rajeswari, N. Preparation, mechanical and antimicrobial properties of SiO2/poly(butylene adipate-co-terephthalate) films for active food packaging. Silicon 2019, 11, 2233–2239. [Google Scholar] [CrossRef]
- Venkatesan, R.; Rajeswari, N. Nanosilica-reinforced poly(butylene adipate-co-terephthalate) nanocomposites: Preparation, characterization and properties. Polym. Bull. 2019, 76, 4785–4801. [Google Scholar] [CrossRef]
- Zhang, W.; Rhim, J.-W. Titanium dioxide (TiO2) for the manufacture of multifunctional active food packaging films. Food Packag. Shelf Life 2022, 31, 100806. [Google Scholar] [CrossRef]
- Remya, R.R.; Julius, A. Titanium dioxide nanomaterials coated films in food packaging: A mini review. Vegetos 2022, 35, 565–570. [Google Scholar] [CrossRef]
- Venkatesan, R.; Rajeswari, N. TiO2 nanoparticles/poly(butylene adipate-co-terephthalate) bionanocomposite films for packaging applications. Polym. Adv. Technol. 2017, 28, 1699–1706. [Google Scholar] [CrossRef]
- Abbas, M.; Buntinx, M.; Deferme, W.; Peeters, R. (Bio)polymer/ZnO nanocomposites for packaging applications: A review of gas barrier and mechanical properties. Nanomaterials 2019, 9, 1494. [Google Scholar] [CrossRef] [PubMed]
- Yari, S.; Mohammadi-Rovshandeh, J.; Shahrousvand, M. Preparation and optimization of starch/poly vinyl alcohol/ZnO nanocomposite films applicable for food packaging. J. Polym. Environ. 2022, 30, 1502–1517. [Google Scholar] [CrossRef]
- Zare, M.; Namratha, K.; Ilyas, S.; Sultana, A.; Hezam, A.; Sunil, L.; Surmeneva, M.A.; Surmenev, R.A.; Nayan, M.B.; Ramakrishna, S.; et al. Emerging trends for ZnO nanoparticles and their applications in food packaging. ACS Food Sci. Technol. 2022, 2, 763–781. [Google Scholar] [CrossRef]
- Kim, I.; Viswanathan, K.; Kasi, G.; Thanakkasaranee, S.; Sadeghi, K.; Seo, J. ZnO nanostructures in active antibacterial food packaging: Preparation methods, antimicrobial mechanisms, safety issues, future prospects, and challenges. Food Rev. Int. 2022, 38, 537–565. [Google Scholar] [CrossRef]
- Venkatesan, R.; Rajeswari, N. ZnO/PBAT nanocomposite films: Investigation on the mechanical and biological activity for food packaging. Polym. Adv. Technol. 2017, 28, 20–27. [Google Scholar] [CrossRef]
- Wang, Y.; Cen, C.; Chen, J.; Fu, L. MgO/carboxymethyl chitosan nanocomposite improves thermal stability, waterproof and antibacterial performance for food packaging. Carbohydr. Polym. 2020, 236, 116078. [Google Scholar] [CrossRef]
- El Sayed, A.M.; Abdelghany, A.M.; Elfadl, A.A. Structural, optical, mechanical and antibacterial properties of MgO/poly(vinyl acetate)/poly(vinyl chloride) nanocomposites. Braz. J. Phys. 2022, 52, 150. [Google Scholar] [CrossRef]
- Kalyani, P.; Muthupandeeswari, T. Investigation on the altered properties of PVA filled magnesium oxide composite (PVA@xMgO) thin films. Polym. Bull. 2022, 79, 10115–10134. [Google Scholar] [CrossRef]
- Ahmed, M.W.; Haque, M.A.; Mohibbullah, M.; Khan, M.S.I.; Islam, M.A.; Mondal, M.H.T.; Ahmmed, R. A review on active packaging for quality and safety of foods: Current trends, applications, prospects and challenges. Food Packag. Shelf Life 2022, 33, 100913. [Google Scholar] [CrossRef]
