Determination of the Mass Fractions of the Heavy Metals in the Recycled Cellulose Pulp
Abstract
1. Introduction
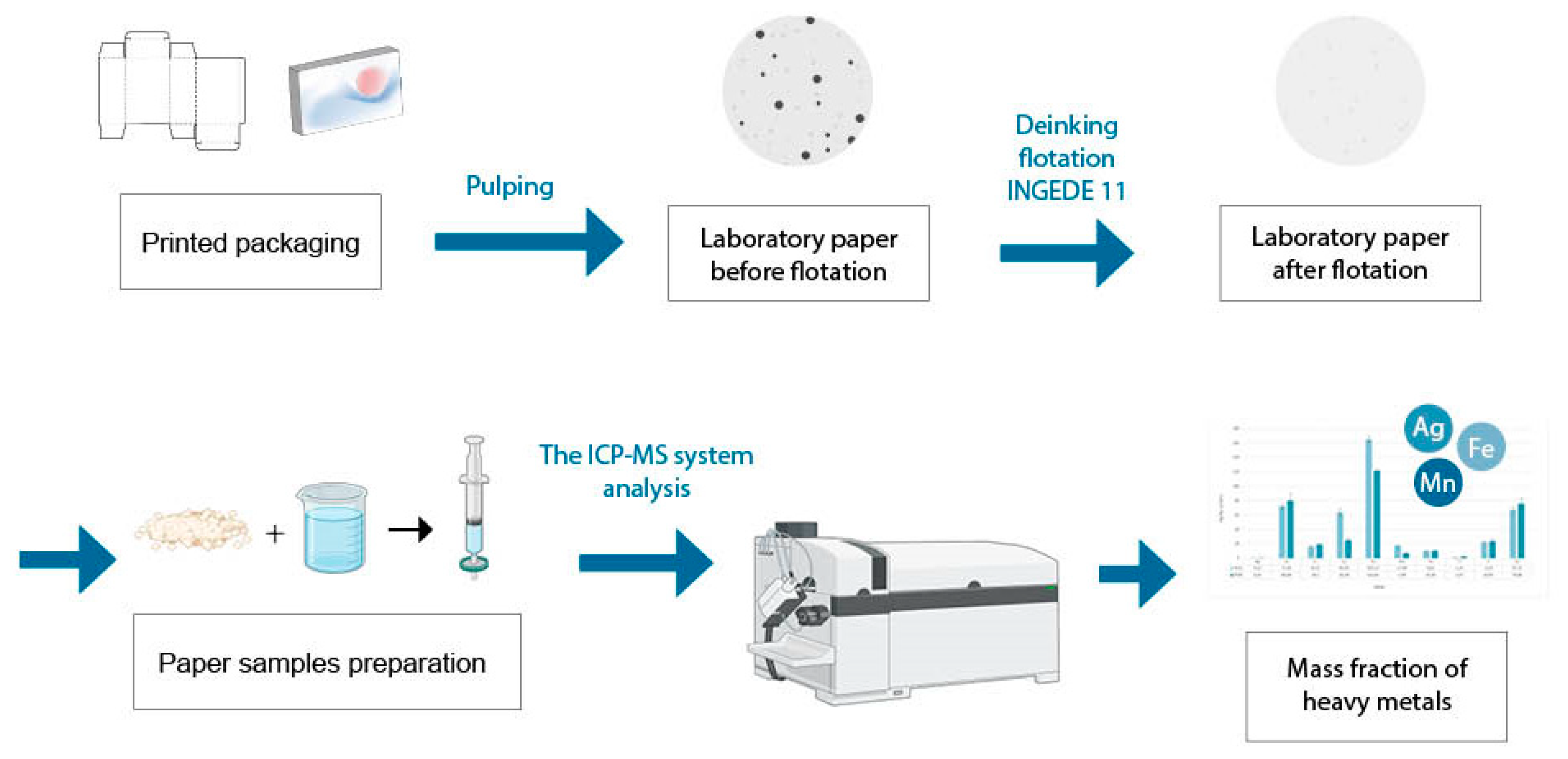
2. Materials and Methods
The ICP-MS Analysis
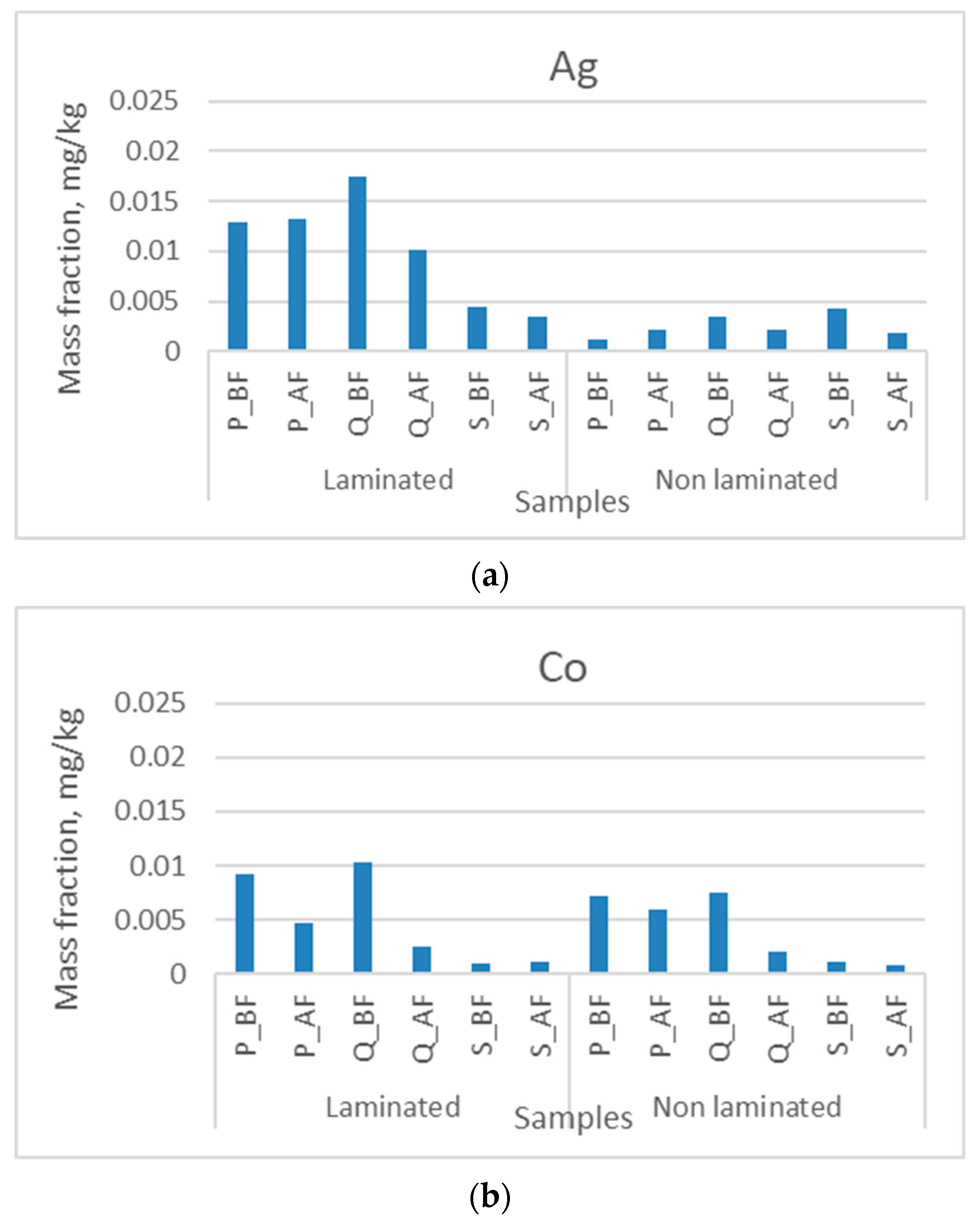
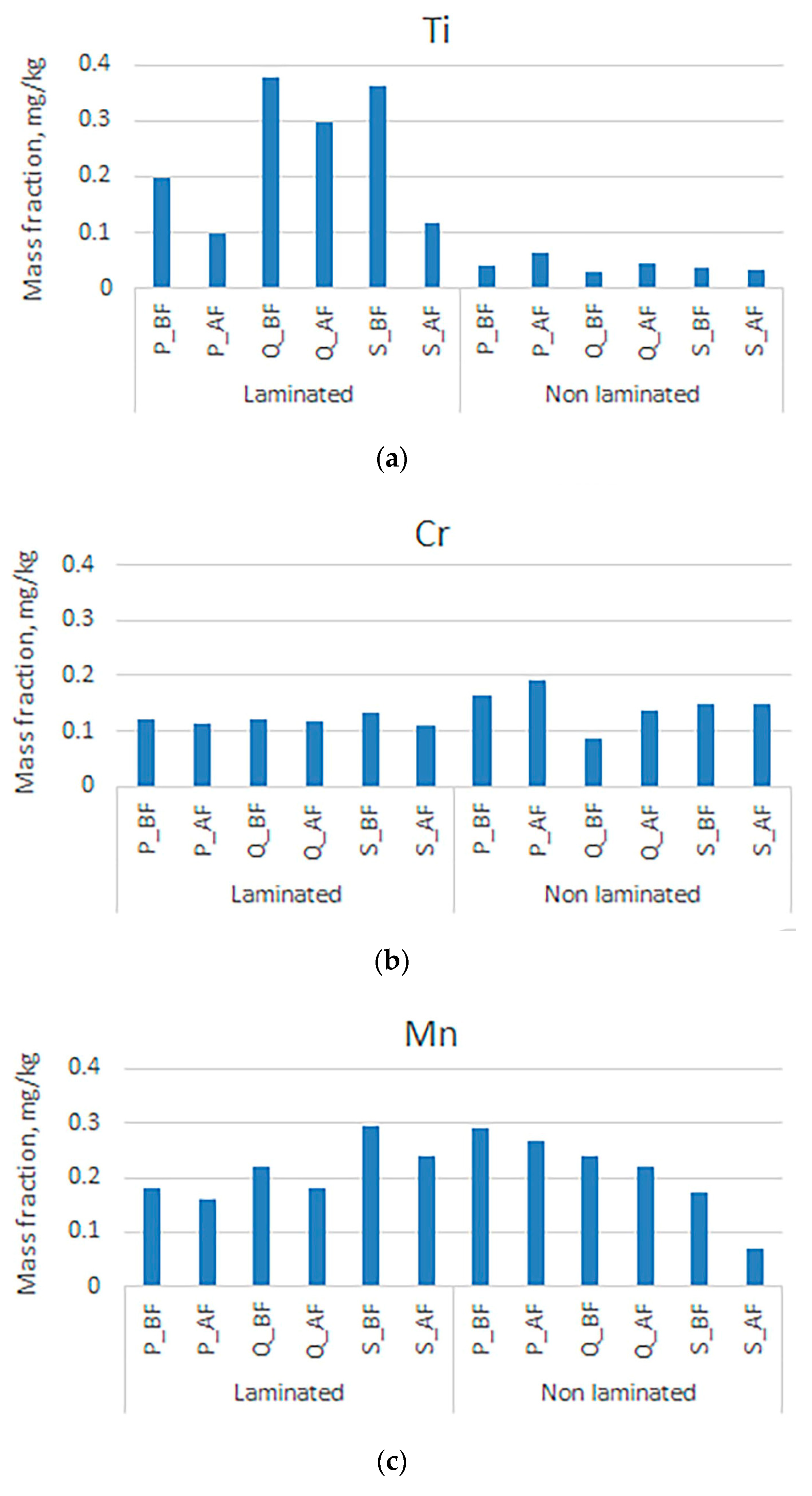
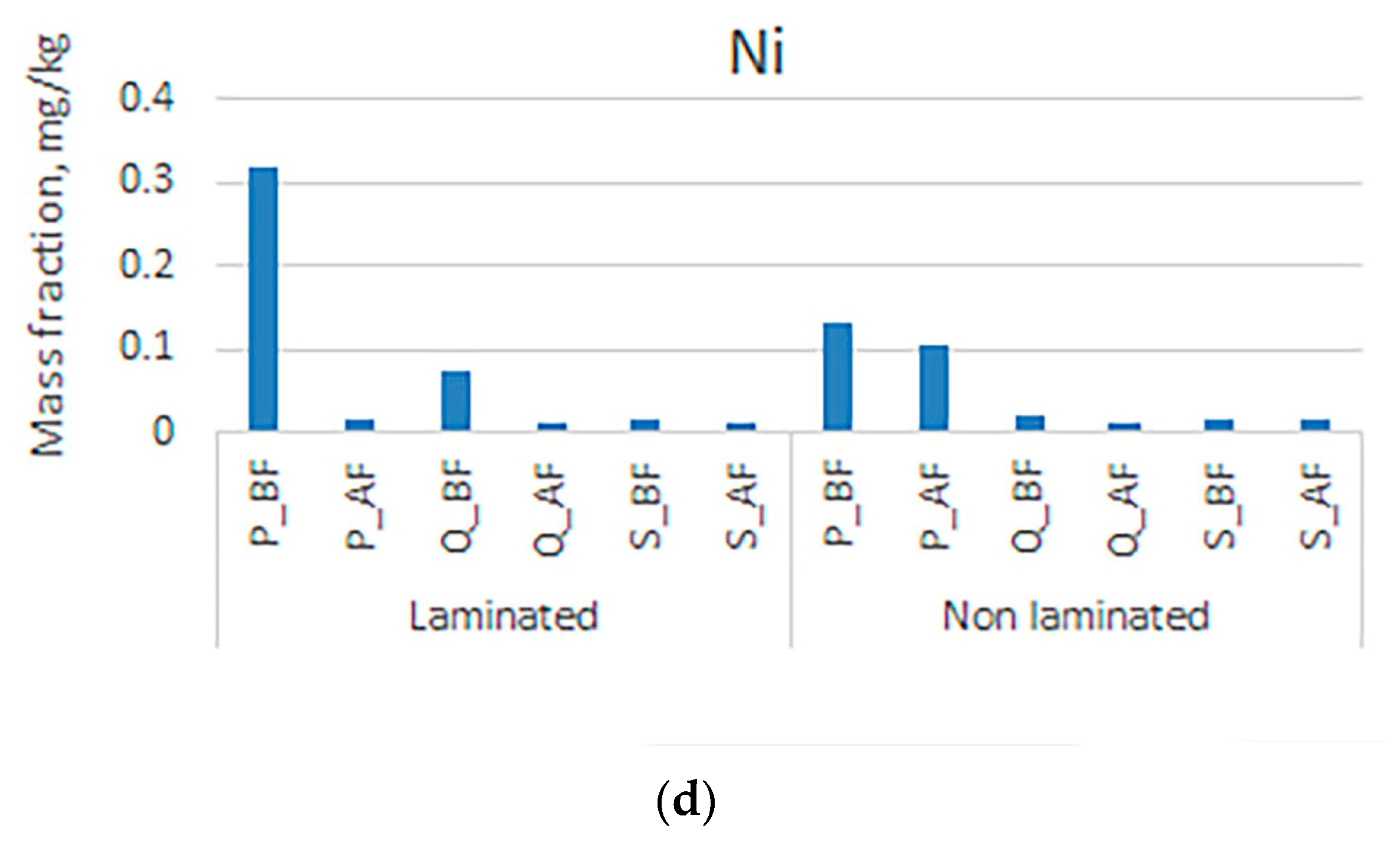
3. Results and Discussion
- Effective nuclear charge (Zeff)
- Actual nuclear charge (Z)
- Shielding constant (S)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, P.; Ji, X.; Zheng, B.; Wang, C.; Hao, W.; Han, W.; Zhang, J.; Xia, G.; Ji, X.; Zhang, J. Eco-Friendly and Complete Recycling of Waste Bamboo-Based Disposable Paper Cups for Value-Added Transparent Cellulose-Based Films and Paper Plastic Composites. Polymers 2022, 14, 1589. [Google Scholar] [CrossRef] [PubMed]
- Venkatarajan, S.; Subbu, C.; Athijayamani, A.; Muthuraja, R. Mechanical Properties of Natural Cellulose Fibers Reinforced Polymer Composites—2015–2020: A Review. Mater. Today Proc. 2021, 47, 1017–1024. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and Sustainable Pretreatment Methods for Cellulose Extraction from Lignocellulosic Biomass and Its Applications: A Review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Huang, D.; Hong, H.; Huang, W.; Zhang, H.; Hong, X. Scalable Preparation of Cellulose Nanofibers from Office Waste Paper by an Environment-Friendly Method. Polymers 2021, 13, 3119. [Google Scholar] [CrossRef]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Lipponen, J.; Ojanen, M.; Puttonen, S.; Vaittinen, H. Trends and Challenges in the Development of Bio-Based Barrier Coating Materials for Paper/Cardboard Food Packaging; a Review. Sci. Total Environ. 2022, 851, 158328. [Google Scholar] [CrossRef] [PubMed]
- Dislaire, C.; Seantier, B.; Muzy, M.; Grohens, Y. Mechanical and Hygroscopic Properties of Molded Pulp Products Using Different Wood-Based Cellulose Fibers. Polymers 2021, 13, 3225. [Google Scholar] [CrossRef]
- de Oliveira, D.M.; de Bomfim, A.S.C.; Benini, K.C.C.d.C.; Cioffi, M.O.H.; Voorwald, H.J.C.; Rodrigue, D. Waste Paper as a Valuable Resource: An Overview of Recent Trends in the Polymeric Composites Field. Polymers 2023, 15, 426. [Google Scholar] [CrossRef]
- Łątka, J.F.; Jasiołek, A.; Karolak, A.; Niewiadomski, P.; Noszczyk, P.; Klimek, A.; Zielińska, S.; Misiurka, S.; Jezierska, D. Properties of Paper-Based Products as a Building Material in Architecture—An Interdisciplinary Review. J. Build. Eng. 2022, 50, 104135. [Google Scholar] [CrossRef]
