Advance Analysis of the Obtained Recycled Materials from Used Disposable Surgical Masks
Abstract
1. Introduction
2. Materials and Methods
2.1. DSMs’ Recycling Process and Test Samples’ Production
2.2. Thermal and Mechanical Properties
2.2.1. Thermogravimetric Analysis and Differential Scanning Calorimetry Measurments
2.2.2. Tensile Test
2.2.3. Bending Test
2.2.4. Density Measurements
2.3. Surface Properties
2.3.1. Color Measurements
2.3.2. Contact Angle Measurements
2.3.3. Zeta Potential
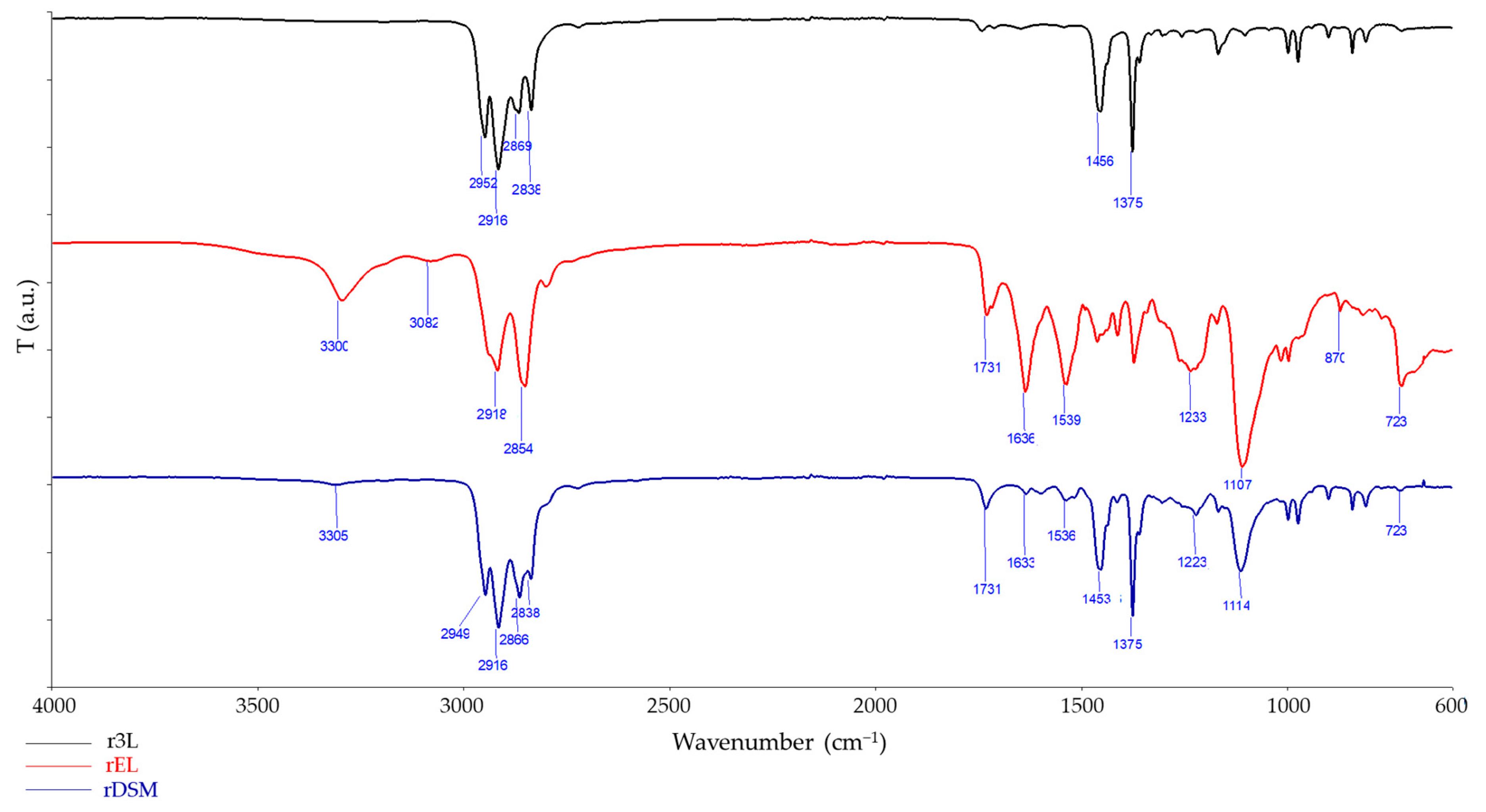
2.3.4. FTIR Spectroscopy and FTIR Microscopy Mapping
2.4. Morphology
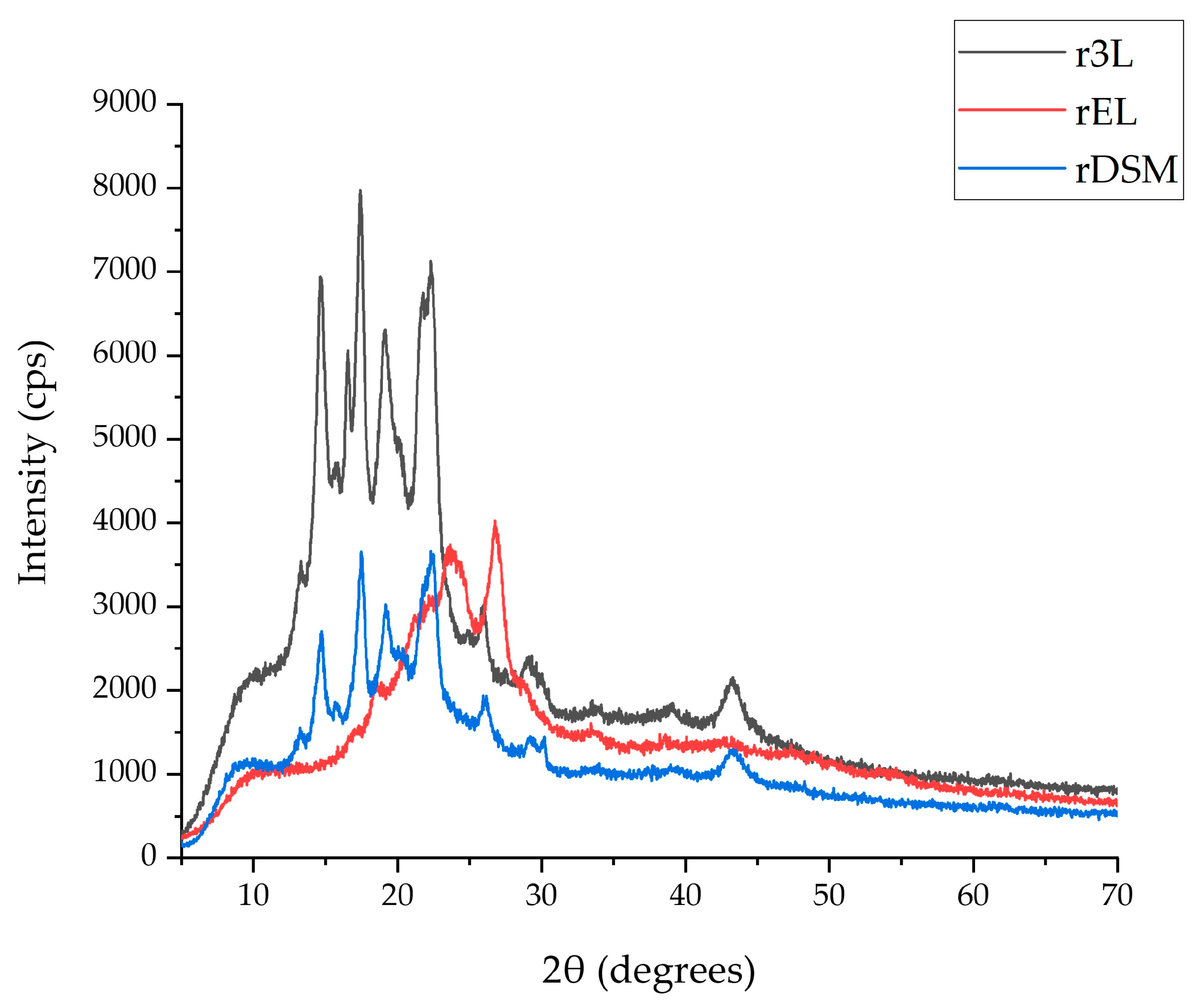
2.4.1. X-ray Diffraction
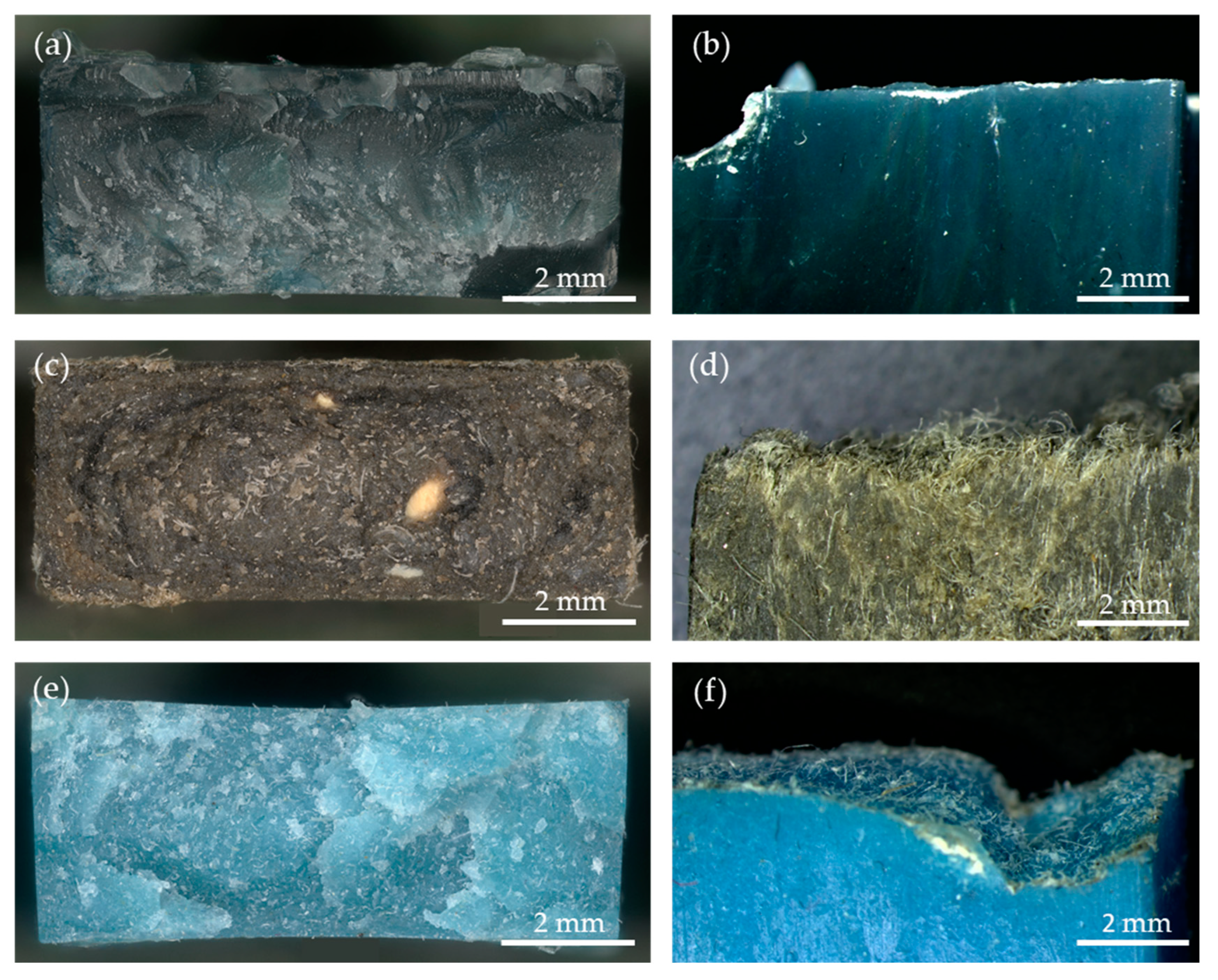
2.4.2. Stereomicroscopy
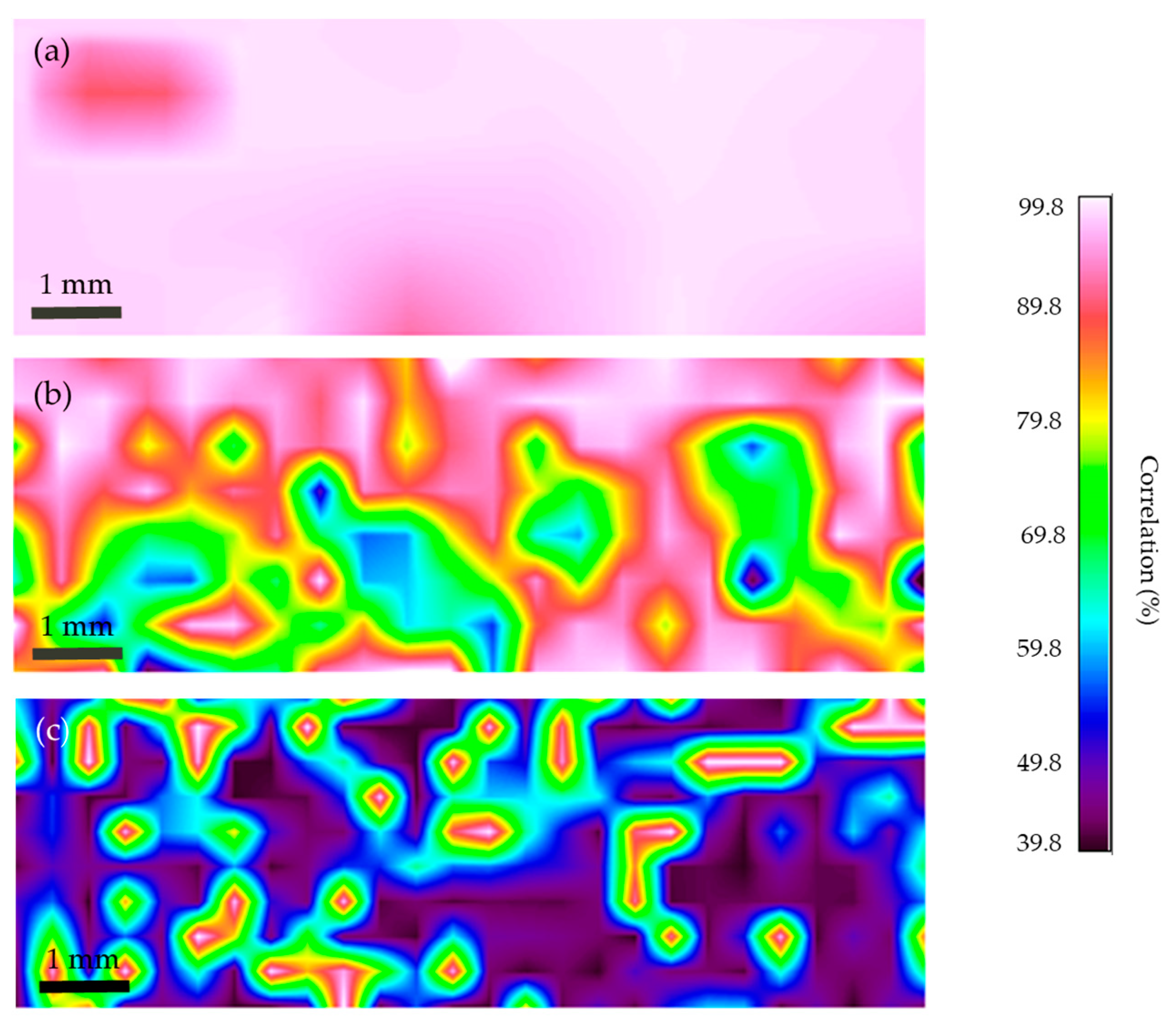
2.4.3. FTIR Microscopy Mapping of the Cross-Sections
3. Results
