Selective Extraction of Diazepam and Its Metabolites from Urine Samples by a Molecularly Imprinted Solid-Phase Extraction (MISPE) Method
Abstract
1. Introduction
2. Experimental Procedure
2.1. Reagents and Materials
2.2. Synthesis of Molecularly Imprinted Polymer
2.3. Urine Sample Treatment
2.4. MISPE Procedure
2.5. Instrumental Analysis
3. Results and Discussion
3.1. Optimization of MISPE Procedure
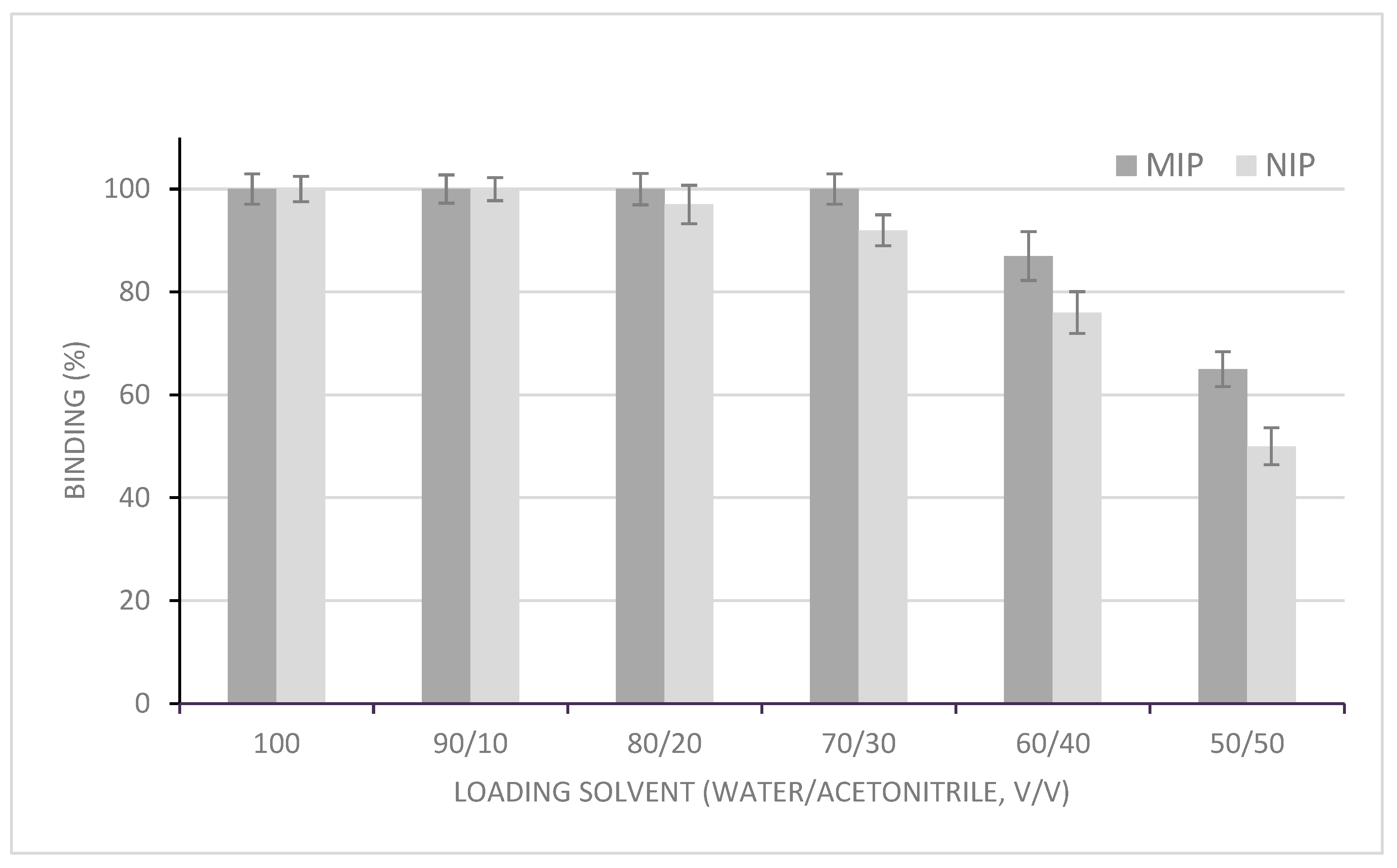
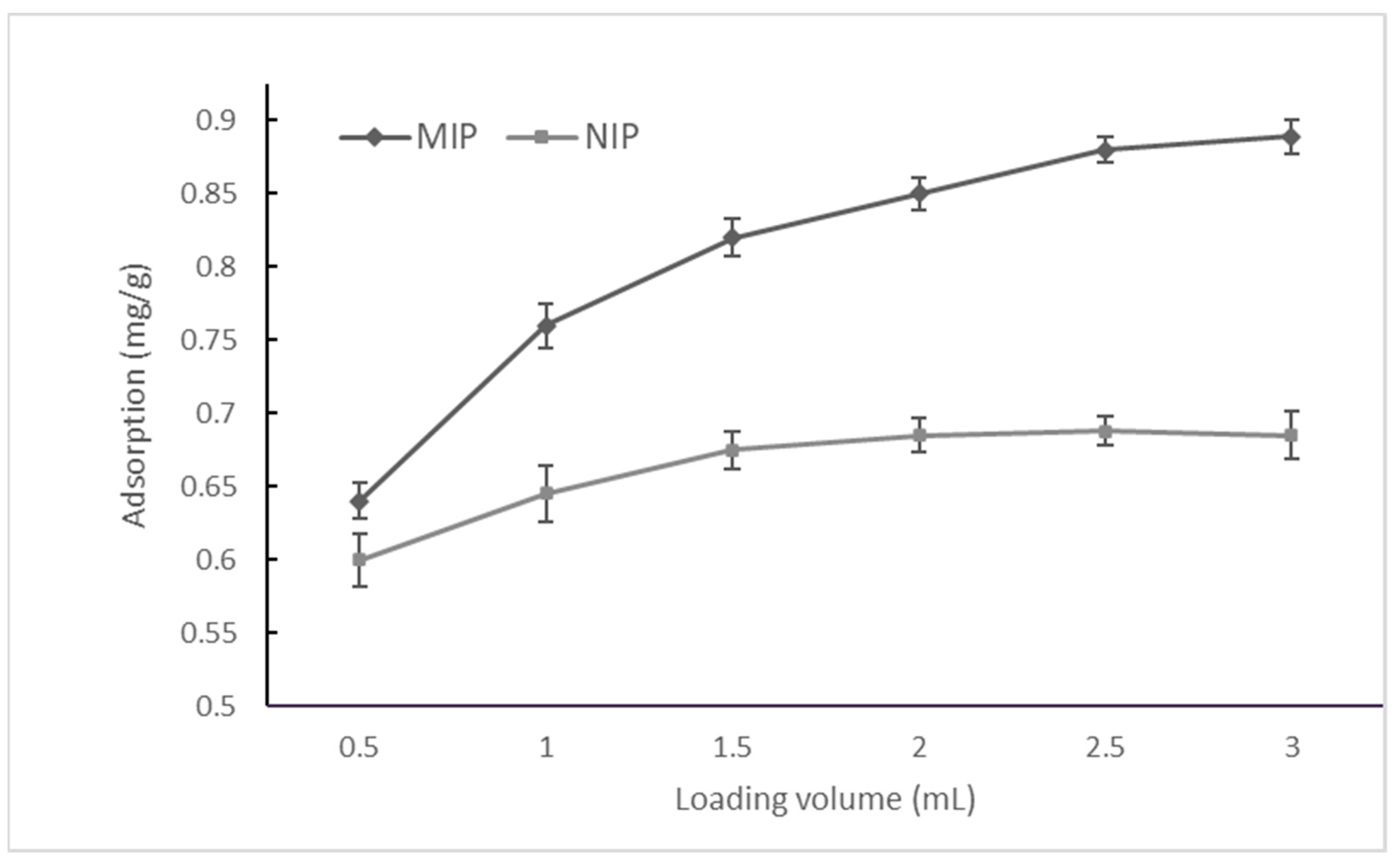
3.1.1. Loading Step
3.1.2. Washing Step
3.1.3. Elution Step
3.2. Imprinting Factor and Specific Adsorption
3.3. Analytical Performance
3.4. Selectivity of MISPE Procedure
3.5. Analysis of Urine Sample
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Maree, R.D.; Marcum, Z.A.; Saghafi, E.; Weiner, D.K.; Karp, J.F. A Systematic Review of Opioid and Benzodiazepine Misuse in Older Adults. Am. J. Geriatr. Psychiatry 2016, 24, 949–963. [Google Scholar] [CrossRef]
- Maust, D.T.; Lin, L.A.; Blow, F.C. Benzodiazepine Use and Misuse among Adults in the United States. Psychiatr. Serv. 2019, 70, 97–106. [Google Scholar] [CrossRef]
- Hood, S.D.; Norman, A.; Hince, D.A.; Melichar, J.K.; Hulse, G.K. Benzodiazepine dependence and its treatment with low dose flumazenil. Br. J. Clin. Pharmacol. 2014, 77, 285–294. [Google Scholar] [CrossRef]
- Longo, L.P.; Johnson, B. Addiction: Part I. Benzodiazepines—Side effects, abuse risk and alternatives. Am. Fam. Physician 2000, 61, 2121–2128. [Google Scholar]
- Diallo, S.; Bugni, E.; Senhadj-Raoul, F.; Gasdeblay, S.; Marot, D.; Dessalles, M.C.; Mahuzier, G. Chromatographic and spectral analytical data for the determination of benzodiazepine abuse in methadone maintenance program. Talanta 2001, 55, 721–732. [Google Scholar] [CrossRef]
- Tanaka, E. Toxicological interactions between alcohol and benzodiazepines. J. Toxicol.-Clin. Toxicol. 2002, 40, 69–75. [Google Scholar] [CrossRef]
- Kurzthaler, I.; Wambacher, M.; Golser, K.; Sperner, G.; Sperner-Unterweger, B.; Haidekker, A.; Pavlic, M.; Kemmler, G.; Fleischhacker, W.W. Alcohol and/or benzodiazepine use in injured road users. Hum. Psychopharmacol.-Clin. Exp. 2003, 18, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.W.; Holmgren, A.; Holmgren, P. High concentrations of diazepam and nordiazepam in blood of impaired drivers: Association with age, gender and spectrum of other drugs present. Forensic Sci. Int. 2004, 146, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pariente, A.; Dartigues, J.-F.; Benichou, J.; Letenneur, L.; Moore, N.; Fourrier-Reglat, A. Benzodiazepines and injurious falls in community dwelling elders. Drugs Aging 2008, 25, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Cheze, M.; Duffort, G.; Deveaux, M.; Pepin, G. Hair analysis by liquid chromatography-tandem mass spectrometry in toxicological investigation of drug-facilitated crimes: Report of 128 cases over the period June 2003 May 2004 in metropolitan Paris. Forensic Sci. Int. 2005, 153, 3–10. [Google Scholar] [CrossRef]