- Deshwal, G.K.; Panjagari, N.R.; Alam, T. An Overview of Paper and Paper Based Food Packaging Materials: Health Safety and Environmental Concerns. J. Food Sci. Technol. 2019, 56, 4391–4403. [Google Scholar] [CrossRef]
- Guan, Y.; Li, W.; Gao, H.; Zhang, L.; Zhou, L.; Peng, F. Preparation of Cellulose Nanocrystals from Deinked Waste Newspaper and Their Usage for Papermaking. Carbohydr. Polym. Technol. Appl. 2021, 2, 100107. [Google Scholar] [CrossRef]
- Vukoje, M. The Influence of Adhesion Parameters on Material and Organic Recycling of Thermochromic Prints. Ph.D. Thesis, Sveučilište u Zagrebu Grafički fakultet, Zagreb, Croatia, 2018. [Google Scholar]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A Review of Cellulose and Its Derivatives in Biopolymer-Based for Food Packaging Application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Muncke, J.; Myers, J.; Scheringer, M.; Porta, M. Food Packaging and Migration of Food Contact Materials: Will Epidemiologists Rise to the Neotoxic Challenge? J. Epidemiol. Community Health 2014, 68, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Trier, X.; Granby, K.; Christensen, J.H. Polyfluorinated Surfactants (PFS) in Paper and Board Coatings for Food Packaging. Environ. Sci. Pollut. Res. Int. 2011, 18, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, M.; Grob, K. Assurance of Safety of Recycled Paperboard for Food Packaging through Comprehensive Analysis of Potential Migrants Is Unrealistic. J. Chromatogr. A 2013, 1293, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Tang, Y.; Liu, J.; Wang, J.; Ye, C. Nanofibrillated Cellulose as Coating Agent for Food Packaging Paper. Int. J. Biol. Macromol. 2021, 168, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xu, H.; Julian McClements, D.; Chen, L.; Jiao, A.; Tian, Y.; Miao, M.; Jin, Z. Recent Advances in Intelligent Food Packaging Materials: Principles, Preparation and Applications. Food Chem. 2022, 375, 131738. [Google Scholar] [CrossRef]
- Weligama Thuppahige, V.T.; Karim, M.A. A Comprehensive Review on the Properties and Functionalities of Biodegradable and Semibiodegradable Food Packaging Materials. Compr. Rev. Food Sci. Food Saf. 2022, 21, 689–718. [Google Scholar] [CrossRef]
- Bernhardt, E.S.; Rosi, E.J.; Gessner, M.O. Synthetic Chemicals as Agents of Global Change. Front. Ecol. Environ. 2017, 15, 84–90. [Google Scholar] [CrossRef]
- Grandjean, P.; Bellanger, M. Calculation of the Disease Burden Associated with Environmental Chemical Exposures: Application of Toxicological Information in Health Economic Estimation. Environ. Health 2017, 16, 123. [Google Scholar] [CrossRef]