3.1. Optical Evaluation of Test Samples
3.2. Thermal and Mechanical Properties
3.2.1. Thermogravimetric Analysis and Differential Scanning Calorimetry
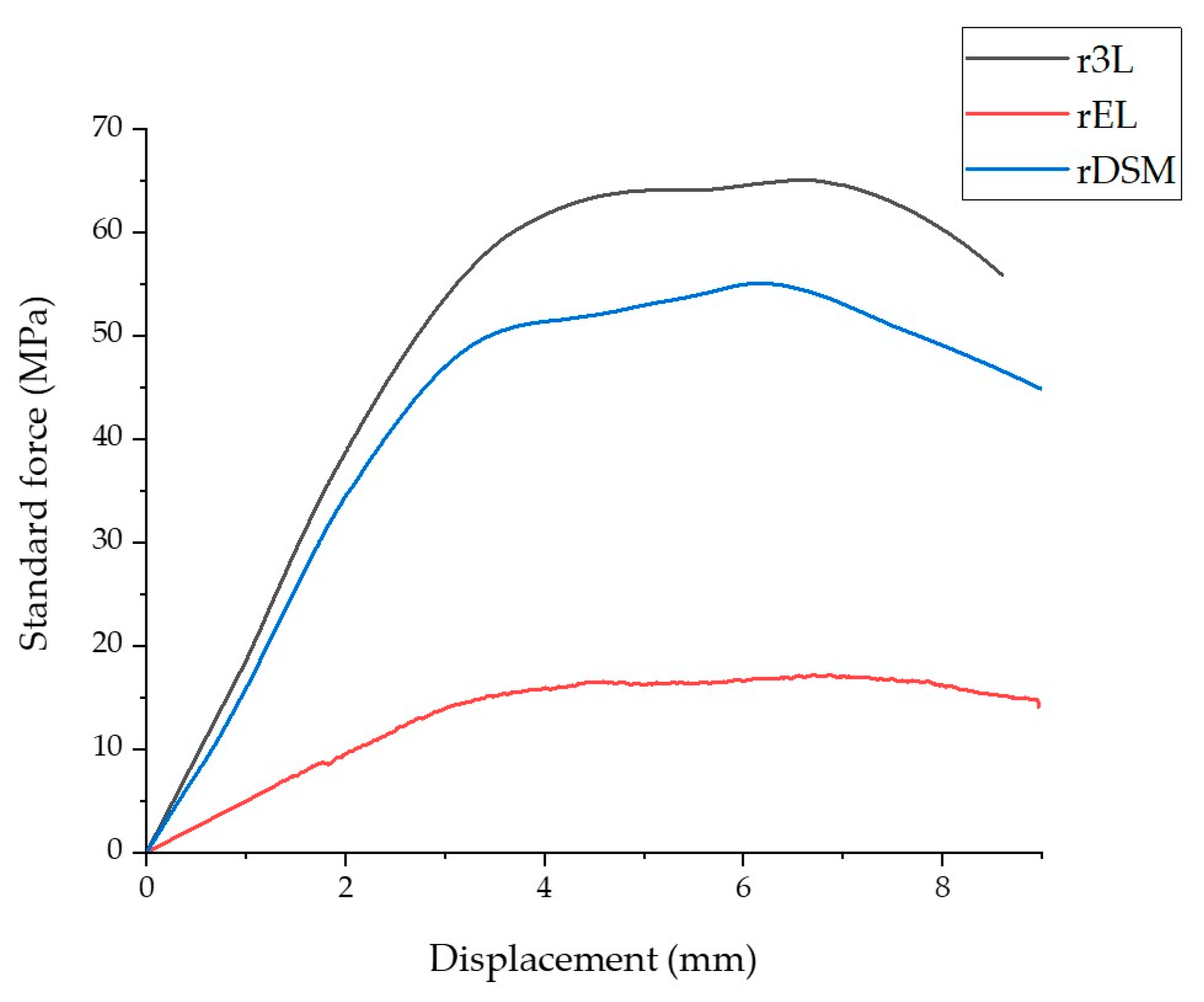
3.2.2. Tensile Test
3.2.3. Bending Test
3.2.4. Density Measurements
3.3. Surface Properties
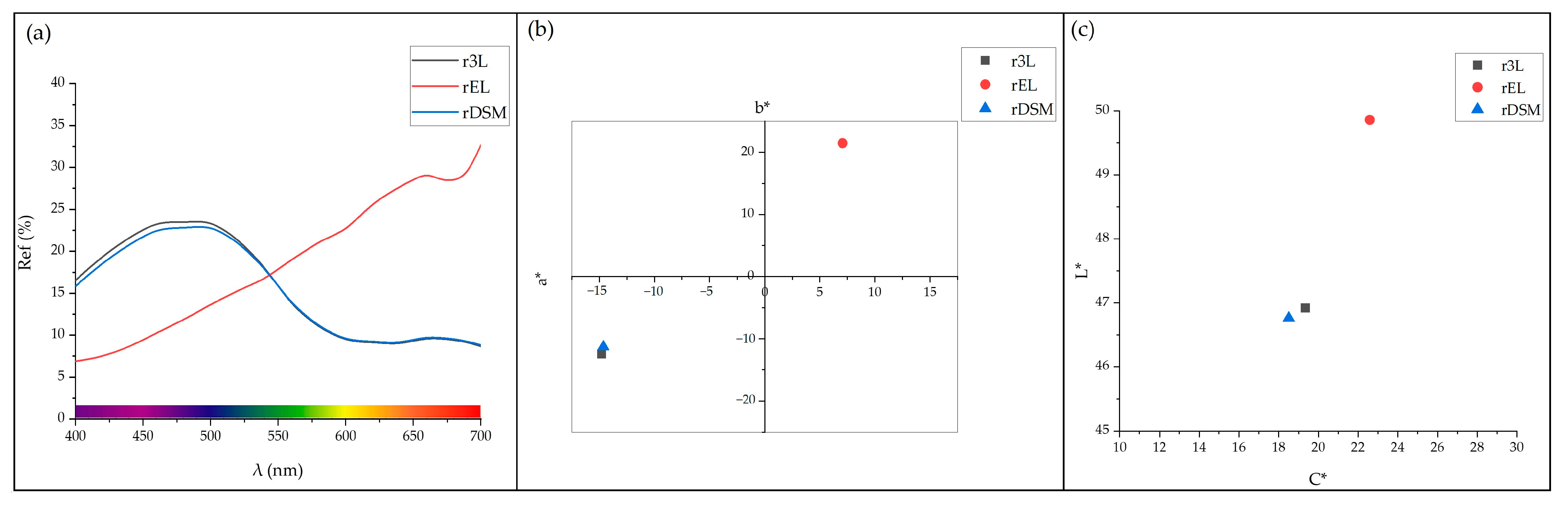
3.3.1. Colorimetric Analysis
3.3.2. Contact Angle Measurements
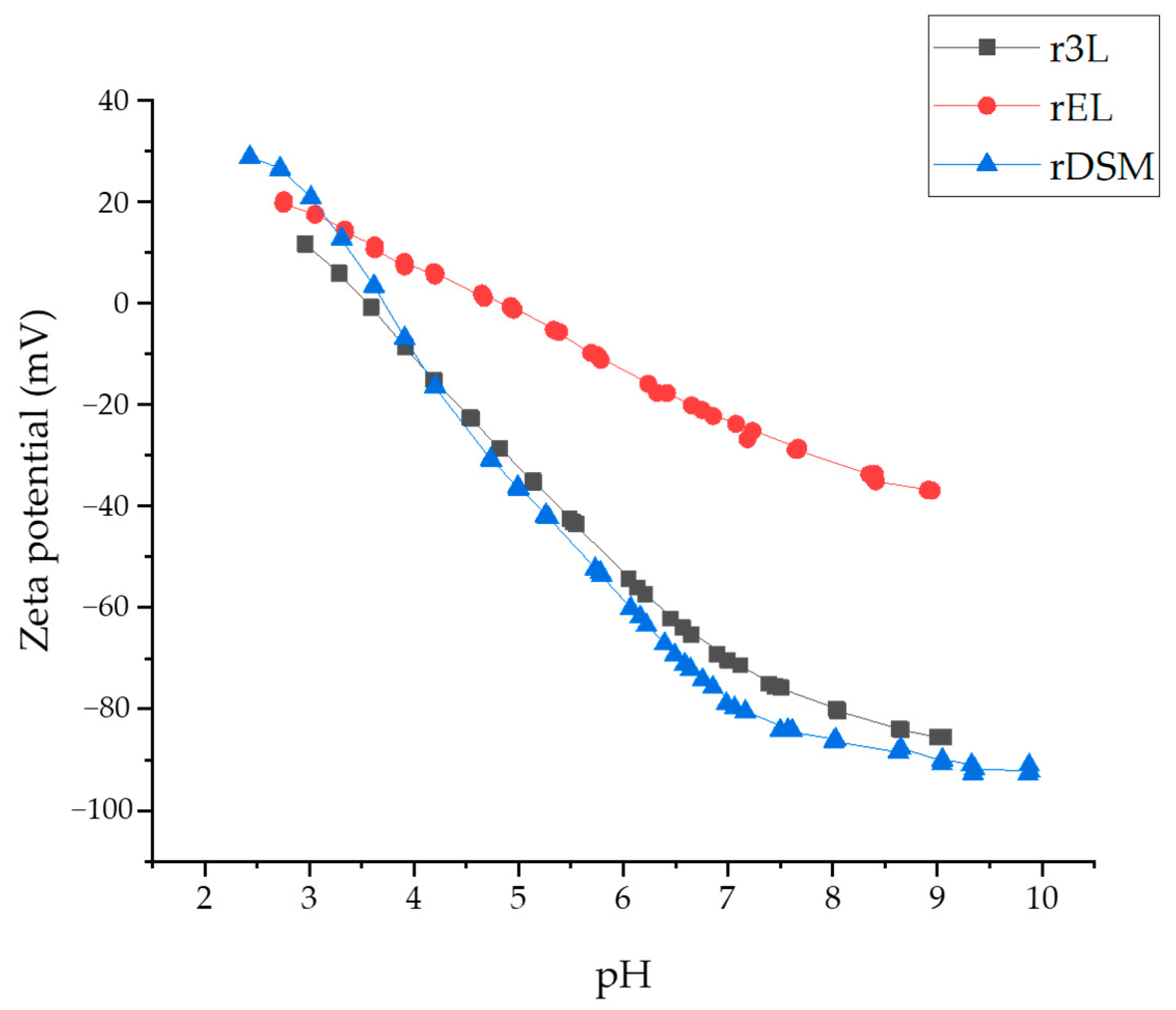
3.3.3. Zeta Potential
3.3.4. FTIR Spectroscopy and FTIR Microscopy Mapping
3.4. Morphology
3.4.1. X-ray Diffraction
3.4.2. Stereomicroscopy
3.4.3. FTIR Microscopy Mapping of the Cross-Sections
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiu, C.K.; Chan, C.Y.W.; Cheung, J.P.Y.; Cheung, P.W.H.; Gani, S.M.A.; Kwan, M.K. Personal protective equipment usage, recycling and disposal among spine surgeons: An Asia Pacific Spine Society survey. J. Orthop. Surg. 2021, 29, 2309499020988176. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Tang, Y.; Ogunseitan, O.A. Environmentally Sustainable Management of Used Personal Protective Equipment. Environ. Sci. Technol. 2020, 54, 8500–8502. [Google Scholar] [CrossRef]
- World Health Organization. Personal Protective Equipment, 4th ed.; World Health Organization: Geneva, Switzerland, 2020.