- Birkler, R.I.D.; Telving, R.; Ingemann-Hansen, O.; Charles, A.V.; Johannsen, M.; Andreasen, M.F. Screening analysis for medicinal drugs and drugs of abuse in whole blood using ultra-performance liquid chromatography time-of-flight mass spectrometry (UPLC-TOF-MS)-Toxicological findings in cases of alleged sexual assault. Forensic Sci. Int. 2012, 222, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, G.A.; Gerhard, T.; Keyes, K.; Hasin, D.; Cerda, M.; Olfson, M. Association of Benzodiazepine Treatment for Sleep Disorders with Drug Overdose Risk among Young People. JAMA Netw. Open 2022, 5, e2243215. [Google Scholar] [CrossRef]
- Bachhuber, M.A.; Hennessy, S.; Cunningham, C.O.; Starrels, J.L. Increasing Benzodiazepine Prescriptions and Overdose Mortality in the United States, 1996–2013. Am. J. Public Health 2016, 106, 686–688. [Google Scholar] [CrossRef]
- Abrahamsson, T.; Berge, J.; Ojehagen, A.; Hakansson, A. Benzodiazepine, z-drug and pregabalin prescriptions and mortality among patients in opioid maintenance treatment—A nation-wide register-based open cohort study. Drug Alcohol Depend. 2017, 174, 58–64. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Sanchis-Gomar, F.; Lippi, G. Benzodiazepines consumption may have increased during the COVID-19 pandemic. J. Affect. Disord. 2022, 314, 124–125. [Google Scholar] [CrossRef] [PubMed]
- de Dios, C.; Fernandes, B.S.; Whalen, K.; Bandewar, S.; Suchting, R.; Weaver, M.F.; Selvaraj, S. Prescription fill patterns for benzodiazepine and opioid drugs during the COVID-19 pandemic in the United States. Drug Alcohol Depend. 2021, 229, 109176. [Google Scholar] [CrossRef]
- Campitelli, M.A.; Bronskill, S.E.; Maclagan, L.C.; Harris, D.A.; Cotton, C.A.; Tadrous, M.; Gruneir, A.; Hogan, D.B.; Maxwell, C.J. Comparison of Medication Prescribing before and after the COVID-19 Pandemic Among Nursing Home Residents in Ontario, Canada. JAMA Netw. Open 2021, 4, e2118441. [Google Scholar] [CrossRef]
- Zaki, N.; Brakoulias, V. The impact of COVID-19 on benzodiazepine usage in psychiatric inpatient units. Australas. Psychiatry 2022, 30, 334–337. [Google Scholar] [CrossRef]