- Geueke, B.; Groh, K.; Muncke, J. Food Packaging in the Circular Economy: Overview of Chemical Safety Aspects for Commonly Used Materials. J. Clean. Prod. 2018, 193, 491–505. [Google Scholar] [CrossRef]
- Ginebreda, A.; Guillén, D.; Barceló, D.; Darbra, R.M. Additives in the Paper Industry. Handb. Environ. Chem. 2012, 18, 11–34. [Google Scholar]
- Muncke, J.; Andersson, A.M.; Backhaus, T.; Boucher, J.M.; Carney Almroth, B.; Castillo Castillo, A.; Chevrier, J.; Demeneix, B.A.; Emmanuel, J.A.; Fini, J.B.; et al. Impacts of Food Contact Chemicals on Human Health: A Consensus Statement. Environ. Health 2020, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Mertoğlu-Elmas, G.; Çınar, G. Toxic Metals in Paper and Paperboard Food Packagings. Bioresources 2018, 13, 7560–7580. [Google Scholar] [CrossRef]
- Osmond, G. Zinc White and the Influence of Paint Composition for Stability in Oil Based Media. In Issues in Contemporary Oil Paint; Springer: Berlin/Heidelberg, Germany, 2014; pp. 263–281. [Google Scholar] [CrossRef]
- Ahmed, H.A.M.; Gouhar, A.S.; Janjua, M.N.; Alhafez, N. Estimation of Some Heavy Metals Contamination in Waste Newspapers. Environ. Monit. Assess. 2022, 194, 711. [Google Scholar] [CrossRef] [PubMed]
- Mertoglu-Elmas, G. The Effect of Colorants on the Content of Heavy Metals in Recycled Corrugated Board Papers. Bioresources 2017, 12, 2690–2698. [Google Scholar] [CrossRef][Green Version]
- Kim, K.C.; Park, Y.B.; Lee, M.J.; Kim, J.B.; Huh, J.W.; Kim, D.H.; Lee, J.B.; Kim, J.C. Levels of Heavy Metals in Candy Packages and Candies Likely to Be Consumed by Small Children. Food Res. Int. 2008, 41, 411–418. [Google Scholar] [CrossRef]
- Mohammadpour, I.; Ahmadkhaniha, R.; Zare Jeddi, M.; Rastkari, N. Heavy Metals in Recycled Pastry Packages and Pastries. Acta Aliment. 2016, 45, 509–514. [Google Scholar] [CrossRef]
- Sood, S.; Sharma, C. Levels of Selected Heavy Metals in Food Packaging Papers and Paperboards Used in India. J. Environ. Prot. 2019, 10, 360–368. [Google Scholar] [CrossRef]
- Chang, H.C.; Chen, Y.J.; Chang, M.H.; Liao, C.D.; Kao, Y.M.; Wang, D.Y.; Cheng, H.F. Novel Multi-Analyte Method for Detection of Thirty Photoinitiators in Breakfast Cereal and Packaged Juice. J. Chromatogr. B 2019, 1130–1131, 121788. [Google Scholar] [CrossRef]
- Tanase, I.G.; Udristioiu, F.M.; Bunaciu, A.A.; Aboul-Enein, H.Y. Trace Elements Analysis in Paper Using Inductively Coupled Plasma-Mass Spectrometry (ICP–MS). Gazi Univ. J. Sci. 2012, 25, 843–851. [Google Scholar]