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—3 March 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- MarketsandMarkets. Market Reports. Personal Protective Equipment Market by Type (Hands & Arm Protection, Protective Clothing, Foot & Leg Protection, Respiratory Protection, Head Protection), End-Use Industry (Manufacturing, Construction, Oil & Gas, Healthcare)—Global Forecast to 2022; MarketsandMarkets: Pune, India, 2022. [Google Scholar]
- Grand View Research. Personal Protective Equipment (PPE) Market Size, Share & Trends Analysis Report by Product (Hand Protection, Eye Protection), by End-Use (Construction, Manufacturing), by Region, and Segment Forecasts, 2023–2030; Grand View Research: Francisco, CA, USA, 2023. [Google Scholar]
- Akber Abbasi, S.; Khalil, A.B.; Arslan, M. Extensive use of face masks during COVID-19 pandemic: (micro-)plastic pollution and potential health concerns in the Arabian Peninsula. Saudi J. Biol. Sci. 2020, 27, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.; Ambrose-Dempster, E.; Domenech, T.; Bawn, M.; Arredondo, M.; Chau, C.; Chandler, K.; Dobrijevic, D.; Hailes, H.; Lettieri, P.; et al. The environmental dangers of employing single-use face masks as part of a COVID-19 exit strategy. UCL Open Environ. 2020, 3, 1–43. [Google Scholar] [CrossRef]
- Czigany, T.; Ronkay, F. The coronavirus and plastics. Express Polym. Lett. 2020, 14, 510–511. [Google Scholar] [CrossRef]
- Erjavec, A.; Plohl, O.; Zemljič, L.F.; Valh, J.V. Significant Fragmentation of Disposable Surgical Masks—Enormous Source for Problematic Micro/Nanoplastics Pollution in the Environment. Sustainability 2022, 14, 12625. [Google Scholar] [CrossRef]
- Rathinamoorthy, R.; Balasaraswathi, S.R. Disposable tri-layer masks and microfiber pollution—An experimental analysis on dry and wet state emission. Sci. Total Environ. 2022, 816, 151562. [Google Scholar] [CrossRef] [PubMed]
- Ammendolia, J.; Saturno, J.; Brooks, A.L.; Jacobs, S.; Jambeck, J.R. An emerging source of plastic pollution: Environmental presence of plastic personal protective equipment (PPE) debris related to COVID-19 in a metropolitan city. Environ. Pollut. 2021, 269, 116160. [Google Scholar] [CrossRef] [PubMed]
- Aragaw, T.A. Surgical face masks as a potential source for microplastic pollution in the COVID-19 scenario. Mar. Pollut. Bull. 2020, 159, 111517. [Google Scholar] [CrossRef] [PubMed]
- Dharmaraj, S.; Ashokkumar, V.; Hariharan, S.; Manibharathi, A.; Show, P.L.; Chong, C.T.; Ngamcharussrivichai, C. The COVID-19 pandemic face mask waste: A blooming threat to the marine environment. Chemosphere 2021, 272, 129601. [Google Scholar] [CrossRef] [PubMed]
- Battegazzore, D.; Cravero, F.; Bernagozzi, G.; Frache, A. Designing a 3D printable polypropylene-based material from after use recycled disposable masks. Mater. Today Commun. 2022, 32, 103997. [Google Scholar] [CrossRef]
- Battegazzore, D.; Cravero, F.; Frache, A. Is it Possible to Mechanical Recycle the Materials of the Disposable Filtering Masks? Polymers 2020, 12, 2726. [Google Scholar] [CrossRef] [PubMed]
- Bratovcic, A. Available Recycling Solutions for Increased Personal Protective Equipment in the Environment Due to the COVID-19 Pandemic. Aswan Univ. J. Environ. Stud. 2021, 2, 1–10. [Google Scholar] [CrossRef]
- Maltings. Reclaim The Mask PPE Recycling; MyGroup: Leeds, UK, 2020. [Google Scholar]
- Marco Tobías, M.; Åhlén, M.; Cheung, O.; Bucknall, D.G.; McCoustra, M.R.S.; Yiu, H.H.P. Plasma degradation of contaminated PPE: An energy-efficient method to treat contaminated plastic waste. Npj Mater. Degrad. 2023, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Price, A.; Cui, Y.; Liao, L.; Xiao, W.; Yu, X.; Wang, H.; Zhao, M.; Wang, Q.; Chu, S.; Chu, L. Is the fit of N95 facial masks effected by disinfection? A study of heat and UV disinfection methods using the OSHA protocol fit test. medRxiv 2020, 2020-04. [Google Scholar] [CrossRef]
- Remic, K.; Erjavec, A.; Volmajer Valh, J.; Šterman, S. Public Handling of Protective Masks from Use to Disposal and Recycling Options to New Products. Stroj. Vestn. J. Mech. Eng. 2022, 68, 281–289. [Google Scholar] [CrossRef]
- KOIN 6 News Staff. French Company Works to Recycle Masks Into PPE. KOIN 2020. Available online: https://www.koin.com/news/health/coronavirus/french-company-works-to-recycle-masks-into-ppe/ (accessed on 15 October 2023).
- Taylor, B. TerraCycle Offers PPE Recycling Program. Recycling Today. 2020. Available online: https://www.recyclingtoday.com/news/terracycle-ppe-recycling-easypak-containers/ (accessed on 21 October 2023).
- Wang, P.; Cutts, W.D.; Ning, H.; Pillay, S.; Liu, S. Effects of chemical and autoclave sterilization treatments on medical personal protective equipment made of nonwoven polypropylene fibers for recycling. J. Polym. Res. 2022, 29, 360. [Google Scholar] [CrossRef]
- ASTM D7209-06; Standard Guide for Waste Reduction, Resource Recovery and Use of Recycled Polymeric Materials and Products (Withdrawn 2015). American Society for Testing and Materials: West Conshohocken, PA, USA, 2006.
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef]
- Ben Amor, I.; Klinkova, O.; Baklouti, M.; Elleuch, R.; Tawfiq, I. Mechanical Recycling and Its Effects on the Physical and Mechanical Properties of Polyamides. Polymers 2023, 15, 4561. [Google Scholar] [CrossRef] [PubMed]
- Zdiri, K.; Elamri, A.; Hamdaoui, M.; Harzallah, O.; Khenoussi, N.; Brendlé, J. Reinforcement of recycled PP polymers by nanoparticles incorporation. Green Chem. Lett. Rev. 2018, 11, 296–311. [Google Scholar] [CrossRef]
- ASTM D638-22; Standard Test Method for Tensile Properties of Plastics. American Society for Testing and Materials: West Conshohocken, PA, USA, 2022.
- ISO 178:2019; Plastics; Determination of Flexural Properties. ISO: Geneva, Switzerland, 2019.
- ISO 1183-1:2019; Plastics; Methods for Determining the Density of Non-Cellular Plastics Part 1. ISO: Geneva, Switzerland, 2019.
- CIE 015:2018; Colorimetry, 4th Edition. CIE International Commission on Illumination: Vienna, Austria, 2018.
