Protective Effects of Carbonated Chitosan Montmorillonite on Vomitoxin-Induced Intestinal Inflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. In Vitro Adsorption of Vomitoxin by Intercalated Complexes
2.2.1. Preparation of the Intercalation Complexes
2.2.2. Characterization of the Intercalation Complexes
2.2.3. In Vitro Adsorption Experiments
2.3. In Vivo Adsorption of Vomitoxin by Intercalated Complexes
2.3.1. Sample Collection
2.3.2. Complete Blood Count
2.3.3. Measurement of Serum Biochemical Indicators
2.3.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.3.5. Histological Examination
2.3.6. Western Blotting Analysis
2.3.7. Statistical Analyses
3. Results
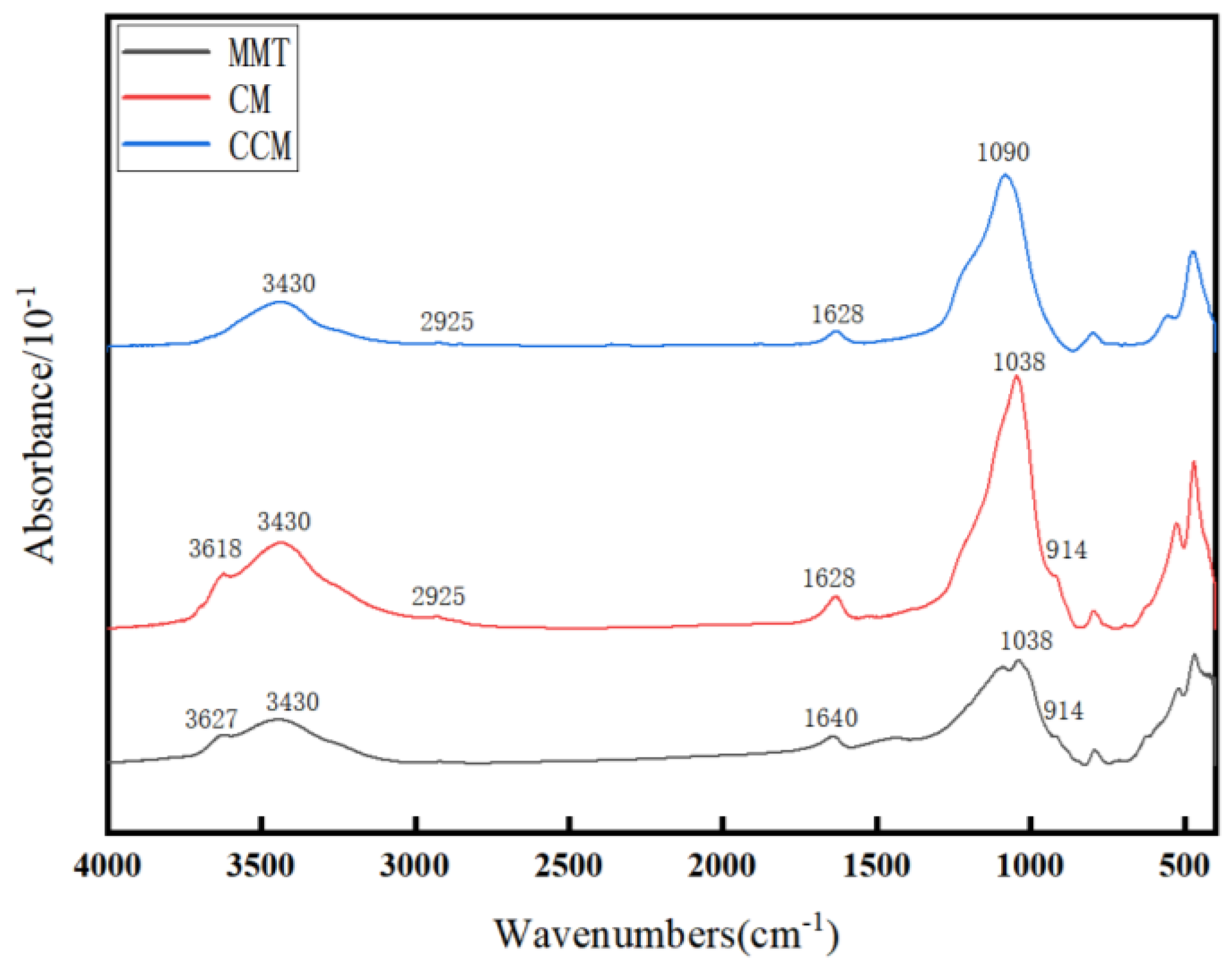
3.1. Characterization of CCM
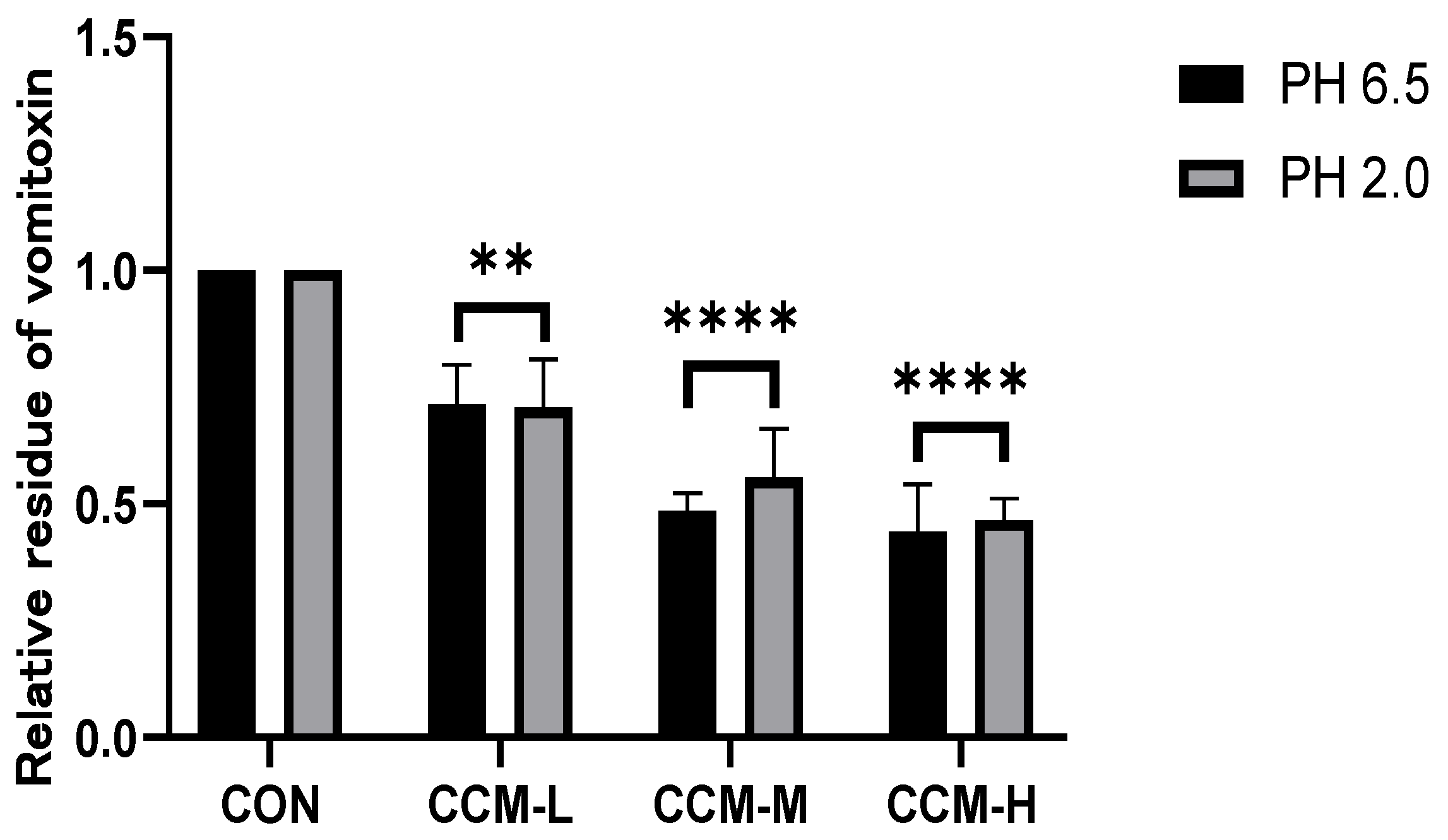
3.2. In Vitro Adsorption Results of DON by CCM
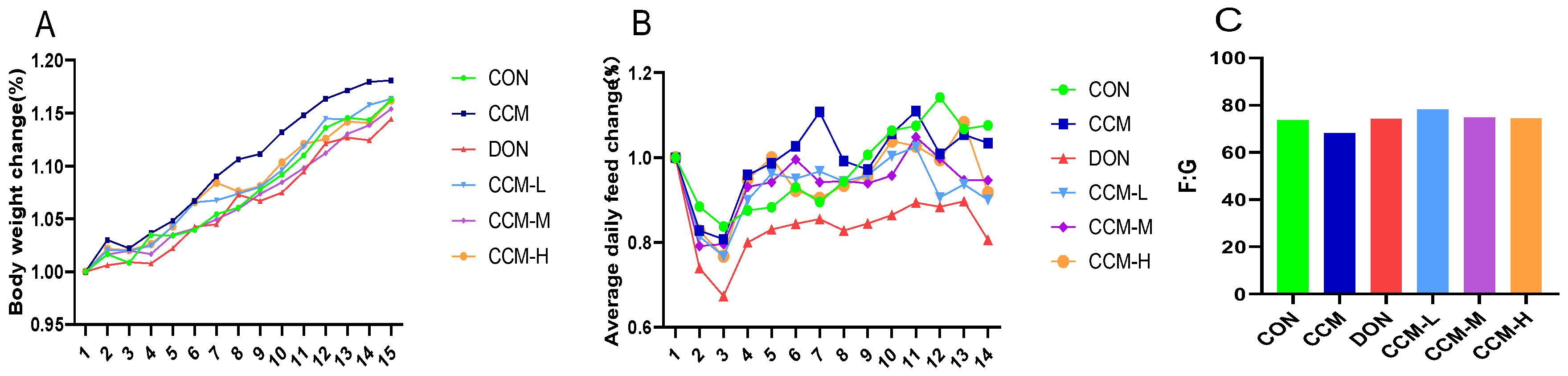
3.3. Effect of CCM on the Growth Performance of Mice
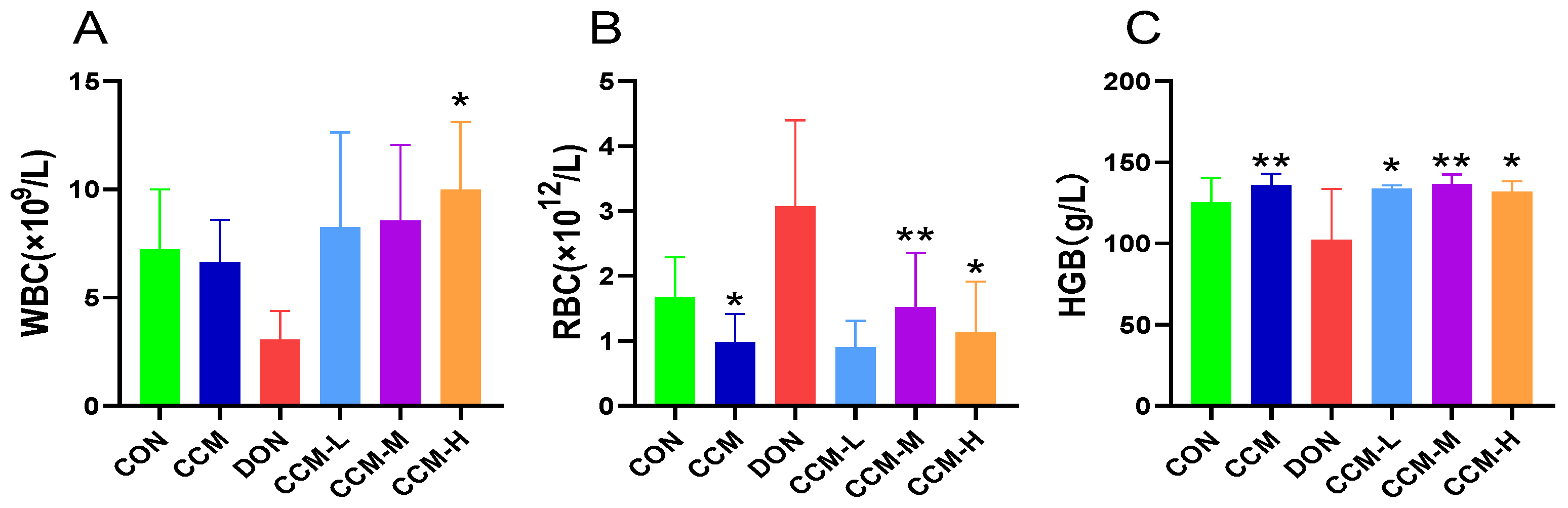
3.4. Effect of CCM on Routine Blood Indices in Mice
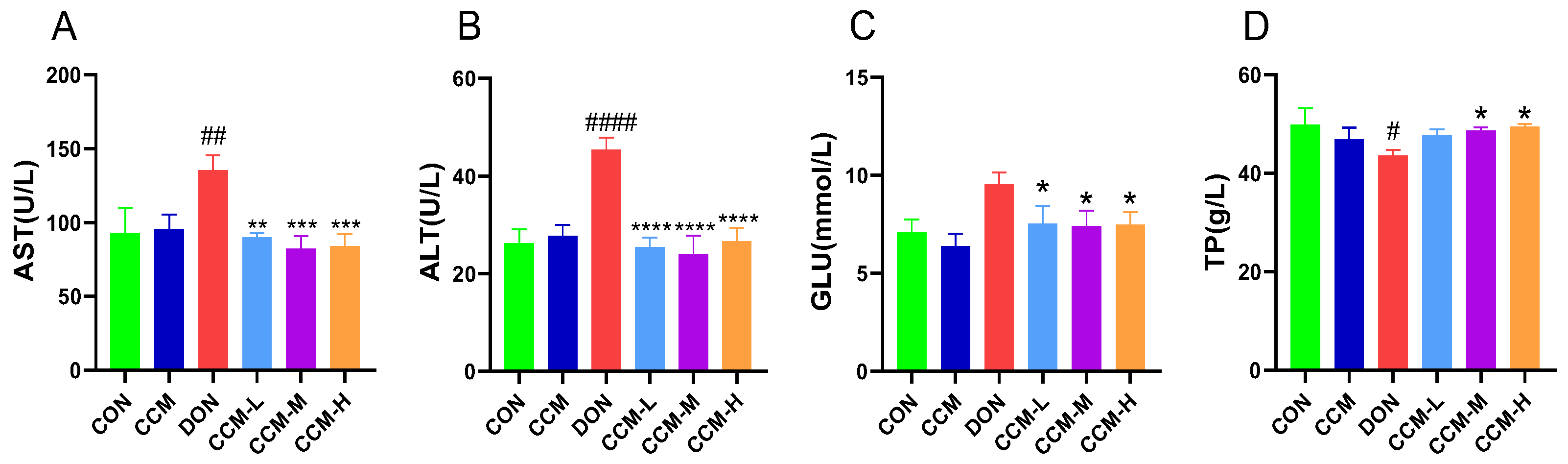
3.5. Effect of CCM on Blood Biochemical Indices in Mice
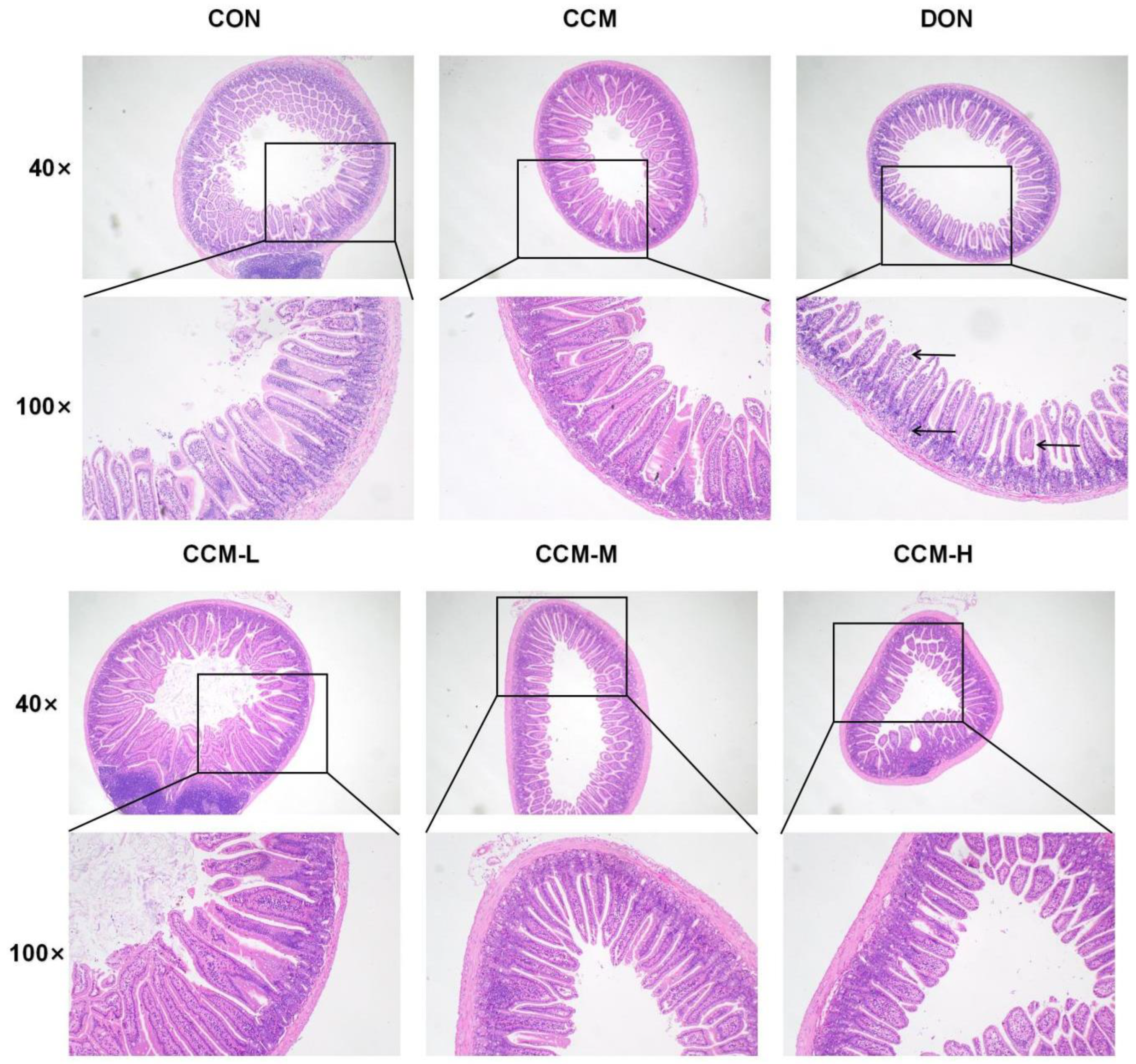
3.6. Histopathological Changes in the Jejunum Due to DON Inhibition by CCM
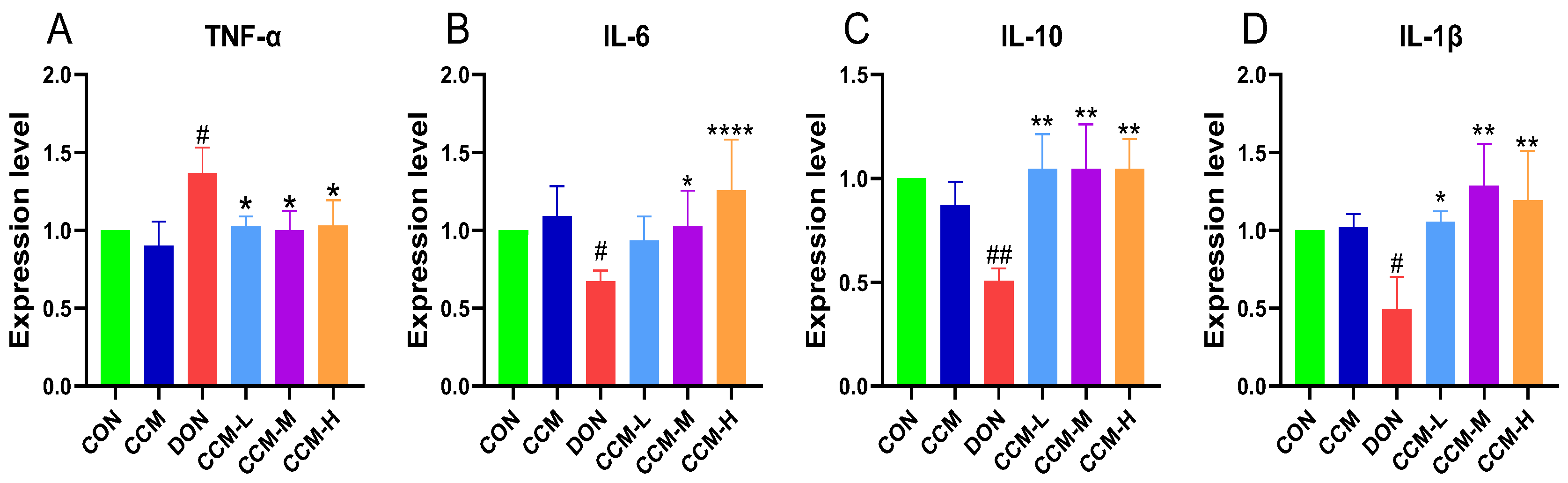
3.7. CCM Inhibits DON-Induced Intestinal Inflammation in Mice
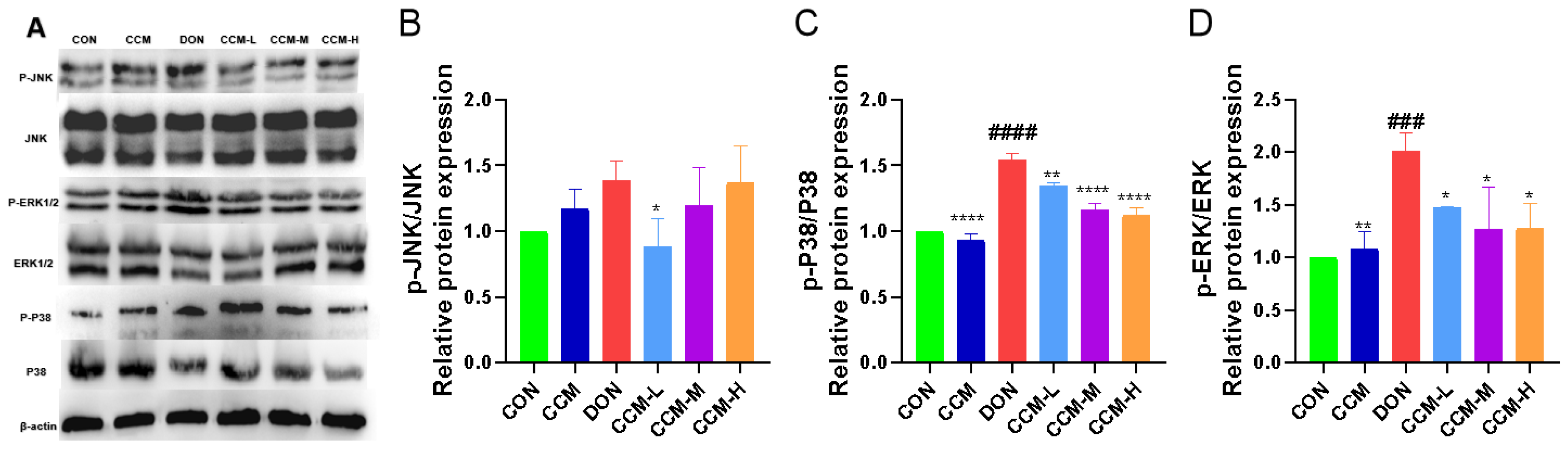
