Increasing the Adhesion of Bitumen to the Surface of Mineral Fillers through Modification with a Recycled Polymer and Surfactant Obtained from Oil Refining Waste
Abstract
1. Introduction
- Investigate the influence of the concentration of additives on the surface tension (σl-g) in binary “bitumen–surfactant”, “bitumen–AG-4I”, and ternary “bitumen–surfactant–AG-4I” systems;
- Study the effect of the concentration of additives in bitumen on the wetting processes of mineral fillers of various types;
- Determine the thermodynamic work of adhesion and the adhesive effectiveness of modifiers in the composition of a bituminous binder in relation to the surface of mineral fillers;
- Derive mathematical models of wetting processes based on the established physical and chemical laws of modification.
2. Materials and Methods
2.1. Materials
- Oxidized bitumen with penetration 90/130 (bitumen brand BND 90/130—oil bitumen for roads in Kazakhstan), manufactured by Gazpromneft-Bitumen Kazakhstan LLP, Shymkent, Kazakhstan.
- 2.
- Modifying additives:
- -
- AS-1 [49] is a product of the amination of distillation residues of petrochemistry (KON-92), which is a mixture of amines of the general formulawhere R’ is n-butyl, R’’-2-ethyl-2-hexenyl.R’-NH2, R’-NH-R’’,
- -
- AG-4I is a used sealant, a product based on high-molecular-weight polyisobutylene (PIB) and petroleum oils (manufactured by “Germetika Research and Production Company”, Moscow, Russia).
- -
- AMDOR-10—a mixture of polyaminoamides and polyaminoimidazolines (manufacturer CJSC “Amdor”, Saint-Petersburg, Russia), a condensation product of polyamines and higher fatty acids.
- 3.
- The following materials were used as substrates for determining the contact angle:
- -
- Polymethyl methacrylate (PMMA), as a solid surface standard with a predominance of basic centers (manufacturer “JiangmenKunxin New Material Technology Co., Ltd.”, Omsk, Russia);
- -
- Polyvinyl chloride (PVC), the standard of a solid surface with a predominance of acid sites (manufactured by “VitaChem” LLC, Dzerzhinsk, Russia);
- -
- Gray crushed stone (plagiogranite);
- -
- Red crushed stone (alaskite).
2.2. Preparation of Modified Bituminous Compositions
2.3. Determination of the Surface Tension of Bituminous Compositions
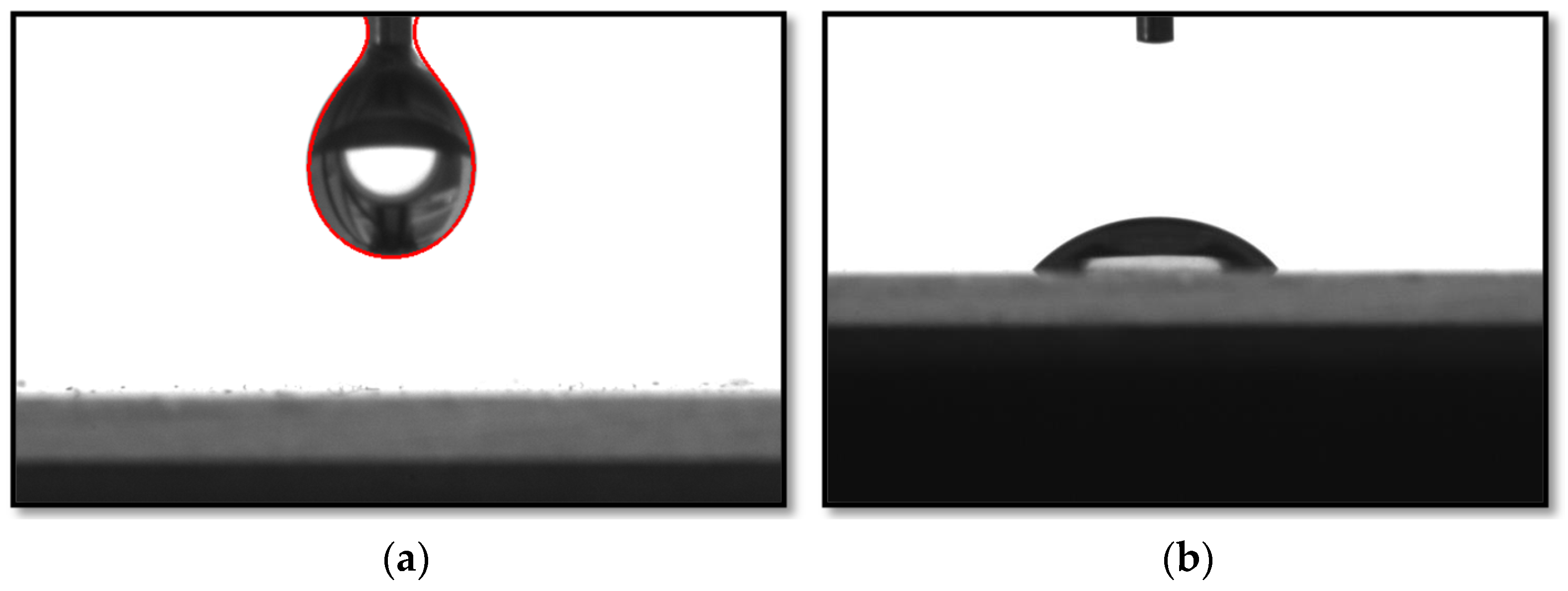
2.4. Measuring the Contact Angle
2.4.1. Method for Preparing the Surface of Crushed Stone
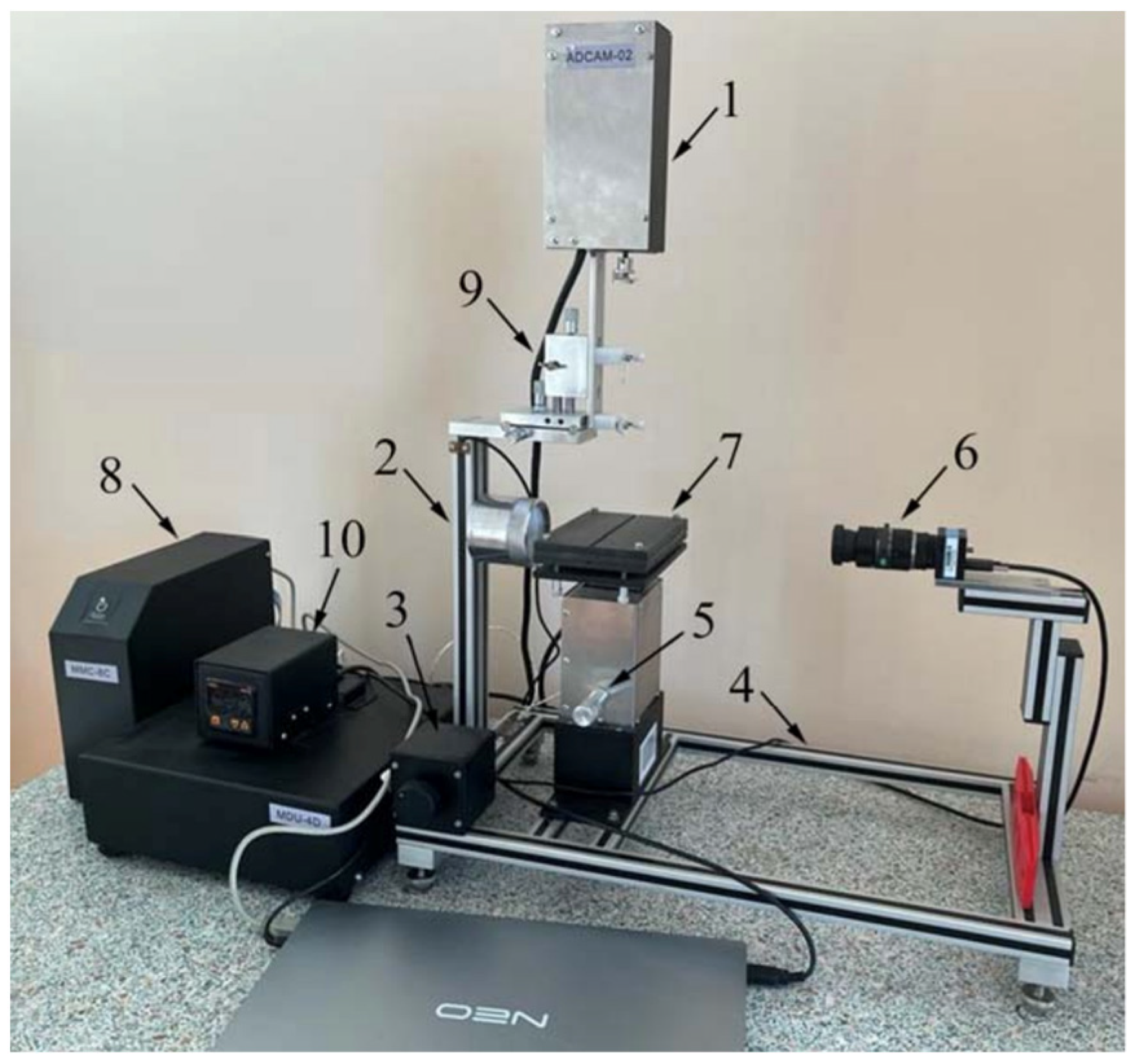
2.4.2. Contact Angle Measurement
2.4.3. Method of Probabilistic–Deterministic Planning
- Determination of factors and levels of their variation.
- Constructing an experimental plan in the form of a plan matrix consisting of m columns corresponding to the number of input parameters (factors) and n rows corresponding to the number of variations in the given levels (numerical value) of the factors. To ensure the orthogonality of the plan matrix, each level of one input parameter was specified only once with each level of another input parameter.
- Conducting an active experiment according to the generated plan matrix and establishing the numerical values of the response function (output parameter).
- Sampling the response function for each level of each factor.
- Construction of partial dependencies of the response function on each factor.
- Approximation of particular dependencies and derivation of a generalized mathematical model.
