Evaluate the Structural and Physicochemical Properties of Exopolysaccharides Produced by Bacillus halotolerans Isolated from Locally Sourced Vegetables
Abstract
1. Introduction
2. Method
2.1. Preserved Dried Cabbages of Tianjin
2.2. Screening for EPS-Producing Microorganisms
2.3. Identification of the Strain
2.4. Isolation and Purification of EPS
2.5. Determination of the Relative Molecular Weight (MW) of EPS
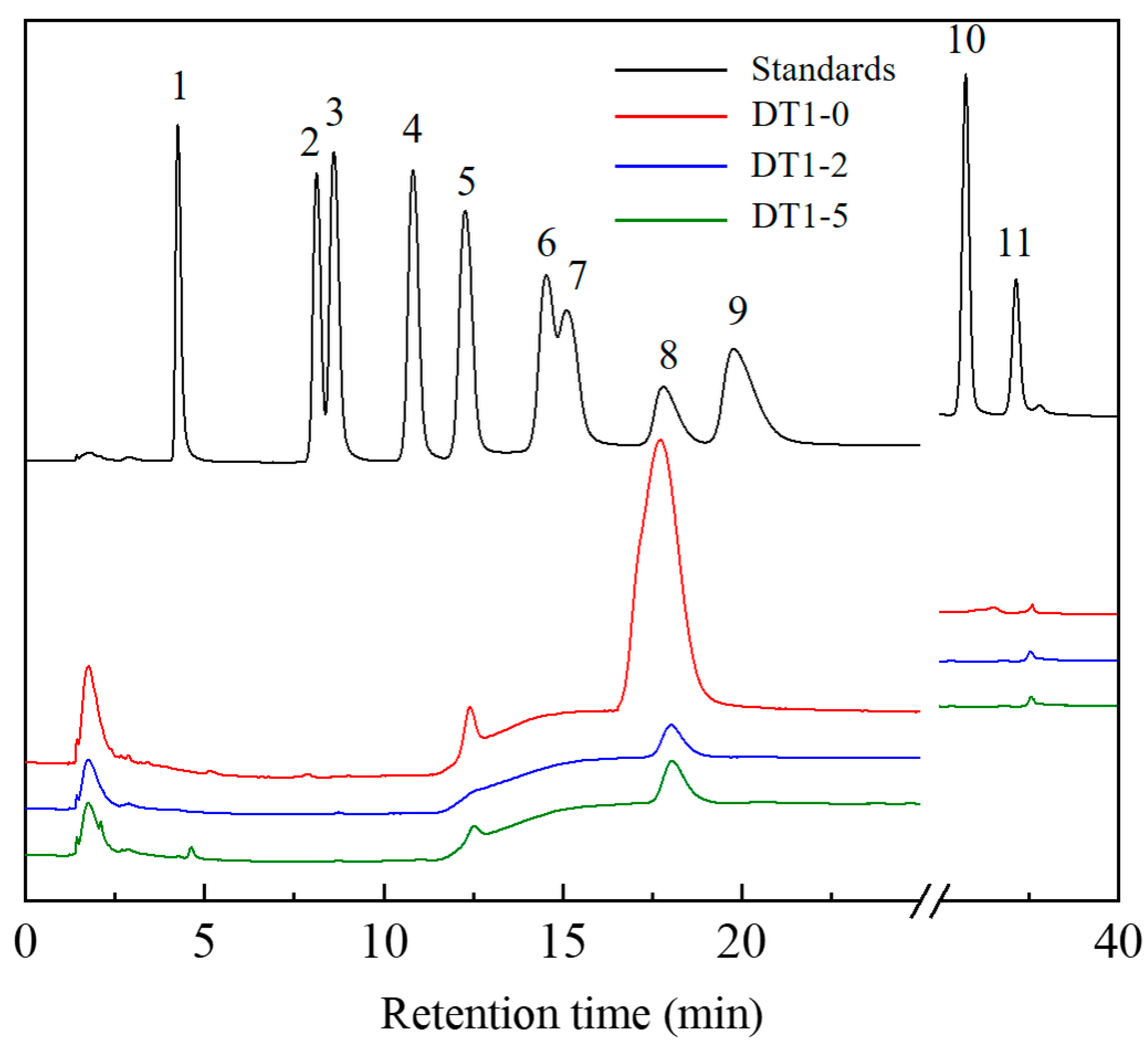
2.6. Monosaccharide Composition Analysis
2.7. Glycosidic Linkage Analysis
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
2.9. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.10. Thermal Stability Assessment
2.11. Scanning Electron Microscopy (SEM) Analysis
2.12. Atomic Force Microscopy (AFM) Analysis
2.13. Water Solubility Index (WSI) Determination
2.14. Water-Holding Capacity (WHC) Determination
2.15. Oil-Holding Capacity (OHC) Determination
3. Results and Discussion
3.1. Identification of the Selected Strains
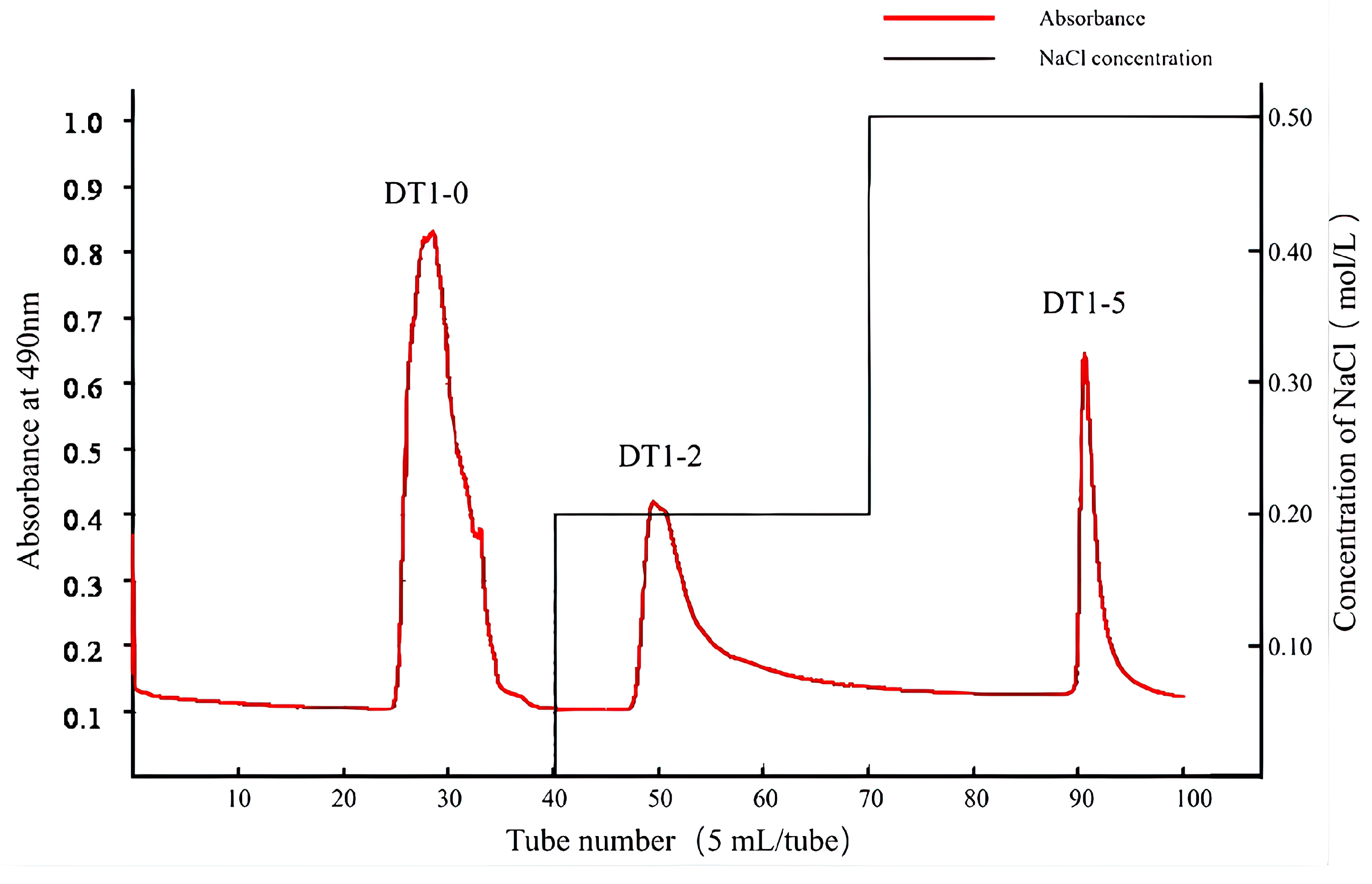
3.2. Purification and Chemical Composition of Polysaccharides DT1-0, DT1-2, and DT1-5
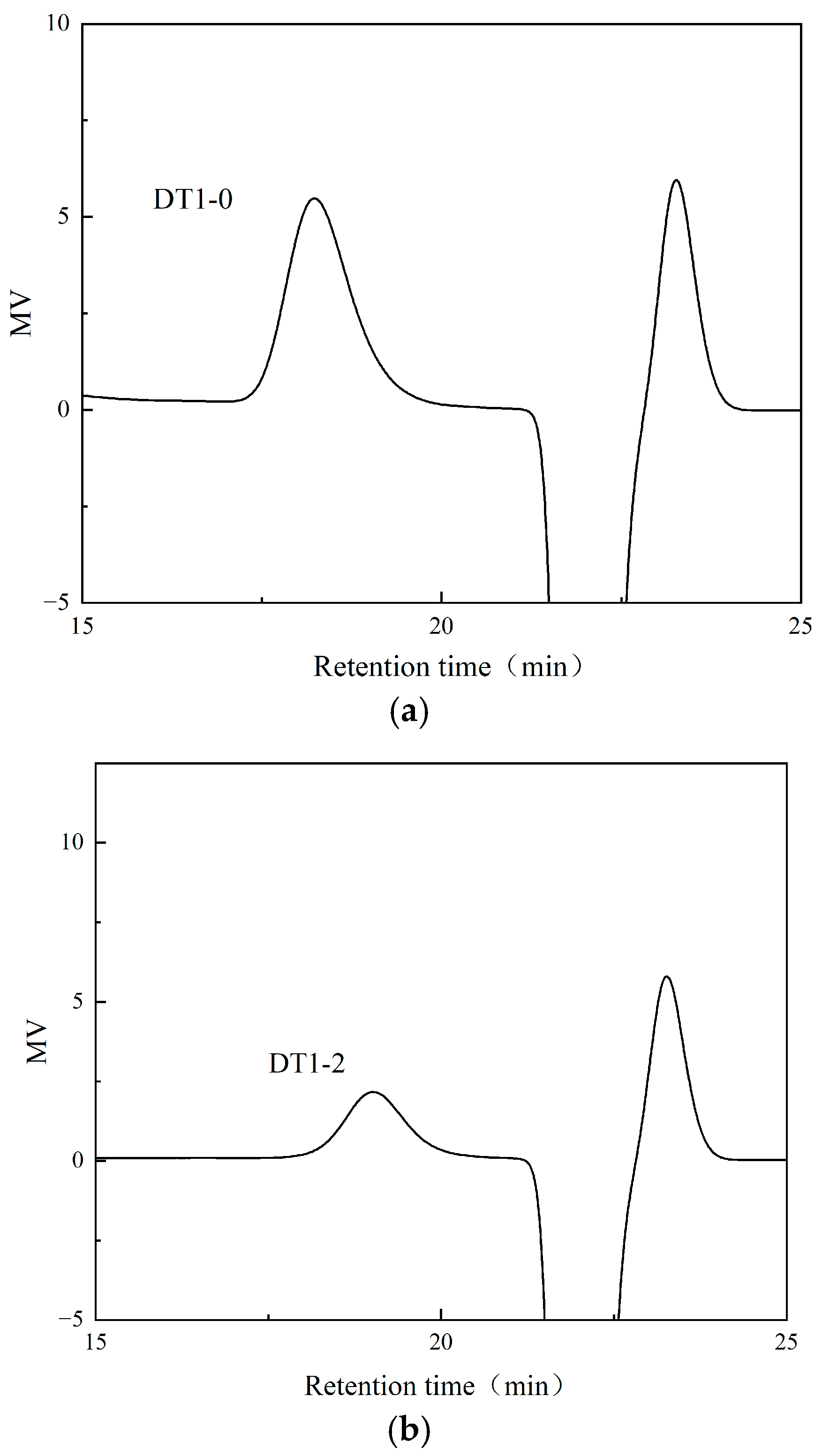
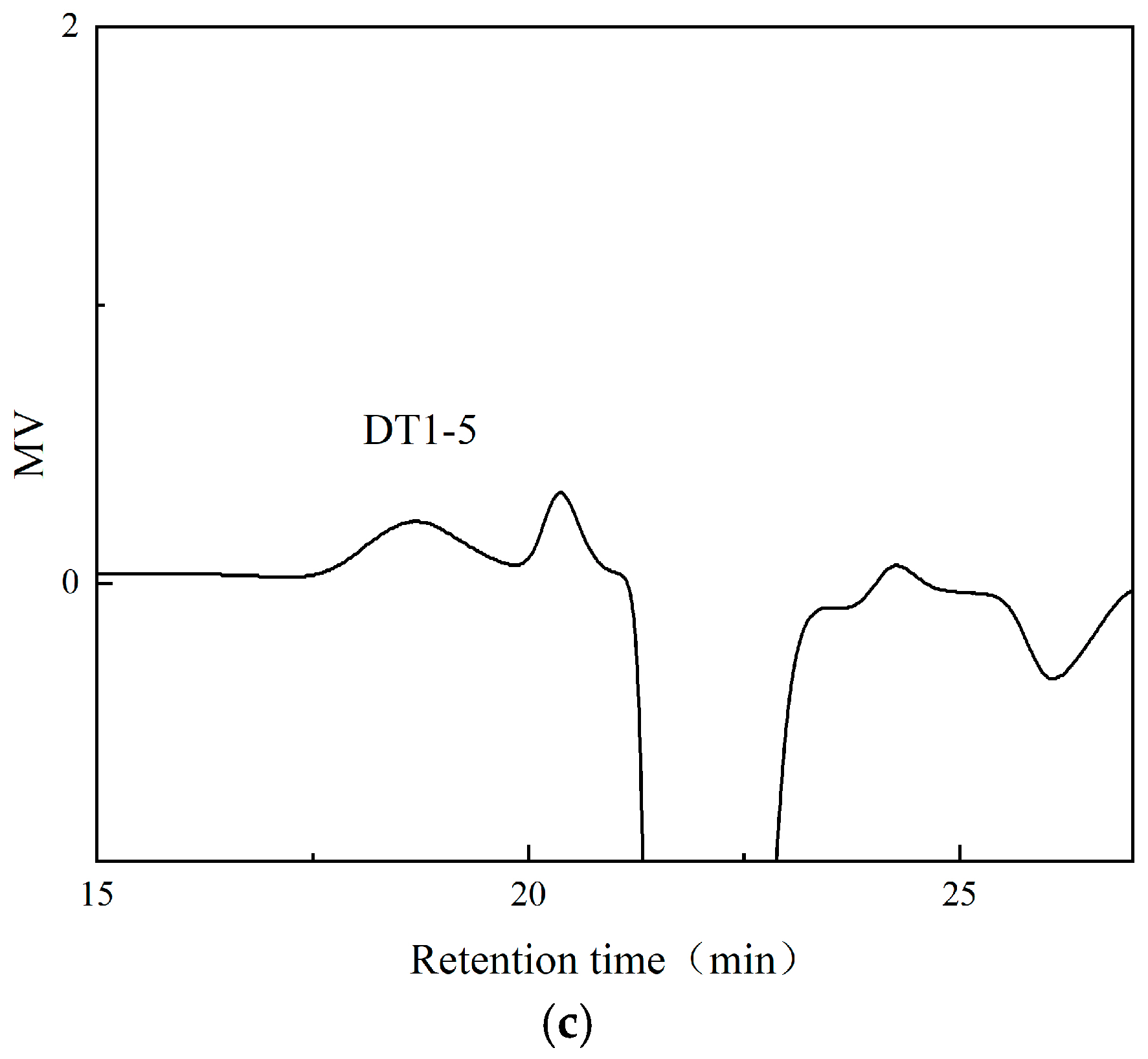
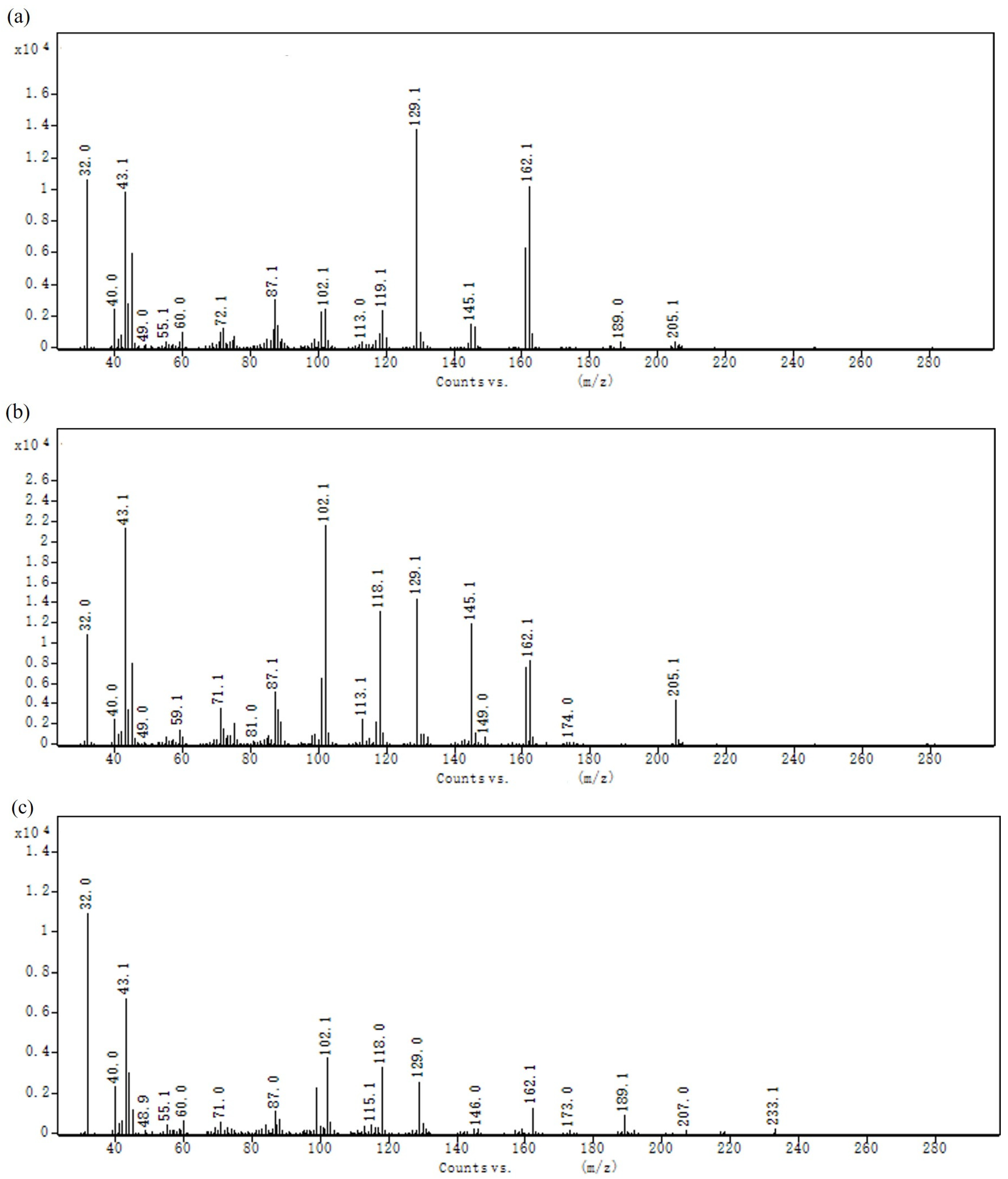
3.3. Analysis of EPS Molecular Weight (Mw) and Monosaccharide Composition and Glycosidic Linkage
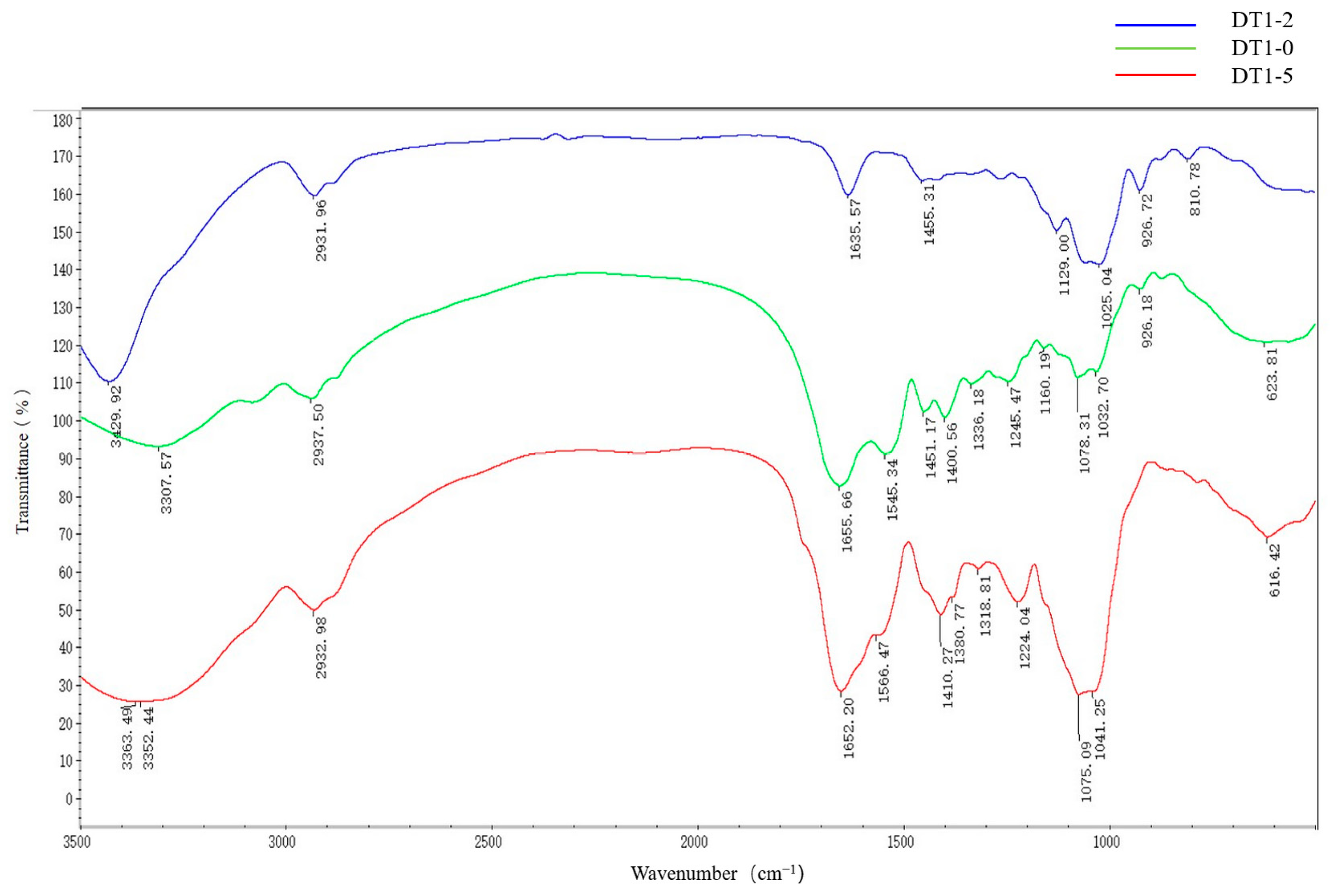