- Arnhard, K.; Schmid, R.; Kobold, U.; Thiele, R. Rapid detection and quantification of 35 benzodiazepines in urine by GC-TOF-MS. Anal. Bioanal. Chem. 2012, 403, 755–768. [Google Scholar] [CrossRef]
- de Bairros, A.V.; de Almeida, R.M.; Pantaleao, L.; Barcellos, T.; Moura e Silva, S.; Yonamine, M. Determination of low levels of benzodiazepines and their metabolites in urine by hollow-fiber liquid-phase microextraction (LPME) and gas chromatography-mass spectrometry (GC-MS). J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2015, 975, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Švidrnoch, M.; Boráňová, B.; Tomková, J.; Ondra, P.; Maier, V. Simultaneous determination of designer benzodiazepines in human serum using non-aqueous capillary electrophoresis–tandem mass spectrometry with successive multiple ionic–polymer layer coated capillary. Talanta 2018, 176, 69–76. [Google Scholar] [CrossRef]
- Salomone, A.; Gerace, E.; Brizio, P.; Gennaro, M.C.; Vincenti, M. A fast liquid chromatography-tandem mass spectrometry method for determining benzodiazepines and analogues in urine. Validation and application to real cases of forensic interest. J. Pharm. Biomed. Anal. 2011, 56, 582–591. [Google Scholar] [CrossRef]
- Montenarh, D.; Wernet, M.P.; Hopf, M.; Maurer, H.H.; Schmidt, P.H.; Ewald, A.H. Quantification of 33 antidepressants by LC-MS/MS-comparative validation in whole blood, plasma, and serum. Anal. Bioanal. Chem. 2014, 406, 5939–5953. [Google Scholar] [CrossRef]
- Wachełko, O.; Szpot, P.; Tusiewicz k Nowak, K.; Chłopaś-Konowałek, A.; Zawadzki, M. An ultra-sensitive UHPLC-QqQ-MS/MS method for determination of 54 benzodiazepines (pharmaceutical drugs, NPS and metabolites) and z-drugs in biological samples. Talanta 2023, 251, 123816. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, P.; Lago, M.; Alvarez, I.; Carro, A.M.; Lorenzo, R.A. Chromatographic determination of benzodiazepines in vitreous humor after microwave-assisted extraction. Anal. Methods 2013, 5, 4999–5004. [Google Scholar] [CrossRef]
- Rezaei, F.; Yamini, Y.; Moradi, M.; Daraei, B. Supramolecular solvent-based hollow fiber liquid phase microextraction of benzodiazepines. Anal. Chim. Acta 2013, 804, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Shahraki, S.; Ahmar, H.; Nejati-Yazdinejad, M. Electrochemical determination of nitrazepam by switchable solvent-based liquid-liquid microextraction combined with differential pulse voltammetry. Microchem. J. 2018, 142, 229–235. [Google Scholar] [CrossRef]