2.4.4. Evaluation of the Operational Properties of the Coating (Contact with Water, Elevated Temperature) by the Adhesive Effectiveness of Modifiers
3. Results and Discussion
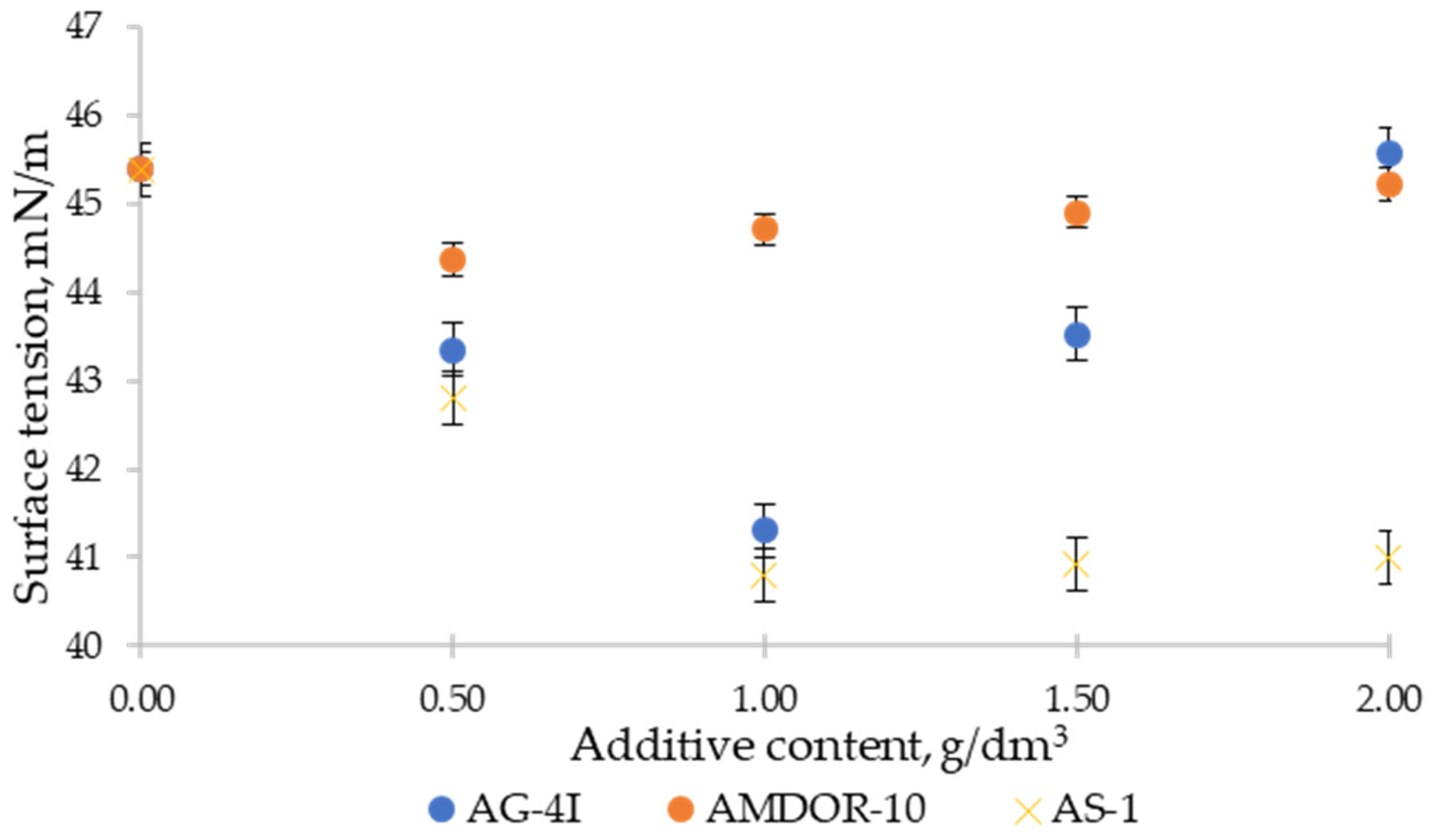
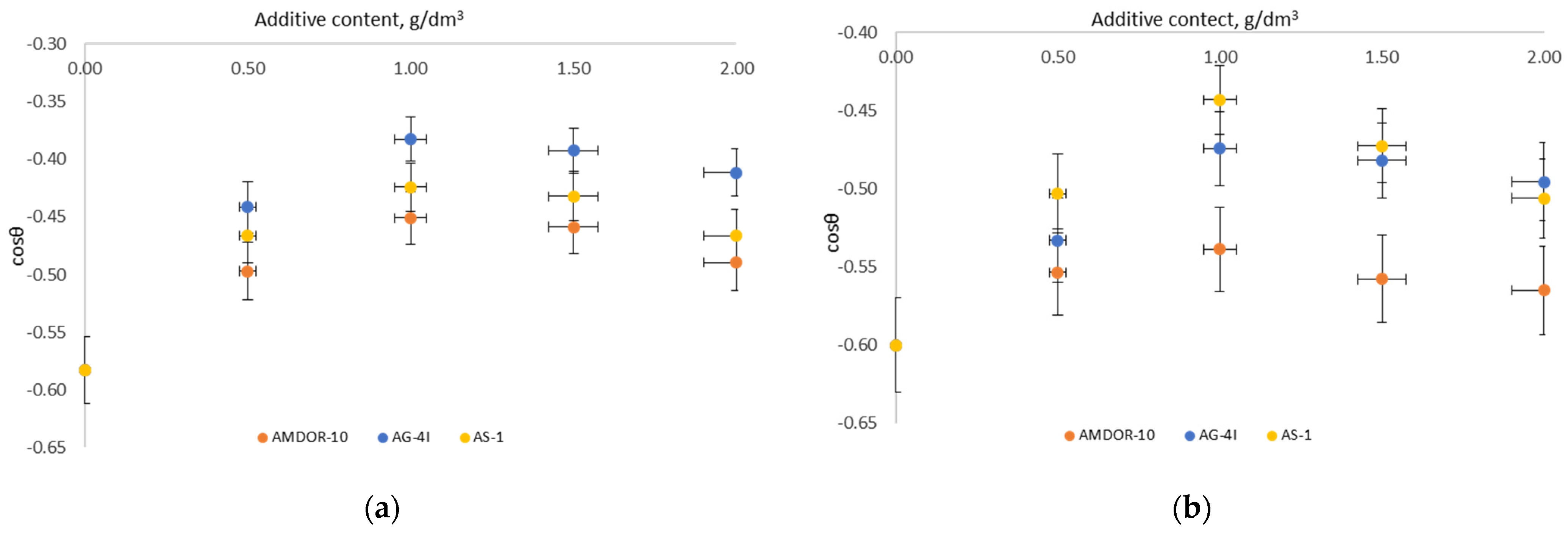
3.1. Surface-Active Properties of Binary Systems “Bitumen–Additive” at Interphase Boundaries with Air
3.2. Surface-Active Properties of Ternary Systems “Bitumen–Polymer–Surfactant” at the Interface with Air
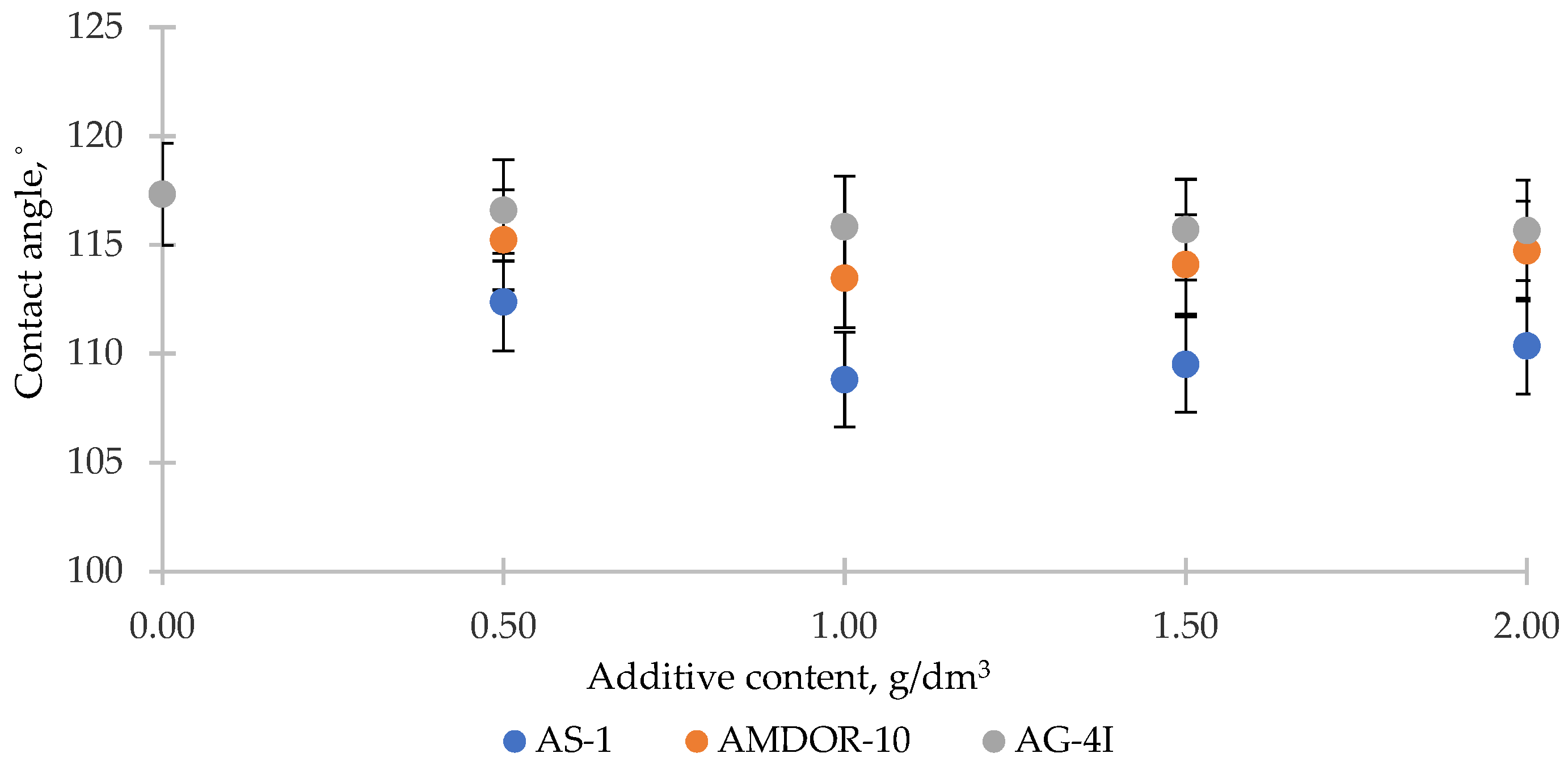
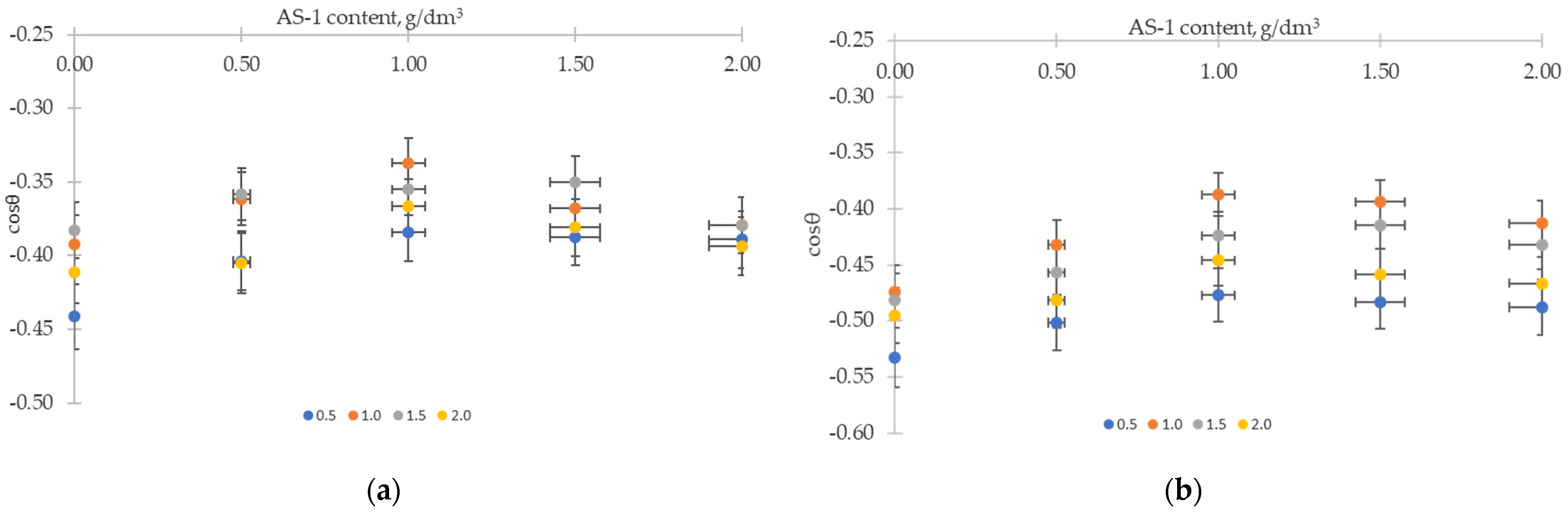
3.3. Investigation of the Processes of Wetting of Solid Surfaces by Binary Systems “Bitumen–Additive”
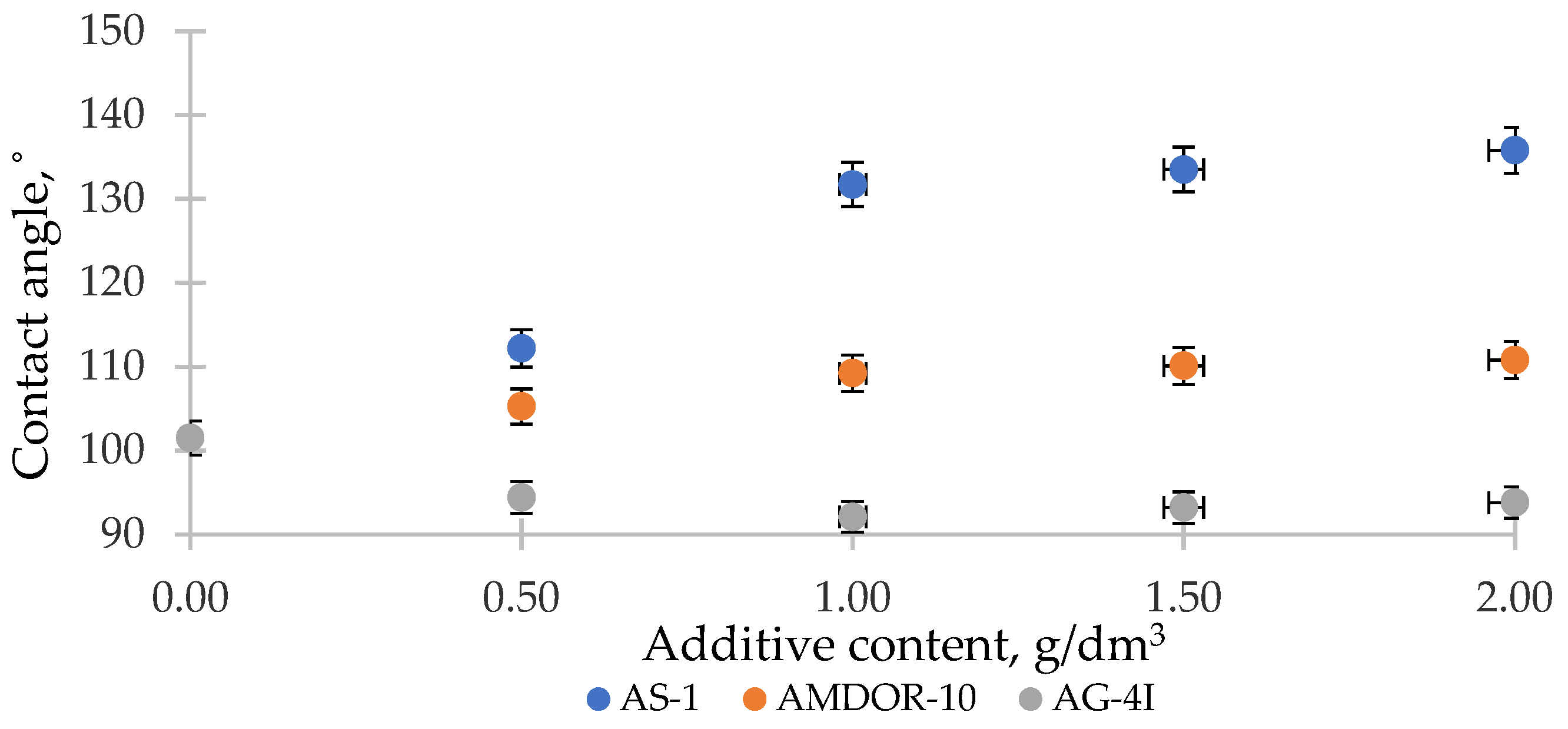
3.4. Investigation of the Processes of Wetting of Solid Surfaces by Ternary Systems “Bitumen–Surfactant–Polymer”
3.5. Evaluation of the Operational Properties of the Coating on the Basis of the Adhesive Effectiveness of Modifying Additives
3.6. Mathematical Modeling of Wetting Processes
4. Conclusions
- In bitumen systems with a limited concentration of AG-4I (C ≤ 1.0 g/dm3), the effect of reducing the surface energy at the interphase boundary with air is achieved due to the concentration of surfactants in the surface layer, which are part of the structure of the bitumen itself, which is facilitated by the development of destructive processes, accompanied by the destruction of associates and the release of active components.
- Differences in the surface activity of nitrogen-containing surfactants are due to their structural and geometric parameters and molecular weight composition. Their surface activity decreases with increasing molecular weight.
- In the “bitumen–AG-4I–AS-1” ternary systems, the change in the specific surface energy at the liquid–gas interface was an additive quantity that took into account the separate contribution of both AG-4I and AS-1. The concentration threshold of additives to achieve the minimum σl-g remained the same (C = 1.0 g/dm3) as in individual compositions. With the joint introduction of AG-4I (C = 1.0 g/dm3) and AS-1 (C = 1.0 g/dm3), the surface tension decreased to a value of 37.20 mN/m compared to unmodified bitumen (Δσ = 8.19 mN/m).
