Abstract
Since the discovery of α-diimine catalysts in 1995, an extensive series of Brookhart-type complexes have shown their excellence in catalyzing ethylene polymerizations with remarkable activity and a high molecular weight. However, although this class of palladium complexes has proven proficiency in catalyzing ethylene copolymerization with various polar monomers, the α-diimine nickel catalysts have generally exhibited a much worse performance in these copolymerizations compared to their palladium counterparts. Recently, Brookhart et al. reported a notable exception, demonstrating that α-diimine nickel catalysts could catalyze the ethylene copolymerization with some vinylalkoxysilanes effectively, producing functionalized polyethylene incorporating trialkoxysilane (-Si(OR)3) groups. This breakthrough is significant since Pd-catalyzed copolymerizations are commercially less usable due to the high cost of palladium. Thus, the utilization of Ni, given its abundance in raw materials and cost-effectiveness, is a landmark in ethylene/polar vinyl monomer copolymerization. Inspired by these findings, we used density functional theory (DFT) calculations to investigate the mechanistic study of ethylene copolymerization with vinyltrimethoxysilane (VTMoS) catalyzed by Brookhart-type nickel catalysts, aiming to elucidate the molecular-level understanding of this unique reaction. Initially, the nickel complexes and cationic active species were optimized through DFT calculations. Subsequently, we explored the mechanisms including the chain initiation, chain propagation, and chain termination of ethylene homopolymerization and copolymerization catalyzed by Brookhart-type complexes. Finally, we conducted an energetic analysis of both the in-chain and chain-end of silane enchainment. It was found that chain initiation is the dominant step in the ethylene homopolymerization catalyzed by the α-diimine Ni complex. The 1,2- and 2,1-insertion of vinylalkoxysilane exhibit similar barriers, explaining the fact that both five-membered and four-membered chelates were identified experimentally. After the VTMoS insertion, the barriers of ethylene reinsertion become higher, indicating that this step is the rate-determining step, which could be attributed to the steric hindrance between the incoming ethylene and the bulky silane substrate. We have also reported the energetic analysis of the distribution of polar substrates. The dominant pathway of chain-end -Si(OR)3 incorporation is suggested as chain-walking → ring-opening → ethylene insertion, and the preference of chain-end -Si(OR)3 incorporation is primarily attributed to the steric repulsion between the pre-inserted silane group and the incoming ethylene molecule, reducing the likelihood of in-chain incorporation.
1. Introduction
Polyolefins stand as a predominant class of plastics, constituting over half of the global plastic production at 360 million tons, playing an important role in modern society [1]. The incorporation of polar functional groups is an effective method to enhance surface properties, thereby widening their range of potential applications [2,3,4,5,6]. Transition-metal-catalyzed copolymerization emerges as a direct and cost-effective method for producing functionalized polyolefins under mild conditions [7]. However, Ziegler–Natta catalysts and metallocene-based catalysts encounter some challenges in ethylene copolymerization related to the poisoning of the metal center (Figure 1) [8,9]. These challenges could come from the deactivation of the Lewis acidic metal center, particularly due to the coordination with Lewis basic polar groups. Furthermore, 2,1-insertion may lead to the formation of stable metal–alkyl chelates, followed by β-X elimination, inhibiting the further insertion. Due to the weaker Lewis acidity and lower oxophilicity, late transition metal catalysts, such as Pd- and Ni-based catalysts, exhibit better tolerance of functional/polar groups, thus having been employed in ethylene copolymerizations for synthesizing polar polymers.
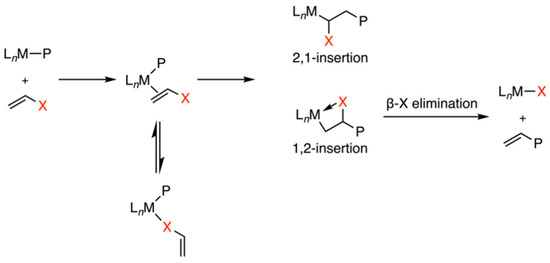
Figure 1.
Several methods for retarding transition-metal-catalyzed ethylene copolymerization [8,9].
Since the discovery of α-diimine catalysts in 1995 for the production of branched polymers [10], an extensive series of Brookhart-type complexes has been reported, demonstrating the excellence in catalyzing ethylene polymerizations with a high activity and high molecular weight (Figure 2a) [6,11,12,13,14,15,16,17,18,19]. However, although this class of palladium complexes has demonstrated proficiency in catalyzing ethylene copolymerization with fundamental polar monomers (Figure 2b) [16] (with a polar group directly attached to the vinyl double bond) such as acrylates [6,11,12,13], vinyl ketones [6], silyl vinyl ethers [14,15], vinyl alkoxysilanes [20], and some other special polar monomers (Figure 2c) (with a spacer between the polar group and the double bond) [17,18,19,21,22,23,24,25], the α-diimine nickel catalysts generally showed much worse properties in these copolymerizations compared with their palladium counterparts [26,27], mainly attributed to the higher oxophilicity of nickel compared to palladium (Figure 2b).