- Herrera-Chacon, A.; Torabi, F.; Faridbod, F.; Ghasemi, J.B.; Gonzalez-Calabuig, A.; del Valle, M. Voltammetric Electronic Tongue for the Simultaneous Determination of Three Benzodiazepines. Sensors 2019, 19, 5002. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, A.M.G.; Fernandez Hernando, P.; Durand Alegria, J.S. A rapid fluorimetric screening method for the 1,4-benzodiazepines: Determination of their metabolite oxazepam in urine. Anal. Chim. Acta 2007, 591, 112–115. [Google Scholar] [CrossRef]
- Furugen, A.; Nishimura, A.; Kobayashi, M.; Umazume, T.; Narumi, K.; Iseki, K. Quantification of eight benzodiazepines in human breastmilk and plasma by liquid-liquid extraction and liquid-chromatography tandem mass spectrometry: Application to evaluation of alprazolam transfer into breastmilk. J. Pharm. Biomed. Anal. 2019, 168, 83–93. [Google Scholar] [CrossRef]
- Fernandez, P.; Vazquez, C.; Lorenzo, R.A.; Carro, A.M.; Bermejo, A.M. Development of a liquid chromatographic method for the simultaneous determination of six benzodiazepines in human plasma after solid-phase extraction. Anal. Lett. 2010, 43, 1075–1084. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, P.; Jin, Q.; Hu, Z.; Wang, J. Multi-residue analysis of sedative drugs in human plasma by ultra-high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2018, 1072, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Du, L.; Zhang, Z.; Li, N.; Wang, M.; Ren, Q. A poly(N,N-dimethylaminoethyl methacrylate-co-ethylene glycol dimethacrylate) monolith for direct solid-phase extraction of benzodiazepines from undiluted human urine. Anal. Methods 2020, 12, 3924–3932. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.M.; Nogueira, J.M.F. High throughput bar adsorptive microextraction: A novel cost-effective tool for monitoring benzodiazepines in large number of biological samples. Talanta 2019, 199, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, R.; Salami, M.; Seidi, S. A silica fiber coated with a ZnO-graphene oxide nanocomposite with high specific surface for use in solid phase microextraction of the antiepileptic drugs diazepam and oxazepam. Microchim. Acta 2018, 185, 312. [Google Scholar] [CrossRef]
- de Carvalho Abrao, L.C.; Figueiredo, E.C. A new restricted access molecularly imprinted fiber for direct solid phase microextraction of benzodiazepines from plasma samples. Analyst 2019, 144, 4320–4330. [Google Scholar] [CrossRef]
- Buzancic, I.; Pejakovic, T.I.; Hadziabdic, M.O. A Need for Benzodiazepine Deprescribing in the COVID-19 Pandemic: A Cohort Study. Pharmacy 2022, 10, 120. [Google Scholar] [CrossRef]
- Lennestal, R.; Lakso, H.-A.; Nilsson, M.; Mjorndal, T. Urine monitoring of diazepam abuse—New intake or not? J. Anal. Toxicol. 2008, 32, 402–407. [Google Scholar] [CrossRef][Green Version]