- The determining factor in the implementation of the wetting action of additives with respect to mineral fillers is the localization of their polar groups on the active centers of the mosaic solid surface in accordance with the principle of acid–base interactions. Nitrogen-containing surfactants (AS-1 and AMDOR-10) are localized on acid centers, and acidic surfactants released from the micellar structure of bitumen with the introduction of a limited (C ≤ 1.0 g/dm3) concentration of AG-4I are localized on basic centers.
- Additives with wetting activity in relation to the gray crushed stone formed the series AG-4I > AS-1 > AMDOR-10. The maximum decrease in the specific surface energy σs-l and the contact angle coincided with the concentration of 1.0 g/dm3.
- Additives in relation to the red crushed stone in a row of decreasing wetting activity formed a different sequence: AS-1 > AG-4I > AMDOR-10. When applying isoconcentration bitumen compositions (C = 1.0 g/dm3), the change in the specific surface energy of the red crushed stone was significantly less in the presence of AG-4I (Δσs = −19.42 mN/m) and AMDOR-10 (Δσs = −24.14 mN/m). Bituminous compositions with AS-1 retained the proximity of reducing the specific surface energy (Δσs = −17.00 mN/m). In comparison with unmodified bitumen (θ = 126.87°), the decrease in the θ values in the presence of AS-1 had indicators close to those of the gray crushed stone surface (Δθ = 10.57°) and was 1.5–2.0 times less for AG-4I (θ = 8.57°) and AMDOR-10 (θ = 4.27°), respectively, at C = 1.0 g/dm3.
- In the ternary systems, the combination of two modifying additives localized on crushed stone adsorption centers of a different nature led to a deeper decrease in the specific surface energy Δσs. The relative changes in the specific surface energy α were 52.37% (gray crushed stone) and 47.06% (red crushed stone), which was 17.47% (gray crushed stone) and 12.98% (red crushed stone) more than in the binary compositions with AS-1 (C = 1.0 g/dm3). The addition of AS-1 shifted the contact angle to lower values and reached a minimum at CAS-1 = 1.0 g/dm3 and CAG-4I = 1.0 g/dm3.
- The introduction of AS-1 into the composition of bitumen with a solution of polyisobutylene increased the adhesion of the bitumen to the surface of both the gray and red crushed stones. The addition of AS-1 increased the adhesive efficiency, reaching a maximum at CAS-1 = 1.0 g/dm3 and CAG-4I = 1.0 g/dm3. In these concentration modes, the composition caused an increase of 52.28% (gray crushed stone) and 36.70% (red crushed stone) in the adhesive efficiency relative to unmodified bitumen.