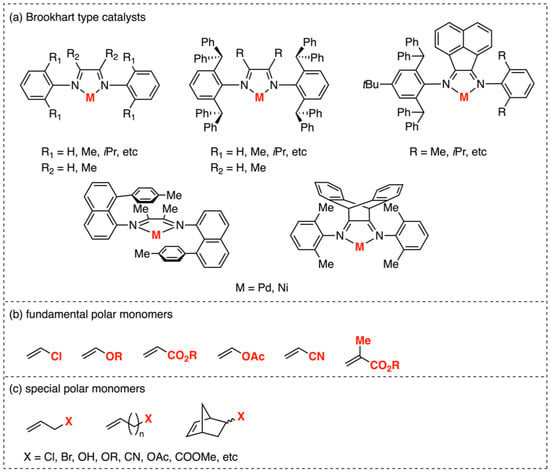
Figure 2.
(a) Structures of Brookhart’s Pd and Ni α-diimine catalysts. (b) Fundamental polar monomers used in copolymerization reactions. (c) Special polar monomers used in copolymerization reactions.
Recently, Brookhart et al. reported that α-diimine nickel catalysts could catalyze the ethylene copolymerization with some vinylalkoxysilanes (Figure 3) [28], producing functionalized polyethylene incorporating trialkoxysilane (-Si(OR)3) groups, which could be cross-linked to form PEX-b, a tough material that is widely used for power cable insulation and hot water piping systems [29,30]. Notably, this breakthrough is significant since Pd-catalyzed copolymerizations are commercially less usable due to the high cost of palladium. Therefore, the utilization of Ni, given its abundance in raw materials and cost-effectiveness, is a landmark in ethylene/polar vinyl monomer copolymerization.
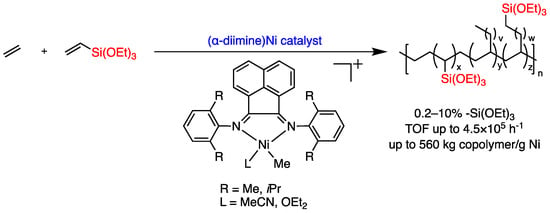
Figure 3.
Reactions of ethylene copolymerization with vinyltrialkoxysilanes catalyzed by (α-diimine) nickel catalyst.
With the rapid evolution of olefin polymerization, the mechanistic study of Brookhart-type catalytic copolymerization systems has become indispensable [9]. The inherent complexity of α-diimine catalytic systems necessitates a thorough exploration of the detailed factors that control catalytic activity, molecular weight, comonomer incorporation ratios, and branch content in these transformations. To advance the late transition-metal-catalyzed olefin copolymerization, many factors of catalysts were considered such as electronic inhibition, steric inhibition, metal center, etc. Despite significant development [9], numerous aspects of late transition-metal-catalyzed olefin copolymerization need further investigation. Catalyst-related factors such as electronic inhibition, steric hindrance, and metal center configuration, have been carefully studied to enhance the efficacy of these processes. However, the fundamental mechanisms driving such catalytic activity and the intricate reaction pathways involved have remained elusive. Clarifying these original aspects is essential for the future advancement and refinement of copolymerization systems, offering insights that could catalyze these transforms in polymerization.
In this manuscript, to elucidate the molecular-level understanding of ethylene polymerization catalyzed by Ni-based α-diimine complexes and to investigate the factors contributing to diverse performances associated with different polar monomers, density functional theory (DFT) calculations were performed to disclose this polymerization mechanism in detail. On the basis of these theoretical studies, we clarified the major pathways and rate-determining steps in this reaction. Furthermore, DFT calculations enabled the identification of active species and transition state structures, enhancing our comprehension of this reaction. The energetic analysis, coupled with an exploration of influencing factors, offers valuable insights for interpreting ethylene copolymerizations. These mechanistic studies could shed light on the future design of nickel-based α-diimine complexes used for ethylene copolymerization with fundamental polar monomers.
2. Computational Methods
All density functional theory (DFT) calculations were performed with the Gaussian 16 program [31]. Geometry optimizations employed the spin-unrestricted dispersion-corrected method (UB3LYP [32,33]-D3 [34,35,36,37]), with the 6-31G(d) basis set for nonmetal atoms (C, N, H, Si, O) and the LANL2DZ basis set along with the associated pseudopotential for metal atoms (Ni), denoted as BSI [38,39,40]. Frequency calculations were also conducted at the same level of theory to obtain vibrational frequencies to determine the identity of stationary points as intermediates (no imaginary frequencies) or transition states (only one imaginary frequency), as well as obtaining the thermal corrections to enthalpy (Hcorrection) and free energy (Gcorrection) at the temperature of 298 K. Single-point energies based on BSI geometries were refined using a higher-level method denoted as BSII. For BSII, the 6-311G(d,p) basis set was applied to nonmetal atoms (C, N, H, Si, O), and the SDD basis set with its associated pseudopotential was used for metal atoms (Ni) [40]. To explore the effect of toluene solvent on the catalytic system, single point energies were performed in implicit toluene solvent through the SMD model [41], labeled as UB3LYP-D3/BSII(SMD)//B3LYP-D3/BSI. Energy profiles were constructed at the UB3LYP-D3/BSII(SMD)//B3LYP-D3/BSI level, including Gibbs free energy corrections taken from frequency calculations in the gas phase. All optimized geometrical figures were generated with CYLview [42], with energies reported in kcal/mol.