- Umezawa, H.; Lee, X.-P.; Arima, Y.; Hasegawa, C.; Marumo, A.; Kumazawa, T.; Sato, K. Determination of diazepam and its metabolites in human urine by liquid chromatography/tandem mass spectrometry using a hydrophilic polymer column. Rapid Commun. Mass Spectrom. 2008, 22, 2333–2341. [Google Scholar] [CrossRef]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerda, V. Solid-phase extraction of organic compounds: A critical review (Part II). Trac-Trends Anal. Chem. 2016, 80, 655–667. [Google Scholar] [CrossRef]
- Hansen, F.A.; Pedersen-Bjergaard, S. Emerging Extraction Strategies in Analytical Chemistry. Anal. Chem. 2020, 92, 2–15. [Google Scholar] [CrossRef]
- Beltran, A.; Borrull, F.; Cormack, P.A.G.; Marce, R.M. Molecularly-imprinted polymers: Useful sorbents for selective extractions. Trac-Trends Anal. Chem. 2010, 29, 1363–1375. [Google Scholar] [CrossRef]
- Kriz, D.; Ramstrom, O.; Mosbach, K. Molecular imprinting—New possibilities for sensor technology. Anal. Chem. 1997, 69, A345–A349. [Google Scholar] [CrossRef]
- Whitcombe, M.J.; Vulfson, E.N. Imprinted polymers. Adv. Mater. 2001, 13, 467–478. [Google Scholar] [CrossRef]
- Ariffin, M.M.; Miller, E.I.; Cormack, P.A.G.; Anderson, R.A. Molecularly imprinted solid-phase extraction of diazepam and its metabolites from hair samples. Anal. Chem. 2007, 79, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Hasanah, A.N.; Soni, D.; Pratiwi, R.; Rahayu, D.; Megantara, S.; Mutakin. Synthesis of Diazepam-Imprinted Polymers with Two Functional Monomers in Chloroform Using a Bulk Polymerization Method. J. Chem. 2020, 2020, 7282415. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Susanti, I.; Marcellino, M.; Maranata, G.J.; Saputri, F.A.; Pratiwi, R. Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol. Open Chem. 2021, 19, 604–613. [Google Scholar] [CrossRef]
- Bakhshi, A.; Daryasari, A.P.; Soleimani, M. A Molecularly Imprinted Polymer as the Adsorbent for the Selective Determination of Oxazepam in Urine and Plasma Samples by High-Performance Liquid Chromatography with Diode Array Detection. J. Anal. Chem. 2021, 76, 1414–1421. [Google Scholar] [CrossRef]
- Varenne, F.; Kadhirvel, P.; Bosman, P.; Renault, L.; Combes, A.; Pichon, V. Synthesis and characterization of molecularly imprinted polymers for the selective extraction of oxazepam from complex environmental and biological samples. Anal. Bioanal. Chem. 2022, 414, 451–463. [Google Scholar] [CrossRef]
- Meatherall, R. Optimal enzymatic-hydrolysis of urinary benzodiazepine conjugates. J. Anal. Toxicol. 1994, 18, 382–384. [Google Scholar] [CrossRef]