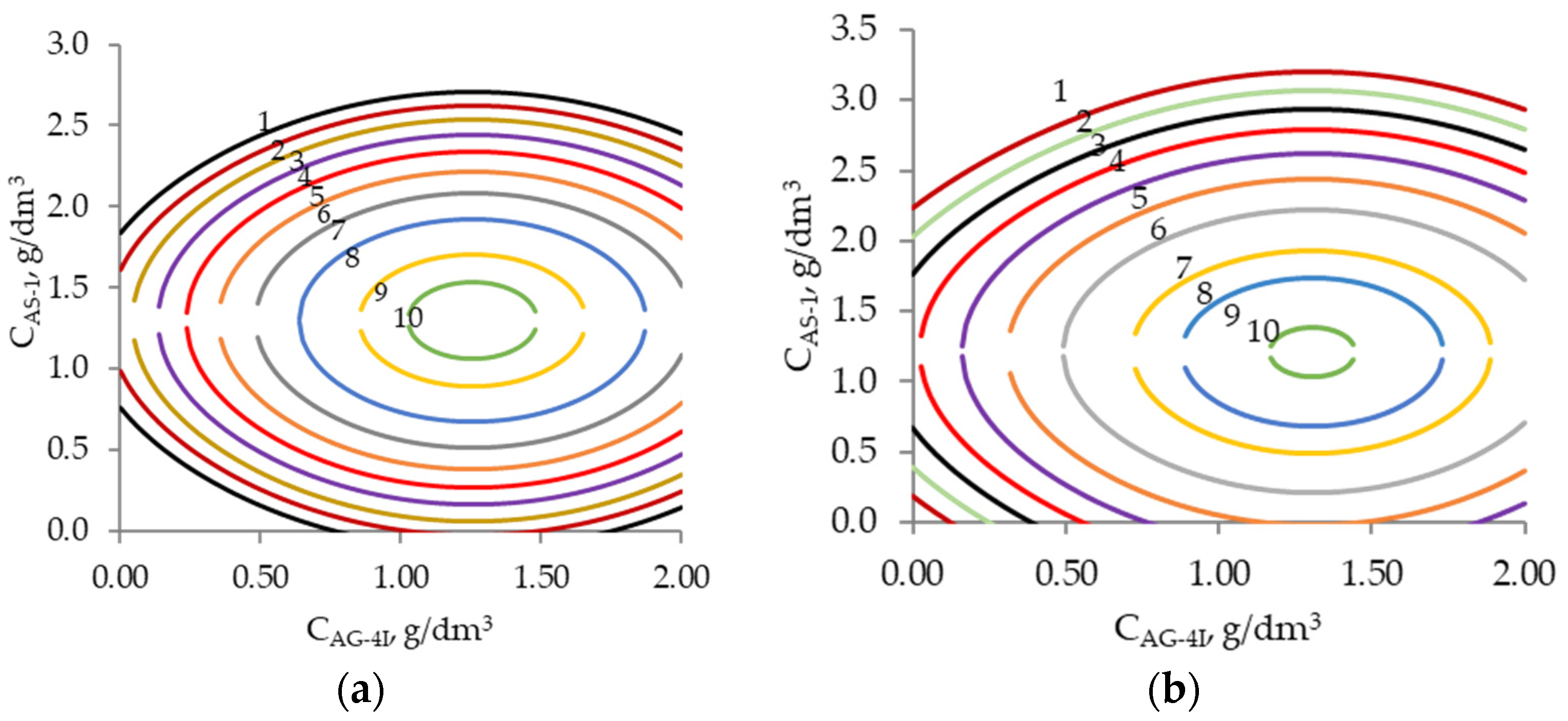
- Based on the results of the mathematical modeling, two-factor nomograms were constructed that allow for solving applied problems, in particular, the optimization of bitumen mineral compositions with predetermined characteristics.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korochkin, A. Impact of rigid pavements with the asphalt-concrete wearing course on road performance and traffic safety. Transp. Res. Procedia 2018, 36, 315–319. [Google Scholar] [CrossRef]
- Porto, M.; Loise, V.; Teltayev, B.; Calandra, P.; De Santo, M.P.; Rossi, C.O.; Caputo, P. Synergic effects between vacuum residue and polymers for preparing high-performance bitumens. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 13214. [Google Scholar] [CrossRef]
- Valentin, J.; Trejbal, J.; Nezerka, V.; Valentova, T.; Vackova, P.; Ticha, P. A comprehensive study on adhesion between modified bituminous binders and mineral aggregates. Constr. Build. Mater. 2021, 305, 124686. [Google Scholar] [CrossRef]
- Dyuryagina, A.; Byzova, Y.; Ostrovnoy, K.; Lutsenko, A. Correlation Dependence between Hydrophobicity of Modified Bitumen and Water Saturation of Asphalt Concrete. Appl. Sci. 2023, 13, 10946. [Google Scholar] [CrossRef]
- Wang, F.; Xiao, Y.; Chen, Z.; Cui, P.; Liu, J.; Wang, N. Morphological characteristics of mineral filler and their influence on active adhesion between aggregates and bitumen. Constr. Build. Mater. 2022, 323, 126520. [Google Scholar] [CrossRef]
- Ren, S.; Liu, X.; Lin, P.; Gao, Y.; Erkens, S. Review on the diffusive and interfacial performance of bituminous materials: From a perspective of molecular dynamics simulation. J. Mol. Liq. 2022, 366, 120363. [Google Scholar] [CrossRef]
- Pasandín, A.R.; Pérez, I. The influence of the mineral filler on the adhesion between aggregates and bitumen. Int. J. Adhes. Adhes. 2015, 58, 53–58. [Google Scholar] [CrossRef]
- Zhu, J.; Birgisson, B.; Kringos, N. Polymer modification of bitumen: Advances and challenges. Eur. Polym. J. 2014, 54, 18–38. [Google Scholar] [CrossRef]
- Alatas, T.; Yilmaz, M. Effects of different polymers on mechanical properties of bituminous binders and hot mixtures. Constr. Build. Mater. 2013, 42, 161–167. [Google Scholar] [CrossRef]
- Porto, M.; Caputo, P.; Loise, V.; Eskandarsefat, S.; Teltayev, B.; Oliviero, R.C. Bitumen and bitumen modification: A review on latest advances. Appl. Sci. 2019, 9, 742. [Google Scholar] [CrossRef]
- Golchin, B.; Meor, O.; Mohd, R. Optimization in producing warm mix asphalt with polymer modified binder and surfactant-wax additive. Constr. Build. Mater. 2017, 141, 578–588. [Google Scholar] [CrossRef]
- Yu, B.; Gu, X.; Ni, F.; Guo, R. Multi-objective optimization for asphalt pavement maintenance plans at project level: Integrating performance, cost and environment. Transp. Res. D Transp. Environ. 2015, 41, 64–74. [Google Scholar] [CrossRef]
- Yu, D.; Yang, E.; Zhang, M.; Zhang, H.; Chen, G.; Di, H.; Huang, B.; Qiu, Y. Chemical modification of sustainable bagasse fiber and its absorption mechanism on SBS-modified emulsified bitumen. Constr. Build. Mater. 2023, 409, 133983. [Google Scholar] [CrossRef]
- Xing, H.; Liang, Y.; Liu, G.; Zhang, Y.; Liu, X.; Fu, W. Organically treating montmorillonite with dual surfactants to modify bitumen. Constr. Build. Mater. 2020, 264, 120705. [Google Scholar] [CrossRef]
- Liu, W.; Jin, Y.; Tan, X.; Yeung, A. Altering the wettability of bitumen-treated glass surfaces with ionic surfactants. Fuel 2011, 90, 2858–2862. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, J.; Xia, H. Analysis of influence of surfactant on the properties of diluted asphalt mixtures. Case Stud. Constr. Mater. 2022, 17, e01335. [Google Scholar] [CrossRef]
- Shi, L.; Chen, Y.; Gong, X.; Yu, X. Synthesis and characterization of quaternary ammonium salt tertiary amide type sodium hydroxypropyl phosphate asphalt emulsifier. Res. Chem. Intermed. 2019, 45, 5183–5520. [Google Scholar] [CrossRef]
- Zhang, D. An Equation-of-State approach to measure the surface free energy (SFE) of bituminous binders. Measurement 2020, 158, 107715. [Google Scholar] [CrossRef]
- Kemalov, A.; Kemalov, R.; Abdrafikova, I.; Fakhretdinov, P.; Valiev, D. Polyfunctional Modifiers for Bitumen and Bituminous Materials with High Performance. Adv. Mater. Sci. Eng. 2018, 2018, 7913527. [Google Scholar] [CrossRef]
- Mercado, R.A.; Salager, J.L.; Sadtler, V.; Marchal, P.; Choplin, L. Breaking of a cationic amine oil-in-water emulsion by pH increasing: Rheological monitoring to modelize asphalt emulsion rupture. Colloids Surf. A Physicochem. Eng. Asp. 2014, 458, 63–68. [Google Scholar] [CrossRef]
- Tan, Y.; Xie, J.; Wang, Z.; Li, X.; He, Z. Effect of surfactant modified nano-composite flame retardant on the combustion and viscosity-temperature properties of asphalt binder and mixture. Powder Technol. 2023, 420, 118188. [Google Scholar] [CrossRef]
- Tyagi, R.; Tyagi, V.K.; Pandey, S.K. Imidazoline and Its Derivatives: An Overview. J. Oleo Sci. 2007, 56, 211–222. [Google Scholar] [CrossRef]
- Gilmore, D.; Kugele, T. Asphalt Antistripping Agents Containing Organic Amines and Portland Cement. Patent No. US4743304A, 10 May 1988. Available online: https://patents.google.com/patent/US4743304A/en (accessed on 15 December 2023).
- Treybig, D.; Chang, D. Asphalt Compositions Containing Anti-Stripping Additives Prepared from Hydrocarbyl Substituted Nitrogen-Containing Aromatic Heterocyclic Compounds, Aldehydes or Ketones and Amines. Patent No. US4724003A, 9 February 1988. Available online: https://patents.google.com/patent/US4724003A/en (accessed on 15 December 2023).