3. Results and Discussion
3.1. Nickel Complexes and Cationic Active Species
As reported in the previous study [28], the ethylene polymerization was catalyzed by α-diimine Ni catalysts. The α-diimine nickel dibromide complex I, the cationic and α-diimine nickel methyl ether complex II, and the nickel methyl acetonitrile complex III were regarded as the initial Ni complexes and active cationic active species, and thus were optimized by DFT calculations (Figure 4). Optimized structures exhibited distinct geometrical characteristics, where the Ni(II)Br2 complex I showed tetrahedral conformation while the Ni(II)(OEt)2 cationic complex II and the Ni(II)(MeCN)+ cationic complex III exhibited square planar conformations. Based on these optimized structures, ethylene polymerizations were investigated computationally in detail as follows.
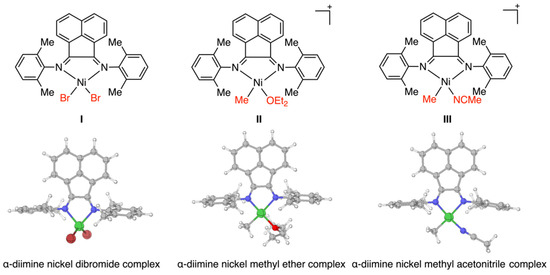
Figure 4.
Optimized structures of complexes I, II, and III.
3.2. Chain Initiation and Chain Propagation (Ethylene Insertion)
Based on previous studies [43,44], DFT calculations were conducted to explore the ethylene polymerization process, including both chain initiation and chain propagation. For all proposed intermediates and transition states, both singlet and triplet spin states were considered, and the lower-energy structures were identified and colored. As shown in Figure 5 (and Figure S1 in Supplementary Materials), starting with the cationic methylated α-diimine Ni complex 3A, one molecule of ethylene coordinated to the Ni center, forming the π-complex 1B and releasing an energy of 2.0 kcal/mol. Subsequently, the π-complex could undergo the first ethylene insertion via transition states 1TS1 with a barrier of 14.4 kcal/mol, leading to the chain initiation product 3C. In turn, the Ni-alkyl species will then be poised to coordinate the second ethylene molecule and undergo the second ethylene insertion via transition states 1TS2 to form the product 3E. Notably, the barrier for the chain propagation step was found to be 13.1 kcal/mol, lower than that of the first step, which suggests that the chain initiation is the rate-determining step in the ethylene homopolymerization catalyzed by this cationic α-diimine Ni complex.
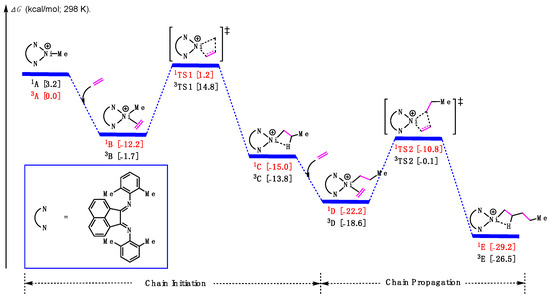
Figure 5.
Calculated relative Gibbs free energies for the insertion of ethylene.
3.3. Chain Propagation (Vinylalkoxysilane Insertion) and Chain Termination
After the chain initiation step to form the Ni-alkyl species, we proceeded to explore the insertion of vinylalkoxysilane (Figure 6). As shown in Figure 6a (and Figure S2a in Supplementary Materials), based on the relative stability of π-complexes and O-complexes [45], the Ni-alkyl complex 3C coordinated with vinyltrimethoxysilane (VTMoS), releasing an energy of 23.0 kcal/mol to generate the O-complex 3F, which could equilibrate to the π-complex 3G. Then, 3G could undergo 1,2-insertion via 1TS3 with an overall barrier of 21.7 kcal/mol, leading to the formation of a Ni(II) five-membered chelate complex 3H (10.8 kcal/mol downhill in energy). Notably, the five-membered chelate complex could be isolated and identified successfully by the NMR spectrum [28]. In turn, excess ethylene induced the chelate opening of 3H, and then another molecule of ethylene inserted into the Ni-alkyl species with a barrier of 23.3 kcal/mol, resulting in the desired product 3J. Considering prior computational studies [43,45,46,47], we also investigated the commonly proposed chain termination pathway that β-H elimination. However, this pathway was found to be much lower in energy (~16 kcal/mol) compared to the 1,2-insertion. Despite this computational result, experimental results [28] supported the predominance of 1,2-insertion over β-H elimination. We attribute this observation to the excess ethylene favoring the chain propagation pathway over the chain termination pathway. And the small energetic difference among 3I, 3K, and 3H allowed for their mutual transfer, thereby giving the possibility of proceeding the chain propagation step.