3.8. CCM Inhibits Activation of the MAPK Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Liao, Y.; Peng, Z.; Chen, L.; Zhang, W.; Nüssler, A.K.; Shi, S.; Liu, L.; Yang, W. Food Raw Materials and Food Production Occurrences of Deoxynivalenol in Different Regions. Trends Food Sci. Technol. 2018, 83, 41–52. [Google Scholar] [CrossRef]
- Maresca, M. From the Gut to the Brain: Journey and Pathophysiological Effects of the Food-Associated Trichothecene Mycotoxin Deoxynivalenol. Toxins 2013, 5, 784–820. [Google Scholar] [CrossRef] [PubMed]
- Vignal, C.; Djouina, M.; Pichavant, M.; Caboche, S.; Waxin, C.; Beury, D.; Hot, D.; Gower-Rousseau, C.; Body-Malapel, M. Chronic ingestion of deoxynivalenol at human dietary levels impairs intestinal homeostasis and gut microbiota in mice. Arch. Toxicol. 2018, 92, 2327–2338. [Google Scholar] [CrossRef] [PubMed]
- Girardet, C.; Bonnet, M.S.; Jdir, R.; Sadoud, M.; Thirion, S.; Tardivel, C.; Roux, J.; Lebrun, B.; Wanaverbecq, N.; Mounien, L.; et al. The food-contaminant deoxynivalenol modifies eating by targeting anorexigenic neurocircuitry. PLoS ONE 2017, 6, e26134. [Google Scholar] [CrossRef]
- Pestka, J.J. Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit. Contam. Part A 2008, 25, 1128–1140. [Google Scholar] [CrossRef]
- Ueno, Y. Toxicological features of T-2 toxin and related trichothecenes. Fundam. Appl. Toxicol. Off. J. Soc. Toxicol. 1984, 4, S124–S132. [Google Scholar] [CrossRef]