- Westlake, J.R.; Tran, M.W.; Jiang, Y.; Zhang, X.; Burrows, A.D.; Xie, M. Biodegradable active packaging with controlled release: Principles, progress, and prospects. ACS Food Sci. Technol. 2022, 2, 1166–1183. [Google Scholar] [CrossRef]
- Jayawardana, B.C.; Liyanage, R.; Lalantha, N.; Iddamalgoda, S.; Weththasinghe, P. Antioxidant and antimicrobial activity of drumstick (Moringa oleifera) leaves in herbal chicken sausages. LWT—Food Sci. Technol. 2015, 64, 1204–1208. [Google Scholar] [CrossRef]
- Xu, Y.X.; Kim, K.M.; Hanna, M.A.; Nag, D. Chitosan-starch composite film: Preparation and characterization. Ind. Crops Prod. 2005, 21, 185–192. [Google Scholar] [CrossRef]
- Kaewkroek, K.; Petchsomrit, A.; Septama, A.W.; Wiwattanapatapee, R. Development of starch/chitosan expandable films as a gastroretentive carrier for ginger extract-loaded solid dispersion. Saudi Pharm. J. 2022, 30, 120–131. [Google Scholar] [CrossRef]
- Li, H.; Gao, X.; Wang, Y.; Zhang, X.; Tong, Z. Comparison of chitosan/starch composite film properties before and after cross-linking. Int. J. Biol. Macromol. 2013, 52, 275–279. [Google Scholar] [CrossRef]
- Pothal, P.; Pathania, K.; Kumar, S.; Kaur, J.; Sah, S.P.; Singh, R.; Pawar, S.V. Lignin-chitosan biocomposite film for antimicrobial activity: Fabrication, characterization and in-vitro evaluation. Mater. Lett. 2023, 337, 133956. [Google Scholar] [CrossRef]
- Rosova, E.; Smirnova, N.; Dresvyanina, E.; Smirnova, V.; Vlasova, E.; Ivan’kova, E.; Sokolova, M.; Maslennikova, T.; Malafeev, K.; Kolbe, K.; et al. Biocomposite materials based on chitosan and lignin: Preparation and characterization. Cosmetics 2021, 8, 24. [Google Scholar] [CrossRef]
- Crouvisier-Urion, K.; Bodart, P.R.; Winckler, P.; Raya, J.; Gougeon, R.D.; Cayot, P.; Domenek, S.; Debeaufort, F.; Karbowiak, T. Biobased composite films from chitosan and lignin: Antioxidant activity related to structure and moisture. ACS Sustain. Chem. Eng. 2016, 4, 6371–6381. [Google Scholar] [CrossRef]
- Fathima, P.E.; Panda, S.K.; Ashraf, P.M.; Varghese, T.O.; Bindu, J. Polylactic acid/chitosan films for packaging of Indian white prawn (Fenneropenaeus indicus). Int. J. Biol. Macromol. 2018, 117, 1002–1010. [Google Scholar] [CrossRef]
- Hijazi, N.; Moigne, N.L.; Rodier, E.; Sauceau, M.; Vincent, T.; Benezet, J.-C.; Fages, J. Biocomposite films based on poly(lactic acid) and chitosan nanoparticles: Elaboration, microstructural and thermal characterization. Polym. Eng. Sci. 2019, 59, E350–E360. [Google Scholar] [CrossRef]
- Pokhrel, S.; Lach, R.; Le, H.H.; Wutzler, A.; Grellmann, W.; Radusch, H.-J.; Dhakal, R.P.; Esposito, A.; Henning, S.; Yadav, P.N.; et al. Fabrication and characterization of completely biodegradable copolyester-chitosan blends: I. Spectroscopic and thermal characterization. Macromol. Symp. 2016, 366, 23–34. [Google Scholar] [CrossRef]
- Wang, S.; Xing, Q. Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders. e-Polymers 2022, 22, 705–718. [Google Scholar] [CrossRef]
- Tan, Y.M.; Lim, S.H.; Tay, B.Y.; Lee, M.W.; Thian, E.S. Functional chitosan-based grapefruit seed extract composite films for applications in food packaging technology. Mater. Res. Bull. 2015, 69, 142–146. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kumar, B.; Negi, Y.S. Chitosan film incorporated with citric acid and glycerol as an active packaging material for extension of green chilli shelf life. Carbohydr. Polym. 2018, 195, 329–338. [Google Scholar] [CrossRef]