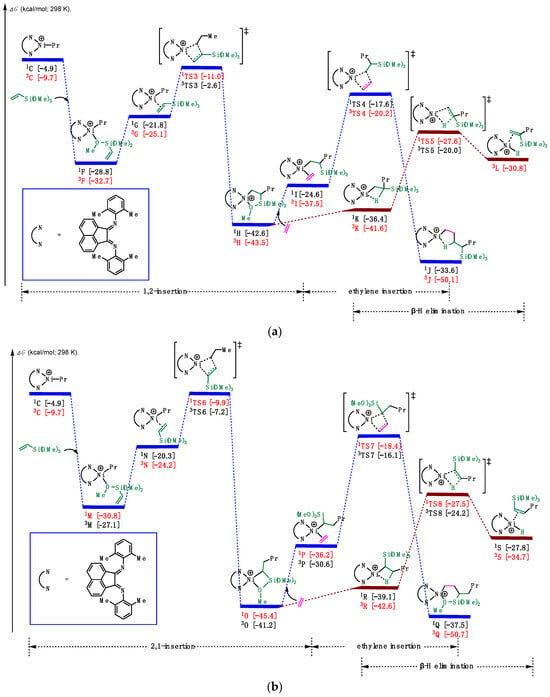
Figure 6.
Calculated relative Gibbs free energies for (a) 1,2-insertion of vinyltrimethoxysilane (b) 2,1-insertion of vinyltrimethoxysilane, ethylene reinsertion, and β-H elimination.
To obtain a comprehensive understanding of this reaction, we also considered the 2,1-insertion pathway (Figure 6b and Figure S2b in Supplementary Materials). Similar to the 1,2-insertion, starting from the coordination of 3C, the Ni(II) species 1N could undergo 2,1-insertion with VTMoS via 1TS6 (the barrier was 20.9 kcal/mol), leading directly to the four-membered chelate complex 1O, accompanied by the release of 14.6 kcal/mol in energy. The four-membered chelate complex 1O could also be detectable by the NMR spectrum [28], supporting the rigidity of the computational results. Then, excess ethylene could foster the ring-opening of 3H, and then another molecule of ethylene inserted into the Ni-C bond with a barrier of 27.0 kcal/mol, resulting in the six-membered chelate product 3Q. Additionally, from 1O, we located the transition state for β-H elimination, denoted as 1TS8, leading to the formation of the Ni(II)-H species 3S. Since the pathways and barriers were similar for both 1,2- and 2,1-insertion pathways, and the chelates could be found in experiments, it is obvious that both 1,2- and 2,1-insertion merged in this reaction, which is special and different from other catalysts.
Given the similarity in pathways and barriers for both 1,2- and 2,1-insertion, combined with the experimental identification of the five-membered and four-membered chelates, it is evident that both 1,2- and 2,1-insertion pathways emerged in this reaction, which is a distinctive feature, setting it apart from reactions involving other catalysts [48,49]. Notably, the barrier of 1,2-insertion was 21.7 kcal/mol, which is higher than that of 2,1-insertion, suggesting that 1,2-insertion is preferred, consistent with the preference of the four-membered chelate experimentally [28]. Additionally, the four-membered and five-membered chelate complexes, 1H and 1O, were favored compared with not only Ni-ethylene complexes 3I and 1P but also O-complexes 3F and 1M.
Notably, according to a pertinent article, the experimentally measured barrier for polar monomer insertion from the π-complex to the insertion product was reported as 15.5 kcal/mol [50], similar to the barrier calculated in our manuscript (14.1 and 14.3 kcal/mol). Despite the difference in the polar monomer studied in the reference (vinyl acetate), we employed the same catalysts in our research, so the minor difference observed is reasonable. This consistency verified the validity and reliability of our computational methodology.
3.4. Energetic Analysis of In-Chain and Chain-End Silane Enchainment
As for the observation indicating copolymers with the incorporation of -Si(OR)3 groups at both in-chain and chain-end positions, the ratios of in-chain to chain-end incorporation ranged from 1.0:1.1 to 1.0:2.8, varying based on temperature and ethylene pressure [28]. We conducted an energetic analysis of both the in-chain and chain-end of silane enchainment (Figure 7 and Figure S4 in Supplementary Materials). We proposed that the silane products emerged through three pathways, starting with the VTMoS insertion products.