- Kishchynskyi, S.; Nagaychuk, V.; Bezuglyi, A. Improving Quality and Durability of Bitumen and Asphalt Concrete by Modification Using Recycled Polyethylene Based Polymer Composition. Procedia Eng. 2016, 143, 119–127. [Google Scholar] [CrossRef]
- Tian, Y.; Li, H.; Sun, L.; Zhang, H.; Harvey, J.; Yang, J.; Yang, B.; Zuo, X. Laboratory investigation on rheological, chemical and morphological evolution of high content polymer modified bitumen under long-term thermal oxidative aging. Constr. Build. Mater. 2021, 303, 124565. [Google Scholar] [CrossRef]
- Diab, A.; Enieb, M.; Singh, D. Influence of aging on properties of polymer-modified asphalt. Constr. Build. Mater. 2019, 96, 54–65. [Google Scholar] [CrossRef]
- Polacco, G.; Filippi, S.; Merusi, F.; Stastna, G. A review of the fundamentals of polymer-modified asphalts: Asphalt/polymer interactions and principles of compatibility. Adv. Colloid Interface Sci. 2015, 224, 72–112. [Google Scholar] [CrossRef]
- Lesueur, D. The colloidal structure of bitumen: Consequences on the rheology and on the mechanisms of bitumen modification. Adv. Colloid Interface Sci. 2009, 145, 42–82. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Lastra-Gonzalez, P.; Rodriguez-Hernandez, J.; González, M.; Castro-Fresno, D. Critical assessment of new polymer-modified bitumen for porous asphalt mixtures. Constr. Build. Mater. 2021, 307, 124957. [Google Scholar] [CrossRef]
- Jiang, Z.; Easa, S.; Hu, C.; Zheng, X. Evaluation of new aspect of styrene-butadiene-styrene modified bitumens: Damping property and mechanism. Constr. Build. Mater. 2020, 242, 118185. [Google Scholar] [CrossRef]
- Soudani, K.; Cerezo, V.; Haddadi, S. Rheological characterization of bitumen modified with waste nitrile rubber (NBR). Constr. Build. Mater. 2016, 104, 126–133. [Google Scholar] [CrossRef]
- Brovelli, C.; Hilliou, L.; Hemar, Y.; Pais, J.; Pereira, P.; Crispino, M. Rheological characteristics of EVA modified bitumen and their correlations with bitumen concrete properties. Constr. Build. Mater. 2013, 48, 1202–1208. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Boom, Y.J.; Giustozzi, F. Sustainable Polymers from Recycled Waste Plastics and Their Virgin Counterparts as Bitumen Modifiers: A Comprehensive Review. Polymers 2021, 13, 3242. [Google Scholar] [CrossRef]
- Gill, Y.; Muhammad, U.; Abdur, R.; Umer, A. High-performance atactic polypropylene modified bitumen blends fabricated via conventional, hot, and in-situ blending techniques. J. Appl. Polym. Sci. 2022, 139, e52378. [Google Scholar] [CrossRef]
- Masad, E.; Roja, K.; Rehman, A.; Abdala, A. A Review of Asphalt Modification Using Plastics: A Focus on Polyethylene; A&M University at Qatar: Doha, Qatar, 2020. [Google Scholar] [CrossRef]
- Xie, X.; Li, C.; Wang, Q. Thermosetting Polymer Modified Asphalts: Current Status and Challenges. Polym. Rev. 2023, 132, 143466. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Jamal, M.; Gravina, R.; Giustozzi, F. Recycled plastic as bitumen modifier: The role of recycled linear low-density polyethylene in the modification of physical, chemical and rheological properties of bitumen. J. Clean. Prod. 2020, 266, 121988. [Google Scholar] [CrossRef]
- Lu, H.; Ye, F.; Yuan, J.; Yin, W. Properties comparison and mechanism analysis of naphthenic oil/SBS and nano-MMT/SBS modified asphalt. Constr. Build. Mater. 2018, 187, 1147–1157. [Google Scholar] [CrossRef]
- Galdina, V. The effect of polymer additives on the properties of bitumen and asphalt concrete. Bull. Sib. St. Automob. R. Ac. 2009, 12, 32–36. [Google Scholar]
- Dyuryagina, A.; Byzova, Y.; Ostrovnoy, K.; Tyukanko, V. Disposal of spent sealing liquid as part of asphalt concrete coatings. Bull. Tomsk. Polytech. Univ. Geo Assets Eng. 2021, 330, 80–86. [Google Scholar]
- Dyuryagina, A.; Lutsenko, A.; Tyukanko, V. Study of the disperse effect of polymeric surface-active substances in acrylic dispersions used for painting oil well armature. Bull. Tomsk Polytech. Univ. Geo Assets Eng. 2019, 330, 37–44. [Google Scholar] [CrossRef]
- Dyuryagina, A.; Lutsenko, A.; Ostrovnoy, K.; Tyukanko, V.; Demyanenko, A.; Akanova, M. Exploration of the Adsorption Reduction of the Pigment Aggregates Strength under the Effect of Surfactants in Water-Dispersion Paints. Polymers 2022, 14, 996. [Google Scholar] [CrossRef]