- Shen, Z.; Kamdem, D.P. Development and characterization of biodegradable chitosan films containing two essential oils. Int. J. Biol. Macromol. 2015, 74, 289–296. [Google Scholar] [CrossRef]
- Perdones, Á.; Vargas, M.; Atarés, L.; Chiralt, A. Physical, antioxidant and antimicrobial properties of chitosan–cinnamon leaf oil films as affected by oleic acid. Food Hydrocoll. 2014, 36, 256–264. [Google Scholar] [CrossRef]
- Hafsa, J.; Smach, M.A.; Khedher, M.R.B.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Physical, antioxidant and antimicrobial properties of chitosan films containing Eucalyptus globulus essential oil. LWT—Food Sci. Tech. 2016, 68, 356–364. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Liang, W.; Qiao, X.; Zhou, Q. Overview of preparation methods for high performance composite materials. IOP Conf. Ser. Mater. Sci. Eng. 2018, 394, 022058. [Google Scholar] [CrossRef]
- Lin, D.; Zheng, Y.; Huang, Y.; Ni, L.; Zhao, J.; Huang, C.; Chen, X.; Chen, X.; Wu, Z.; Wu, D.; et al. Investigation of the structural, physical properties, antioxidant, and antimicrobial activity of chitosan-nano-silicon aerogel composite edible films incorporated with okara powder. Carbohydr. Polym. 2020, 250, 116842. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Fabrication of bioactive binary composite film based on gelatin/chitosan incorporated with cinnamon essential oil and rutin. Colloids Surf. B 2021, 204, 111830. [Google Scholar] [CrossRef]
- Fan, S.; Wang, D.; Wen, X.; Li, X.; Fang, F.; Richel, A.; Xiao, N.; Fauconnier, M.-L.; Hou, C.; Zhang, D. Incorporation of cinnamon essential oil-loaded Pickering emulsion for improving antimicrobial properties and control release of chitosan/gelatin films. Food Hydrocoll. 2023, 138, 108438. [Google Scholar] [CrossRef]
- Riaz, A.; Aadil, R.M.; Amoussa, A.M.O.; Bashari, M.; Abid, M.; Hashim, M. Application of chitosan-based apple peel polyphenols edible coating on the preservation of strawberry (Fragaria ananassa cv Hongyan) fruit. J. Food Process. 2021, 45, e15018. [Google Scholar] [CrossRef]
- Irikura, K.; Ekapakul, N.; Choochottiros, C.; Chanthaset, N.; Yoshida, H.; Ajiro, H. Fabrication of flexible blend films using a chitosan derivative and poly(trimethylene carbonate). Polym. J. 2021, 53, 823–833. [Google Scholar] [CrossRef]
- Li, L.-H.; Deng, J.-C.; Deng, H.-R.; Liu, Z.-L.; Li, X.-L. Preparation, characterization and antimicrobial activities of chitosan/Ag/ZnO blend films. Chem. Eng. J. 2010, 160, 378–382. [Google Scholar] [CrossRef]
- Murali, S.; Kumar, S.; Koh, J.; Seena, S.; Singh, P.; Ramalho, A.; Sobral, A.J.F.N. Bio-based chitosan/gelatin/Ag@ZnO bionanocomposites: Synthesis and mechanical and antibacterial properties. Cellulose 2019, 26, 5347–5361. [Google Scholar] [CrossRef]
- Lu, Z.; Gao, J.; He, Q.; Wu, J.; Liang, D.; Yang, H.; Chen, R. Enhanced antibacterial and wound healing activities of microporous chitosan-Ag/ZnO composite dressing. Carbohydr. Polym. 2017, 156, 460–469. [Google Scholar] [CrossRef]
- Sarojini, S.K.; Indumathi, M.P.; Rajarajeswari, G.R. Mahua oil-based polyurethane/chitosan/nano ZnO composite films for biodegradable food packaging applications. Int. J. Biol. Macromol. 2019, 124, 163–174. [Google Scholar] [CrossRef]