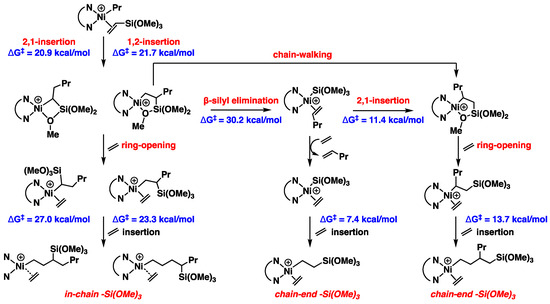
Figure 7.
Calculated relative Gibbs free energies for the silane enchainment.
After the VTMoS insertion, chelate complexes become the major resting states but are in rapid equilibrium with (α-diimine)Ni(R)(C2H4)+ complexes. Starting from the four-membered and five-membered chelate complexes, they undergo ring-opening via ethylene coordination followed by chain propagation to form the in-chain -Si(OR)3 structures with barriers of 27.0 and 23.3 kcal/mol. Additionally, β-silyl elimination from the five-membered chelate complex generates the Ni-Si(OR)3 species (the barrier was 30.2 kcal/mol), followed by butene displacement and ethylene insertion, leading to the formation of the chain-end copolymer. Moreover, after facial chain-walking from the five-membered chelate complex or 2,1-insertion of the Ni-Si(OR)3 species, the “new” five-membered chelate complex underwent ring-opening, followed by ethylene insertion to produce the chain-end product with a lower barrier of 13.7 kcal/mol, compared to ethylene insertion in in-chain silane copolymer formation. Notably, the pathway of chain-walking → ring-opening → ethylene insertion is considered the dominant one for chain-end -Si(OR)3 incorporation [28], while β-silyl elimination → olefin displacement → ethylene insertion is a minor pathway [28] due to the higher barrier (30.2 kcal/mol) of β-silyl elimination though this is the dominant pathway in the Pd system [20]. The higher barriers (27.0 and 23.3 kcal/mol) for in-chain product formation are consistent with lower ratios of in-chain silane groups observed in experimental results. We assumed the steric repulsion between the pre-inserted silane group and the incoming ethylene molecule contributes to the higher barriers for in-chain product formation, making the incorporation of the in-chain less likely. These calculations align with the difference caused by temperature and concentration [28]. Higher ethylene pressure enhances the ethylene trapping and subsequent insertion relative to the chain-walking and β-silyl elimination, increasing the ratio of in-chain -Si(OR)3 incorporation. Higher temperature led to the increase in the ratio of chain-end -Si(OR)3 incorporation, likely because the higher temperature caused the β-silyl elimination more facial, which accelerated the minor chain-end -Si(OR)3 incorporation pathway.
4. Conclusions
In this manuscript, DFT calculations were performed to explore the mechanistic study of ethylene copolymerization with vinyltrimethoxysilane (VTMoS) catalyzed by α-diimine nickel catalysts. It was found that chain initiation is the dominant step in the ethylene homopolymerization catalyzed by the α-diimine Ni complex. After the insertion of the first molecule of ethylene, the subsequent insertion of vinylalkoxysilane in both 1,2-mode and 2,1-mode exhibits similar barriers, being consistent with the experimental results that five-membered and four-membered chelates were all isolated. After the VTMoS insertion, the barriers of ethylene reinsertion became higher (23.3 and 27.0 kcal/mol), which indicates that this step is the rate-determining step, similar to the previous studies [43,47]. This phenomenon could be attributed to the steric hindrance between the incoming ethylene and the bulky silane substrate.
Furthermore, we considered the distribution of polar substrates. Experimental ratios of in-chain to chain-end incorporation of -Si(OR)3 groups ranging from 1.0:1.1 to 1.0:2.8 are aligned with the trend of calculated results: higher computational barriers for in-chain product formation result in lower experimental ratios of in-chain incorporation. Additionally, as ethylene pressure increases, the 1,2-insertion product, a five-membered chelate, shows a preference for coordinating with ethylene, meanwhile decreasing the chain-walking to form the chain-end product. Subsequently, it undergoes ethylene reinsertion to form the in-chain product, thereby increasing the ratio of in-chain products. Moreover, with increasing temperature, in-chain incorporation with higher barriers becomes more feasible, leading to a higher ratio. The experimental results mentioned above provide solid evidence for our computational findings.
The dominant pathway for chain-end -Si(OR)3 incorporation is suggested as chain-walking → ring-opening → ethylene insertion, with a lower barrier compared to other pathways. The preference of the chain-end -Si(OR)3 incorporation is primarily attributed to the steric repulsion between the pre-inserted silane group and the incoming ethylene molecule, reducing the likelihood of in-chain incorporation.