- Park, J.H.; Shin, H.J.; Kim, M.H.; Kim, J.S.; Kang, N.; Lee, J.Y.; Kim, K.T.; Lee, J.I.; Kim, D.D. Application of montmorillonite in bentonite as a pharmaceutical excipient in drug delivery systems. J. Pharm. Investig. 2016, 46, 363–375. [Google Scholar] [CrossRef]
- Ophir, A.; Zonder, L.; Rios, P.F. Thermodynamic characterization of hybrid polymer blend systems. Polym. Eng. Sci. 2009, 49, 1168–1176. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymer–layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Navrátilová, Z.; Mucha, M. Organo-montmorillonites as carbon paste electrode modifiers. J. Solid State Electrochem. 2015, 19, 2013–2022. [Google Scholar] [CrossRef]
- Demir, B.; Seleci, M.; Ag, D.; Cevik, S.; Yalcinkaya, E.E.; Demirkol, D.O.; Anik, U.; Timur, S. Amine intercalated clay surfaces for microbial cell immobilization and biosensing applications. RSC Adv. 2013, 3, 7513–7519. [Google Scholar] [CrossRef]
- An, N.; Zhou, C.H.; Zhuang, X.Y.; Tong, D.S.; Yu, W.H. Immobilization of enzymes on clay minerals for biocatalysts and biosensors. Appl. Clay Sci. 2015, 114, 283–296. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.; Thanou, M. Chitin and chitosan: Sources, production and medical applications. In Renewable Resources for Functional Polymers and Biomaterials; Royal Society of Chemistry Publishing: London, UK, 2011; pp. 292–318. [Google Scholar]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels As Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Xiao, F.; Guan, Y.; Yang, D.; Li, Z.; Yao, K. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar]