- Peters, R.J.B.; Groeneveld, I.; Sanchez, P.L.; Gebbink, W.; Gersen, A.; de Nijs, M.; van Leeuwen, S.P.J. Review of Analytical Approaches for the Identification of Non-Intentionally Added Substances in Paper and Board Food Contact Materials. Trends Food Sci. Technol. 2019, 85, 44–54. [Google Scholar] [CrossRef]
- Biedermann, M.; Grob, K. Advantages of Comprehensive Two-Dimensional Gas Chromatography for Comprehensive Analysis of Potential Migrants from Food Contact Materials. Anal. Chim. Acta 2019, 1057, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Leeman, W.; Krul, L. Non-Intentionally Added Substances in Food Contact Materials: How to Ensure Consumer Safety. Curr. Opin. Food Sci. 2015, 6, 33–37. [Google Scholar] [CrossRef]
- Geueke, B. Dossier—Non-Intentionally Added Substances (NIAS). Food Packag. Forum 2018, 7, 1265331. [Google Scholar] [CrossRef]
- Rennen, M.A.J.; Koster, S.; Krul, C.A.M.; Houben, G.F. Application of the Threshold of Toxicological Concern (TTC) Concept to the Safety Assessment of Chemically Complex Food Matrices. Food Chem. Toxicol. 2011, 49, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Koster, S.; Rennen, M.; Leeman, W.; Houben, G.; Muilwijk, B.; van Acker, F.; Krul, L. A Novel Safety Assessment Strategy for Non-Intentionally Added Substances (NIAS) in Carton Food Contact Materials. Food Addit. Contam. Part A 2014, 31, 422–443. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Zubiaguirre, L.; Zabaleta, I.; Usobiaga, A.; Prieto, A.; Olivares, M.; Zuloaga, O.; Elizalde, M.P. Target and Suspect Screening of Substances Liable to Migrate from Food Contact Paper and Cardboard Materials Using Liquid Chromatography-High Resolution Tandem Mass Spectrometry. Talanta 2020, 208, 120394. [Google Scholar] [CrossRef] [PubMed]
- Rusko, J.; Perkons, I.; Rasinger, J.D.; Bartkevics, V. Non-Target and Suspected-Target Screening for Potentially Hazardous Chemicals in Food Contact Materials: Investigation of Paper Straws. Food Addit. Contam. Part A 2020, 37, 649–664. [Google Scholar] [CrossRef]
- Szulejko, J.E.; Kim, Y.-H.; Kim, K.-H. Method to Predict Gas Chromatographic Response Factors for the Trace-Level Analysis of Volatile Organic Compounds Based on the Effective Carbon Number Concept. J. Sep. Sci. 2013, 36, 3356–3365. [Google Scholar] [CrossRef]
- Aparicio, J.; Elizalde, M. Migration of Photoinitiators in Food Packaging: A Review. Packag. Technol. Sci. 2014, 28, 181–203. [Google Scholar] [CrossRef]
- Sapozhnikova, Y. Non-Targeted Screening of Chemicals Migrating from Paper-Based Food Packaging by GC-Orbitrap Mass Spectrometry. Talanta 2021, 226, 122120. [Google Scholar] [CrossRef] [PubMed]