- Turiel, E.; Martin-Esteban, A. Molecularly imprinted polymers for sample preparation: A review. Anal. Chim. Acta 2010, 668, 87–99. [Google Scholar] [CrossRef]
- He, C.; Long, Y.; Pan, J.; Li, K.; Liu, F. Application of molecularly imprinted polymers to solid-phase extraction of analytes from real samples. J. Biochem. Biophys. Methods 2007, 70, 133–150. [Google Scholar] [CrossRef]
- Bui, B.T.S.; Haupt, K. Molecularly imprinted polymers: Synthetic receptors in bioanalysis. Anal. Bioanal. Chem. 2010, 398, 2481–2492. [Google Scholar] [CrossRef]
- Su, Q.; Zeng, C.; Tang, Y.; Finlow, D.E.; Cao, M. Evaluation of diazepam-molecularly imprinted microspheres for the separation of diazepam and its main metabolite from body fluid samples. J. Chromatogr. Sci. 2012, 50, 608–614. [Google Scholar] [CrossRef][Green Version]
- Bazmi, E.; Behnoush, B.; Akhgari, M.; Bahmanabadi, L. Quantitative analysis of benzodiazepines in vitreous humor by high-performance liquid chromatography. SAGE Open Med. 2016, 4, 2050312116666243. [Google Scholar] [CrossRef]
- Saldanhaa, G.A.; Bezerra, A.L.; Fonseca, A.M.; Valle, A. Analysis of benzodiazepines in plasma samples by dllme and lc-dad: Critical aspects, flaws and issues encountered—A discussion. Quim. Nova. 2022, 45, 867–874. [Google Scholar] [CrossRef]
- Su, H.L.; Kao, W.C.; Lin, K.W.; Lee, C.Y.; Hsieh, Y.Z. 1-Butyl-3-methylimidazolium-based ionic liquids and an anionic surfactant: Excellent background electrolyte modifiers for the analysis of benzodiazepines through capillary electrophoresis. J. Chromatogr. A 2010, 1217, 2973–2979. [Google Scholar] [CrossRef]
- Mandrioli, R.; Mercolini, L.; Raggi, M.A. Benzodiazepine Metabolism: An Analytical Perspective. Curr. Drug Metab. 2008, 9, 827–844. [Google Scholar] [CrossRef] [PubMed]
- Chouinard, G.; Lefko-Singh, K.; Teboul, E. Metabolism of anxiolytics and hypnotics: Benzodiazepines, buspirone, zoplicone, and zolpidem. Cell. Mol. Neurobiol. 1999, 19, 533–552. [Google Scholar] [CrossRef] [PubMed]


| Analyte | Linear Equation | R | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|---|
| OZ | y = 0.219x + 0.0012 | 0.9984 | 16.2 | 53.5 |
| TZ | y = 0.4179x − 0.0026 | 0.9982 | 21.1 | 63.9 |
| NZ | y = 0.4576x + 0.002 | 0.9993 | 13.5 | 44.5 |
| DZP | y = 0.4823x − 4 × 10−5 | 0.9985 | 21.0 | 69.3 |
| Analyte | 1000 ng/mL | 250 ng/mL | ||||||
|---|---|---|---|---|---|---|---|---|
| MIP | NIP | MIP | NIP | |||||
| Recovery | RSD | Recovery | RSD | Recovery | RSD | Recovery | RSD | |
| OZ | 91.5 | 5.7 | 67.3 | 14.2 | 92.4 | 2.4 | 66.5 | 8.8 |
| TZ | 90.8 | 6.0 | 72.1 | 10.4 | 91.4 | 5.3 | 74.8 | 3.7 |
| NZ | 89.1 | 7.9 | 72.9 | 10.1 | 89.5 | 8.1 | 70.1 | 6.6 |