3.4. Fourier-Transform Infrared (FTIR) Analysis
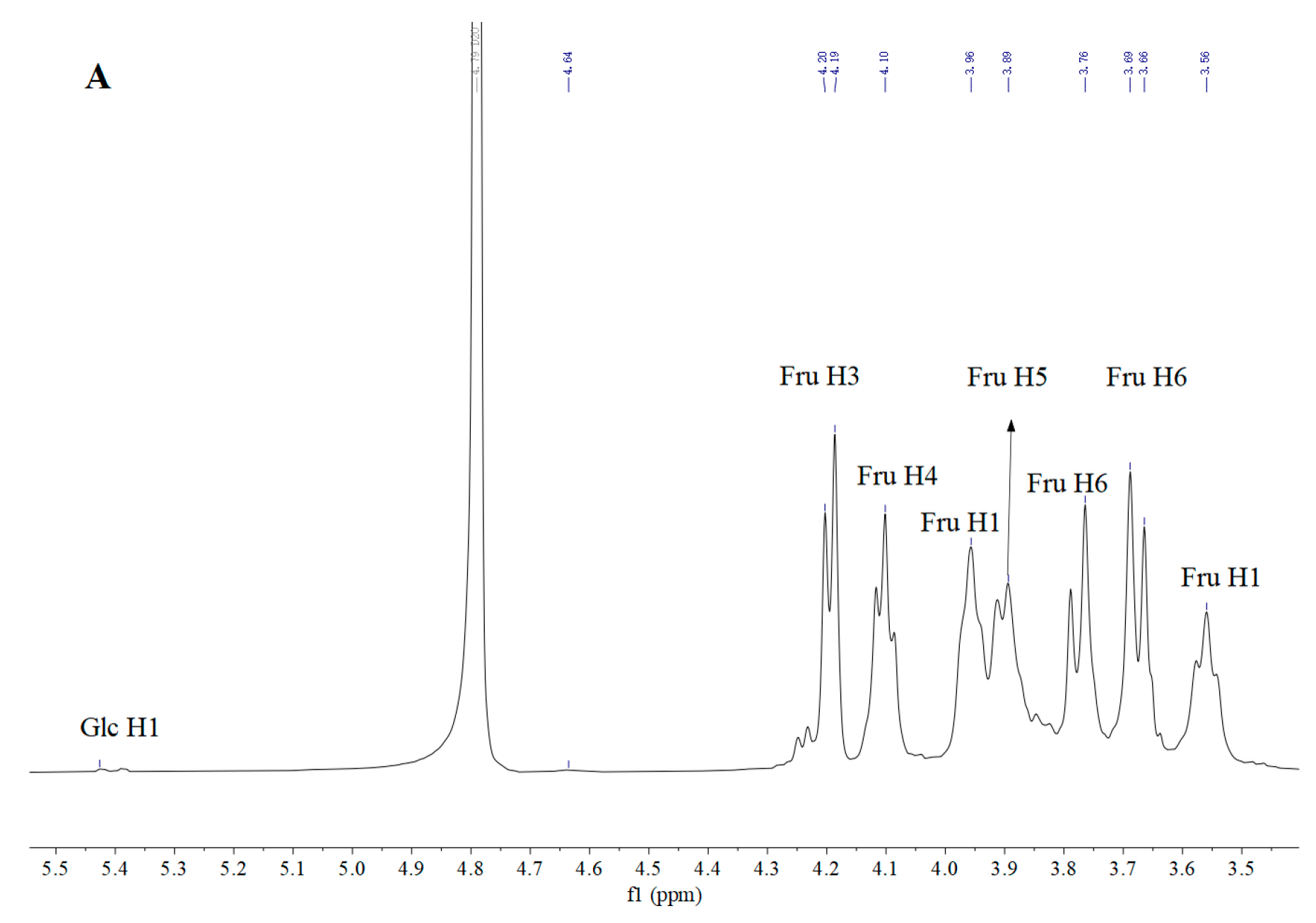
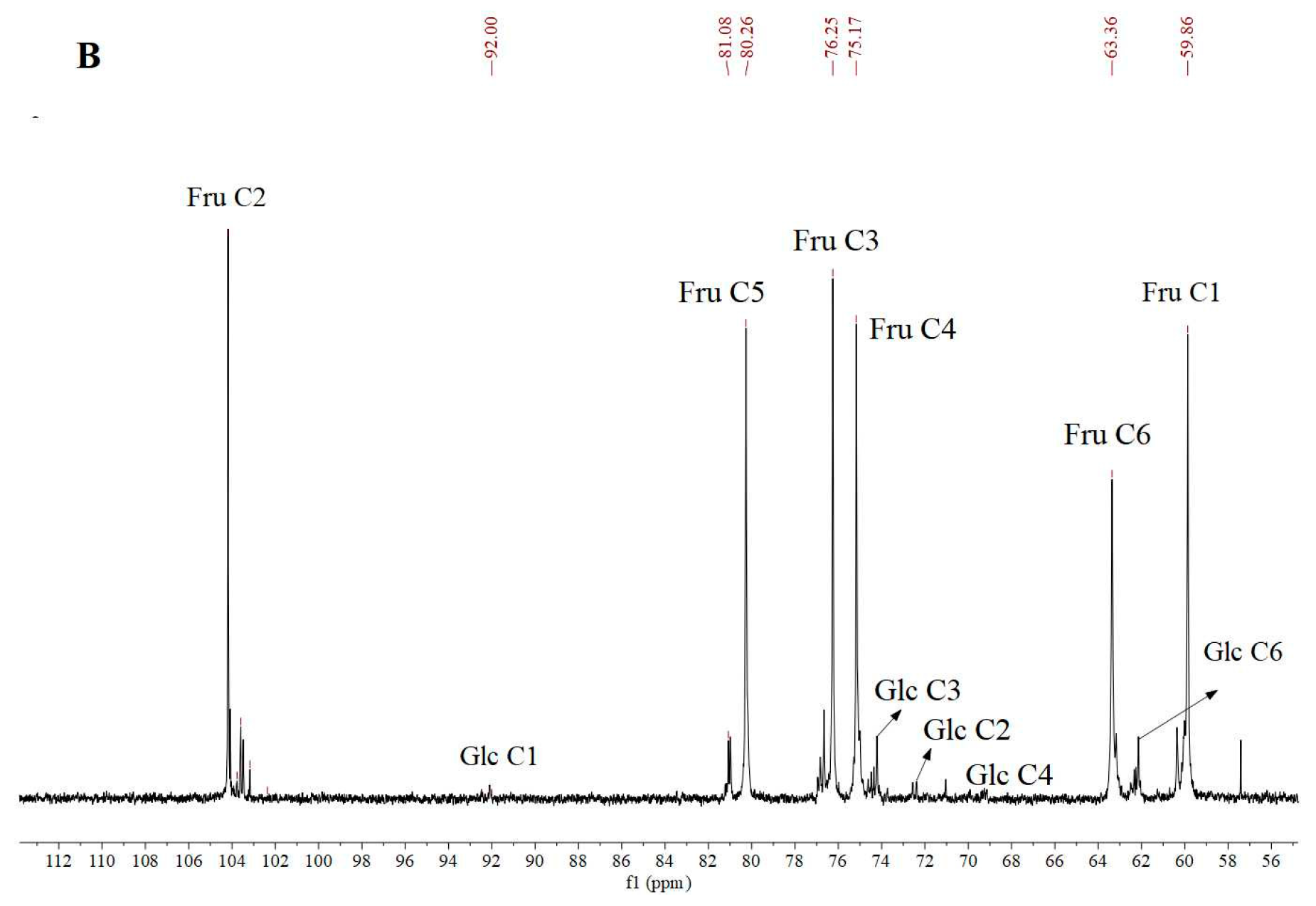
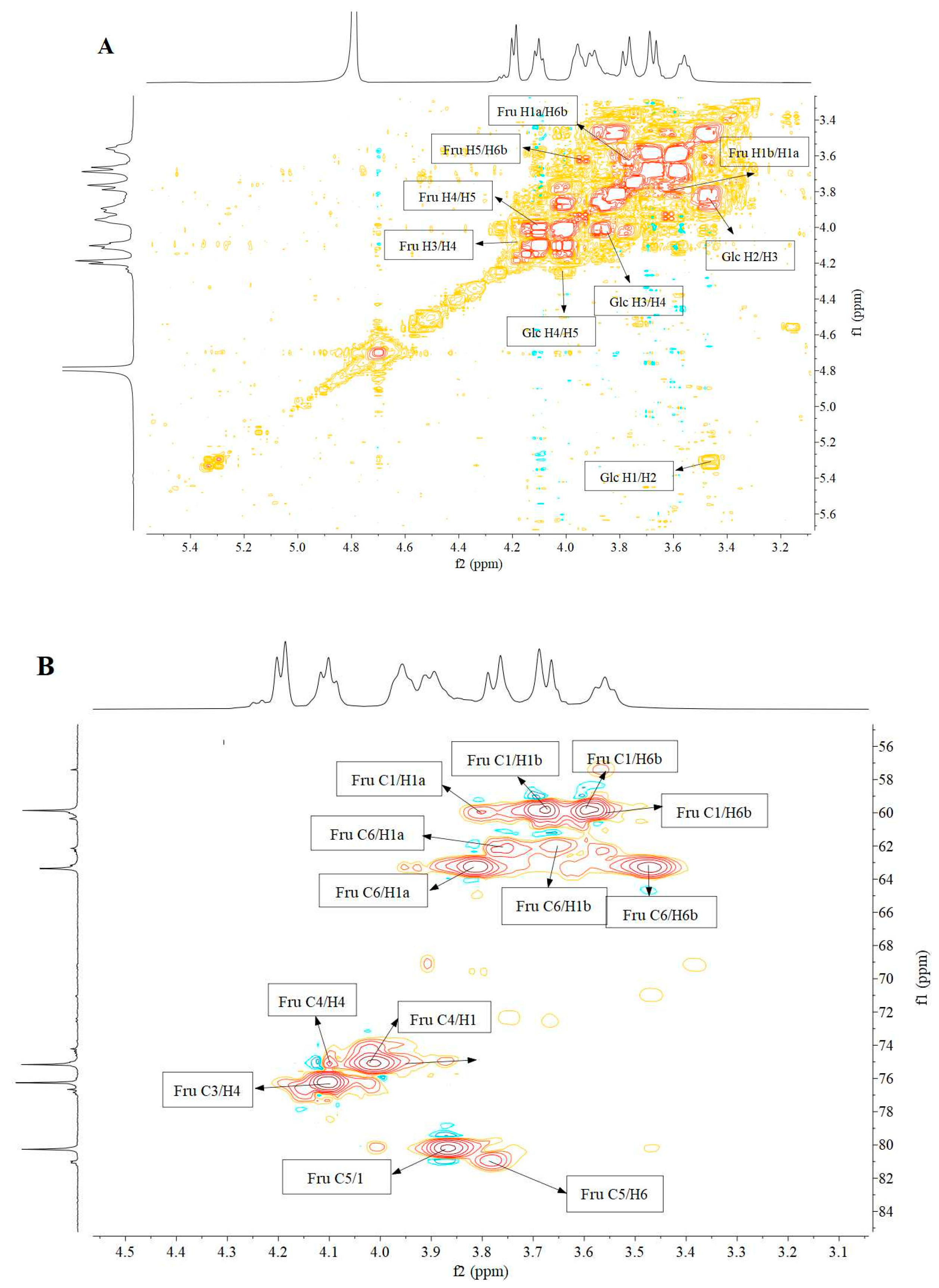
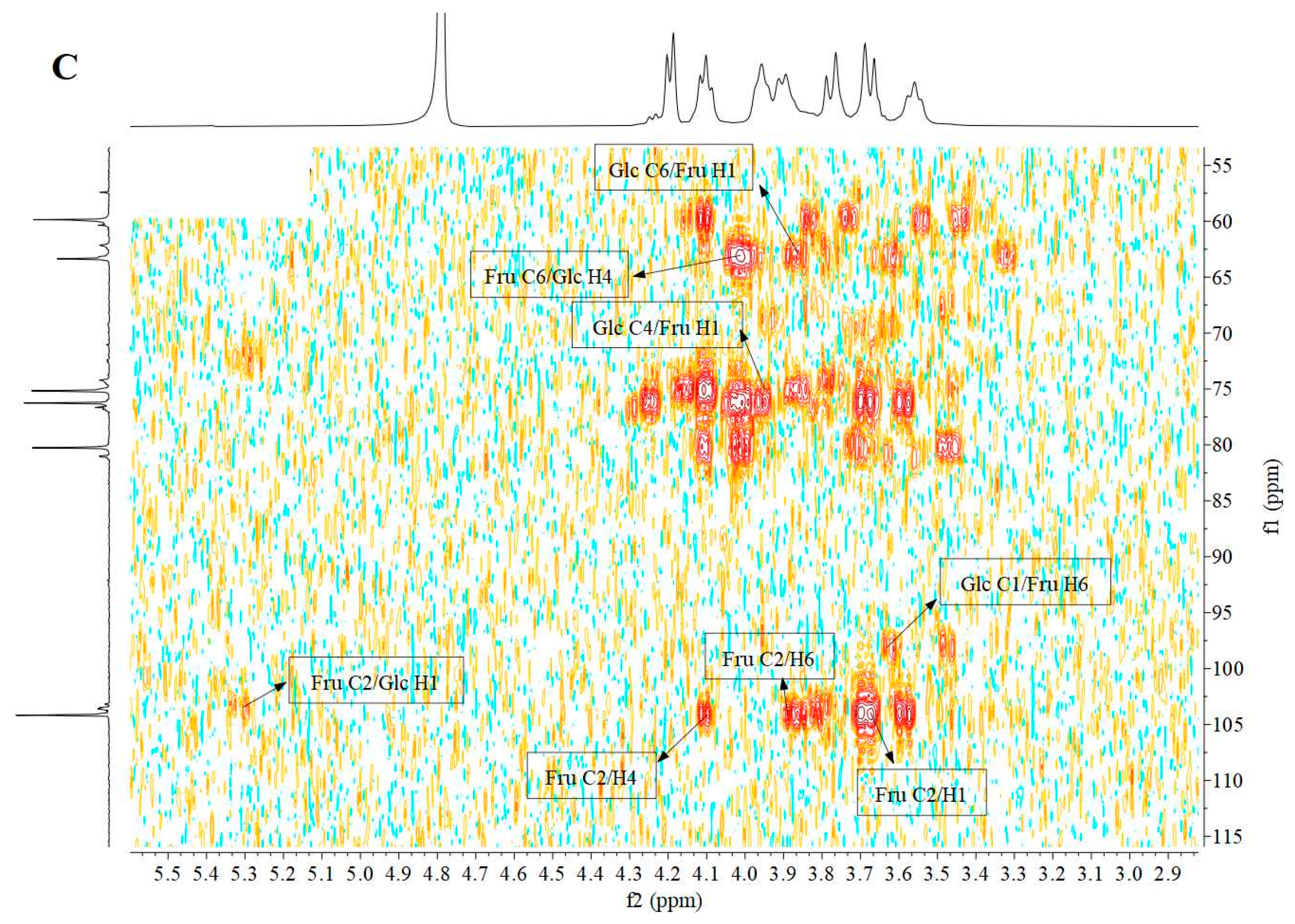
3.5. Nuclear Magnetic Resonance (NMR) Analysis
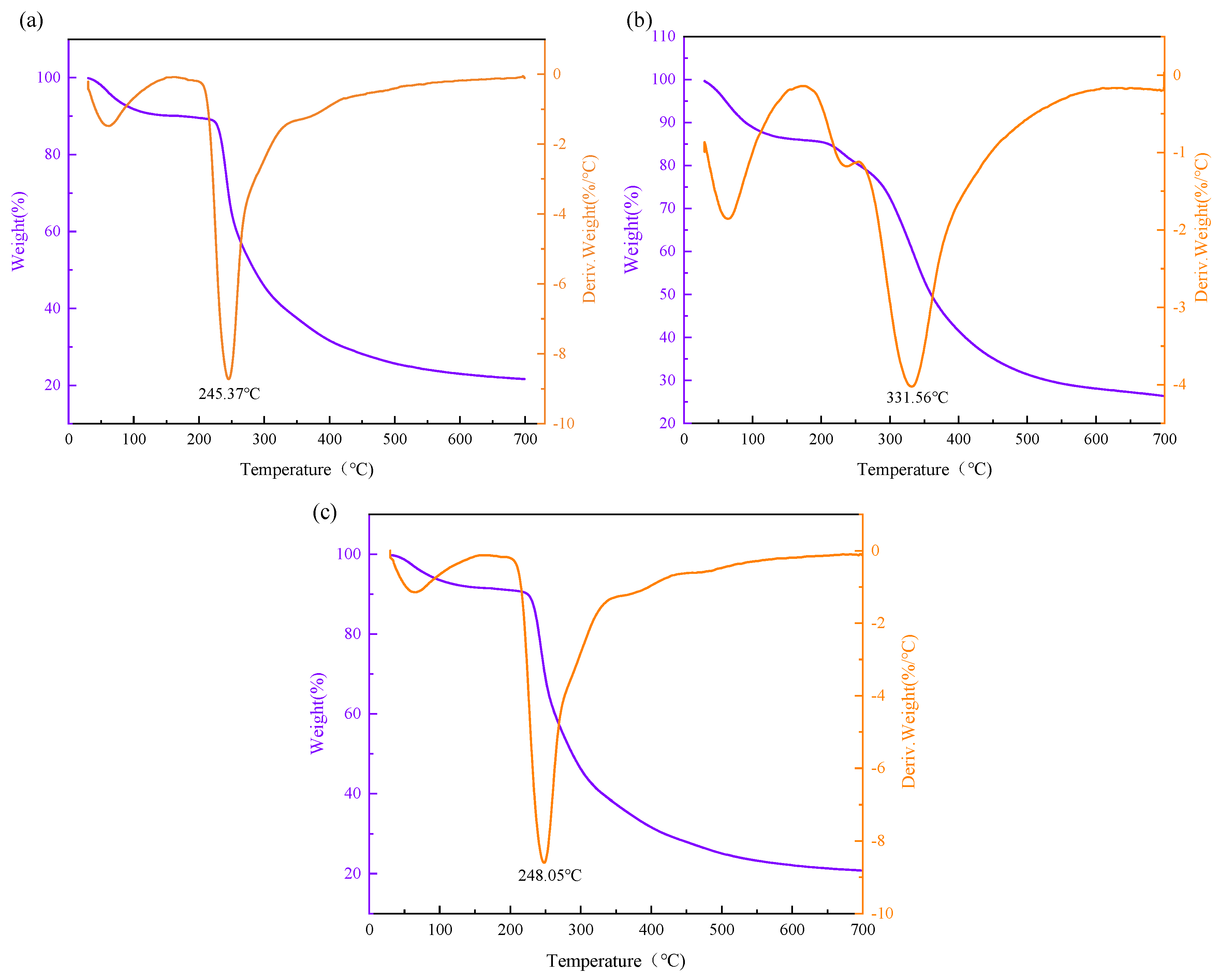
3.6. Thermal Stability Analysis
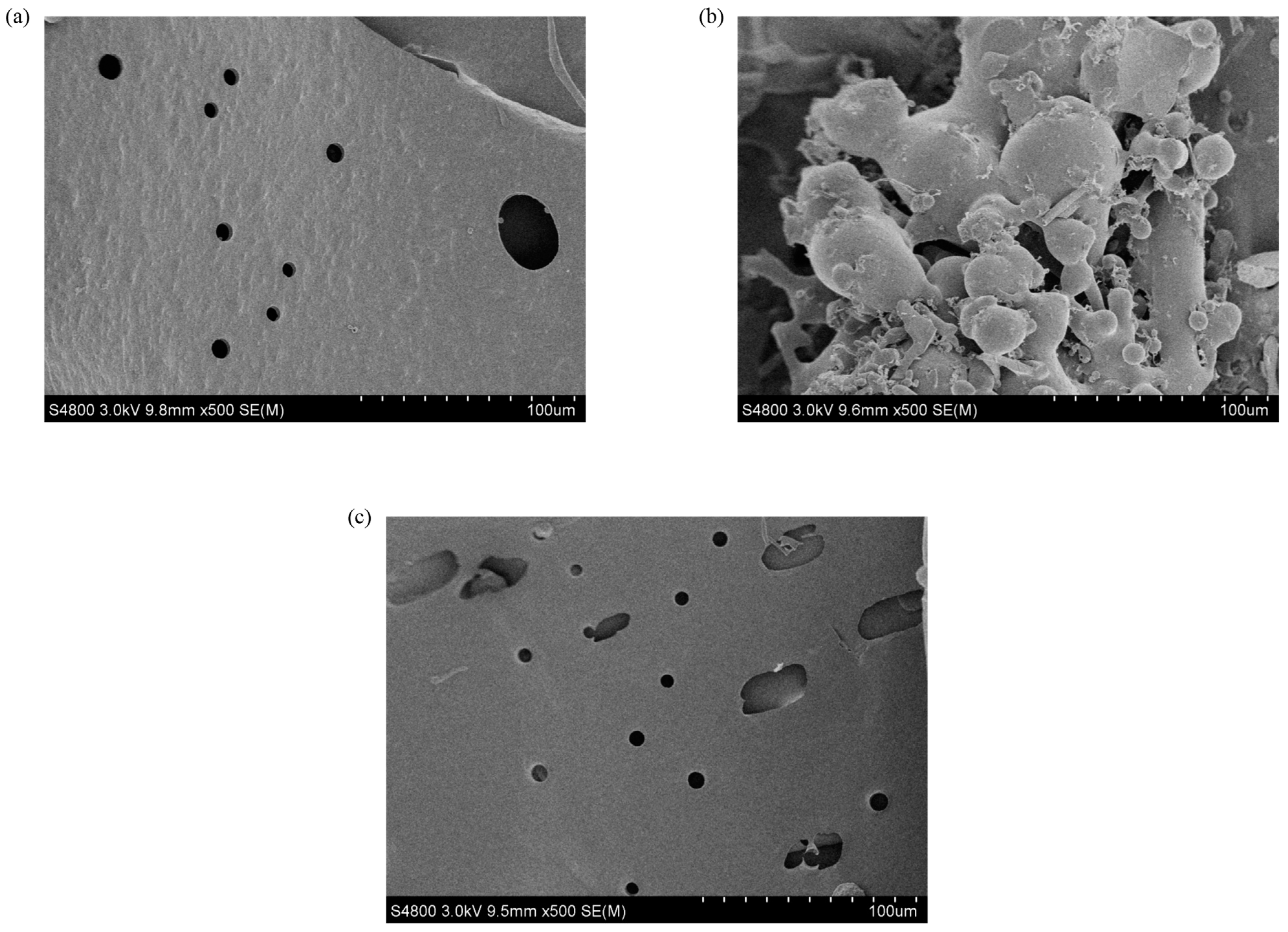
3.7. Scanning Electron Microscopy (SEM)
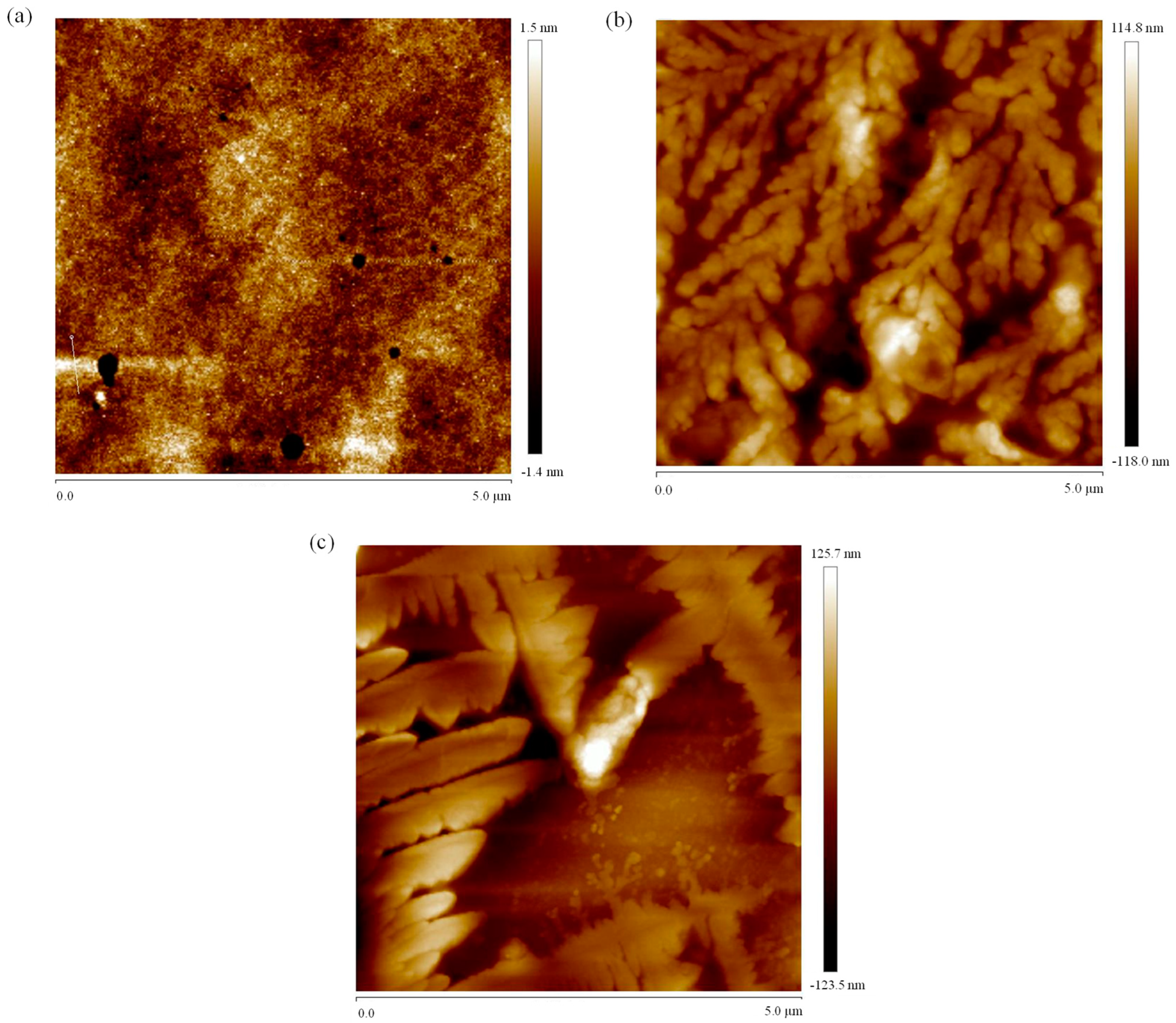
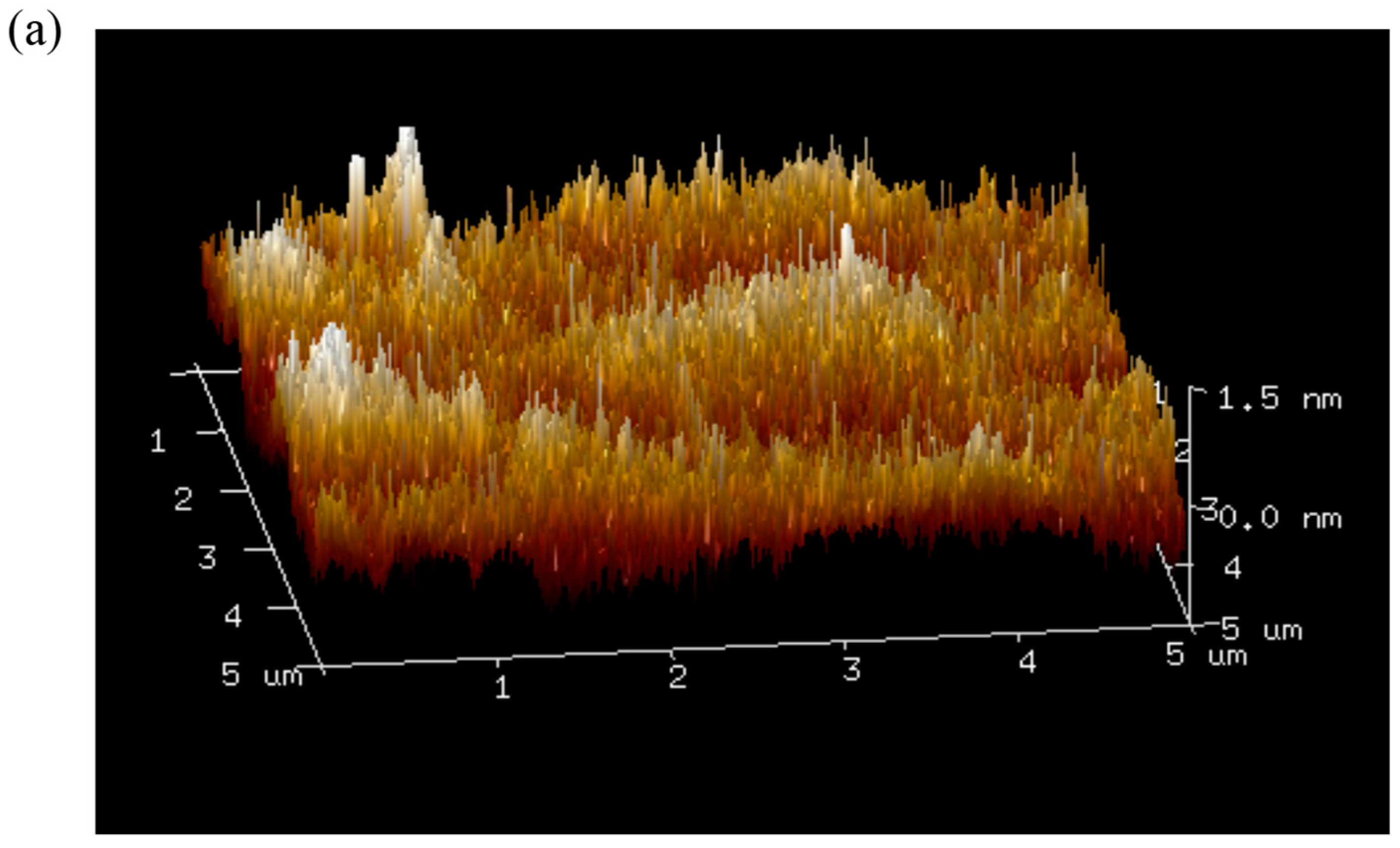
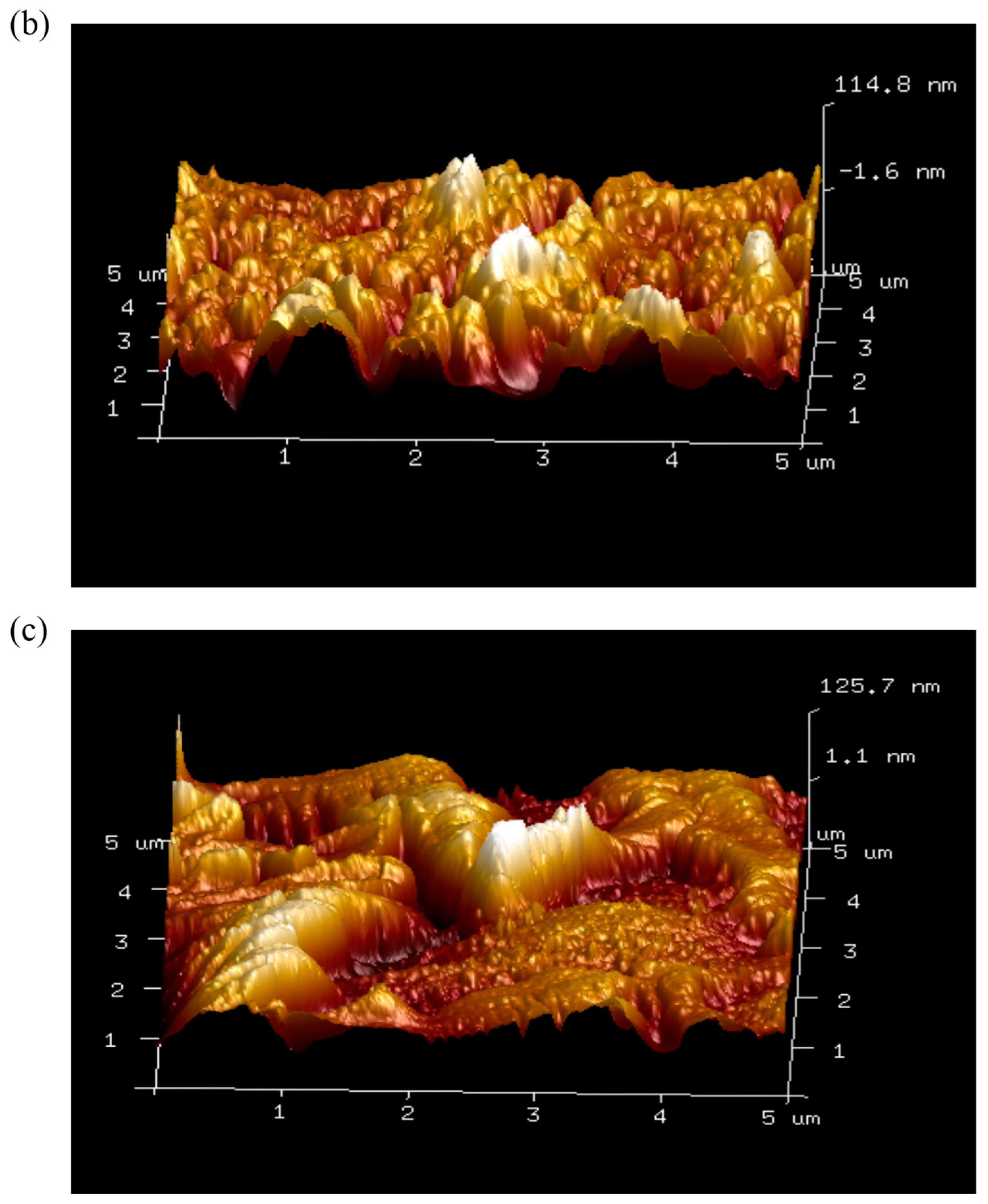
3.8. Atomic Force Microscopy Imaging
3.9. Water Solubility Index (WSI)
3.10. Water-Holding Capacity Evaluation
3.11. Lipophilicity Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Song, C. Tianjin winter vegetable traditional production technology. Nongcun Baishitong 2019, 20, 44–45. (In Chinese) [Google Scholar] [CrossRef]
- Park, W.J.; Kong, S.J.; Park, J.H. Kimchi bacteriophages of lactic acid bacteria: Population, characteristics, and their role in watery kimchi. Food Sci. Biotechnol. 2021, 30, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Ragul, K.; Syiem, I.; Sundar, K.; Shetty, P.H. Characterization of probiotic potential of Bacillus species isolated from a traditional brine pickle. J. Food Sci. Technol. 2017, 54, 4473–4483. [Google Scholar] [CrossRef]