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Mao, J.; Zhou, Y.; Lv, G.; Zhou, R. Simultaneous Detoxification of Aflatoxin B1, Zearalenone and Deoxynivalenol by Modified Montmorillonites. Molecules 2022, 27, 315. [Google Scholar] [CrossRef]
- Bryden, L.W. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Technol. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Bočarov-Stančić, A.; Adamović, M.; Salma, N.; Bodroža-Solarov, M.; Vučković, J.; Pantić, V. In vitro efficacy of mycotoxins adsorption by natural mineral adsorbents. Biotechnol. Anim. Husb. 2011, 27, 1241–1251. [Google Scholar] [CrossRef]
- Zeng, L.; Yan, C.; Zhan, G.; Yu, C. Study on the adsorption properties of different silicate mineral materials on mycotoxins. Chin. J. Anim. Sci. 2011, 47, 64–66. [Google Scholar]
- Wu, Y. A Primary Research on Adsorption of Mycotoxins by Modified Clays. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2009. [Google Scholar]
- Razzazi, J.B.E. Effects of feeding deoxynivalenol contaminated wheat to piglets. Mycotoxin Res. 2003, 19, 176–179. [Google Scholar]
- Jiang, Z.; Fan, J.; Chen, M.; Li, L.; Wang, S.; Yin, Y.; Li, T. Effects of Diets Contaminated by Deoxynivalenol on Blood Physiological and Biochemical ndexes and lntervention Effects of Bamboo-Carbon and Bamboo Vinegar in Weaner Piglets. Chin. J. Anim. Nutr. 2012, 24, 2459–2468. [Google Scholar]
- Feng, Z.; Que, F.; Yu, F. Protective Effects of Bioactive Peptides from Mytlus coruscus on Alcohol-induced Liver Injury and Their Regulation of Gut Microbiota in Mice. J. Zhanjiang Ocean Univ. 2023, 43, 10–18. [Google Scholar]
- Wang, L.; Zhang, C.; Gao, J.; Zheng, H.; Cao, W.; Qin, X.; Chen, J. lmproving Effect of Pinctada martensii Meat Extract on Acute Alcoholic Liver Injury in Mice. J. Zhanjiang Ocean Univ. 2021, 41, 99–107. [Google Scholar]
- Ji, J.; Zhu, P.; Cui, F.; Pi, F.; Zhang, Y.; Li, Y.; Wang, J.; Sun, X. The Antagonistic Effect of Mycotoxins Deoxynivalenol and Zearalenone on Metabolic Profiling in Serum and Liver of Mice. Toxins 2017, 9, 28. [Google Scholar] [CrossRef]
- Parent-Massin, D. Haematotoxicity of trichothecenes. Toxicol. Lett. 2004, 153, 75–81. [Google Scholar] [CrossRef]
- Pinto, A.C.S.M.; De Pierri, C.R.; Evangelista, A.G.; Gomes, A.S.D.L.P.B.; Luciano, F.B. Deoxynivalenol: Toxicology, Degradation by Bacteria, and Phylogenetic Analysis. Toxins 2022, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Q.; Xue, Z.; Zhang, S.; Ren, Z.; Chen, S.; Zhou, A.; Chen, H.; Liu, Y. Deoxynivalenol induces endoplasmic reticulum stress-associated apoptosis via the IRE1/JNK/CHOP pathway in porcine alveolar macrophage 3D4/21 cells. Food Chem. Toxicol. 