- Suciu, N.A.; Tiberto, F.; Vasileiadis, S.; Lamastra, L.; Trevisan, M. Recycled Paper–Paperboard for Food Contact Materials: Contaminants Suspected and Migration into Foods and Food Simulant. Food Chem. 2013, 141, 4146–4151. [Google Scholar] [CrossRef]
- Xue, M.; Chai, X.S.; Li, X.; Chen, R. Migration of Organic Contaminants into Dry Powdered Food in Paper Packaging Materials and the Influencing Factors. J. Food Eng. 2019, 262, 75–82. [Google Scholar] [CrossRef]
- Zülch, A.; Piringer, O. Measurement and Modelling of Migration from Paper and Board into Foodstuffs and Dry Food Simulants. Food Addit. Contam. Part A 2010, 27, 1306–1324. [Google Scholar] [CrossRef]
- Directive—94/62—EN–EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A31994L0062 (accessed on 24 February 2024).
- Prop 65 Compliance. Available online: https://www.z2data.com/landing/lpa/prop-65-compliance?utm_source=Google&utm_medium=PPC&utm_campaign=20103637910&utm_term=california%20prop%2065&gad_source=1&gclid=Cj0KCQiAxOauBhCaARIsAEbUSQRtTikRX2lHLSuJrLQ2SUptXp8ex0Q4iVIskMsJz7KKH4idUsNWtkUaArshEALw_wcB (accessed on 24 February 2024).
- (PDF) Survey of Alkylphenols and Alkylphenol Ethoxylates. Part of the LOUS-Review. Available online: https://www.researchgate.net/publication/299235359_Survey_of_alkylphenols_and_alkylphenol_ethoxylates_Part_of_the_LOUS-review (accessed on 25 November 2023).
- Regulation—1935/2004—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32004R1935 (accessed on 24 February 2024).
- Regulation—10/2011—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32011R0010 (accessed on 24 February 2024).
- ISO 5263-2:2004; Pulps—Laboratory Wet Disintegration—Part 2: Disintegration of Mechanical Pulps at 20 Degrees C. ISO: Geneve, Switzerland, 2004. Available online: https://www.iso.org/standard/37894.html (accessed on 8 November 2023).
- INGEDE (2018): International Association of the Deinking Industry; INGEDE Method 11p—Assessment of Print Product Recyclability—Deinkability Test. 2018. Available online: https://ingede.com/ingindxe/methods/ingede-method-11-2018.pdf (accessed on 24 February 2024).
- INGEDE (2014): International Association of the Deinking Industry; INGEDE Method 1—Test Sheet Preparation of Pulps and Filtrates from Deinking Processes. 2014. Available online: https://ingede.com/ingindxe/methods/ingede-method-01-2014.pdf (accessed on 24 February 2024).
- ISO 5269-2:2004; Pulps—Preparation of Laboratory Sheets for Physical Testing—Part 2: Rapid-Köthen Method. ISO: Geneve, Switzerland, 2004. Available online: https://www.iso.org/obp/ui/#iso:std:iso:5269:-2:ed-3:v1:en (accessed on 18 January 2023).