- Dyuryagina, A.; Lutsenko, A.; Demyanenko, A.; Tyukanko, V.; Ostrovnoy, K.; Yanevich, A. Modeling the wetting of titanium dioxide and steel substrate in water-borne paint and varnish materials in the presence of surfactants. East.-Eur. J. Enterp. Technol. 2022, 6, 31–42. [Google Scholar] [CrossRef]
- Tyukanko, V.; Duryagina, A.; Ostrovnoy, K.; Demyanenko, A. Study of wetting of aluminum and steel substrates with polyorganosiloxanes in the presence of nitrogen-containing surfactants. Bull. Tomsk Polytech. Univ. Geo Assets Eng. 2017, 328, 75–81. [Google Scholar]
- Lvovskiy, E.N. Statistical Methods for Empirical Formulas Constructing; Vysshaya Shkola: Moscow, Russia, 1982. [Google Scholar]
- Malyshev, V.P. Probabilistic-Deterministic Planning of the Experiment; Nauka: Alma-Ata, Kazakhstan, 1981. [Google Scholar]
- Tyukanko, V.; Demyanenko, A.; Dyuryagina, A.; Ostrovnoy, K.; Aubakirova, G. Optimizing the Composition of Silicone Enamel to Ensure Maximum Aggregative Stability of Its Suspensions Using Surfactant Obtained from Oil Refining Waste. Polymers 2022, 14, 3819. [Google Scholar] [CrossRef] [PubMed]
- Bolatbaev, K.N.; Dyuryagina, A.N.; Nurushov, A.K.; Korytina, O.G. Sposob Polucheniya Ingibitorov Kislotnoj Korrozii Metallov (Variantỳ). Patent No. RK 14467, 25 February 2004. [Google Scholar]
- Tang, H.; Cheng, X. Measurement of liquid surface tension by fitting the lying droplet profile. Measurement 2022, 188, 110379. [Google Scholar] [CrossRef]
- Pradhan, S.; Bikkina, P. Influence of step-down pressure on wettability-controlled heterogeneous bubble nucleation of sparingly soluble gases in water. Sep. Purif. Technol. 2024, 328, 125098. [Google Scholar] [CrossRef]
- Jafari, M.; Jung, J. Direct Measurement of Static and Dynamic Contact Angles Using a Random Micromodel Considering Geological CO2 Sequestration. Sustainability 2017, 9, 2352. [Google Scholar] [CrossRef]
- Zhao, M. Wetting on soft gels. Chemical Physics. Ph.D. Thesis, Université Paris Sciences et Lettres, Paris, France, 2017. Available online: https://pastel.hal.science/tel-02356275/file/ESPCI_MenghuaZHAO_2017.pdf (accessed on 15 December 2023).
- Paliukaitė, M.; Vorobjovas, V.; Bulevicius, M.; Andrejevas, V. Evaluation of Different Test Methods for Bitumen Adhesion Properties. Transp. Res. Procedia 2016, 14, 724–731. [Google Scholar] [CrossRef]
- Evdokimova, N.; Galiev, R.; Karteshkov, A. Vlijanie dobavok-modifikatorov na nizkotemperaturnye i adgezionnye svojstva dorozhnyh bitumov. Neft. I Gaz 2001, 6, 78–79. [Google Scholar]
- Cai, H.; Wang, Y.; Wu, K.; Guo, W. Enhanced Hydrophilic and Electrophilic Properties of Polyvinyl Chloride (PVC) Biofilm Carrier. Polymers 2020, 12, 1240. [Google Scholar] [CrossRef]
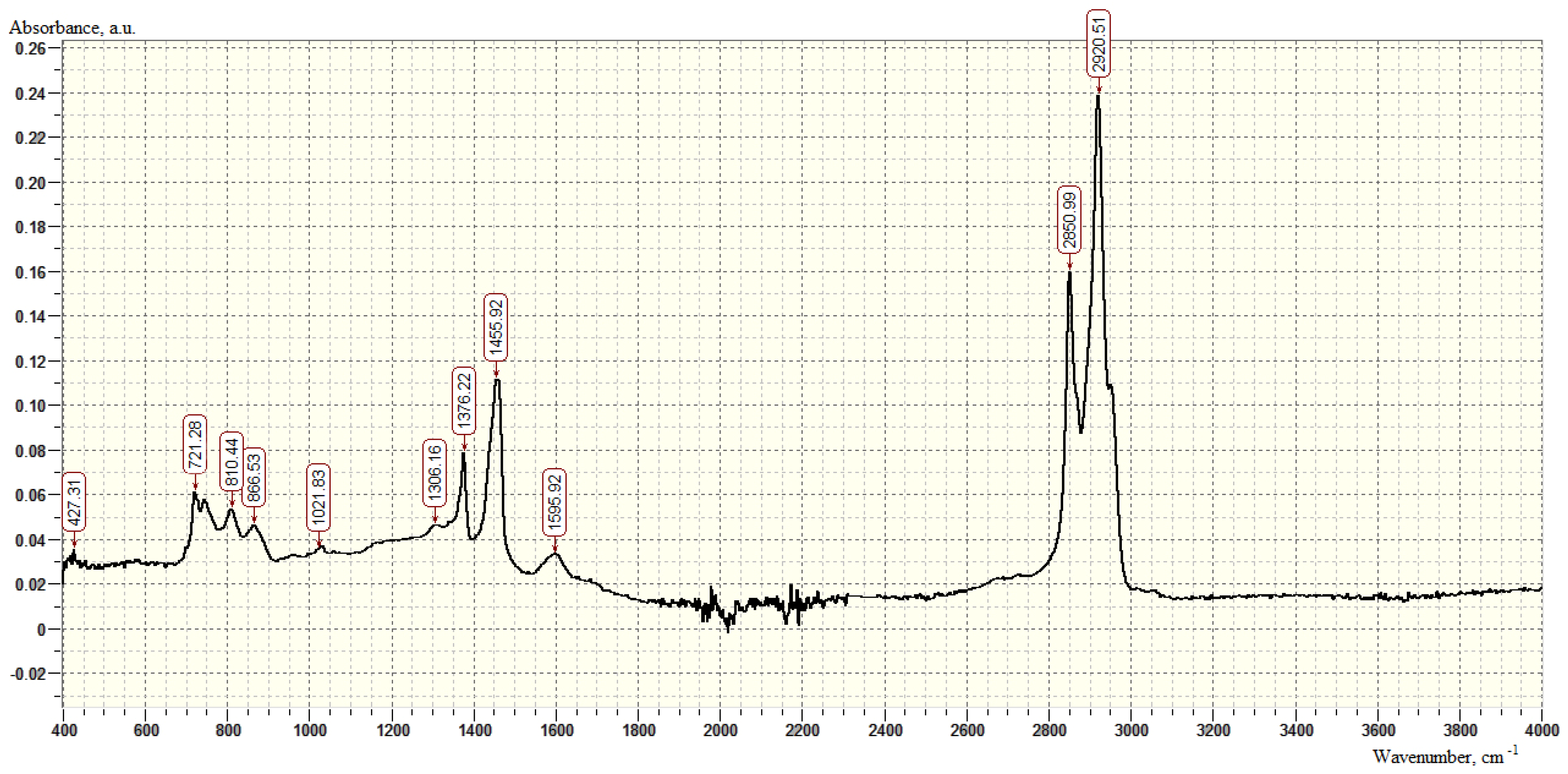
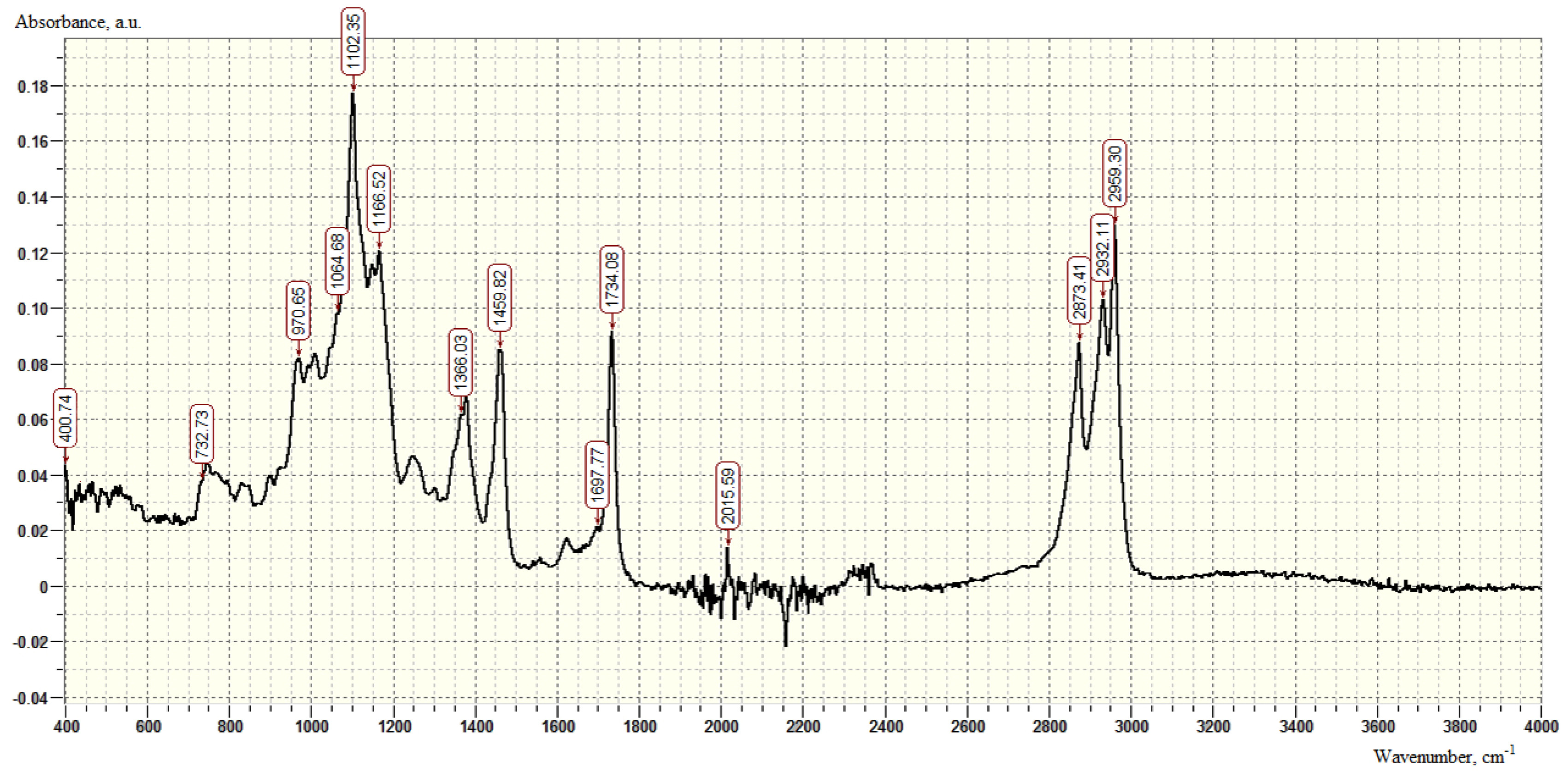
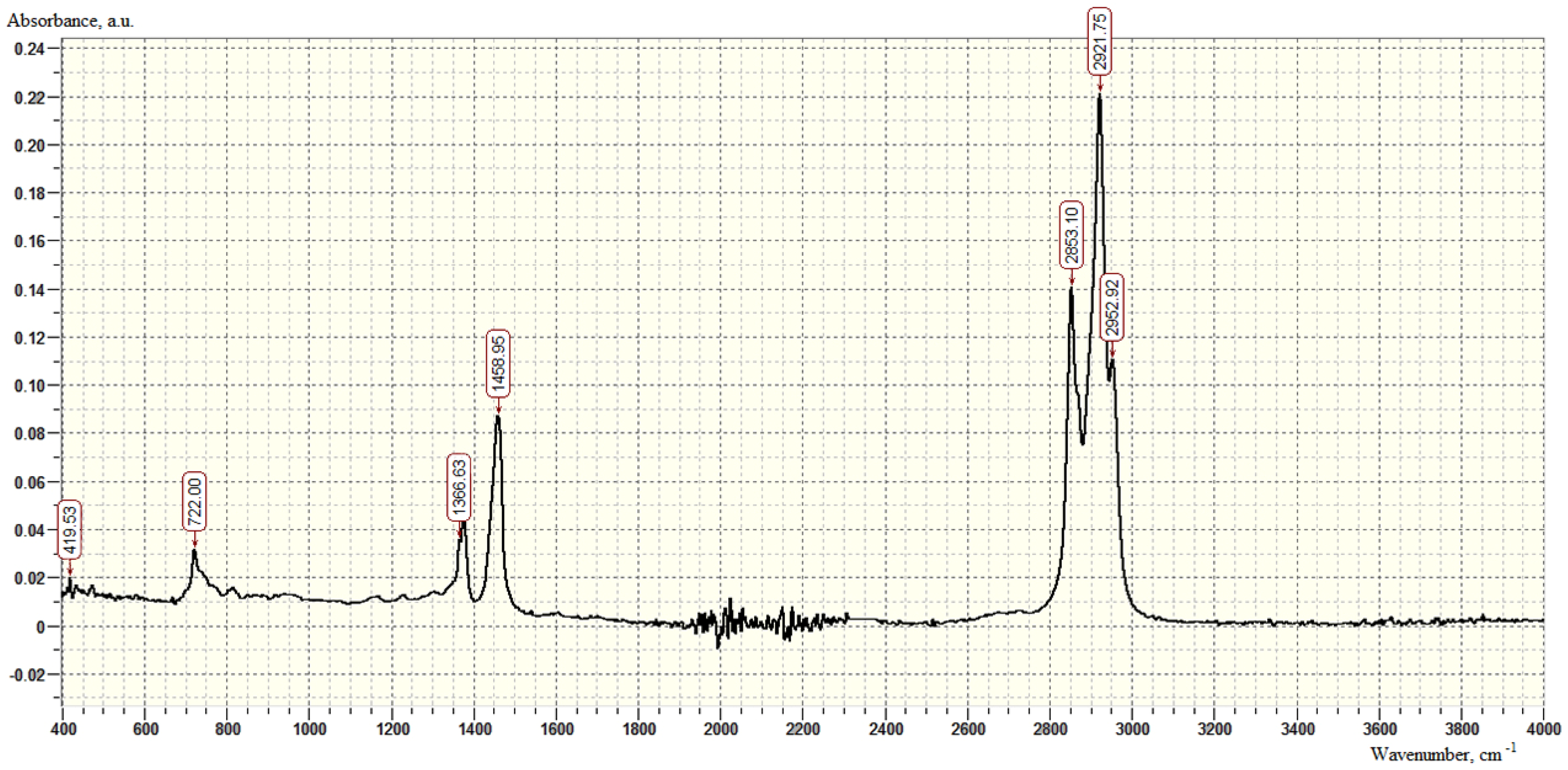
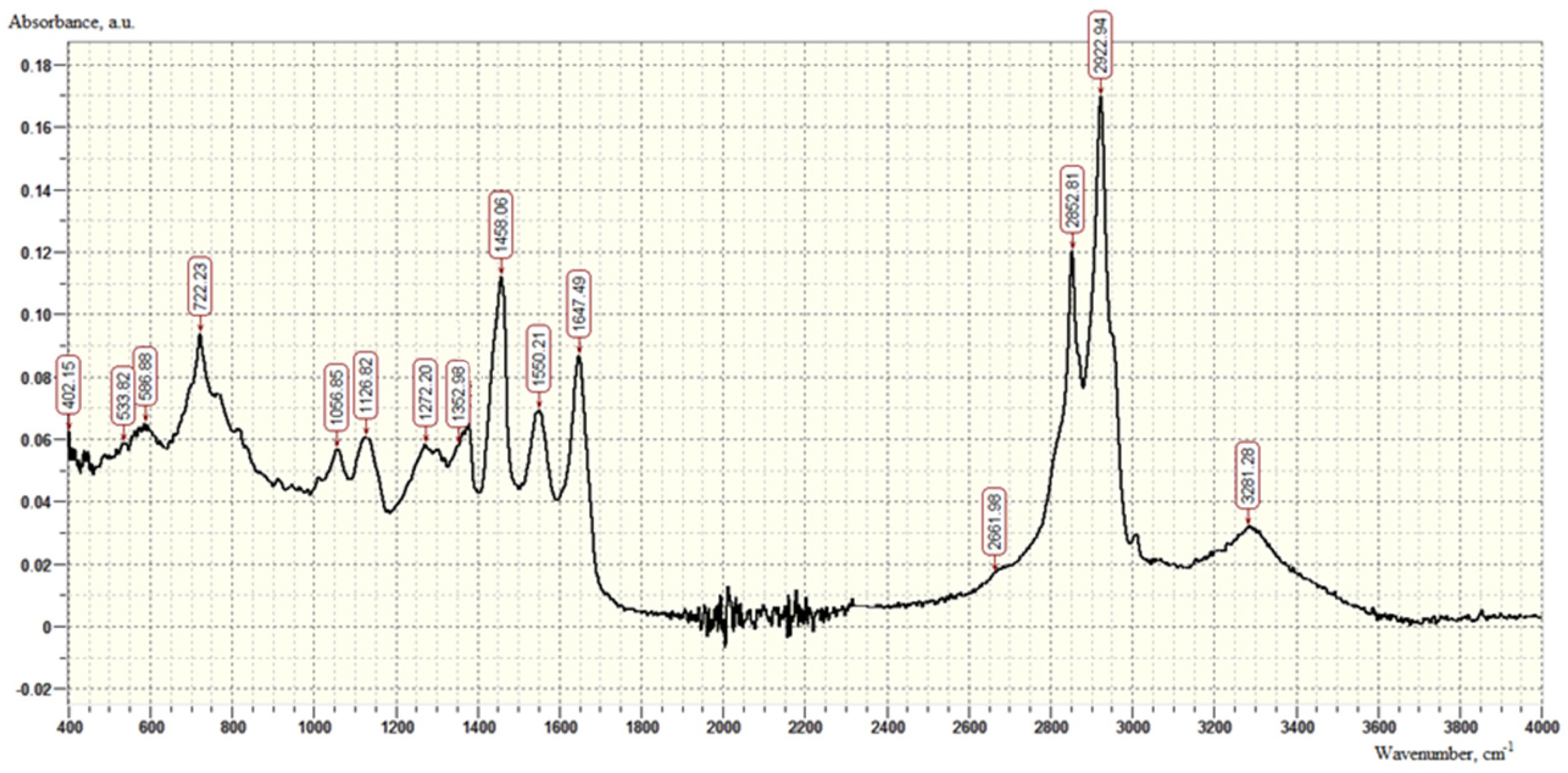

| SiO2 % | TiO2 % | Al2O3 % | Fe2O3 % | CaO % | MgO % | S % | Mn ppm | K2O % | Na2O % |
|---|---|---|---|---|---|---|---|---|---|
| 75.37 | 0.98 | 12.04 | 2.67 | 0.78 | 0.31 | - | 774 | 5.27 | 2.58 |
| SiO2 % | TiO2 % | Al2O3 % | Fe2O3 % | CaO % | MgO % | S % | Mn ppm | K2O % | Na2O % |
|---|---|---|---|---|---|---|---|---|---|
| 73.20 | 0.98 | 13.87 | 2.29 | 1.14 | 0.39 | - | 1051 | 4.63 | 3.50 |
| Substrate | Roughness, Ra, µm | Hardness, Rockwell Scale, HRC |
|---|---|---|
| Gray crushed stone | 4.3 | 69.2 |
| Red crushed stone | 4.3 | 76.8 |
| Factors | Level 1 | Level 2 | Level 3 | Level 4 | Level 5 |
|---|---|---|---|---|---|
| CAS-1, g/dm3 (x1) | 0 | 0.5 | 1.0 | 1.5 | 2.0 |
| CAG-4I, g/dm3 (x2) | 0 | 0.5 | 1.0 | 1.5 | 2.0 |
| X1 Factor Levels | X2 Factor Levels | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| 1 | y1 | y6 | y11 | y16 | y21 |
| 2 | y2 | y7 | y12 | y17 | y22 |
| 3 | y3 | y8 | y13 | y18 | y23 |
| 4 | y4 | y9 | y14 | y19 | y24 |
| 5 | y5 | y10 | y15 | y20 | y25 |
| X1 Factor Levels CAS-1, g/dm3 | Sample | X2 Factor Levels CAG-4I, g/dm3 | Sample |
|---|---|---|---|
| 0 | (y1 + y6 + y11 + y16 + y21)/5 | 0 | (y1 + y2 + y3 + y4 + y5)/5 |
| 0.5 | (y2 + y7 + y12 + y17 + y22)/5 | 0.5 | (y6 + y7 + y8 + y9 + y10)/5 |
| 1.0 | (y3 + y8 + y13 + y18 + y23)/5 | 1.0 | (y11 + y12 + y13 + y14 + y15)/5 |
| 1.5 | (y4 + y9 + y14 + y19 + y24)/5 | 1.5 | (y16 + y17 + y18 + y19 + y20)/5 |
| 2.0 | (y5 + y10 + y15 + y20 + y25)/5 | 2.0 | (y21 + y22 + y23 + y24 + y25)/5 |
| AG-4I | AAMDOR-10 | AS-1 | |||
|---|---|---|---|---|---|