In summary, our DFT calculations provide a molecular understanding of Ni-catalyzed ethylene copolymerization, offering insights into the copolymerization details and inspiring the design of catalytic systems and modification of transition-metal catalysts. Future investigations will focus on the comparison of more transition-metal systems and the behavior of different polar monomers. In the forthcoming research, we intend to conduct a comparative analysis between this current nickel-based system and a palladium-based counterpart. Our objective is to deepen our understanding of catalytic mechanisms and optimize reactions by investigating the impact of diverse substrates. Through this investigation, we aim to build the internal relationship between catalyst structures and reactivity, thereby improving synthetic methodologies.
Furthermore, we plan to explore the behavior of the polar monomer utilized in this manuscript, vinyltrimethoxysilane, in comparison with other polar monomers such as methyl methacrylate and methyl acrylate. This comparative study is motivated by the observed differences in reactivity among these monomers. By elucidating the underlying factors contributing to these variations, we look forward to shedding light on the mechanistic intricacies governing polymerization processes, advancing the understanding of polymer chemistry and facilitating the development of synthetic catalytic systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym16060762/s1, Figure S1: Calculated relative Gibbs free energies of optimization for the insertion of ethylene. Free energies (kcal/mol) were computed at the B3LYP-D3/BSI level of theory with the 6-31G(d) basis set for nonmetal atoms (C, N, H, Si, O) and the LANL2DZ basis set along with the associated pseudopotential for metal atoms (Ni), denoted as BSI.; Figure S2: Calculated relative Gibbs free energies of optimization for the (a) 1,2-insertion of vinyltrimethoxysilane (b) 2,1-insertion of vinyltrimethoxysilane, ethylene reinsertion, and β-H elimination. Free energies (kcal/mol) were computed at the B3LYP-D3/BSI level of theory with the 6-31G(d) basis set for nonmetal atoms (C, N, H, Si, O) and the LANL2DZ basis set along with the associated pseudopotential for metal atoms (Ni), denoted as BSI.; Figure S3: Calculated relative Gibbs free energies of optimization for the silane enchainment. Free energies (kcal/mol) were computed at the B3LYP-D3/BSI level of theory with the 6-31G(d) basis set for nonmetal atoms (C, N, H, Si, O) and the LANL2DZ basis set along with the associated pseudopotential for metal atoms (Ni), denoted as BSI; Figure S4: Calculated relative Gibbs free energies for the silane enchainment. Free energies (kcal/mol) were computed at the UB3LYP-D3/BSII(SMD)//B3LYP-D3/BSI level of theory with the 6-311G(d,p) basis set for nonmetal atoms (C, N, H, Si, O) and the SDD basis set along with the associated pseudopotential for metal atoms (Ni), denoted as BSII. And the Supplementary Materials include the optimized Cartesian coordinates (XYZ) with the relative energies.
Author Contributions
Conceptualization, Z.S. and R.G.; methodology, Z.S.; formal analysis, Z.S.; investigation, Z.S.; writing—original draft preparation, Z.S.; writing—review and editing, Z.S.; supervision, C.W., Q.G., R.G. and G.Z.; software, J.L., S.Y. and H.F.; funding acquisition, C.W., Q.G., R.G. and G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
Authors Zhihui Song, Rong Gao, Qingqiang Gou, Gang Zheng, Junjie Liu, Shifang Yang, and Huasheng Feng and Changjiang Wu were employed by the company SINOPEC (Beijing) Research Institute of Chemical Industry Co., Ltd. The all authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Annual Production of Plastics Worldwide from 1950 to 2021. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 11 October 2023).
- Dong, J.-Y.; Hu, Y. Design and synthesis of structurally well-defined functional polyolefins via transition metal-mediated olefin polymerization chemistry. Coord. Chem. Rev. 2006, 250, 47–65. [Google Scholar] [CrossRef]
- Rünzi, T.; Mecking, S. Saturated Polar- Substituted Polyethylene Elastomers from Insertion Polymerization. Adv. Funct. Mater. 2014, 24, 387–395. [Google Scholar] [CrossRef]
- Dai, S.Y.; Chen, C.L. Palladium-Catalyzed Direct Synthesis of Various Branched, Carboxylic Acid-Functionalized Polyolefins: Characterization, Derivatization, and Properties. Macromolecules 2018, 51, 6818–6824. [Google Scholar] [CrossRef]