- A Beginner’s Guide to ICP-MS, Mass Spectrometry Basics|Agilent. Available online: https://www.agilent.com/en/product/atomic-spectroscopy/inductively-coupled-plasma-mass-spectrometry-icp-ms/what-is-icp-ms-icp-ms-faqs (accessed on 19 March 2024).
- Subedi, K.; Trejos, T.; Almirall, J. Forensic Analysis of Printing Inks Using Tandem Laser Induced Breakdown Spectroscopy and Laser Ablation Inductively Coupled Plasma Mass Spectrometry. Spectrochim. Acta Part B Spectrosc. 2015, 103–104, 76–83. [Google Scholar] [CrossRef]
- Tanase, I.G.; Popa, D.E.; Udriştioiu, G.E.; Bunaciu, A.A.; Aboul-Enein, H.Y. Estimation of the Uncertainty of the Measurement Results of Some Trace Levels Elements in Document Paper Samples Using ICP-MS. RSC Adv. 2015, 5, 11445–11457. [Google Scholar] [CrossRef]
- Mirkovic, I.B.; Majnaric, I.; Bolanca, Z. Ecological Sustainability and Waste Paper Recycling. Procedia Eng. 2015, 100, 177–186. [Google Scholar] [CrossRef]
- Vukoje, M.; Bolanča Mirković, I.; Bolanča, Z. Influence of Printing Technique and Printing Conditions on Prints Recycling Efficiency and Effluents Quality. Sustainability 2022, 14, 335. [Google Scholar] [CrossRef]
- Vukoje, M.; Mirković, I.B.; Bešlić, M.; Petković, G. The Influence of Artificial Aging on Recyclability and Mechanical Stability of Pharmaceutical Packaging. In Proceedings of the 10th International Symposium on Graphic Engineering and Design, Novi Sad, Serbia, 12–14 November 2020; pp. 251–259. [Google Scholar] [CrossRef]
- Jamnicki, S.; Serra, M.À.P.; Lozo, B.; Stanić, M.; Barušič, L. Deinking Flotation of Recycled Linerboard for Food Packaging Applications. Cellul. Chem. Technol. 2010, 44, 481–488. [Google Scholar]
- Faul, A.M. Quality Requirements in Graphic Paper Recycling. Cellul. Chem. Technol. 2010, 44, 451–460. [Google Scholar]
- Runte, S.; Putz, H.J.; Bussini, D.; Limongi, L.; Elegir, G. Recyclability Criteria for Paper Based Packaging Products. Cellul. Chem. Technol. 2015, 49, 667–676. [Google Scholar]
- (PDF) Roles of Surfactants in Flotation Deinking. Available online: https://www.researchgate.net/publication/228852362_Roles_of_surfactants_in_flotation_deinking (accessed on 24 February 2024).
- Deinking Chemistry: Part 1. Available online: https://www.researchgate.net/publication/281477434_Deinking_chemistry_Part_1 (accessed on 24 February 2024).
- Yokoyama, H.; Katoh, N.; Hotta, T.; Naito, M.; Hirano, S.I. Effect of Ink Properties of Titanium Nitride for Ink Jet Printing. J. Ceram. Soc. Jpn. 2003, 111, 262–266. [Google Scholar] [CrossRef]