- Sanuja, S.; Agalya, A.; Umapathy, M.J. Synthesis and characterization of zinc oxide–neem oil–chitosan bionanocomposite for food packaging application. Int. J. Biol. Macromol. 2015, 74, 76–84. [Google Scholar] [CrossRef]
- Patehkhor, H.A.; Fattahi, M.; Khosravi-Nikou, M. Synthesis and characterization of ternary chitosan–TiO2–ZnO over graphene for photocatalytic degradation of tetracycline from pharmaceutical wastewater. Sci. Rep. 2021, 11, 24177. [Google Scholar] [CrossRef]
- Wang, S.F.; Shen, L.; Tong, Y.J.; Chen, L.; Phang, I.Y.; Lim, P.Q.; Liu, T.X. Biopolymer chitosan/montmorillonite nanocomposites: Preparation and characterization. Polym. Degrad. Stab. 2005, 90, 123–131. [Google Scholar] [CrossRef]
- Bhagath, S.; Vivek, A.; Krishna, V.V.; Mittal, S.S.; Balachandran, M. Synthesis and characteristics of MMT reinforced chitosan nanocomposite. Mater. Today: Proc. 2021, 46, 4487–4492. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, D. Synthesis and characterization of AlCl3-chitosan composite aerogels incorporating polyvinyl alcohol and bentonite clay. J. Sol-Gel Sci. Technol. 2022, 103, 722–729. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, H.; Xiao, N.; Ma, X.; Liu, C.; Zhong, L.; Xiao, G. Preparation and properties of epichlorohydrin-cross-linked chitosan/hydroxyethyl cellulose based CuO nanocomposite films. Cellulose 2022, 29, 4413–4426. [Google Scholar] [CrossRef]
- Vidal, C.P.; Luzi, F.; Puglia, D.; López-Carballo, G.; Rojas, A.; Galotto, M.J.; de Dicastillo, L. Development of a sustainable and antibacterial food packaging material based in a biopolymeric multilayer system composed by polylactic acid, chitosan, cellulose nanocrystals and ethyl lauroyl arginate. Food Packag. Shelf Life 2023, 36, 101050. [Google Scholar] [CrossRef]
- Li, H.-Z.; Chen, S.-C.; Wang, Y.-Z. Preparation and characterization of nanocomposites of polyvinyl alcohol/cellulose nanowhiskers/chitosan. Compos. Sci. Technol. 2015, 115, 60–65. [Google Scholar] [CrossRef]
- Dhanalekshmi, K.I.; Magesan, P.; Umapathy, M.J.; Zhang, X.; Srinivasan, N.; Jayamoorthy, K. Enhanced photocatalytic and photodynamic activity of chitosan and garlic loaded CdO–TiO2 hybrid bionanomaterials. Sci. Rep. 2021, 11, 20790. [Google Scholar] [CrossRef]
- Ehsani, A.; Bigdeloo, M.; Assefi, F.; Kiamehr, M.; Alizadeh, R. Ternary nanocomposite of conductive polymer/chitosan biopolymer/metal organic framework: Synthesis, characterization and electrochemical performance as effective electrode materials in pseudocapacitors. Inorg. Chem. Commun. 2020, 115, 107885. [Google Scholar] [CrossRef]
- Venkatesan, R.; Surya, S.; Suganthi, S.; Muthuramamoorthy, M.; Pandiaraj, S.; Kim, S.-C. Biodegradable composites from poly(butylene adipate-co-terephthalate) with carbon nanoparticles: Preparation, characterization and performances. Environ. Res. 2023, 235, 116634. [Google Scholar] [CrossRef]
- ASTM D3985; Standard Test Method for Oxygen Gas Transmission Rate through Plastic Film and Sheeting Using a Coulometric Sensor. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM F1249-90; Standard Test Method for Water Vapor Transmission Rate through Plastic Film and Sheeting Using a Modulated Infrared Sensor. ASTM International: West Conshohocken, PA, USA, 2022.