- Na, Y.N.; Dai, S.Y.; Chen, C.L. Direct Synthesis of Polar-Functionalized Linear Low-Density Polyethylene (LLDPE) and Low-Density Polyethylene (LDPE). Macromolecules 2018, 51, 4040–4048. [Google Scholar] [CrossRef]
- Sui, X.; Hong, C.; Pang, W.; Chen, C. Unsymmetrical α-diimine palladium catalysts and their properties in olefin (co)polymerization. Mater. Chem. Front. 2017, 1, 967–972. [Google Scholar] [CrossRef]
- Chen, E.Y.X. Coordination Polymerization of Polar Vinyl Monomers by Single-Site Metal Catalysts. Chem. Rev. 2009, 109, 5157–5214. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gao, Y.; Marks, T.J. Early Transition Metal Catalysis for Olefin-Polar Monomer Copolymerization. Angew. Chem. Int. Ed. 2020, 59, 14726–14735. [Google Scholar] [CrossRef]
- Song, Z.; Wang, S.; Gao, R.; Wang, Y.; Gou, Q.; Zheng, G.; Feng, H.; Fan, G.; Lai, J. Recent Advancements in Mechanistic Studies of Palladium- and Nickel-Catalyzed Ethylene Copolymerization with Polar Monomers. Polymers 2023, 15, 4343. [Google Scholar] [CrossRef]
- Johnson, L.K.; Killian, C.M.; Brookhart, M. New Pd(II)-Based and Ni(II)-Based Catalysts for Polymerization of Ethylene and alpha-Olefins. J. Am. Chem. Soc. 1995, 117, 6414–6415. [Google Scholar] [CrossRef]
- Johnson, L.K.; Mecking, S.; Brookhart, M. Copolymerization of ethylene and propylene with functionalized vinyl monomers by palladium(II) catalysts. J. Am. Chem. Soc. 1996, 118, 267–268. [Google Scholar] [CrossRef]
- Dai, S.Y.; Sui, X.L.; Chen, C.L. Highly Robust Palladium(II) α-Diimine Catalysts for Slow-Chain-Walking Polymerization of Ethylene and Copolymerization with Methyl Acrylate. Angew. Chem. Int. Ed. 2015, 54, 9948–9953. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, S.; Gong, Q.; Zhang, S.; Liu, B.; Dai, S. Systematic Investigations of Ligand Steric Effects on α-Diimine Nickel Catalyzed Olefin Polymerization and Copolymerization. Organometallics 2019, 38, 2919–2926. [Google Scholar] [CrossRef]
- Luo, S.; Jordan, R.F. Copolymerization of silyl vinyl ethers with olefins by (α-diimine)PdR+. J. Am. Chem. Soc. 2006, 128, 12072–12073. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Luo, S.; Jordan, R.F. Cationic Polymerization and Insertion Chemistry in the Reactions of Vinyl Ethers with (α-Diimine)PdMe+ Species. J. Am. Chem. Soc. 2010, 132, 5273–5284. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, W.; Daugulis, O.; Brookhart, M. Mechanistic studies of Pd(II)-catalyzed copolymerization of ethylene and vinylalkoxysilanes: Evidence for a β-silyl elimination chain transfer mechanism. J. Am. Chem. Soc. 2016, 138, 16120–16129. [Google Scholar] [CrossRef]
- Camacho, D.H.; Guan, Z. Designing late-transition metal catalysts for olefin insertion polymerization and copolymerization. Chem. Commun. 2010, 46, 7879–7893. [Google Scholar] [CrossRef]
- Dong, Z.; Ye, Z. Hyperbranched polyethylenes by chain walking polymerization: Synthesis, properties, functionalization, and applications. Polym. Chem. 2012, 3, 286–301. [Google Scholar] [CrossRef]
- Takeuchi, D. Stereo-controlled synthesis of polyolefins with cycloalkane groups by using late transition metals. Polym. J. 2012, 44, 919–928. [Google Scholar] [CrossRef]
- Tan, C.; Chen, C. Emerging Palladium and Nickel Catalysts for Copolymerization of Olefins with Polar Monomers. Angew. Chem. Int. Ed. 2019, 58, 7192–7200. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Yu, H.; Zhao, Y.; Sun, R.; Jing, G.; Huang, J.; Khalid, H.; Abbasi, N.M.; Akram, M. Synthesis and application of polyethylene-based functionalized hyperbranched polymers. Prog. Polym. Sci. 2015, 45, 23–43. [Google Scholar] [CrossRef]
- Guo, L.; Chen, C. (α-Diimine)palladium catalyzed ethylene polymerization and (co)polymerization with polar comonomers. Sci. China Chem. 2015, 58, 1663–1673. [Google Scholar] [CrossRef]
- Guo, L.; Dai, S.; Sui, X.; Chen, C. Palladium and Nickel Catalyzed Chain Walking Olefin Polymerization and Copolymerization. ACS Catal. 2016, 6, 428–441. [Google Scholar] [CrossRef]
- Dai, S.; Chen, C. Direct Synthesis of Functionalized High-Molecular-Weight Polyethylene by Copolymerization of Ethylene with Polar Monomers. Angew. Chem. Int. Ed. 2016, 55, 13281–13285. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Liu, W.; Chen, C. Late transition metal catalyzed α-olefin polymerization and copolymerization with polar monomers. Mater. Chem. Front. 2017, 1, 2487–2494. [Google Scholar] [CrossRef]