- Boschmeier, E.; Archodoulaki, V.; Schwaighofer, A.; Lendl, B.; Bartl, A. A novel quantification tool for elastane in textiles using thermal treatment. Polym. Test. 2022, 118, 107920. [Google Scholar] [CrossRef]
- Mohammadi, A.; Barikani, M.; Barmar, M. Synthesis and investigation of thermal and mechanical properties of in situ prepared biocompatible Fe3O4/polyurethane elastomer nanocomposites. Polym. Bull. 2014, 72, 219–234. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, L.; Fischer, A.; Wang, L.; Drummer, D.; Wu, W. The effect of hBN on the flame retardancy and thermal stability of P-N flame retardant PA6. J. Macromol. Sci. Part A 2017, 55, 17–23. [Google Scholar] [CrossRef]
- Miandad, R.; Rehan, M.; Barakat, M.; Aburizaiza, A.; Khan, H.; Ismail, I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, D.A.-S. Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based Biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef]
- Blanco, I.; Siracusa, V. The Use of Thermal Techniques in the Characterization of Bio-Sourced Polymers. Materials 2021, 14, 1686. [Google Scholar] [CrossRef] [PubMed]
- Ünal, H.; Kaya, A.U.; Esmer, K.; Mimaroglu, A.; Poyraz, B. Influence of wax content on the electrical, thermal and tribological behaviour of a polyamide 6/graphite composite. J. Polym. Eng. 2015, 36, 279–286. [Google Scholar] [CrossRef]
- Demirel, B.; Yaraş, A.; Elçiçek, H. Crystallization Behavior of PET Materials. Balıkesir Üniversitesi Fen Bilim. Enstitü Derg. 2011, 13, 26–35. [Google Scholar]
- Boschmeier, E.; Archodoulaki, V.-M.; Schwaighofer, A.; Lendl, B.; Ipsmiller, W.; Bartl, A. New separation process for elastane from polyester/elastane and polyamide/elastane textile waste. Resour. Conserv. Recycl. 2023, 198, 107215. [Google Scholar] [CrossRef]
- Saechtling, H. International Plastics Handbook for the Technologist, Engineer and User; Hanser-Gardner Publications: Liberty Township, OH, USA, 1987. [Google Scholar]
- Mills, K. ASM Handbook: Fractography; ASM International: Almere, The Netherlands, 1987. [Google Scholar]
- Silva, L.; Munhoz, A.; Peres, R.; Machado, M. Characterization and Evaluation of Employment in Rigid Packaging of Polypropylene Composites with Bamboo Fiber. Mater. Res. 2022, 25, e20220316. [Google Scholar] [CrossRef]
- Campanale, C.; Savino, I.; Pojar, I.; Massarelli, C.; Uricchio, V. A Practical Overview of Methodologies for Sampling and Analysis of Microplastics in Riverine Environments. Sustainability 2020, 12, 6755. [Google Scholar] [CrossRef]
- Plastics Europe. Plastics—The Facts 2022; Plastics Europe: Brussels, Belgium, 2022. [Google Scholar]
- Plohl, O.; Erjavec, A.; Fras Zemljič, L.; Vesel, A.; Čolnik, M.; Škerget, M.; Fan, Y.V.; Čuček, L.; Trimmel, G.; Volmajer Valh, J. Morphological, surface and thermal properties of polylactic acid foils, melamine-etherified resin, and polyethylene terephthalate fabric during (bio)degradation in soil. J. Clean. Prod. 2023, 421, 138554. [Google Scholar] [CrossRef]
- Hyun-Jung, C.; Kim, M.; Ahn, D.; Yeo, S.Y.; Lee, S. Electrical percolation threshold of carbon black in a polymer matrix and its application to antistatic fibre. Sci. Rep. 2019, 9, 6338. [Google Scholar] [CrossRef]
- Mahi, H.; Alemdar, A.; Rodrigue, D. Polypropylene reinforced with nanocrystalline cellulose: Coupling agent optimization. J. Appl. Polym. Sci. 2015, 132, 42438. [Google Scholar] [CrossRef]
- Xu, C.; Cao, L.; Cao, X. Highly toughened polypropylene/ethylene–propylene-diene monomer/zinc dimethacrylate ternary blends prepared via peroxide-induced dynamic vulcanization. Mater. Chem. Phys. 2013, 138, 63–71. [Google Scholar] [CrossRef]
- Tan, M.; Feng, Y.; Wang, H.; Zhang, L.; Khan, M.; Guo, J.; Chen, Q.; Liu, A. Immobilized Bioactive Agents onto Polyurethane Surface with Heparin and Phosphorylcholine Group. Macromol. Res. 2013, 21, 541–549. [Google Scholar] [CrossRef]
- Gupta, V.; Trivedi, P. Chapter 15—In vitro and in vivo characterization of pharmaceutical topical nanocarriers containing anticancer drugs for skin cancer treatment. In Lipid Nanocarriers for Drug Targeting; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 563–627. [Google Scholar]
- Plohl, O.; Zemljič, L.; Potrč, S.; Luxbacher, T. Applicability of electro-osmotic flow for the analysis of the surface zeta potential. RSC Adv. 2020, 10, 6777–6789. [Google Scholar] [CrossRef] [PubMed]
- Gorassini, A.; Adami, G.; Calvini, P.; Giacomello, A. ATR-FTIR characterization of old pressure sensitive adhesive tapes in historic papers. J. Cult. Herit. 2016, 21, 775–785. [Google Scholar] [CrossRef]
- Patel, B.; Mandot, A.; Jha, P. Extraction, characterization and application of Azadirachta indica leaves for development of hygienic lycra filament. J. Int. Acad. Res. Multidiscip. 2014, 1, 65–84. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Peets, P.; Kaupmees, K.; Vahur, S.; Leito, I. Reflectance FT-IR spectroscopy as a viable option for textile fiber identification. Herit. Sci. 2019, 7, 93. [Google Scholar] [CrossRef]