- Moshabaki Isfahani, F.; Tahmourespour, A.; Hoodaji, M.; Ataabadi, M.; Mohammadi, A. Characterizing the new bacterial isolates of high yielding exopolysaccharides under hypersaline conditions. J. Clean. Prod. 2018, 185, 922–928. [Google Scholar] [CrossRef]
- Hu, X.; Pang, X.; Wang, P.G.; Chen, M. Isolation and characterization of an antioxidant exopolysaccharide produced by Bacillus sp. S-1 from Sichuan Pickles. Carbohydr. Polym. 2019, 204, 9–16. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, G.; Wang, F.; Zhao, H.; Wei, Y.; Liu, L.; Zhang, H. Extraction, characterization, antioxidant activity and rheological behavior of a polysaccharide produced by the extremely salt tolerant Bacillus subtilis LR-1. LWT 2022, 162, 113413. [Google Scholar] [CrossRef]
- Gan, L.; Jiang, G.; Li, X.; Zhang, S.; Tian, Y.; Peng, B. Structural elucidation and physicochemical characteristics of a novel high-molecular-weight fructan from halotolerant Bacillus sp. SCU-E108. Food Chem. 2021, 365, 130496. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Dong, F.; Yan, F.; Cheng, M.; Li, W.; Chang, Q.; Song, T.; Liu, A.; Song, B. Therapeutic Effect of Calcipotriol Pickering Nanoemulsions Prepared by Exopolysaccharides Produced by Bacillus halotolerans FYS Strain on Psoriasis. Int. J. Nanomed. 2020, 15, 10371–10384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Ni, Z.J.; Zhang, F.; Thakur, K.; Zhang, J.G.; Khan, M.R.; Busquets, R.; Wei, Z.J. Physicochemical and antioxidant properties of Lycium barbarum seed dreg polysaccharides prepared by continuous extraction. Food Chem. X 2022, 14, 100282. [Google Scholar] [CrossRef]