- Vidal, E.; Torres, D.; Guillem-Marti, J.; Scionti, G.; Manero, J.M.; Ginebra, M.P.; Rodríguez, D.; Rupérez, E. Titanium Scaffolds by Direct Ink Writing: Fabrication and Functionalization to Guide Osteoblast Behavior. Metals 2020, 10, 1156. [Google Scholar] [CrossRef]
- Klemenčić, M.; Bolanča Mirković, I.; Bolf, N. The Influence of the Production Stages of Cardboard Pharmaceutical Packaging on the Circular Economy. Sustainability 2023, 15, 16882. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Li, Y.; Li, H.; Wang, W.; Ye, B. Impacts of Lead/Zinc Mining and Smelting on the Environment and Human Health in China. Environ. Monit. Assess. 2012, 184, 2261–2273. [Google Scholar] [CrossRef]
- Conti, M.E.; Boccacci Mariani, M.; Milana, M.R.; Gramiccioni, L. Heavy Metals and Optical Whitenings as Quality Parameters of Recycled Paper for Food Packaging. J. Food Process. Preserv. 1996, 20, 1–11. [Google Scholar] [CrossRef]


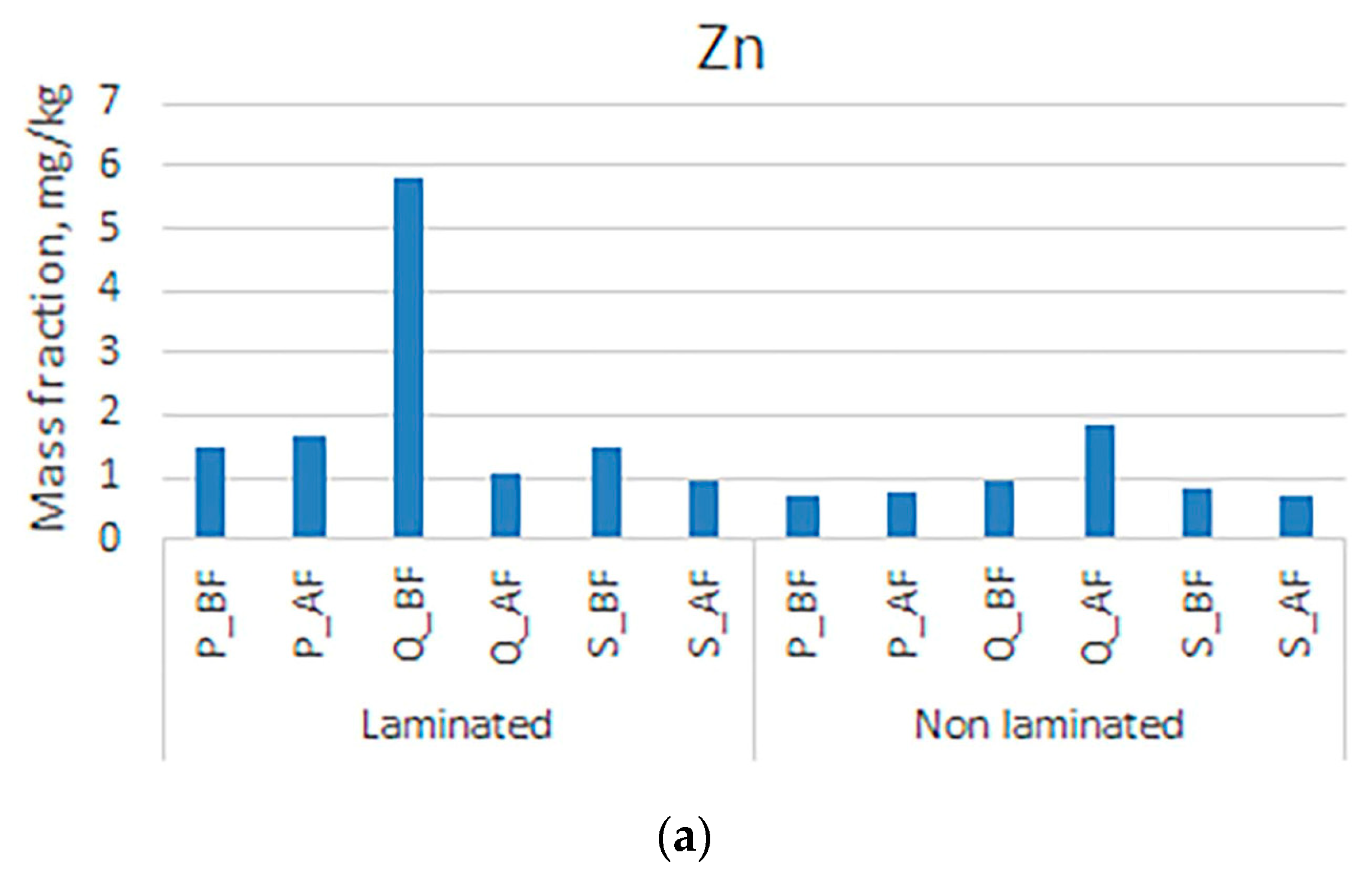
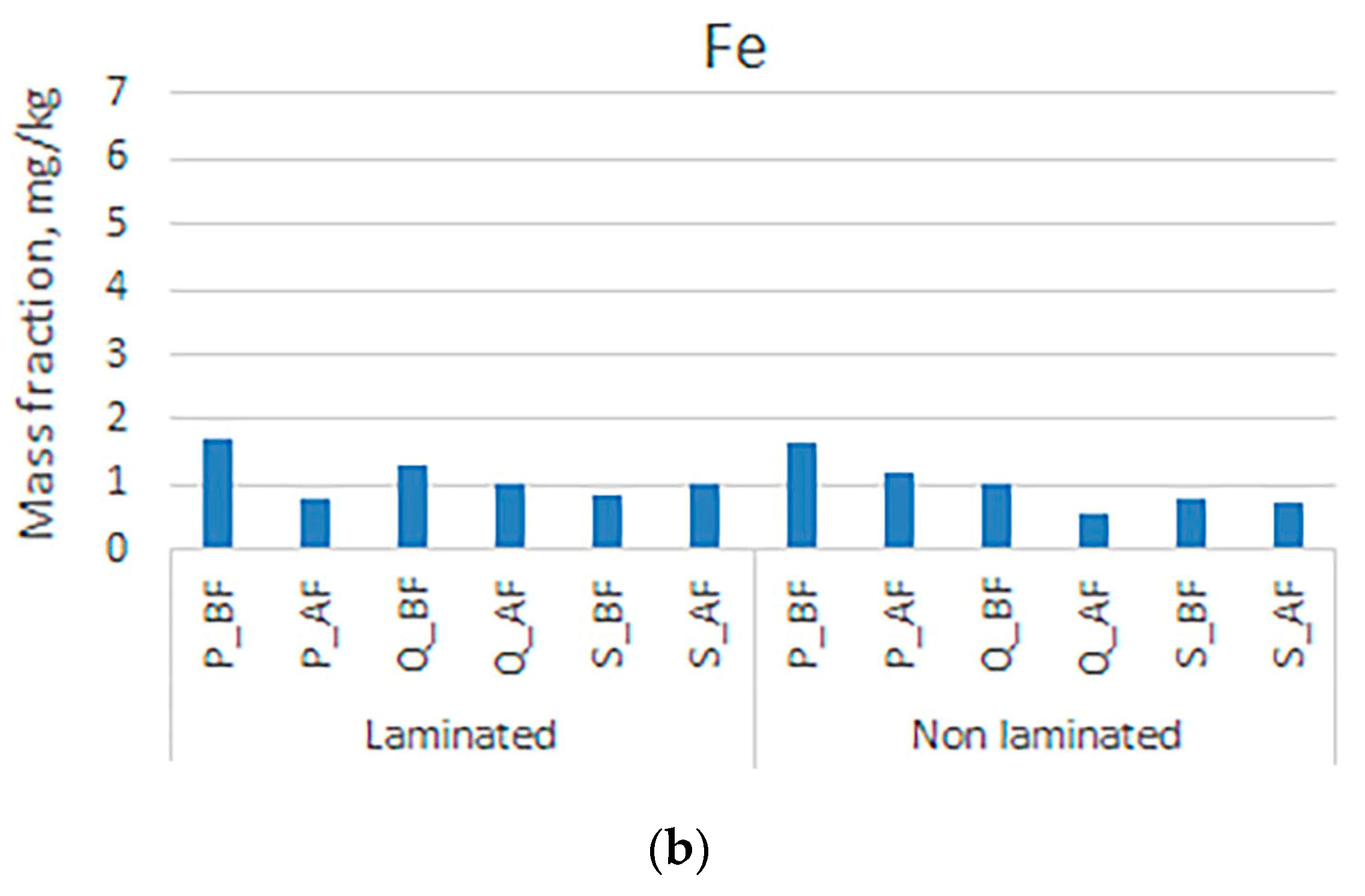
| Symbol | Sample |
|---|---|
| P_BF | printed packaging before flotation |
| P_AF | printed packaging after flotation |
| Q_BF | printed quire before flotation |
| Q_AF | printed quire after flotation |
| S_BF | printing substrate before flotation |
| S_AF | printing substrate after flotation |
| Parameters | Working Conditions |
|---|---|
| Spray gas flow rate | 0.85 L/min |
| Auxiliary gas flow rate | 1.2 L/min |
| Plasma flow rate | 14 L/min; |
| Lens Voltage | 8.5 V; ICP RF |
| Power supply | 1100 W; CeO/Ce = 0.016; Ba++/Ba+ = 0.015 |
| Ag | Co | Cu | Cr | Ni | Fe | Mn | Ti | V | Zn | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Printed packaging before flotation | SD | 0.031 | 0.125 | 0.025 | 0.125 | 0.025 | 0.045 | 0.035 | 0.025 | 0.055 | 0.125 |
| σ2 | 0.025 | 0.101 | 0.001 | 0.081 | 0.001 | 0.031 | 0.021 | 0.001 | 0.041 | 0.078 | |
| Printed packaging after flotation | SD | 0.030 | 0.106 | 0.006 | 0.096 | 0.016 | 0.036 | 0.026 | 0.016 | 0.032 | 0.099 |
| σ2 | 0.021 | 0.101 | 0.001 | 0.071 | 0.011 | 0.021 | 0.021 | 0.011 | 0.029 | 0.077 | |
| Printed quire before flotation | SD | 0.020 | 0.085 | 0.015 | 0.055 | 0.025 | 0.025 | 0.025 | 0.025 | 0.025 | 0.085 |
| σ2 | 0.015 | 0.081 | 0.001 | 0.041 | 0.011 | 0.011 | 0.011 | 0.011 | 0.016 | 0.074 | |
| Printed quire after flotation | SD | 0.020 | 0.076 | 0.011 | 0.056 | 0.016 | 0.016 | 0.016 | 0.016 | 0.032 | 0.075 |
| σ2 | 0.015 | 0.061 | 0.001 | 0.041 | 0.011 | 0.011 | 0.011 | 0.011 | 0.016 | 0.068 | |
| Printed substrate before flotation | SD | 0.025 | 0.055 | 0.015 | 0.035 | 0.025 | 0.025 | 0.025 | 0.025 | 0.025 | 0.042 |