- Marjo, C.E.; Gatenby, S.; Rich, A.M.; Gong, B.; Chee, S. ATR-FTIR as a tool for assessing potential for chemical ageing in Spandex/Lycra®/elastane-based fabric collections. Stud. Conserv. 2017, 62, 343–353. [Google Scholar] [CrossRef]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, C.V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.-J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Ioakeimidis, C.; Fotopoulou, K.N.; Karapanagioti, H.K.; Geraga, M.; Zeri, C.; Papathanassiou, E.; Galgani, F.; Papatheodorou, G. The degradation potential of PET bottles in the marine environment: An ATR-FTIR based approach. Sci. Rep. 2016, 6, 23501. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; He, S.; Yan, Y.; Zhang, J.; Dong, J. Efficient approach to produce functional polypropylene via solvent assisted solid-phase free radical grafting of multi-monomers. Appl. Petrochem. Res. 2021, 11, 99–111. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; Niu, Y.; Hsiao, B.S.; Piccarolo, S. Phase Transitions in Prequenched Mesomorphic Isotactic Polypropylene during Heating and Annealing Processes as Revealed by Simultaneous Synchrotron SAXS and WAXD Technique. J. Phys. Chem. B 2012, 116, 147–153. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Q.; Huang, J.; Ding, D. Friction and wear behaviors of polyamide-based composites blended with polyphenylene sulfide. J. Thermoplast. Compos. Mater. 2012, 27, 977–991. [Google Scholar] [CrossRef]
- Fabia, J.; Gawłowski, A.; Rom, M.; Ślusarczyk, C.; Brzozowska-Stanuch, A.; Sieradzka, M. PET Fibers Modified with Cloisite Nanoclay. Polymers 2020, 12, 774. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, R.; Nam, K.T.; Navamathavan, R.; Park, S.-J.; Kim, H.Y. Hydroxyapatite Mineralization on the Calcium Chloride Blended Polyurethane Nanofiber via Biomimetic Method. Nanoscale Res. Lett. 2010, 6, 2. [Google Scholar] [CrossRef] [PubMed]








| Sample [Material] (Abbreviation) | Temperature Zones (°C) | Screw Speed (RPM) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Die | 8 | 7 | 6 | 5 | 4 | 3 | 2 | ||
| Recycled three-layered part of the DSM [PP] (r3L) | 165 | 170 | 180 | 190 | 200 | 195 | 190 | 185 | 125 |
| Recycled ear loops [PET, PU and PA6] (rEL) | 235 | 230 | 230 | 230 | 225 | 220 | 220 | 210 | 50 |
| Recycled combined materials [86% r3L and 14% rEL] (rDSM) | 165 | 170 | 180 | 190 | 200 | 195 | 190 | 185 | 125 |
| Sample | Cylinder Temperature (°C) | Mold Temperature (°C) | Injection Pressure (bar) | Injection Time (s) | Post Injection Pressure (bar) | Post Injection Time (s) |
|---|---|---|---|---|---|---|
| r3L | 180 | 65 | 600 | 5 | 450 | 5 |
| rEL | 240 | 70 | 500 | 5 | 450 | 5 |
| rDSM | 180 | 65 | 600 | 5 | 450 | 5 |
| Material | Average E (MPa) ± STD | (MPa) ± STD | (%) ± STD | Average Z (%) ± STD |
|---|---|---|---|---|
| r3L | 2339.5 ±8 5.6 | 30.5 ± 1.7 | 3.2 ± 0.2 | 2.9 ± 0.2 |
| rEL | 858.2 ± 56 | 10.7 ± 0.4 | 3.1 ± 0.2 | 0.2 ± 0.03 |
| rDSM | 2150.7 ± 71.5 | 22.7 ± 0.8 | 4.1 ± 0.4 | 2.2 ± 0.17 |
| Material | Average Ef (MPa) ± STD | (MPa) ± STD |
|---|---|---|
| r3L | 1927.2 ± 80.1 | 63.8 ± 4.3 |
| rEL | 439.9 ± 61.4 | 16.4 ± 1.9 |
| rDSM | 1655.3 ± 60.7 | 54.7 ± 1.3 |
| Sample | Average Density (kg/m3) ± STD |
|---|---|
| r3L | 0.905 ± 0.002 |
| rEL | 1.156 ± 0.014 |
| rDSM | 0.969 ± 0.004 |
| Sample | L* | a* | b* | C* | Hue | ΔE* |
|---|---|---|---|---|---|---|
| r3L | 46.92 | −14.81 | −12.45 | 19.35 | 220.05 | / |
| rEL | 49.86 | 7.06 | 21.46 | 22.59 | 71.80 | / |
| rDSM | 46.76 | −14.65 | −11.31 | 18.51 | 217.67 | 1.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erjavec, A.; Volmajer Valh, J.; Hribernik, S.; Kraševac Glaser, T.; Fras Zemljič, L.; Vuherer, T.; Neral, B.; Brunčko, M. Advance Analysis of the Obtained Recycled Materials from Used Disposable Surgical Masks. Polymers 2024, 16, 935. https://doi.org/10.3390/polym16070935
Erjavec A, Volmajer Valh J, Hribernik S, Kraševac Glaser T, Fras Zemljič L, Vuherer T, Neral B, Brunčko M. Advance Analysis of the Obtained Recycled Materials from Used Disposable Surgical Masks. Polymers. 2024; 16(7):935. https://doi.org/10.3390/polym16070935
Chicago/Turabian StyleErjavec, Alen, Julija Volmajer Valh, Silvo Hribernik, Tjaša Kraševac Glaser, Lidija Fras Zemljič, Tomaž Vuherer, Branko Neral, and Mihael Brunčko. 2024. "Advance Analysis of the Obtained Recycled Materials from Used Disposable Surgical Masks" Polymers 16, no. 7: 935. https://doi.org/10.3390/polym16070935
APA StyleErjavec, A., Volmajer Valh, J., Hribernik, S., Kraševac Glaser, T., Fras Zemljič, L., Vuherer, T., Neral, B., & Brunčko, M. (2024). Advance Analysis of the Obtained Recycled Materials from Used Disposable Surgical Masks. Polymers, 16(7), 935. https://doi.org/10.3390/polym16070935