| DZP | 94.0 | 4.7 | 77.7 | 8.9 | 99.4 | 4.3 | 76.7 | 3.7 |
| Analyte | Recovery (%) | RSD (%) |
|---|---|---|
| OZ | 91.5 | 5.7 |
| TZ | 90.8 | 6.0 |
| NZ | 89.1 | 7.9 |
| DZP | 94.0 | 4.7 |
| BRZ | 50.0 | 2.1 |
| HZ | 69.1 | 2.0 |
| TTZ | 63.4 | 1.8 |
| Sample Matrix | Method of Analysis | Template | Recovery (%) | Linear Range (ng/mL) | Det. Limit (ng/mL) | Quant. Limit (ng/mL) | Ref |
|---|---|---|---|---|---|---|---|
| Blood serum | MISPE + HPLC-UV | DZP | DZP 95.3 | - | DZP 3.5 | [46] | |
| Blood serum | MISPE + HPLC-UV | DZP | DZP 105.6 | - | - | - | [47] |
| Urine | MIP + HPLC-DAD | OZ | OZ 88 | 2–600 | OZ 0.5 | OZ 2.0 | [48] |
| Urine | MISPE + HPLC-MS | NZ | - | - | - | OZ 0.357 | [49] |
| Urine | MISPE + HPLC-DAD | DZP | DZP 87.2–87.8 NZ 88.6–90.4 | 50–1600 | DZP 21.5 NZ 24.5 | [54] | |
| Vitreous humor | LLE + HPLC-DAD | - | DZP 83.8 | 30–3000 | DZP 30.0 | DZP 100.0 | [55] |
| Plasma | DLLE + HPLC-DAD | - | - | 50–1500 | DZP 50.0 NZ 50.0 | DZP 60.0 NZ 60.0 | [56] |
| Urine | Oasis MCX-SPE + CE-DAD | - | DZP 78.0 | 10,000–150,000 | DZP 2740 | DZP 9140 | [57] |
| Urine | MISPE + HPLC-DAD | OZ | OZ 91.5–92.4 TZ 90.8–91.4 NZ 89.1–89.5 DZP 94.0–99.4 | 10–1500 | OZ 16.2 TZ 21.1 NZ 13.5 DZP 21.0 | OZ 53.5 TZ 63.9 NZ 44.5 DZP 69.3 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil Tejedor, A.M.; Bravo Yagüe, J.C.; Paniagua González, G.; Garcinuño Martínez, R.M.; Fernández Hernando, P. Selective Extraction of Diazepam and Its Metabolites from Urine Samples by a Molecularly Imprinted Solid-Phase Extraction (MISPE) Method. Polymers 2024, 16, 635. https://doi.org/10.3390/polym16050635
Gil Tejedor AM, Bravo Yagüe JC, Paniagua González G, Garcinuño Martínez RM, Fernández Hernando P. Selective Extraction of Diazepam and Its Metabolites from Urine Samples by a Molecularly Imprinted Solid-Phase Extraction (MISPE) Method. Polymers. 2024; 16(5):635. https://doi.org/10.3390/polym16050635
Chicago/Turabian StyleGil Tejedor, Ana María, Juan Carlos Bravo Yagüe, Gema Paniagua González, Rosa María Garcinuño Martínez, and Pilar Fernández Hernando. 2024. "Selective Extraction of Diazepam and Its Metabolites from Urine Samples by a Molecularly Imprinted Solid-Phase Extraction (MISPE) Method" Polymers 16, no. 5: 635. https://doi.org/10.3390/polym16050635
APA StyleGil Tejedor, A. M., Bravo Yagüe, J. C., Paniagua González, G., Garcinuño Martínez, R. M., & Fernández Hernando, P. (2024). Selective Extraction of Diazepam and Its Metabolites from Urine Samples by a Molecularly Imprinted Solid-Phase Extraction (MISPE) Method. Polymers, 16(5), 635. https://doi.org/10.3390/polym16050635