2023, 180, 114033. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Puel, O.; Pinton, P.; Cossalter, A.M.; Chou, T.C.; Oswald, I.P. Co-exposure to low doses of the food contaminants deoxynivalenol and nivalenol has a synergistic inflammatory effect on intestinal explants. Arch. Toxicol. 2017, 91, 2677–2687. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chang, J.; Wang, P.; Liu, C.; Liu, M.; Zhou, T.; Yin, Q.; Yan, G. Combination of glycyrrhizic acid and compound probiotics alleviates deoxynivalenol-induced damage to weaned piglets. Ecotoxicol. Environ. Saf. 2023, 256, 114901. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef]
- Aguzey, H.A.; Gao, Z.; Haohao, W.; Guilan, C.; Zhengmin, W.; Junhong, C. The Effects of Deoxynivalenol (DON) on the Gut Microbiota, Morphology and Immune System of Chicken—A Review. Ann. Anim. Sci. 2019, 19, 305–318. [Google Scholar] [CrossRef]
- Del Favero, G.; Janker, L.; Neuditschko, B.; Hohenbichler, J.; Kiss, E.; Woelflingseder, L.; Gerner, C.; Marko, D. Exploring the dermotoxicity of the mycotoxin deoxynivalenol: Combined morphologic and proteomic profiling of human epidermal cells reveals alteration of lipid biosynthesis machinery and membrane structural integrity relevant for skin barrier function. Arch. Toxicol. 2021, 95, 2201–2221. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol-Induced Proinflammatory Gene Expression: Mechanisms and Pathological Sequelae. Toxins 2010, 2, 1300–1317. [Google Scholar] [CrossRef]