- Xu, M.; Pan, L.; Zhou, Z.; Han, Y. Structural characterization of levan synthesized by a recombinant levansucrase and its application as yogurt stabilizers. Carbohydr. Polym. 2022, 291, 119519. [Google Scholar] [CrossRef]
- Liang, L. Structure and Characterization of Dextran Produced by Leuconostoc citreum Using Molasses as a Carbon Source. Master’s thesis, Tianjin University, Tianjin, China, 2022. [Google Scholar]
- Lin, X.; Ji, X.; Wang, M.; Yin, S.; Peng, Q. An alkali-extracted polysaccharide from Zizyphus jujuba cv. Muzao: Structural characterizations and antioxidant activities. Int. J. Biol. Macromol. 2019, 136, 607–615. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Tian, Z.; Yang, Y.; Yang, Z. Characterization of an exopolysaccharide produced by Lactobacillus plantarum YW11 isolated from Tibet Kefir. Carbohydr. Polym. 2015, 125, 16–25. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Rui, X.; Chen, X.; Jiang, M.; Dong, M. Structural characterization and bioactivity of released exopolysaccharides from Lactobacillus plantarum 70810. Int. J. Biol. Macromol. 2014, 67, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Pang, D.J.; Huang, C.; Chen, M.L.; Chen, Y.L.; Fu, Y.P.; Paulsen, B.S.; Rise, F.; Zhang, B.Z.; Chen, Z.L.; Jia, R.Y.; et al. Characterization of Inulin-Type Fructan from Platycodon grandiflorus and Study on Its Prebiotic and Immunomodulating Activity. Molecules 2019, 24, 1199. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Xiong, Y.; Liu, M.; Zeng, D.; Song, C.; Baranenko, D.; Cheng, D.; Lu, W. Structural characterization and immunomodulatory activity of a new polysaccharide isolated from the radix of Platycodon grandiflorum. Int. J. Food Sci. Technol. 2021, 56, 2662–2674. [Google Scholar] [CrossRef]
- Gupta, J.; Rathour, R.; Dupont, C.L.; Kaul, D.; Thakur, I.S. Genomic insights into waste valorized extracellular polymeric substances (EPS) produced by Bacillus sp. ISTL8. Environ. Res. 2021, 192, 110277. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lin, L.; Zhao, M. Preparation, chemical composition, glycolipid-lowering activity and functional property of high-purity polysaccharide from Moringa oleifera Lam. leaf: A novel plant-based functional hydrophilic colloid. Food Hydrocoll. 2023, 142, 108857. [Google Scholar] [CrossRef]
- Cai, G.; Liu, Y.; Li, X.; Lu, J. New Levan-Type Exopolysaccharide from Bacillus amyloliquefaciens as an Antiadhesive Agent against Enterotoxigenic Escherichia coli. J. Agric. Food Chem. 2019, 67, 8029–8034. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, S.; Rajendran, K. Structural and Functional Characterization of Exopolysaccharide Produced by a Novel Isolate Bacillus sp. EPS003. Appl. Biochem. Biotechnol. 2023, 195, 4583–4601. [Google Scholar] [CrossRef]