- Sanuja, S.; Agalya, A.; Umapathy, M.J. Studies on magnesium oxide reinforced chitosan bionanocomposite incorporated with clove oil for active food packaging application. Int. J. Polym. Mater. 2014, 63, 733–740. [Google Scholar] [CrossRef]
- Saran, M.; Vyas, S.; Mathur, M.; Bagaria, A. Green synthesis and characterization of CuNPs: Insights into their potential bioactivity. IET Nanobiotechnol. 2018, 12, 357–364. [Google Scholar] [CrossRef]
- Kebede, T.G.; Dube, S.; Nindi, M.M. Fabrication and characterization of electrospun nanofibers from Moringa stenopetala seed protein. Mater. Res. Express 2018, 5, 125015. [Google Scholar] [CrossRef]
- Araújo, C.S.; Alves, V.N.; Rezende, H.C.; Almeida, I.L.; Assuncao, D.R.; Tarley, C.R.; Segatelli, M.G.; Coelho, N.M. Characterization and use of Moringa oleifera seeds as biosorbent for removing metal ions from aqueous effluents. Water. Sci. Technol. 2010, 62, 2198–2203. [Google Scholar] [CrossRef]
- Shirani, Z.; Santhosh, C.; Iqbal, J.; Bhatnagar, A. Waste Moringa oleifera seed pods as green sorbent for efficient removal of toxic aquatic pollutants. J. Environ. Manag. 2018, 227, 95–106. [Google Scholar] [CrossRef]
- Siddique, A.B.; Singh, V.P.; Pramanick, A.K.; Ray, M. Amorphous carbon dot and chitosan-based composites as fluorescent inks and luminescent films. Mater. Chem. Phys. 2020, 249, 122984. [Google Scholar] [CrossRef]
- Mallakpour, S.; Behranvand, V. Nanocomposites based on biosafe nano ZnO and different polymeric matrixes for antibacterial, optical, thermal and mechanical applications. Eur. Polym. J. 2016, 84, 377–403. [Google Scholar] [CrossRef]
- Thomas, R.; Vincent, V.T.; Umapathy, M.J.; Rajagopal, L.; Jay, S. Preparation, characterization, and antimicrobial activity of novel chitosan blended almond gum–nanosilica bionanocomposite film for food packaging applications. Mate. Test. 2023, 65, 1805–1816. [Google Scholar] [CrossRef]
- Lan, W.; Li, S.; Shama, S.; Zhao, Y.; Sameen, D.E.; He, L.; Liu, Y. Investigation of ultrasonic treatment on physicochemical, structural and morphological properties of sodium alginate/AgNPs/apple polyphenol films and its preservation effect on strawberry. Polymers 2020, 12, 2096. [Google Scholar] [CrossRef]
- Dhaka, R.K.; Kumar, N.; Pratibha; Upadhyay, A. Optimization, characterization, and influence of microfluidization on almond gumbased composite edible film. Starch—Stärke 2021, 73, 2000101. [Google Scholar] [CrossRef]
- Rekik, S.B.; Gassara, S.; Bouaziz, J.; Deratani, A.; Baklouti, S. Development and characterization of porous membranes based on kaolin/chitosan composite. Appl. Clay Sci. 2017, 143, 1–9. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A natural biopolymer with a wide and varied range of applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Mohd Abdull Majid, M.A.H.; Osman, N.H.; Tamchek, N.; Asyikin, N.; Sukri, A.; Mazlan, H.I.; Mazu, N.N.; Idris, A.; Liew, J.Y.C.; Ramil, M.M. Physical, mechanical and electrical properties of chitosan/graphene oxide composite films for copper ions (Cu2+) detection. J. Polym. Environ. 2023, 31, 3565–3572. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Aguilar, J.M.; Bengoechea, C.; López-Castejón, M.L.; Guerrero, A. Rheology and water absorption properties of alginate–soy protein composites. Polymers 2021, 13, 1807. [Google Scholar] [CrossRef]
- González, K.; Iturriaga, L.; González, A.; Eceiza, A.; Gabilondo, N. Improving mechanical and barrier properties of thermoplastic starch and polysaccharide nanocrystals nanocomposites. Eur. Polym. J. 2020, 123, 109415. [Google Scholar] [CrossRef]