- Wang, X.; Chu, X.; Cao, L.; Zhao, J.; Zhu, L.; Rahman, S.U.; Hu, Z.; Zhang, Y.; Feng, S.; Li, Y.; et al. The role and regulatory mechanism of autophagy in hippocampal nerve cells of piglet damaged by deoxynivalenol. Toxicol. Vitr. 2020, 66, 104837. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Horn, N.; Ajuwon, K.M. Mechanisms of deoxynivalenol-induced endocytosis and degradation of tight junction proteins in jejunal IPEC-J2 cells involve selective activation of the MAPK pathways. Arch. Toxicol. 2021, 95, 2065–2079. [Google Scholar] [CrossRef] [PubMed]
- Hooft, J.M.; Bureau, D.P. Deoxynivalenol: Mechanisms of action and its effects on various terrestrial and aquatic species. Food Chem. Toxicol. 2021, 157, 112616. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, Y.J.; Kim, M.-H.; Kwak, J.M. MAPK cascades in guard cell signal transduction. Front. Plant Sci. 2016, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.J. Compromised MAPK signaling in human diseases: An update. Arch. Toxicol. 2015, 89, 867–882. [Google Scholar] [CrossRef] [PubMed]

| Group | DON Treatment | CCM Processing |
|---|---|---|
| CON | - | - |
| DON | 2 mg/kg | - |
| CCM | - | 2 mg/kg |
| CCM-L | 2 mg/kg | 1 mg/kg |
| CCM-M | 2 mg/kg | 2 mg/kg |
| CCM-H | 2 mg/kg | 4 mg/kg |