- Ye, G.; Chen, Y.; Wang, C.; Yang, R.; Bin, X. Purification and characterization of exopolysaccharide produced by Weissella cibaria YB-1 from pickle Chinese cabbage. Int. J. Biol. Macromol. 2018, 120 Pt A, 1315–1321. [Google Scholar] [CrossRef]
- Ye, G.; Li, G.; Wang, C.; Ling, B.; Yang, R.; Huang, S. Extraction and characterization of dextran from Leuconostoc pseudomesenteroides YB-2 isolated from mango juice. Carbohydr. Polym. 2019, 207, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Kumari, S.; Kurmi, S.; Pinnaka, A.K.; Choudhury, A.R. Isolation, purification, and characterization of a novel exopolysaccharide isolated from marine bacteria Brevibacillus borstelensis M42. Arch. Microbiol. 2022, 204, 399. [Google Scholar] [CrossRef]
- Malick, A.; Khodaei, N.; Benkerroum, N.; Karboune, S. Production of exopolysaccharides by selected Bacillus strains: Optimization of media composition to maximize the yield and structural characterization. Int. J. Biol. Macromol. 2017, 102, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]


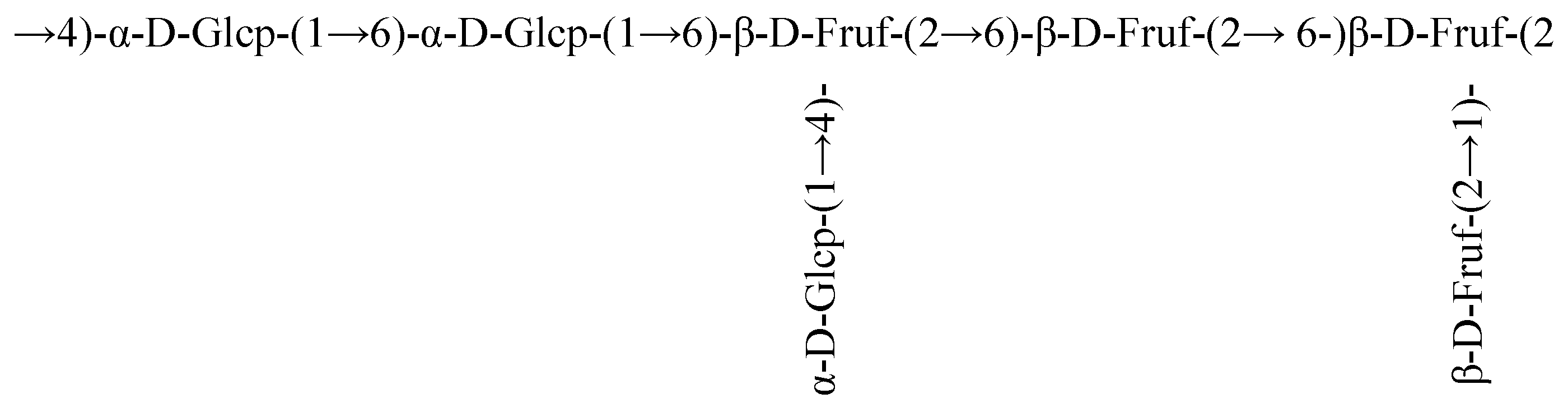
| Types of Polysaccharides | Mw | Mn | Mz | Mp |
|---|---|---|---|---|
| DT1-0 | 4253 | 3996 | 4098 | 3876 |
| DT1-2 | 1722 | 1326 | 2164 | 1636 |