- Venkatesan, R.; Sekar, S.; Raorane, C.J.; Raj, V.; Kim, S.-C. Hydrophilic composites of chitosan with almond gum: Characterization and mechanical, and antimicrobial activity for compostable food packaging. Antibiotics 2022, 11, 1502. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kumar, B.; Deeba, F.; Kulshreshtha, A.; Negi, Y.S. Chitosan films incorporated with Apricot (Prunus armeniaca) kernel essential oil as active food packaging material. Food Hydrocoll. 2018, 85, 158–166. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Kaewklin, P. Fabrication and characterization of chitosan-titanium dioxide nanocomposite film as ethylene scavenging and antimicrobial active food packaging. Food Hydrocoll. 2018, 84, 125–134. [Google Scholar] [CrossRef]





| S. No. | Formulated Films | Nomenclature | CS (wt.%) | TA (wt.%) | MOSP (wt.%) |
|---|---|---|---|---|---|
| 1. | Chitosan (100.0) | CTM-0 | 100.0 | -- | -- |
| 2. | CS/TA/MOSP (69.0:30.0:1.0) | CTM-1 | 69.0 | 30.0 | 1.0 |
| 3. | CS/TA/MOSP (67.0:30.0:3.0) | CTM-2 | 67.0 | 30.0 | 3.0 |
| 4. | CS/TA/MOSP (65.0:30.0:5.0) | CTM-3 | 65.0 | 30.0 | 5.0 |
| 5. | CS/TA/MOSP (60.0:30.0:10.0) | CTM-4 | 60.0 | 30.0 | 10.0 |
| S. No. | Samples | Tensile Strength (MPa) | Elongation at Break (wt.%) | Solubility in Water (wt.%) | Swelling (wt.%) |
|---|---|---|---|---|---|
| 1. | CTM-0 | 26.1 ± 1.6 | 12.0 ± 2.5 | 64.52 | 21.00 |
| 2. | CTM-1 | 31.4 ± 1.1 | 18.8 ± 1.3 | 51.10 | 15.30 |
| 3. | CTM-2 | 37.0 ± 2.7 | 24.3 ± 2.2 | 44.54 | 11.94 |
| 4. | CTM-3 | 40.9 ± 1.0 | 28.5 ± 1.9 | 35.20 | 5.36 |
| 5. | CTM-4 | 51.6 ± 3.0 | 37.1 ± 2.8 | 29.25 | 2.60 |
| S. No. | Samples | OTR (cc/m2/24 h) | WVTR (g/m2/24 h) |
|---|---|---|---|
| 1. | CTM-0 | 82.6 ± 3.0 | 22.0 ± 2.5 |
| 2. | CTM-1 | 71.9 ± 1.0 | 18.2 ± 1.3 |
| 3. | CTM-2 | 51.0 ± 2.7 | 16.3 ± 2.2 |
| 4. | CTM-3 | 43.4 ± 1.1 | 12.5 ± 1.9 |
| 5. | CTM-4 | 31.1 ± 1.6 | 10.1 ± 2.8 |
| Microorganisms/Samples | Antimicrobial Activity [Zone of Inhibition (dia. in mm)] | ||||
|---|---|---|---|---|---|
| CTM-0 | CTM-1 | CTM-2 | CTM-3 | CTM-4 | |
| E. coli | 10.0 ± 1.05 a | 10.8 ± 2.50 c | 12.1 ± 3.31 c | 13.9 ± 1.10 a | 17.4 ± 3.05 b |
| S. aureus | 10.3 ± 2.84 c | 11.8 ± 3.15 b | 14.5 ± 1.85 b | 17.4 ± 1.00 a | 20.2 ± 2.22 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesan, R.; Vetcher, A.A.; Al-Asbahi, B.A.; Kim, S.-C. Chitosan-Based Films Blended with Tannic Acid and Moringa Oleifera for Application in Food Packaging: The Preservation of Strawberries (Fragaria ananassa). Polymers 2024, 16, 937. https://doi.org/10.3390/polym16070937
Venkatesan R, Vetcher AA, Al-Asbahi BA, Kim S-C. Chitosan-Based Films Blended with Tannic Acid and Moringa Oleifera for Application in Food Packaging: The Preservation of Strawberries (Fragaria ananassa). Polymers. 2024; 16(7):937. https://doi.org/10.3390/polym16070937
Chicago/Turabian StyleVenkatesan, Raja, Alexandre A. Vetcher, Bandar Ali Al-Asbahi, and Seong-Cheol Kim. 2024. "Chitosan-Based Films Blended with Tannic Acid and Moringa Oleifera for Application in Food Packaging: The Preservation of Strawberries (Fragaria ananassa)" Polymers 16, no. 7: 937. https://doi.org/10.3390/polym16070937
APA StyleVenkatesan, R., Vetcher, A. A., Al-Asbahi, B. A., & Kim, S.-C. (2024). Chitosan-Based Films Blended with Tannic Acid and Moringa Oleifera for Application in Food Packaging: The Preservation of Strawberries (Fragaria ananassa). Polymers, 16(7), 937. https://doi.org/10.3390/polym16070937