| Group | Specific Surface Area | Pore Volume | Average Pore Size |
|---|---|---|---|
| CM | 21.745 m2/g | 3.794 nm | 10.78 nm |
| CCM | 18.786 m2/g | 3.969 nm | 15.18 nm |
| Group | CCM-L | CCM-M | CCM-H |
|---|---|---|---|
| Desorption Ratio (%) | - | - | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, R.; Ju, X.; Niu, X.; Liu, X.; Li, Y.; Yu, Z.; Ma, X.; Gao, Y.; Li, Y.; Xie, H.; et al. Protective Effects of Carbonated Chitosan Montmorillonite on Vomitoxin-Induced Intestinal Inflammation. Polymers 2024, 16, 715. https://doi.org/10.3390/polym16050715
Tang R, Ju X, Niu X, Liu X, Li Y, Yu Z, Ma X, Gao Y, Li Y, Xie H, et al. Protective Effects of Carbonated Chitosan Montmorillonite on Vomitoxin-Induced Intestinal Inflammation. Polymers. 2024; 16(5):715. https://doi.org/10.3390/polym16050715
Chicago/Turabian StyleTang, Ruifan, Xianghong Ju, Xueting Niu, Xiaoxi Liu, Youquan Li, Zhichao Yu, Xingbin Ma, Yuan Gao, Yin Li, Huili Xie, and et al. 2024. "Protective Effects of Carbonated Chitosan Montmorillonite on Vomitoxin-Induced Intestinal Inflammation" Polymers 16, no. 5: 715. https://doi.org/10.3390/polym16050715
APA StyleTang, R., Ju, X., Niu, X., Liu, X., Li, Y., Yu, Z., Ma, X., Gao, Y., Li, Y., Xie, H., Zhou, Q., & Yong, Y. (2024). Protective Effects of Carbonated Chitosan Montmorillonite on Vomitoxin-Induced Intestinal Inflammation. Polymers, 16(5), 715. https://doi.org/10.3390/polym16050715