| σ2 | 0.015 | 0.031 | 0.001 | 0.021 | 0.009 | 0.011 | 0.011 | 0.011 | 0.011 | 0.031 | |
| Printed substrate after flotation | SD | 0.020 | 0.036 | 0.006 | 0.026 | 0.016 | 0.016 | 0.016 | 0.016 | 0.022 | 0.032 |
| σ2 | 0.011 | 0.021 | 0.001 | 0.015 | 0.011 | 0.011 | 0.011 | 0.011 | 0.011 | 0.016 |
| Ag | Co | Cu | Cr | Ni | Fe | Mn | Ti | V | Zn | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Printed packaging before flotation | SD | 0.025 | 0.089 | 0.025 | 0.095 | 0.025 | 0.035 | 0.025 | 0.025 | 0.045 | 0.093 |
| σ2 | 0.020 | 0.077 | 0.001 | 0.075 | 0.001 | 0.022 | 0.011 | 0.001 | 0.031 | 0.067 | |
| Printed packaging after flotation | SD | 0.025 | 0.086 | 0.006 | 0.066 | 0.016 | 0.026 | 0.016 | 0.006 | 0.022 | 0.073 |
| σ2 | 0.011 | 0.071 | 0.001 | 0.041 | 0.011 | 0.011 | 0.011 | 0.011 | 0.013 | 0.056 | |
| Printed quire before flotation | SD | 0.017 | 0.065 | 0.015 | 0.035 | 0.025 | 0.020 | 0.025 | 0.020 | 0.020 | 0.070 |
| σ2 | 0.011 | 0.041 | 0.001 | 0.021 | 0.010 | 0.005 | 0.001 | 0.001 | 0.011 | 0.055 | |
| Printed quire after flotation | SD | 0.016 | 0.056 | 0.010 | 0.045 | 0.015 | 0.010 | 0.010 | 0.010 | 0.021 | 0.062 |
| σ2 | 0.010 | 0.040 | 0.001 | 0.032 | 0.011 | 0.001 | 0.005 | 0.004 | 0.015 | 0.044 | |
| Printed substrate before flotation | SD | 0.020 | 0.050 | 0.015 | 0.030 | 0.021 | 0.015 | 0.020 | 0.020 | 0.015 | 0.035 |
| σ2 | 0.011 | 0.028 | 0.001 | 0.015 | 0.007 | 0.007 | 0.001 | 0.010 | 0.005 | 0.025 | |
| Printed substrate after flotation | SD | 0.020 | 0.030 | 0.004 | 0.020 | 0.010 | 0.011 | 0.010 | 0.010 | 0.015 | 0.025 |
| σ2 | 0.009 | 0.017 | 0.001 | 0.011 | 0.007 | 0.008 | 0.007 | 0.008 | 0.007 | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klemenčić, M.; Bolanča Mirković, I.; Bolf, N.; Markić, M. Determination of the Mass Fractions of the Heavy Metals in the Recycled Cellulose Pulp. Polymers 2024, 16, 934. https://doi.org/10.3390/polym16070934
Klemenčić M, Bolanča Mirković I, Bolf N, Markić M. Determination of the Mass Fractions of the Heavy Metals in the Recycled Cellulose Pulp. Polymers. 2024; 16(7):934. https://doi.org/10.3390/polym16070934
Chicago/Turabian StyleKlemenčić, Mia, Ivana Bolanča Mirković, Nenad Bolf, and Marinko Markić. 2024. "Determination of the Mass Fractions of the Heavy Metals in the Recycled Cellulose Pulp" Polymers 16, no. 7: 934. https://doi.org/10.3390/polym16070934
APA StyleKlemenčić, M., Bolanča Mirković, I., Bolf, N., & Markić, M. (2024). Determination of the Mass Fractions of the Heavy Metals in the Recycled Cellulose Pulp. Polymers, 16(7), 934. https://doi.org/10.3390/polym16070934