| g, mN×dm3/m×g | R2 | g, mN×dm3/m×g | R2 | g, mN×dm3/m×g | R2 |
| 4.08 | 0.99 | 2.04 | 0.99 | 5.16 | 0.99 |
| Additive, g/dm3 | ||||||
|---|---|---|---|---|---|---|
| AS-1 | AG-4I | ΔσAS-1 | ΔσAG-4I | σc, mN/m | σex, mN/m | Δ, mN/m |
| 0.5 | 0.5 | 2.58 | 2.04 | 40.77 | 41.20 | 0.43 |
| 1.0 | 0.5 | 4.59 | 2.04 | 38.76 | 38.20 | −0.56 |
| 1.5 | 0.5 | 4.49 | 2.04 | 38.86 | 38.75 | −0.11 |
| 2.0 | 0.5 | 4.39 | 2.04 | 38.96 | 39.40 | 0.44 |
| 0.5 | 1.0 | 2.58 | 4.08 | 38.73 | 38.50 | −0.23 |
| 1.0 | 1.0 | 4.59 | 4.08 | 36.72 | 37.20 | 0.48 |
| 1.5 | 1.0 | 4.49 | 4.08 | 36.82 | 37.00 | 0.18 |
| 2.0 | 1.0 | 4.39 | 4.08 | 36.92 | 37.30 | 0.38 |
| 0.5 | 1.5 | 2.58 | 3.09 | 39.72 | 39.20 | −0.52 |
| 1.0 | 1.5 | 4.59 | 3.09 | 37.71 | 37.50 | −0.21 |
| 1.5 | 1.5 | 4.49 | 3.09 | 37.81 | 37.80 | −0.01 |
| 2.0 | 1.5 | 4.39 | 3.09 | 37.91 | 38.20 | 0.29 |
| 0.5 | 2.0 | 2.58 | −0.17 | 42.98 | 42.50 | −0.48 |
| 1.0 | 2.0 | 4.59 | −0.17 | 40.97 | 40.50 | −0.47 |
| 1.5 | 2 | 4.49 | −0.17 | 41.07 | 40.70 | −0.37 |
| 2 | 2 | 4.39 | −0.17 | 41.17 | 40.90 | −0.27 |
| C, g/dm3 | θ, ° | θ, ° | ||||
|---|---|---|---|---|---|---|
| Gray Crushed Stone | Red Crushed Stone | |||||
| AG-4I | AS-1 | AMDOR-10 | AG-4I | AS-1 | AMDOR-10 | |
| 0 | 125.66 | 125.66 | 125.66 | 126.87 | 126.87 | 126.87 |
| 0.5 | 116.22 | 117.81 | 119.83 | 122.20 | 120.21 | 123.63 |
| 1.0 | 112.50 | 115.10 | 116.80 | 118.30 | 116.30 | 122.60 |
| 1.5 | 113.11 | 115.63 | 117.32 | 118.82 | 118.22 | 123.94 |
| 2.0 | 114.32 | 117.84 | 119.35 | 119.71 | 120.44 | 124.41 |
| Additive | AG-4I | AMDOR-10 | AS-1 | |||
|---|---|---|---|---|---|---|
| The nature of crushed stone | a, dm3/g | R2 | a, dm3/g | R2 | a, dm3/g | R2 |
| Gray crushed stone | 0.19 | 0.93 | 0.12 | 0.95 | 0.15 | 0.91 |
| Red crushed stone | 0.13 | 0.99 | 0.09 | 0.99 | 0.16 | 0.98 |
| Gray Crushed Stone | Red Crushed Stone | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AG-4I | AS-1 | AMDOR-10 | AG-4I | AS-1 | AMDOR-10 | |||||||
| C, g/dm3 | Δσs, mN/m | α, % | Δσs, mN/m | α, % | Δσs, mN/m | α, % | Δσs, mN/m | α, % | Δσs, mN/m | α, % | Δσs, mN/m | α, % |
| 0 | −26.33 | −26.33 | −26.33 | −27.23 | −27.23 | −27.23 | ||||||
| 0.5 | −19.07 | 27.57 | −20.12 | 23.59 | −22.19 | 15.72 | −22.98 | 15.61 | −21.41 | 21.37 | −24.40 | 10.39 |
| 1.0 | −15.70 | 40.37 | −17.14 | 34.90 | −20.12 | 23.59 | −19.42 | 28.68 | −17.95 | 34.08 | −24.14 | 11.35 |
| 1.5 | −16.69 | 36.61 | −17.59 | 33.19 | −20.65 | 21.57 | −20.89 | 23.28 | −19.23 | 29.38 | −25.14 | 7.68 |
| 2.0 | −18.68 | 29.05 | −19.27 | 26.81 | −22.16 | 15.84 | −22.78 | 16.34 | −20.91 | 23.21 | −25.50 | 6.35 |
| Gray Crushed Stone | |||||||||
| CAG-4I, g/dm3 | θ, ° | CAS-1, g/dm3 | θ, ° | CAS-1, g/dm3 | θ, ° | CAS-1, g/dm3 | θ, ° | CAS-1, g/dm3 | θ, ° |
| 0 | 125.66 | 0.5 | 117.83 | 1.0 | 115.14 | 1.5 | 115.62 | 2.0 | 117.82 |
| 0.5 | 116.23 | 0.5 | 113.81 | 1.0 | 112.60 | 1.5 | 112.82 | 2.0 | 112.93 |
| 1.0 | 113.10 | 0.5 | 111.0 | 1.0 | 109.70 | 1.5 | 111.61 | 2.0 | 112.34 |
| 1.5 | 112.53 | 0.5 | 111.22 | 1.0 | 110.82 | 1.5 | 110.54 | 2.0 | 112.30 |
| 2.0 | 114.30 | 0.5 | 113.91 | 1.0 | 111.52 | 1.5 | 112.43 | 2.0 | 113.20 |
| Red Crushed Stone | |||||||||
| CAG-4I, g/dm3 | θ, ° | CAS-1, g/dm3 | θ, ° | CAS-1, g/dm3 | θ, ° | CAS-1, g/dm3 | θ, ° | CAS-1, g/dm3 | θ, ° |
| 0 | 126.87 | 0.5 | 120.23 | 1.0 | 116.33 | 1.5 | 118.20 | 2.0 | 120.42 |
| 0.5 | 122.22 | 0.5 | 120.15 | 1.0 | 118.52 | 1.5 | 118.92 | 2.0 | 119.24 |
| 1.0 | 118.35 | 0.5 | 115.61 | 1.0 | 112.81 | 1.5 | 113.21 | 2.0 | 114.43 |
| 1.5 | 118.81 | 0.5 | 117.22 | 1.0 | 115.14 | 1.5 | 114.51 | 2.0 | 115.65 |
| 2.0 | 119.72 | 0.5 | 118.84 | 1.0 | 116.51 | 1.5 | 117.30 | 2.0 | 117.82 |
| Gray Crushed Stone | ||||||||
| CAG-4I = 0.5 g/dm3 | CAG-4I = 1.0 g/dm3 | CAG-4I = 1.5 g/dm3 | CAG-4I = 2.0 g/dm3 | |||||
| CAS-1, g/dm3 | Δσs, mN/m | α, % | Δσs, mN/m | α, % | Δσs, mN/m | α, % | Δσs, mN/m | α, % |
| 0 | −26.33 | −26.33 | −26.33 | −26.33 | ||||
| 0.5 | −16.63 | 36.86 | −13.92 | 47.12 | −14.05 | 46.65 | −17.22 | 34.61 |
| 1.0 | −14.68 | 44.25 | −12.54 | 52.37 | −13.32 | 49.42 | −14.84 | 43.63 |
| 1.05 | −15.02 | 42.97 | −13.62 | 48.27 | −13.24 | 49.72 | −15.51 | 41.10 |
| 2.0 | −15.33 | 41.77 | −14.15 | 46.25 | −14.50 | 44.95 | −16.11 | 38.81 |
| Red Crushed Stone | ||||||||
| CAG-4I = 0.5 g/dm3 | CAG-4I = 1.0 g/dm3 | CAG-4I = 1.5 g/dm3 | CAG-4I = 2.0 g/dm3 | |||||