| DT1-5 | 2582 | 2069 | 3181 | 2416 |
| Residue | (PMAA) | Mw | Peak Area | Peak Height | Peak Width | Relative Hazard Molecular Weight | Relative Molar Ratio (%) |
|---|---|---|---|---|---|---|---|
| Fruf-(2→ | 2,5-di-O-acetyl-1,3,4,6-tetra-O-methyl mannitol | 323 | 1,516,418.11 | 457,566.68 | 0.383 | 4694.793 | 7.815 |
| 323 | |||||||
| Glcp-(1→ | 1,5-di-O-acetyl-2,3,4,6-tetra-O-methyl glucitol | 323 | 2,132,435.17 | 611,101.17 | 0.104 | 6601.966 | 10.990 |
| →6)-Fruf-(2→ | 2,5,6-tri-O-acetyl-1,3,4-tri-O-methyl mannitol | 351 | 15,878,216.23 | 4,374,779.81 | 0.376 | 45,237.083 | 75.304 |
| 351 | |||||||
| →4)-Glcp-(1→ | 1,4,5-tri-O-acetyl-2,3,6-tri-O-methyl glucitol | 351 | 289,025 | 77,234.48 | 0.150 | 823.433 | 1.371 |
| →6)-Glcp-(1→ | 1,5,6-tri-O-acetyl-2,3,4-tri-O-methyl gluctiol | 351 | 189,399.83 | 49,851.83 | 0.181 | 539.601 | 0.898 |
| →1,6)-Fruf-(2→ | 1,2,5,6-tetra-O-acetyl-3,4-di-O-methyl-hexitol (mannitol, glucitol) | 379 | 824,629.15 | 136,153.77 | 0.239 | 2175.803 | 3.622 |
| Residue | δ13C/1H (ppm) | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| β-D-Fruf-(2→ | 59.96 3.77, 3.67 | 104.08 | 76.71 4.20 | 74.90 4.08 | 80.91 3.96 | 63.18 3.89, 3.55 |
| α-D-Glcp-(1→ | 92.12 5.42 | 74.21 | 72.37 4.23 | 71.04 4.09 | 61.21 3.94 | 57.42 3.90 |
| →6)-β-D-Fruf-(2→ | 59.81 3.76, 3.69 | 104.18 | 76.24 4.18 | 75.11 4.10 | 80.25 3.96 | 63.33 3.89, 3.56 |