- Johnson, L.; Wang, L.; McLain, S.; Bennett, A.; Dobbs, K.; Hauptman, E.; Ionkin, A.; Ittel, S.; Kunitsky, K.; Marshall, W.; et al. Copolymerization of ethylene and acrylates by nickel catalysts. In Beyond Metallocenes: Next Generation Polmerization Catalysts; ACS Publications: Washington, DC, USA, 2003; Volume 857, pp. 131–142. [Google Scholar]
- Long, B.K.; Eagan, J.M.; Mulzer, M.; Coates, G.W. Semi-Crystalline Polar Polyethylene: Ester-Functionalized Linear Polyolefins Enabled by a Functional-Group-Tolerant, Cationic Nickel Catalyst. Angew. Chem. Int. Ed. 2016, 55, 7106–7110. [Google Scholar] [CrossRef]
- Chen, Z.; Leatherman, M.D.; Daugulis, O.; Brookhart, M. Nickel-Catalyzed Copolymerization of Ethylene and Vinyltrialkoxysilanes: Catalytic Production of Cross-Linkable Polyethylene and Elucidation of the Chain-Growth Mechanism. J. Am. Chem. Soc. 2017, 139, 16013–16022. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, P.; Baione, F.; Guerra, G.; Martinotto, L.; Albizzati, E. Polyethylene unit cell and crystallinity variations as a consequence of different cross-linking processes. Macromolecules 2001, 34, 5175–5179. [Google Scholar] [CrossRef]
- Khonakdar, H.A.; Morshedian, J.; Wagenknecht, U.; Jafari, S.H. An investigation of chemical crosslinking effect on properties of high-density polyethylene. Polymer 2003, 44, 4301–4309. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Grimme, S. Accurate description of van der Waals complexes by density functional theory including empirical corrections. J. Comput. Chem. 2004, 25, 1463–1473. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S. Density functional theory with London dispersion corrections. WIREs Comput. Mol. Sci. 2011, 1, 211–228. [Google Scholar] [CrossRef]
- Ehrlich, S.; Moellmann, J.; Grimme, S. Dispersion-Corrected Density Functional Theory for Aromatic Interactions in Complex Systems. Acc. Chem. Res. 2013, 46, 916–926. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Performance of SM6, SM8, and SMD on the SAMPL1 Test Set for the Prediction of Small-Molecule Solvation Free Energies. J. Phys. Chem. B 2009, 113, 4538–4543. [Google Scholar] [CrossRef]
- Legault, C.Y. CYLview20. Available online: http://www.cylview.org (accessed on 31 October 2020).
- Sun, J.; Chen, M.; Luo, G.; Chen, C.; Luo, Y. Diphosphazane-monoxide and Phosphine-sulfonate Palladium Catalyzed Ethylene Copolymerization with Polar Monomers: A Computational Study. Organometallics 2019, 38, 638–646. [Google Scholar] [CrossRef]
- Yan, M.; Kang, X.; Li, S.; Xu, X.; Luo, Y.; He, S.; Chen, C. Mechanistic Studies on Nickel-Catalyzed Ethylene Polymerization: Ligand Effects and Quantitative Structure–Activity Relationship Model. Organometallics 2022, 41, 3212–3218. [Google Scholar] [CrossRef]
- Nozaki, K.; Kusumoto, S.; Noda, S.; Kochi, T.; Chung, L.W.; Morokuma, K. Why Did Incorporation of Acrylonitrile to a Linear Polyethylene Become Possible? Comparison of Phosphine−Sulfonate Ligand with Diphosphine and Imine−Phenolate Ligands in the Pd-Catalyzed Ethylene/Acrylonitrile Copolymerization. J. Am. Chem. Soc. 2010, 132, 16030–16042. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Luo, Y.; Chen, C. A Second-Coordination-Sphere Strategy to Modulate Nickel- and Palladium-Catalyzed Olefin Polymerization and Copolymerization. Angew. Chem. Int. Ed. 2017, 56, 11604–11609. [Google Scholar] [CrossRef]
- Mehmood, A.; Xu, X.W.; Raza, W.; Kim, K.H.; Luo, Y. Mechanistic Studies for Palladium Catalyzed Copolymerization of Ethylene with Vinyl Ethers. Polymers 2020, 12, 2401. [Google Scholar] [CrossRef]
- Chen, M.; Chen, C. A Versatile Ligand Platform for Palladium- and Nickel-Catalyzed Ethylene Copolymerization with Polar Monomers. Angew. Chem. Int. Ed. 2018, 57, 3094–3098. [Google Scholar] [CrossRef]
- Rünzi, T.; Guironnet, D.; Göttker-Schnetmann, I.; Mecking, S. Reactivity of methacrylates in insertion polymerization. J. Am. Chem. Soc. 2010, 132, 16623–16630. [Google Scholar] [CrossRef]
- Williams, B.S.; Leatherman, M.D.; White, P.S.; Brookhart, M. Reactions of Vinyl Acetate and Vinyl Trifluoroacetate with Cationic Diimine Pd(II) and Ni(II) Alkyl Complexes: Identification of Problems Connected with Copolymerizations of These Monomers with Ethylene. J. Am. Chem. Soc. 2005, 127, 5132–5146. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).