| CAS-1, g/dm3 | Δσs, mN/m | α, % | Δσs, mN/m | α, % | Δσs, mN/m | α, % | Δσs, mN/m | α, % |
| 0 | −26.33 | −26.33 | −26.33 | −26.33 | ||||
| 0.5 | −20.66 | 24.12 | −16.64 | 38.91 | −17.92 | 34.20 | −20.47 | 24.81 |
| 1.0 | −18.23 | 33.06 | −14.42 | 47.06 | −15.91 | 41.58 | −18.07 | 33.64 |
| 1.5 | −18.73 | 31.23 | −14.58 | 46.47 | −15.68 | 42.43 | −18.67 | 31.45 |
| 2.0 | −19.22 | 29.41 | −15.41 | 43.41 | −16.51 | 39.38 | −19.08 | 29.95 |
| CAG-4I | A, % | CAS-1 | A, % | CAMDOR-10 | A, % |
|---|---|---|---|---|---|
| Gray crushed stone | |||||
| 0.5 | 30.52 | 0.5 | 28.02 | 0.5 | 24.15 |
| 1.0 | 38.67 | 1.0 | 29.32 | 1.0 | 25.06 |
| 1.5 | 34.92 | 1.5 | 28.51 | 1.5 | 24.92 |
| 2.0 | 32.88 | 2.0 | 26.90 | 2.0 | 23.33 |
| Red crushed stone | |||||
| 0.5 | 20.98 | 0.5 | 26.55 | 0.5 | 20.82 |
| 1.0 | 24.25 | 1.0 | 28.76 | 1.0 | 21.06 |
| 1.5 | 22.67 | 1.5 | 24.21 | 1.5 | 20.32 |
| 2.0 | 20.56 | 2.0 | 23.02 | 2.0 | 20.00 |
| Gray Crushed Stone | |||||||||
| CAG-4I, g/dm3 | A, % | CAS-1, g/dm3 | A, % | CAS-1, g/dm3 | A, % | CAS-1, g/dm3 | A, % | CAS-1, g/dm3 | A, % |
| 0 | 0 | 0.5 | 28.02 | 1.0 | 29.32 | 1.5 | 28.51 | 2.0 | 26.90 |
| 0.5 | 30.52 | 0.5 | 36.52 | 1.0 | 43.43 | 1.5 | 42.01 | 2.0 | 43.34 |
| 1.0 | 38.67 | 0.5 | 44.89 | 1.0 | 52.28 | 1.5 | 43.47 | 2.0 | 39.26 |
| 1.5 | 34.92 | 0.5 | 47.87 | 1.0 | 43.76 | 1.5 | 43.15 | 2.0 | 39.82 |
| 2.0 | 32.88 | 0.5 | 41.03 | 1.0 | 48.75 | 1.5 | 45.78 | 2.0 | 37.91 |
| Red Crushed Stone | |||||||||
| CAG-4I, g/dm3 | A, % | CAS-1, g/dm3 | A, % | CAS-1, g/dm3 | A, % | CAS-1, g/dm3 | A, % | CAS-1, g/dm3 | A, % |
| 0 | 0 | 0.5 | 26.55 | 1.0 | 28.76 | 1.5 | 24.21 | 2.0 | 23.02 |
| 0.5 | 20.98 | 0.5 | 28.70 | 1.0 | 32.67 | 1.5 | 31.00 | 2.0 | 31.20 |
| 1.0 | 24.25 | 0.5 | 35.98 | 1.0 | 36.70 | 1.5 | 30.48 | 2.0 | 30.81 |
| 1.5 | 22.67 | 0.5 | 35.05 | 1.0 | 36.15 | 1.5 | 37.18 | 2.0 | 35.76 |
| 2.0 | 20.56 | 0.5 | 30.15 | 1.0 | 31.03 | 1.5 | 30.00 | 2.0 | 29.25 |
| CAS-1, g/dm3 | CAG-4I, g/dm3 | ||||
|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | |
| Gray Crushed Stone | |||||
| 0.0 | −0.583 | −0.442 | −0.392 | −0.383 | −0.412 |
| 0.5 | −0.466 | −0.404 | −0.362 | −0.358 | −0.405 |
| 1.0 | −0.424 | −0.384 | −0.337 | −0.355 | −0.367 |
| 1.5 | −0.432 | −0.388 | −0.368 | −0.350 | −0.381 |
| 2.0 | −0.466 | −0.389 | −0.379 | −0.379 | −0.394 |
| Red Crushed Stone | |||||
| 0.0 | −0.600 | −0.533 | −0.474 | −0.482 | −0.495 |
| 0.5 | −0.503 | −0.502 | −0.432 | −0.457 | −0.482 |
| 1.0 | −0.443 | −0.477 | −0.388 | −0.424 | −0.446 |
| 1.5 | −0.473 | −0.483 | −0.394 | −0.415 | −0.459 |
| 2.0 | −0.506 | −0.488 | −0.413 | −0.432 | −0.466 |
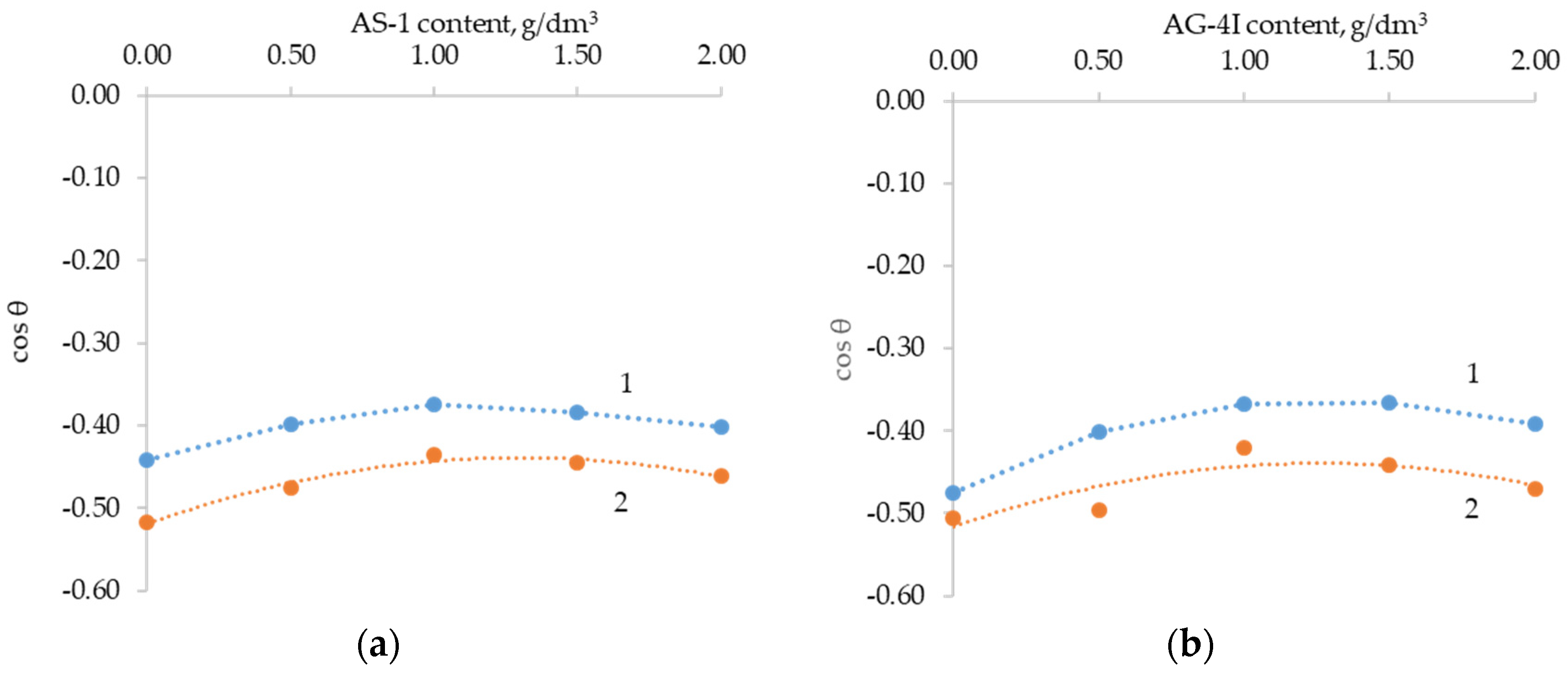
| CAS-1, g/dm3 | Gray Crushed Stone | Red Crushed Stone |
| 0.0 | −0.442 | −0.517 |
| 0.5 | −0.399 | −0.475 |
| 1.0 | −0.373 | −0.436 |
| 1.5 | −0.384 | −0.445 |
| 2.0 | −0.402 | −0.461 |
| CAG-4I, g/dm3 | Gray Crushed Stone | Red Crushed Stone |
| 0.0 | −0.474 | −0.505 |
| 0.5 | −0.401 | −0.497 |
| 1.0 | −0.368 | −0.420 |
| 1.5 | −0.365 | −0.442 |
| 2.0 | −0.392 | −0.470 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyuryagina, A.; Byzova, Y.; Ostrovnoy, K.; Demyanenko, A.; Lutsenko, A.; Shirina, T. Increasing the Adhesion of Bitumen to the Surface of Mineral Fillers through Modification with a Recycled Polymer and Surfactant Obtained from Oil Refining Waste. Polymers 2024, 16, 714. https://doi.org/10.3390/polym16050714
Dyuryagina A, Byzova Y, Ostrovnoy K, Demyanenko A, Lutsenko A, Shirina T. Increasing the Adhesion of Bitumen to the Surface of Mineral Fillers through Modification with a Recycled Polymer and Surfactant Obtained from Oil Refining Waste. Polymers. 2024; 16(5):714. https://doi.org/10.3390/polym16050714
Chicago/Turabian StyleDyuryagina, Antonina, Yuliya Byzova, Kirill Ostrovnoy, Alexandr Demyanenko, Aida Lutsenko, and Tatyana Shirina. 2024. "Increasing the Adhesion of Bitumen to the Surface of Mineral Fillers through Modification with a Recycled Polymer and Surfactant Obtained from Oil Refining Waste" Polymers 16, no. 5: 714. https://doi.org/10.3390/polym16050714
APA StyleDyuryagina, A., Byzova, Y., Ostrovnoy, K., Demyanenko, A., Lutsenko, A., & Shirina, T. (2024). Increasing the Adhesion of Bitumen to the Surface of Mineral Fillers through Modification with a Recycled Polymer and Surfactant Obtained from Oil Refining Waste. Polymers, 16(5), 714. https://doi.org/10.3390/polym16050714