| →4)-α-D-Glcp-(1→ | 92.53 5.38 | 74.35 | 72.58 4.23 | 70.96 4.11 | 61.15 3.94 | 57.27 3.90 |
| →6)-α-D-Glcp-(1→ | 92.27 5.26 | 74.47 | 72.55 4.25 | 71.16 4.09 | 61.36 3.94 | 57.48 3.90 |
| →1,6)-β-D-Fruf-(2→ | 60.17 3.79, 3.67 | 103.59 | 76.97 4.20 | 75.37 4.11 | 81.17 3.96 | 63.50 3.89, 3.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.; Xu, M.; Zhou, Z.; Han, Y. Evaluate the Structural and Physicochemical Properties of Exopolysaccharides Produced by Bacillus halotolerans Isolated from Locally Sourced Vegetables. Polymers 2024, 16, 759. https://doi.org/10.3390/polym16060759
Dai Y, Xu M, Zhou Z, Han Y. Evaluate the Structural and Physicochemical Properties of Exopolysaccharides Produced by Bacillus halotolerans Isolated from Locally Sourced Vegetables. Polymers. 2024; 16(6):759. https://doi.org/10.3390/polym16060759
Chicago/Turabian StyleDai, Yutian, Min Xu, Zhijiang Zhou, and Ye Han. 2024. "Evaluate the Structural and Physicochemical Properties of Exopolysaccharides Produced by Bacillus halotolerans Isolated from Locally Sourced Vegetables" Polymers 16, no. 6: 759. https://doi.org/10.3390/polym16060759
APA StyleDai, Y., Xu, M., Zhou, Z., & Han, Y. (2024). Evaluate the Structural and Physicochemical Properties of Exopolysaccharides Produced by Bacillus halotolerans Isolated from Locally Sourced Vegetables. Polymers, 16(6), 759. https://doi.org/10.3390/polym16060759






